CHAPTER 318 Diagnosis and Management of Thoracic Spine Fractures
The thoracic spine between T1 and T10 is unique in that considerable stiffness and stability are conferred by the connecting ribs, sternum, and chest wall musculature. The clinical notion that thoracic spinal trauma requires high-velocity impact and tremendous force has been buttressed by biomechanical studies demonstrating that the rib cage alone augments the spine’s load-bearing capacity by twofold to threefold.1 Nevertheless, this added osteoligamentous stabilization is offset by increased vulnerability to neurological deficits because the thoracic spine exhibits the narrowest canal-to-cord relationship and a relatively sparse blood supply to the spinal cord.2 Furthermore, the thoracic spinal cord, when compared with other spinal regions, has the poorest prognosis for functional recovery.
Historical Perspective
Surgical management of thoracic spine fractures before introduction of the Harrington internal fixation system in the 1960s was limited to laminectomy and often associated with neurological deterioration secondary to spinal cord manipulation or increased instability.3 However, the success of surgical treatment of thoracic fractures has grown in parallel with technologic advancements in spinal instrumentation. Krengel and colleagues were among the first to demonstrate that early stabilization plus decompression of incomplete thoracic spinal cord injuries was associated with improved neurological function.4 Further data have suggested that early internal fixation may decrease hospital stay, prevent bony deformity, and accelerate transfer to rehabilitation.5
The past 2 decades have witnessed a shift away from hook constructs as pedicle screw systems have been shown to be a safe, reliable, and biomechanically superior choice for internal fixation in the thoracic spine.6–8 The presumed clinical benefit of operative intervention continues to be the impetus behind surgical intervention despite a lack of class I evidence. As the emphasis on evidence-based clinical decision making continues, new methods of comparison have been championed to supplant the costly, lengthy, and often impractical randomized trial in the traumatic spine patient population. Recently, a novel parallel cohort study with blinded inclusion based on clinical equipoise was used to compare operative and nonoperative treatment strategies for traumatic lumbar and thoracic fractures; a trend toward better neurological recovery, although not reaching statistical significance, was found in the operative group.9
Epidemiology
Thoracic spine fractures are relatively rare in comparison to fractures of the cervical, lumbar, and thoracolumbar regions; in a large multicenter study, only 16% of spinal fractures occurred in the T1-10 region.10 A wedge compression fracture is the most common thoracic spine injury, and multiple noncontiguous fractures in the thoracic spine are not rare. Spinal cord injury accompanies about 10% of thoracic vertebral body injuries, as opposed to a 39% incidence of concurrent spinal cord injury seen with cervical injuries.11 Approximately 15% of all spinal cord injuries occur in the thoracic spinal cord, and 17% of upper thoracic spine injuries cause paraplegia.12–14
The midthoracic spine is the most rigid and consequently at increased risk for fracture; the upper thoracic and T10 levels are less frequently injured, as noted later. The high-energy impacts causing thoracic spine fractures can generate severe concomitant injuries, including dissection or rupture of the aorta, intra-abdominal solid organ injury, hemothorax, pulmonary contusion, esophageal perforation, thoracic duct injury with chylothorax, and tracheal injury (Fig. 318-1).15–17 Thoracic fracture-dislocations are very unstable and lead to complete paraplegia in 80% of patients; however, there have been several well-documented reports of neurologically intact patients in whom accompanying bilateral pedicular fractures caused autodecompression and spared the spinal cord.18
Anatomic and Biomechanical Considerations
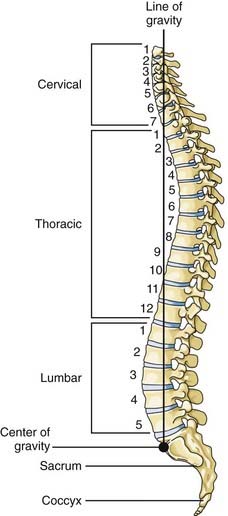
The thoracic spine consists of 12 vertebral bodies aligned in a prominent kyphotic curvature. These load-bearing elements of the spinal column are 2 to 3 mm shorter in anterior body height than in posterior body height.19 This is the primary cause of the normal kyphotic curve. There is also a dorsal arch that consists of relatively small pedicles, transverse processes, articulating facets, broad laminae, and a downward-pointing spinous process. The center of gravity of an upright individual falls through the anterior body of T1, passes anterior to the thoracic spine, and falls through the midbody of T12 (Fig. 318-2).
The thoracic spine is connected to the rib cage by body and transverse process articulations to individual ribs. The rib cage restricts the motion of the thoracic spinal column—especially in extension, which is reduced by 70%. The effects of the rib cage on flexion and lateral rotation are less prominent. The rib cage also adds stiffness to the spine, and experimental data have demonstrated a fourfold increase in tolerance of the spinal column to compression with an intact rib cage. Stabilization of the thoracic spine by the rib cage results in greater force being required to disrupt the spinal column at these levels than in other regions of the spinal column.20
The net result of the aforementioned anatomic considerations is that the mean of the range of flexion and extension at each segment in the upper thoracic spine is 4 degrees whereas that of the midthoracic spine is 6 degrees. The range of lateral bending is 6 degrees of motion per segment, whereas the range of axial rotation is 8 to 9 degrees.21 The rigidity of the midportion of the thoracic spine apparently places the midthoracic levels at increased risk for fracture. The upper thoracic and T10 levels are the most infrequently injured levels of the thoracic spine because they are protected by the adjacent, flexible cervical and thoracolumbar junctions (Fig. 318-3).22
Spinal Instability
Clinical instability is loss of the ability of the spine to maintain its pattern of displacement under physiologic loads so that there is no initial or additional neurological deficit, no major deformity, and no incapacitating pain.23 Although this definition is very inclusive, it has rather limited utility to a clinician faced with an acutely injured patient.
Various authors have tried to define stability and then recommend treatment based on the presumed injury mechanism. In 1949, Nicoll attempted to define stable versus unstable fractures by using an anatomic classification based on analysis of a series of 166 fractures or fracture-dislocations of the thoracolumbar spine. In his view, the major determinant of stability was the integrity of the interspinous ligament.24
Holdsworth introduced the first modern classification of fractures and attempted to use radiography to identify specific patterns of spinal fractures associated with the development of early as well as late instability.25 This classification scheme is based on the two-column theory of spinal column stability and is essentially a biomechanical interpretation of the injury mechanisms determined by a review of plain radiography. The integrity of the posterior bony elements and the posterior ligamentous complex, including the interspinous and supraspinous ligaments and the ligamentum flavum, is the major determinant of stability. A flexion-rotation injury is the most unstable of spinal column injuries in this classification scheme because both the bony and ligamentous elements of the posterior spinal column are compromised. Holdsworth acknowledged that this system was flawed and that it did not predict the presence of an unstable subset of compression fractures.25
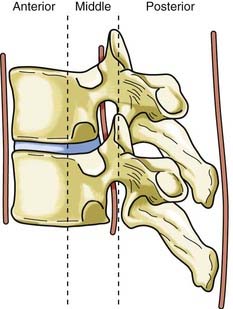
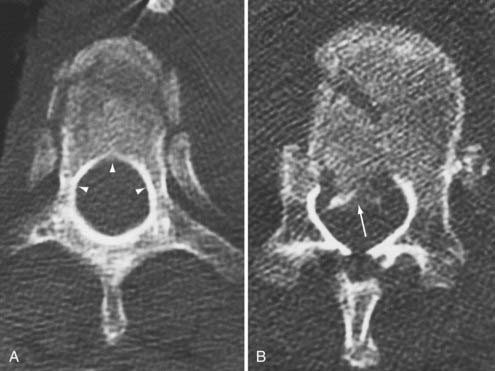
Disruption of the posterior ligamentous structures alone, as posited by Nicoll and Holdsworth, is an insufficient view of instability of the spinal column. Denis modified the two-column theory of Holdsworth by dividing the anterior column into an anterior and a middle column, the latter consisting of the posterior longitudinal ligament and the posterior third of the vertebral body (Fig. 318-4).26 He used computed tomography (CT) of the spinal column injury to establish the integrity of the middle column. The more common anterior wedge compression fracture is distinguished by continuity of the posterior cortical rim from one pedicle medially over the posterior vertebral body to the opposite pedicle. If what appears to be a compression fracture also features disruption of the posterior vertebral body, it is actually a burst fracture (Fig. 318-5). The integrity of the middle column became the basis of a new classification of spinal stability, with injury to the anterior and the middle or the posterior and the middle columns resulting in an “unstable” fracture.
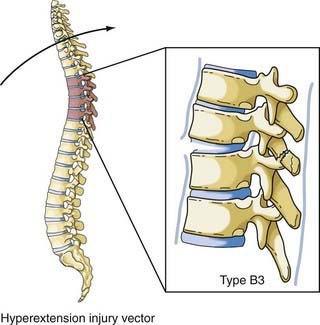
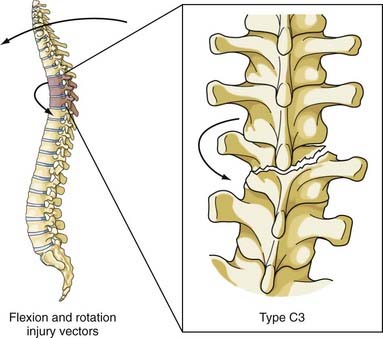
The most comprehensive classification scheme was published in 1994 by Magerl and associates, whose initial report described 53 distinct fracture patterns.22 The classification system of Magerl is based on the mechanism of failure of the spinal column and is accepted, with modifications, as a research tool by members of the major spine societies. The main categories in the Magerl system are type A, compression injuries; type B, distraction injuries; and type C, multidirectional torque injuries with translation (Fig. 318-6).27 These types are ordered in terms of increasing severity of the resultant spinal column injury and subdivided into groups according to the injury mechanism. The division into three groups is based on the morphologic features of the injury and the severity of the injury as it progresses through both the types and the groups, the most stable being an A1 fracture and the least stable being C3. Although the original classification contains further subdivisions, the simplified system reported by Gertzbein stops at the 3-by-3 grid (Fig. 318-6).27
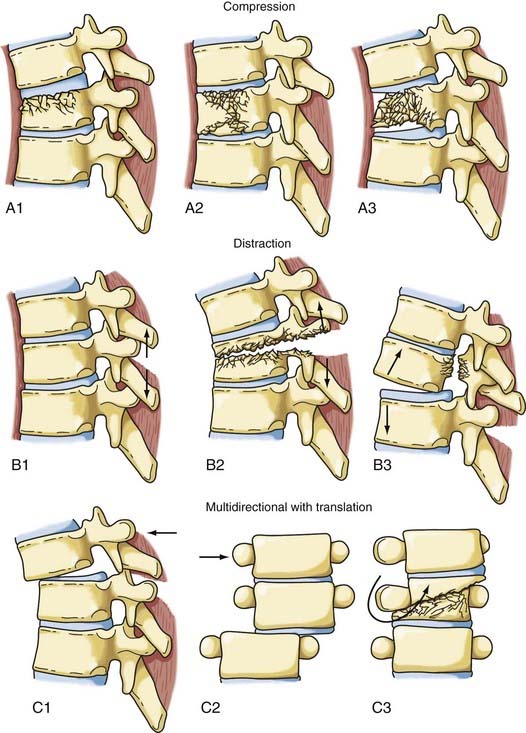
FIGURE 318-6 Fracture classification system as proposed by Magerl and coworkers and modified by Gertzbein. Fractures are divided into types according to major injury force: A1 to A3, compression; B1 to B3, distraction; and C1 to C3, multidirectional with translation (see text for details). The classification system is arranged in order of increasing severity: A1 is the most stable, whereas C3 is the least stable. The classification system can be used to determine the need for instrumentation and the optimal type of fixation. It represents a uniform method of classification of fractures for clinical research.22,27
Although perhaps useful in the research setting, the Magerl system has been criticized as being too complex for routine clinical use and has performed poorly in tests of interobserver agreement.28,29 Furthermore, although the Denis model remains popular, its original tenet—that instability ensues when two of the three columns are disrupted—has been recognized as an oversimplification that fails to incorporate the biomechanical importance of the spinal ligaments.
With spinal experts agreeing that most thoracolumbar fractures can be managed conservatively, the ideal classification system would be easy to apply, be highly reproducible, and assist in identifying the minority of patients who would benefit from operative intervention. This need for a practical thoracolumbar classification system directly linked to a clinical decision-making algorithm prompted the Spine Trauma Study Group to develop the thoracolumbar injury classification and severity score (TLICS).30,31 The TLICS scale is a point-based system founded on the triad of injury mechanism/morphology, integrity of the posterior ligamentous complex, and neurological status of the patient (Table 318-1).32 The total number of points is calculated for each thoracolumbar fracture based on these three major categories, and the final score is linked to an algorithm to help guide management: injuries with a total TLICS score of 3 or less should be considered for primary nonoperative management, fractures with a total TLICS score of 4 may be treated operatively or nonoperatively at the surgeon’s discretion, and injuries with a total TLICS score of 5 or higher should undergo primary operative management.30 Further studies have shown that TLICS exhibits excellent validity and interobserver reliability, unlike older classifications.33–35 By shifting emphasis away from the nebulous concept of spinal stability in favor of a quantitative assessment of operative necessity, the Spine Trauma Study Group has designed a seemingly robust classification that has thus far successfully met several stepwise validation challenges. Future studies will reveal whether the TLICS algorithm becomes widespread and whether the fracture score and its associated management recommendation are correlated with patient outcomes.
TABLE 318-1 Thoracolumbar Injury Classification and Severity Score
| PARAMETER | POINTS |
|---|---|
| Morphology | |
| Compression | 1 |
| Burst | 2 |
| Translational/rotational | 3 |
| Distraction | 4 |
| Neurological Status | |
| Intact | 0 |
| Nerve root injury | 2 |
| Spinal Cord/Conus Medullaris Injury | |
| Complete | 2 |
| Incomplete | 3 |
| Cauda equina | 3 |
| Posterior Ligamentous Complex | |
| Intact | 0 |
| Indeterminate | 2 |
| Disrupted | 3 |
The maximum score is 10. Treatment recommendation: total score of 3 or less, nonoperative treatment; total score of 4, surgeon discretion regarding nonoperative versus operative treatment; total score of 5 or higher, operative treatment.
Mechanism of Injury
Surgical therapy for an injured thoracic spine must take into account the injury force or forces that acted on the bony column and the pattern of instability associated with the specific injury. The forces responsible for a spinal column injury are generally accepted as compression, flexion, extension, rotation, shear, distraction, or a combination thereof.36 Magerl and colleagues formulated a classification system by dividing the pathomorphologic characteristics of the action of injury moments into three patterns: compressive, tensile/distractive, and axial torque, which correspond to the three injury types in the system—A, B, and C, respectively (Fig. 318-6).22
Compression or Axial Loading Fractures
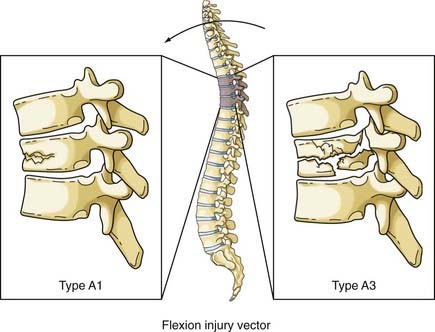
The normal kyphosis of the upper thoracic spine results in an axial compression load being converted into an anterior flexion load on the vertebral body. The resultant fracture of the vertebral body is a wedge compression fracture; the same injury is produced by a flexion vector (Fig. 318-7).36 Magerl classified these fractures as types A1 to A3, depending on the injury pattern.
Flexion
Flexion and compression or axial loads produce an anterior force (flexion-compression)—the most common load—on the anterior vertebral body and a simultaneous tensile force on the posterior column. The injury produced is an anterior wedge fracture with an intact posterior vertebral body and therefore an intact middle column. This fracture is stable and not usually associated with spinal cord injury. If the anterior vertebral body is compressed in excess of 50%, posterior ligamentous disruption or facet joint failure often occurs and renders the injury unstable even in the absence of a fracture of the bony elements of the posterior column. A facet fracture or capsule injury can allow the superior body to sublux on the inferior body and thereby result in a fracture-dislocation. The posterior elements are injured more frequently than generally appreciated, and disruption of the posterior ligaments often occurs before failure of the vertebral body.37 Severe injuries that compromise the posterior vertebral body in addition to the anterior vertebral body result in a distinctive pattern of fracture in which the superomedial portion of the posterior vertebral body is fragmented from the remainder of the body; the fragment then rotates into the spinal canal and compromises the intracanalicular neural elements (Fig. 318-7, A3). Although the resultant burst fracture is a type of compression fracture, the Magerl system differentiates between a stable burst fracture, type A3, which is similar to the more common wedge compression fracture, and an unstable burst fracture, type B1 or C1. The latter types of injuries are often associated with a spinal cord injury and are prone to long-term instability if not surgically reconstructed.
Shear
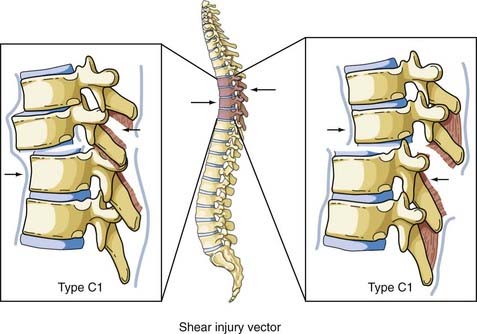
Shear forces can be directed in any direction along the longitudinal axis of the spine (Fig. 318-8). Roaf demonstrated that shear force can produce severe ligamentous disruption similar to that generated by the combination of flexion and rotation considered earlier.38 Shear forces acting in a posterolateral direction cause the superior vertebral body to sublux anteriorly on the inferior vertebral body, with a complete spinal cord injury resulting from movement of the vertebral bodies. There have been occasions when anterior movement of the superior body results in disruption of the pars interarticularis or the pedicles, thereby “decompressing” the spinal cord and protecting it from serious injury.39 The more common result of this load is an anterior spondylolisthesis; however, the anterior body can be displaced posteriorly or laterally on the inferior body. The Magerl classification of this fracture pattern is type C1.
Flexion-Distraction
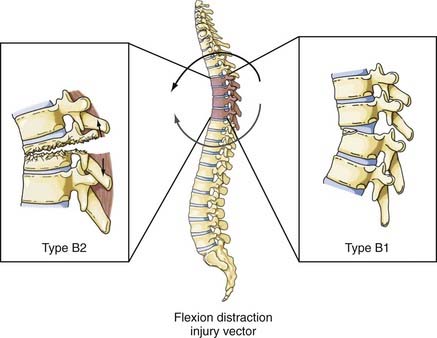
Although this type of injury was first described radiographically by Chance in 1948,40 the mechanism of injury was not defined until later.41 These injuries are commonly associated with the use of seat belts, especially lap belts, were once called seat belt injuries, but are more appropriately designated flexion-distraction injuries. Denis distinguished between “seat belt–type” injuries, of which the fracture described by Chance is only one, and fracture-distraction injury, which is a subtype of fracture-dislocation injury.26 Although the mechanism of injury is similar in both injuries, that is, a flexion movement produces a tensile force on the posterior and middle columns that acts on an axis of rotation and is translated anteriorly (by a seat belt holding the anterior abdominal wall to the seat), the latter injury is characterized by failure of the anterior column along with disruption of the anulus and stripping of the anterior longitudinal ligament from the inferior vertebral body. Loss of the anterior column allows anterior spondylolisthesis to develop. Although none of the fracture-dislocations of the flexion-distraction type occurred in the upper thoracic levels in the series reported by Denis, Eismont and associates stated that flexion-distraction can result in a bilateral facet dislocation in the thoracic spine.26,36 The fracture described by Chance is designated type B2 in the Magerl classification system, whereas fracture-distractions involving ligamentous disruption of the posterior column are designated type B1 (Fig. 318-9).22
Extension
The vector in this injury is the opposite of the flexion vector acting on the trunk, and it results in forced posterior movement. Although the majority of the injuries resulting from this force are isolated fractures of the posterior elements (facet, lamina, or spinous process), if a severe force is applied, the anterior longitudinal ligament and anulus will fail and result in a highly unstable shear fracture because retrolisthesis of the superior vertebral body on the inferior vertebral segment can occur. Denis and Burkus reported 12 of these types of fractures, 7 of which were in the upper thoracic spine, in a report after Denis’ original paper (Fig. 318-10).42 The Magerl classification of this fracture type is type B3.
Flexion-Rotation
This combination of injury vectors produces an extremely unstable injury of the vertebral column (Fig. 318-11). The flexion load causes an anterior vertebral body compression fracture, whereas the rotational load disrupts the facet joints by fracturing the superior facet on one side and tearing the facet capsule on the other. In addition, the anulus may fail, the posterior ligaments are disrupted, and the posterior vertebral body may fracture, with retropulsion of bone into the canal. The fracture-dislocation that results from this combination of load on the vertebral column was recognized by Holdsworth in his original paper as a highly unstable fracture that was “so constantly associated with paraplegia.”25 Magerl and colleagues classified this fracture as type C3, the most unstable fracture in their classification system.22
Initial Management of a Thoracic Spine Injury
After the patient is immobilized in the field with a collar and hard board and stabilized in accordance with the advanced trauma life support guidelines, the primary survey is conducted by the trauma team, including palpation of the entire posterior spinal axis to detect any bony step-off (and tenderness if the patient is responsive) during a logroll procedure to remove the hard board. In a modern trauma center, the initial thoracic spine imaging is typically generated as reconstructed images from thoracoabdominal CT scans in patients with a sufficient mechanism or clinical concern for spinal trauma. Any spinal fractures or bony displacement seen on such reconstructed CT images triggers neurosurgical consultation. Although trauma to the posterior ligamentous complex may be inferred from bony imaging findings such as splaying of the spinous processes on CT, MRI is performed for all thoracolumbar fractures as the preferred modality to assess the spinal ligaments.43 MRI is ordered on an emergency basis when the neurological examination reveals a focal motor or sensory deficit or if there is a history or signs of bowel/bladder dysfunction (e.g., elevated postvoid residual). The prevalence of wrong-level operations in the thoracic spine is significant,44 and we therefore insist on concomitant lumbar imaging so that one can count up from the sacrum to localize the thoracic pathology.
All patients with a neurological deficit from a spinal fracture undergo a physician-documented neurological examination based on the American Spinal Injury Association (ASIA) Standard Classification of Spinal Cord Injury Scale, which has very good interrater reliability.45,46 Although an ASIA score is documented on admission, many subsequent scores are obtained because evidence demonstrates that the first ASIA score with prognostic capability occurs at 72 hours after injury. The use of high-dose intravenous methylprednisolone for acute spinal cord injury is highly controversial, and many centers are moving away from the practice.47,48 An important consideration—which remains at the level of option—is that patients with an acute spinal cord injury should be monitored in the intensive care unit with a minimum mean arterial pressure goal of 85 to 90 mm Hg and adjunctive use of vasopressors to limit spinal cord hypoperfusion.49,50
Surgical versus Nonsurgical Management
Since the introduction of Harrington distraction rods, enthusiasm has been growing for surgical treatment of thoracic fractures. This is due in part to the ongoing introduction of effective internal fixation technology and the perceived clinical benefits of operative spinal stabilization, such as early mobilization of the patient. Another impetus for surgery is to prevent neurological deterioration, chronic pain, or the development of spinal deformity. Before the TLICS scale (see earlier), classification systems for thoracic and lumbar fractures provided minimal assistance in helping physicians decide between nonoperative and operative management. With surgeons clinging to the simple dictum that instability merits operative intervention, broad definitions of instability, such as that of White and Panjabi, have become popular ways to encompass the concept that surgery for thoracic and lumbar fractures not only immediately protects the neuraxis but also provides prophylactic protection from future deformity, pain, or deficit. As noted earlier, White and Panjabi defined clinical instability as loss of the ability of the spine to maintain its pattern of displacement under physiologic loads so that there is no initial or additional neurological deficit, no major deformity, and no incapacitating pain.23
The results reported by Frankel and associates remain the “gold standard” for nonoperative therapy to which alternative treatments and final outcomes are measured; in 371 patients with thoracic and lumbar fractures, there was only a 0.5% incidence of neurological worsening in those treated by postural reduction and recumbency.51 Similarly, excellent results with long-term follow-up are available for nonoperatively treated burst fractures of the thoracic and lumbar spine (primarily the thoracolumbar junction), with or without neurological deficit.52–57 However, other series have demonstrated a much higher rate of neurological worsening during nonoperative treatment, such as the 18% incidence reported by Denis and coauthors.58 Moreover, a thoracic or lumbar burst fracture—particularly one with a rotational component—has been associated with a higher risk for early neurological deterioration, even while patients are still hospitalized.59
Whether surgery for patients with a thoracic fracture and spinal cord injury can increase the likelihood of neurological improvement remains a matter of debate. Neurological improvement of incomplete spinal cord lesions after anterior or posterior surgical decompression has been reported.60–63 Krengel and colleagues favored early posterior decompression and instrumented fusion and demonstrated that surgery was safe and improved neurological recovery in comparison to historical controls managed nonoperatively or by late surgical intervention.4 With a focus on incomplete upper thoracic injuries, Bohlman and coauthors reported an improvement of 2.0 Frankel grades per patient with anterior surgical decompression.64
Although two randomized prospective studies have compared operative with nonoperative management of neurologically intact patients with thoracolumbar burst fractures, there are no comparable studies for thoracic fractures.65,66 The multiple hurdles encountered when performing randomized trials involving surgical intervention have prompted some to explore novel methods of cohort comparison in the trauma population. Most recently, Stadhouder and coworkers performed a parallel cohort study based on clinical equipoise in which operative and nonoperative treatment of traumatic thoracic and lumbar fractures were compared. With a mean follow-up of 6.2 years, pain scores, disability indices, and general health outcomes were comparable between the two groups; however, there was a nonstatistically significant trend toward better neurological recovery in the surgical group.9
The preponderance of evidence points to the importance of restoring anatomic alignment. Whitesides was adamant that persistent or progressive kyphosis inhibited neurological recovery.20 In their studies of fractures of the thoracolumbar junction, Shuman, Jacobs, and colleagues found a direct correlation between progressive posttraumatic kyphosis and progression of neurological symptoms.67,68 Similarly, in a follow-up study of patients with unstable thoracic and lumbar fractures, Soreff and associates showed a significant correlation between spinal deformity and symptomatology, and Edwards and coworkers’ data on patients with posttraumatic paraplegia suggested that anatomic restoration was important for good long-term results.69,70 Nevertheless, Nicoll did not find any link between the degree of spinal deformity and symptoms, and McAfee and colleagues, in reviewing anterior decompression and fusion in patients with incomplete deficits, found that residual kyphosis did not preclude neurological improvement.24,71 In the absence of cystic degeneration of the spinal cord, neurological deterioration in the setting of spinal deformity has been associated with progressive kyphosis, stenosis, arachnoiditis, and spinal cord tethering.72 The potential morbidity of untreated deformity and the increased neurological risks encountered with delayed surgical management have caused some to argue that long-term outcomes may be more satisfactory when posttraumatic deformity is corrected with earlier surgical intervention.73
Nonoperative management of most thoracic and lumbar fractures has been fairly successful and almost as heterogeneous as surgical interventions—bed rest alone versus some combination of postural reduction, bracing, casting, early mobilization, and physical therapy.52,74–76 Most of the bracing literature focuses on treating thoracolumbar junction fractures with thoracolumbosacral orthoses (TLSOs). Some have argued that many thoracic fractures do not require bracing because an intact rib cage and sternum will splint the affected portion of the spinal column. Occasionally, fractures of the upper thoracic spine are braced by obtaining purchase from the cervical spine via mandible or occipital pads or with a halo ring; such braces are categorized as cervicothoracic orthoses or cervicothoracolumbosacral orthoses.2 Unstable, purely upper thoracic spine fractures have been successfully treated with orthotic bracing alone; in one series of 49 patients with upper thoracic fractures treated with bracing, no failure of arthrodesis was seen, yet complications included 1 case of neurological worsening, 8 cases of brace-related skin necrosis, and 12 cases of deep venous thrombosis.77
Clearly, the risk for complications must also weigh into the decision of whether to proceed to the operating room. Gertzbein and associates, in a large multicenter study of 1019 thoracolumbar fractures, found a complication rate of 3% in the nonoperative group and 25% in surgically managed patients.10 However, one must be wary of such comparison studies given the lack of randomization and the realization that surgery is usually offered to patients with more severe injuries and neurological deficits. Proponents of surgery argue that it facilitates mobilization and rehabilitation. There is evidence that hospitalization time can be shortened with surgical stabilization in patients with thoracic and lumbar fractures causing paralysis.78–80
Mirza and associates offer specific treatments for spinal fractures based on the classification of McAfee and colleagues and the presence or absence of a neurological injury. The specific recommendations are based on the authors’ collective experience and represent more traditional, principally orthopedic approaches to the treatment of spinal fractures. The recommendations have not been scientifically validated (Table 318-2).81,82
TABLE 318-2 Treatment Guidelines as Identified by Mirza and Associates
| INJURY TYPE | SUGGESTED TREATMENT |
|---|---|
| Compression fracture | Observation with follow-up or prefabricated brace immobilization for 12 wk |
| Burst fracture, stable | Custom-fitted orthosis or cast immobilization for 12 wk |
| TLSO, L4 and above | |
| TLSO or HTLSO, L5 and below | |
| Hyperextension cast or brace if kyphosis >15 degrees | |
| Burst fracture, unstable | Approach for surgical decompression and stabilization is controversial |
| Emergency posterior short-segment decompression and fusion with immobilization in a custom TLSO for 12 wk | |
| Delayed anterior decompression and fusion if the patient has residual cord compression in the setting of a neurological deficit | |
| Flexion-distraction (Chance) | Hyperextension cast if osseous injury with no associated neurological deficit and no abdominal injury (Chance injury) |
| Posterior short-segment stabilization and fusion if associated neurological injury or abdominal injury or when the spinal injury is primarily ligamentous | |
| Fracture-dislocation | Posterior long-segment surgical stabilization with pedicle screw fixation of two to three levels above and below the injury and fusion with local bone graft |
HTLSO, hip thoracolumbosacral orthosis; TLSO, thoracolumbosacral orthosis.
Modified from Mirza SK, Mirza AJ, Chapman JR, et al. Classification of thoracic and lumbar fractures: rationale and supporting data. J Am Acad Orthop Surg. 2002;10:364.
Surgical Management of Thoracic Injuries
General Principles
The timing of surgery for patients with thoracic fractures and spinal cord injury is as controversial as the role of surgery. The preponderance of animal studies demonstrates that neurological recovery is directly correlated with the duration of external spinal cord compression, and there are multiple clinical reports of immediate neurological improvement in humans after closed reduction of devastating cervical spinal cord injuries.83–85 Thus, we treat spinal cord injury—incomplete and complete—with imaging evidence of persistent spinal cord compression as a neurosurgical emergency. In the absence of other severe injuries that might preclude surgery, patients are taken to the operating room urgently for decompression and internal fixation once hemodynamically stabilized. Vigilance on the part of the surgical team is required to ensure that any secondary spinal cord insult from hypotension is prevented during the induction and maintenance of general anesthesia.
Once the patient is properly positioned, the baseline neurophysiologic test battery is repeated to ensure that no deterioration has taken place. A nitrous-narcotic balanced anesthetic is usually given because even low concentrations of inhaled anesthetic have been shown to impair evoked waveforms.86 We prefer the Jackson table (Orthopaedic Systems, Inc., Union City, CA) for prone positioning and use careful foam padding to reduce the risk for brachial plexus injuries or meralgia paresthetica. The Mayfield Skull Clamp (Ohio Medical Instrument Co., Cincinnati, OH) is routinely used for cervicothoracic junction/upper thoracic instrumentation, and a Cell Saver system (Haemonetics, Braintree, MA) is often used to reduce the need for heterologous blood products. C-arm fluoroscopy is used initially to localize the skin incision, and we have been accruing a favorable experience with an image guidance fiducial system (Stryker, Kalamazoo, MI) to assist in the placement of thoracic pedicle screws.
Thoracic spine fractures occur in complex polytrauma patients, and such patients are best treated by a multidisciplinary team coordinated by general trauma surgeons and surgical intensivists. The timing of neurosurgical intervention, the choice of approach, and the decision whether to stage the operation must take into account not only the other injuries suffered and comorbid conditions of the patient but also the operative plans of other surgical consultants. For example, given the importance of the rib cage and costovertebral ligaments to thoracic spinal integrity, multiple rib fractures may render a thoracic fracture more likely to fail nonoperative management.87 Moreover, the acute condition of these patients often requires the coordination of multiple imaging studies and operations, and therefore continual communication between the surgical services, anesthesiologists, medical consultants, and critical care intensivists is crucial for optimizing care and preventing unnecessary testing and transport of critically ill patients.
Surgical Approaches for Thoracic Fractures
The surgical options for thoracic spine fractures requiring operative stabilization fall into four basic categories: anterior/anterolateral transthoracic procedures, short-segment posterior fixation, long-segment posterior fixation, and combined anterior/posterior (the so-called 360-degree) approaches (Fig. 318-12).
The surgical technique for anterior and posterior instrumentation of the thoracic spine is described in other chapters (see Chapter 294, Chapter 303, and Chapter 304). In the following section we discuss the technique of anterior transthoracic decompression of the thoracic spinal cord. In a later section we discuss selection of treatment options for specific types of thoracic spine fractures.
Anterior Transthoracic Decompression
Transthoracic decompression was first described for the treatment of tuberculosis of the spine in 1956 by Hodgson and Stock, who stated that “the region from C.6 to D.4 is reached without undue difficulty” through resection of a third rib.88 Hodgson and Stock also advised performing rib resection above the level of the lesion with T4-12 lesions because “it is much easier to work downwards than upwards on the spine” and the rib was available for reconstructive fusion. Although Chou and Selijeskog described transthoracic osteotomy for a variety of lesions,89 Eismont and associates credit Paul and colleagues with the first report of using this technique for decompression and fusion of a traumatic injury to the thoracic spine.36,90 Bohlman and coauthors published the long-term results and details of the surgical techniques.64,91 In one review of acute injuries to the upper thoracic spine, eight patients were treated by transthoracic decompression and fusion for residual neural compression after spinal column and cord injuries. All eight patients had reached a plateau in their neurological recovery before the anterior decompression. After transthoracic decompression and fusion, five patients achieved independent ambulation, two recovered ambulatory status with assistive devices, and one gained increased motor function without restoration of ambulatory status. In all patients the anterior strut graft fused without instrumentation, but no data were given regarding initial or final angulation at the decompression site.91
Transthoracic decompression may cause destabilizing effects. Gurr and coworkers demonstrated experimentally that spinal strength is markedly reduced in axial, flexion, or rotational loading after a corpectomy when compared with an intact spine.92 The addition of an anterior iliac graft still allowed 3 times the displacement with axial loading, whereas torsional stiffness was less than a third that of an intact spine. Our experience with a variable-length titanium cage has been positive, especially when combined with a rod-screw or screw-plate anterior fixation system (TSLP, AO Synthes, Davos, Switzerland) (Fig. 318-13).
Surgical Technique
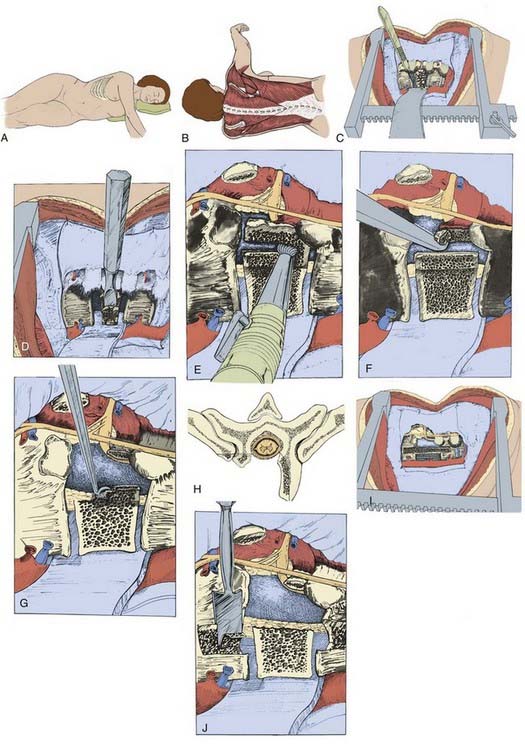
An axillary roll is placed to prevent stretch palsy of the lower brachial plexus, and the superior portion of the arm is held in a neutral position with an arm support, with the arm being held in forward flexion of no greater than 90 degrees at the shoulder to minimize the risk for upper brachial plexopathy. Wide adhesive tape can be used to secure the position of the patient and should be placed at the level of the greater trochanter and across the shoulder. The tape should be securely affixed to the table. Some surgeons prefer to place a beanbag under the patient, but we are inclined not to do so because not using this device provides additional posterior exposure. This is an advantage in the rare event that simultaneous anterior decompression and posterior stabilization are necessary.93 Care should be taken to prepare an adequate amount of skin: from the midline anteriorly to beyond the midline posteriorly and from just inferior to the axilla down to the iliac crest.
The skin incision is made over the rib one to two vertebral levels superior to the level of the lesion when dealing with lesions from T6 to T10. At the most superior thoracic levels, the skin incision should extend inferior to the tip of the scapula and halfway between the medial border of the scapula and the midline spinous processes (Fig. 318-14A and B). The incision should be extended to the subcutaneous tissues down to the deep fascia and muscles, which are divided in line with the skin incision. The rib to be resected is then stripped subperiosteally and divided with a rib cutter. The inner rib bed is divided to expose the chest cavity.
The parietal pleura is then divided between the rib insertions on the vertebral body and the great vessels until one level superior and one level inferior to the injury levels are exposed. The segmental vessels are then identified in the middle of the vertebral body and are clamped, divided, and tied. Extraperiosteal dissection of the soft tissues overlying the vertebral body can be performed with dissectors or periosteal elevators. The dissection can be carried across the anterior midline of the vertebral body and a malleable retractor placed to protect the great vessels and the esophagus (Fig. 318-14C).
The proximal portion of the rib inserting at the level of decompression has to be removed to expose the pedicle and neural foramen. The disk spaces immediately superior and inferior to the vertebrectomy site should be evacuated with a scalpel and rongeurs. A small periosteal elevator is useful for detaching the cartilaginous end plate, which can aid in disk removal. An osteotome is then used to remove a portion of the vertebral body while taking care to leave the anterior-most portion of the vertebral body attached to the anterior longitudinal ligament, unlike the removal shown in Figure 318-14H. The dura can be exposed by using a power bur, rongeurs, curets, and osteotomes once the dura is identified at the neural foramina by partial resection of the pedicle (Fig. 318-14D-G). The dissection should be extended across the full width of the canal until the pedicle on the distal side is identified. Measurements of vertebral body width from the preoperative imaging studies are valuable. One should explore the superior disk space to verify that any bone fragments attached to the anulus have been removed from the canal. If there is any question of residual bone fragments having penetrated the posterior longitudinal ligament, the ligament must be divided or a window cut made to allow exploration of the epidural space.
The vertebrectomy site should be grafted to reconstruct the spinal column. Iliac crest can be harvested, but we use tibial allograft struts or titanium cages packed with salvaged fragments of the resected vertebral bodies and the resected rib from the thoracotomy site. If a tibial strut is used, the superior and inferior vertebral bodies are notched to lock the graft in place. More recently, a titanium cage (see Fig. 318-12D) packed with salvaged vertebral body and rib has been used to fill the defect in the anterior spinal column. The cage can be expanded to engage the adjacent vertebral bodies and reduce the kyphotic deformity. The space between the strut and the anterior longitudinal ligament is packed with salvaged autologous bone. A rib strut may also be used to fill the defect if there is minimal instability. There should be a space between the strut graft and the dura covering the neural elements; the graft should be locked into the superior and inferior vertebral bodies to prevent posterior migration into the spinal canal (see Fig. 318-14I and J).
Treatment of Specific Injuries
Correct treatment of thoracic spine fractures depends on accurate diagnosis. Accurate diagnosis is dependent on data garnered from the physical and neurological examinations, appropriate diagnostic studies, and proper imaging studies. Table 318-2 lists the specific recommendations of one group for thoracic spine fractures. A more detailed discussion of factors influencing the selection of specific treatment interventions is provided here.
Compression Fractures
Compression fractures are the most common thoracic spine injury (Fig. 318-15A). The mechanism is an axial load, which when applied to the kyphotic thoracic vertebral bodies leads to an anterior flexion vector and a resulting wedge compression deformity of the anterior vertebral body. In the Denis classification, a compression fracture is an isolated anterior column injury that was considered stable because the middle column was spared. These injuries are almost never associated with spinal cord injury. However, it was soon recognized that loss of anterior body height of 50% or greater or a kyphotic deformity of 30 degrees or greater may indicate concomitant posterior ligamentous failure and be associated with an increased risk for long-term deformity.37,91 The compression fracture morphology persists in TLICS, where direct MRI assessment of the integrity of the posterior ligamentous complex replaces such indirect bony clues to ligamentous incompetence. A history of thoracic laminectomy must be weighed into the interpretation of thoracic compression injuries inasmuch as previous removal of posterior column bony elements may render a seemingly benign compression deformity unstable.
When the anterior column is compressed more than 40% or the kyphosis exceeds 25 degrees, surgical management is indicated.65,66 Consideration should be given to the force of the injury and the age of the patient in borderline cases. An elderly patient with osteoporosis who suffered a low-velocity injury would not necessarily undergo surgery, whereas a young patient involved in a high-velocity injury would be considered for surgery because of the possibility of posterior ligamentous injury.
Burst Fractures
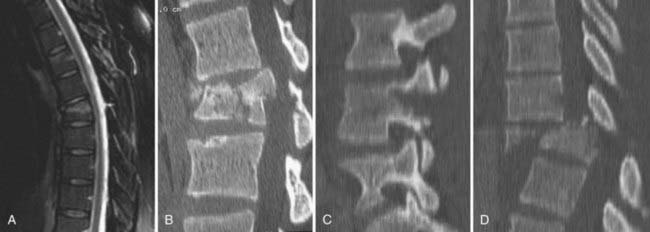
A burst fracture is a form of compression fracture also caused by an axial load. Thoracolumbar burst fractures were first described by Holdsworth in 1970.25 Denis defined a burst fracture as one involving both the anterior and middle columns and considered all burst fractures to be unstable.26 The radiographic hallmark of a burst fracture is disruption of the posterior vertebral body cortex with retropulsion of a bony fragment into the spinal canal (Fig. 318-15B); however, regardless of any bony retropulsion, the sine qua non for a burst fracture is middle column involvement. The other radiographic findings commonly observed with a burst fracture include comminution of the vertebral body, increased interpedicular distance, vertical lamina fractures, and loss of posterior vertebral body height.94 A burst fracture is considered a more severe form of a compression fracture and is weighted with an extra point in the direction of operative management within the TLICS. In contrast to a wedge compression fracture, neurological deficits often accompany burst fractures in the thoracic and lumbar spine.95 MRI is typically indicated for evaluating burst fractures because the posterior ligamentous complex, in particular the supraspinous ligament, may be disrupted in up to 28% of patients, even in the absence of bony radiographic signs of ligamentous injury on CT.96
By definition, both the anterior and middle columns are disrupted in burst fractures, but the posterior column may or may not be injured. As discussed previously, some authors believe that the integrity of the posterior column is the determinant of stability in this type of vertebral injury.24,25,97 Other important factors to consider in rendering treatment are the percentage of canal compromise, the degree of angulation, and the neurological status of the patient. No consensus exists regarding the treatment of burst fractures. We do not decompress lesions of the spine when there is less than 40% spinal canal compromise and the patient is neurologically intact. If the degree of angulation of the kyphotic deformity is less than 25 degrees, the patient may be treated with a total-contact TLSO and allowed to ambulate. Upright radiographs are necessary to verify that the fracture is stable when the patient bears weight.74
We believe that surgical therapy is preferred for burst fractures if the patient has greater than 40% canal compromise, greater than 25-degree kyphosis at the injury site, a neurological deficit, or a combination of these conditions. Potential neurological deficits from the injury include lower extremity motor and sensory abnormalities, decreased perineal sensation, and bowel and bladder dysfunction. A rectal examination should be performed, voluntary sphincter function documented, and bilateral perianal sensory function examined. Absence of the latter sensory and motor functions was found to correlate with a less favorable functional outcome and is the basis of the ASIA classification of a complete neurological injury.98 For patients who are neurologically intact or who have an incomplete spinal cord injury, greater consideration should be given to surgery to prevent deterioration from structural incompetence of the bony column and to alleviate continuing compression of neural tissues.
DeWald reported that burst fractures that were severely compressed preoperatively failed to reconstitute to a functional support structure after posterior reduction and fixation and failed to fuse.99 Although DeWald did not provide a system to measure the amount of disruption of the vertebral body, he stated that “in a clinical situation it can almost be predicted which body will need anterior grafting by the amount of compression initially present.”
The load-sharing classification scoring system described by McCormack and associates is a classification system that can be used to predict the load-sharing capability of anterior column spine fractures.100 Parker and associates use a modification of this system to assist in the identification of fractures that should be decompressed and fused anteriorly, in addition to placement of a posterior construct.101 The anterior procedure allows the anterior column to be properly reconstructed by placement of an adequate graft, thereby ensuring the ability of the column to share load while the fusion is maturing.
Seat Belt–Type Injuries
The British radiologist G. Q. Chance described a fracture in which horizontal splitting occurred through the spinous process, posterior neural arch, pedicles, and posterior part of the vertebral body.40 The Chance fracture was later dubbed the “seat belt fracture” because it became a relatively common injury in passengers restrained with a lap belt. This type of injury results from a flexion-distraction mechanism in which a flexion vector acts on an anteriorly located axis of rotation. The seat belt creates this anteriorly displaced axis by acting as a fulcrum about which spinal motion leads to tension failure of both ligaments and bone (Fig. 318-15C). Denis considered seat belt fractures unstable three-column injuries and described four different types. Seat belt fractures in the thoracic spine are rare but commonly associated with neurological deficits, as well as severe intra-abdominal injuries such as bowel, spleen, or liver rupture.102
Fracture-Dislocations
Fracture-dislocations, in which severe three-column bony and ligamentous disruption is heralded by displacement of adjacent vertebrae, represent the most unstable form of thoracic fracture. The affected facet joints are typically jumped, perched, or impacted, and significant trauma is also sustained by the paravertebral soft tissues. Three patterns of facet dislocation have been reported: (1) anterior subluxation from anterior facet dislocation (Fig. 318-15D), (2) lateral subluxation from lateral facet dislocation, and (3) acute kyphosis from superior facet subluxation.103 Most patients with thoracic fracture-dislocations suffer significant spinal cord injury, and operative fixation is mandatory, even in the rare patients without neurological deficit.
With a fracture-dislocation injury, all three columns of the spine are disrupted and the spine is rendered unstable (Fig. 318-15D). This is undoubtedly the reason for the high incidence of spinal cord injury associated with fracture-dislocation injuries. It is also the reason that most fracture-dislocation injuries require surgical treatment. If after a fracture-dislocation injury of the spine the patient has normal findings on neurological examination, the spine needs to be stabilized by internal fixation and fusion to prevent a spinal injury from occurring and to allow the patient to be rapidly mobilized. When the patient has an incomplete spinal cord injury from a fracture-dislocation injury to the spine, the spinal canal should be decompressed and the spine stabilized. Even a patient with a complete neurological deficit from a fracture-dislocation injury requires stabilization of the spine to minimize the length of the acute hospital stay and to begin rehabilitation promptly.
Flexion-rotation injuries are associated with the highest incidence of early neurological deterioration and should therefore be managed with extreme care until the spinal column can be stabilized.59 The anterior longitudinal ligament is usually intact in fracture-dislocation injuries, and the flexion-rotation and flexion-distraction subtypes can be reduced and fixed with posterior segmental instrumentation.
Penetrating Thoracic Spinal Cord Injuries
The literature on civilian gunshot wounds to the thoracic spine has generally advocated restraint in the surgical treatment of these injuries, regardless of the extent of neurological injury. Studies of patients with spinal cord injuries in conjunction with gunshot injuries have failed to show any recovery of neurological function after primary treatment with laminectomy.104–107 Stauffer and associates demonstrated that after primary treatment of gunshot wounds to the spine by laminectomy, the incidence of spinal instability increased from 0% to 6%, the incidence of CSF leakage increased from 0% to 6%, and the incidence of infection increased from 0% to 4% when compared with nonsurgical management.105 In a collaborative study of the National Spinal Cord Injury Model Systems, bullet removal from the canal between T1 and T11 had no effect on motor recovery, and their data showed no significant difference in the development of late pain and sensation between surgically and nonsurgically managed patients.106 (Note: in patients with lesions between vertebral levels T12 and L4, there was better motor recovery in those from whom the bullet was removed than in those who did not have the bullet removed.) The authors suggest that surgical treatment of patients with gunshot wounds to the thoracic spine be limited to those in whom neurological deterioration in the presence of neural compression can be demonstrated. Instability of the spine is rarely associated with thoracic-level gunshot wounds.
Miscellaneous Injuries
Treatment of a symptomatic thoracic disk herniation is surgical. There are a number of potential approaches for treatment of these lesions, including transthoracic, transpedicular, costotransversectomy, and endoscopic. A standard laminectomy should not be used because the disk cannot be removed without manipulation of the spinal cord and such manipulation can be associated with neurological deterioration. Logue reported neurological deterioration in 45% of patients undergoing thoracic disk excision via a standard laminectomy.108 Reports of transthoracic, transpedicular, or costotransversectomy approaches have documented that 80% to 90% of patients improve whereas the remainder are unchanged.109–112 Bohlman and associates reviewed 19 patients who underwent surgical resection of a thoracic disk herniation (8 treated by transthoracic opening and 11 by costotransversectomy). They preferred the transthoracic approach because the lesion and the neural structures were visualized more easily, and all patients with paralysis before surgery improved with this surgical treatment.113
Complications
Neurological deterioration during or after surgery is a very serious complication that accompanies approximately 1% of operations for treatment of a spine injury.114–116 Overdistraction, inadvertent introduction of instrumentation into the spinal canal, overcompression, or loss of reduction can all lead to neurological deterioration. Overdistraction has been associated with Harrington distraction rod constructs but is less of a concern with posterior segmental fixation systems that do not depend on the competence of ligamentous tissues. Compression can cause herniation of a disk or bone fragment into the spinal canal, with resultant compression of the spinal cord. This injury can be minimized by using this type of construct only when the posterior vertebral body is intact and after anterior decompression and fusion/fixation, ventral decompression and intraoperative somatosensory monitoring, and the use of intraoperative ultrasound. Hooks can compromise the spinal canal, and the close proximity of the spinal cord to the laminae does not leave much space for hooks. The use of pedicle screws, when properly placed, and pedicle and transverse process hooks can reduce but not entirely obviate this complication.
Bohlman HH. Treatment of fractures and dislocations of the thoracic and lumbar spine. J Bone Joint Surg Am. 1985;67:165.
Cantor JB, Lebwohl NH, Garvey T, et al. Nonoperative management of stable thoracolumbar burst fractures with early ambulation and bracing. Spine. 1993;18:971.
Dai LY, Jiang SD, Wang XY, et al. A review of the management of thoracolumbar burst fractures. Surg Neurol. 2007;67:221.
Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg Am. 1995;77:1042.
Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817.
Denis F, Armstrong GW, Searls K, et al. Acute thoracolumbar burst fractures in the absence of neurologic deficit. A comparison between operative and nonoperative treatment. Clin Orthop Relat Res. 1984;189:142.
Dickson JH, Harrington PR, Erwin WD. Results of reduction and stabilization of the severely fractured thoracic and lumbar spine. J Bone Joint Surg Am. 1978;60:799.
Lee JY, Vaccaro AR, Schweitzer KMJr, et al. Assessment of injury to the thoracolumbar posterior ligamentous complex in the setting of normal-appearing plain radiography. Spine J. 2007;7:422.
Magerl F, Aebi M, Gertzbein SD, et al. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184.
Maiman DJ, Larson SJ, Luck E, et al. Lateral extracavitary approach to the spine for thoracic disc herniation: report of 23 cases. Neurosurgery. 1984;14:178.
McAfee PC, Bohlman HH, Yuan HA. Anterior decompression of traumatic thoracolumbar fractures with incomplete neurological deficit using a retroperitoneal approach. J Bone Joint Surg Am. 1985;67:89.
McAfee PC, Yuan HA, Fredrickson BE, et al. The value of computed tomography in thoracolumbar fractures. An analysis of one hundred consecutive cases and a new classification. J Bone Joint Surg Am. 1983;65:461.
McCormack T, Karaikovic E, Gaines RW. The load sharing classification of spine fractures. Spine. 1994;19:1741.
Parker JW, Lane JR, Karaikovic EE, et al. Successful short-segment instrumentation and fusion for thoracolumbar spine fractures: a consecutive 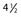 -year series. Spine. 2000;25:1157.
-year series. Spine. 2000;25:1157.
Sayer FT, Kronvall E, Nilsson OG. Methylprednisolone treatment in acute spinal cord injury: the myth challenged through a structured analysis of published literature. Spine J. 2006;6:335.
Siebenga J, Leferink VJ, Segers MJ, et al. Treatment of traumatic thoracolumbar spine fractures: a multicenter prospective randomized study of operative versus nonsurgical treatment. Spine. 2006;31:2881.
Spencer DL, Dewald RL. Simultaneous anterior and posterior surgical approach to the thoracic and lumbar spine. Spine. 1979;4:29.
Tropiano P, Huang RC, Louis CA, et al. Functional and radiographic outcome of thoracolumbar and lumbar burst fractures managed by closed orthopaedic reduction and casting. Spine. 2003;28:2459.
Vaccaro AR, Baron WM, Sanfilippo J, et al. Reliability of a novel classification system for thoracolumbar injuries: the thoracolumbar injury severity score. Spine. 2006;31:s62.
Vaccaro AR, Lehman RAJr, Hurlbert RJ, et al. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine. 2005;30:2325.
Vaccaro AR, Silber JS. Post-traumatic spinal deformity. Spine. 2001;26:s111.
Vaccaro AR, Zeiller SC, Hulbert RJ, et al. The thoracolumbar injury severity score: a proposed treatment algorithm. J Spinal Disord Tech. 2005;18:209.
Waters RL, Adkins RH. The effects of removal of bullet fragments retained in the spinal canal. A collaborative study by the National Spinal Cord Injury Model Systems. Spine. 1991;16:934.
Whang PG, Vaccaro AR, Poelstra KA, et al. The influence of fracture mechanism and morphology on the reliability and validity of two novel thoracolumbar injury classification systems. Spine. 2007;32:791.
Wood K, Buttermann G, Mehbod A, et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg Am. 2003;85:773.
1 Vialle LR, Vialle E. Thoracic spine fractures. Injury. 2005;36(suppl 2):B65.
2 Quinlan JF, Harty JA, O’Byrne JM. The need for multidisciplinary management of patients with upper thoracic spine fractures caused by high-velocity impact: a review of 32 surgically stabilised cases. J Orthop Surg (Hong Kong). 2005;13:34.
3 Morgan TH, Wharton GW, Austin GN. The results of laminectomy in patients with incomplete spinal cord injuries. Paraplegia. 1971;9:14.
4 Krengel WF3rd, Anderson PA, Henley MB. Early stabilization and decompression for incomplete paraplegia due to a thoracic-level spinal cord injury. Spine. 1993;18:2080.
5 Braakman R, Fontijne WP, Zeegers R, et al. Neurological deficit in injuries of the thoracic and lumbar spine. A consecutive series of 70 patients. Acta Neurochir (Wien). 1991;111:11.
6 Liljenqvist U, Hackenberg L, Link T, et al. Pullout strength of pedicle screws versus pedicle and laminar hooks in the thoracic spine. Acta Orthop Belg. 2001;67:157.
7 Roy-Camille R, Saillant G, Mazel C. Plating of thoracic, thoracolumbar, and lumbar injuries with pedicle screw plates. Orthop Clin North Am. 1986;17:147.
8 Yue JJ, Sossan A, Selgrath C, et al. The treatment of unstable thoracic spine fractures with transpedicular screw instrumentation: a 3-year consecutive series. Spine. 2002;27:2782.
9 Stadhouder A, Buskens E, de Klerk LW, et al. Traumatic thoracic and lumbar spinal fractures: operative or nonoperative treatment: comparison of two treatment strategies by means of surgeon equipoise. Spine. 2008;33:1006.
10 Gertzbein SD. Scoliosis Research Society. Multicenter spine fracture study. Spine. 1992;17:528.
11 Riggins RS, Kraus JF. The risk of neurologic damage with fractures of the vertebrae. J Trauma. 1977;17:126.
12 Calenoff L, Chessare JW, Rogers LF, et al. Multiple level spinal injuries: importance of early recognition. AJR Am J Roentgenol. 1978;130:665.
13 Rogers LF, Thayer C, Weinberg PE, et al. Acute injuries of the upper thoracic spine associated with paraplegia. AJR Am J Roentgenol. 1980;134:67.
14 Tator C. Epidemiology and general characteristics of the spinal cord injured patient. In: Tator CH, Benzel EC, editors. Contemporary Management of Spinal Cord Injury. Park Ridge, IL: American Association of Neurological Surgeons, 1994.
15 Argenson C, Boileau P, de Peretti F, et al. Fractures of the thoracic spine (T1-T10). Apropos of 105 cases. Rev Chir Orthop Reparatrice Appar Mot. 1989;75:370.
16 Chilimindris CP. Rupture of the thoracic esophagus from blunt trauma. J Trauma. 1977;17:968.
17 Sturm JT, Hynes JT, Perry JFJr. Thoracic spinal fractures and aortic rupture: a significant and fatal association. Ann Thorac Surg. 1990;50:931.
18 Shapiro S, Abel T, Rodgers RB. Traumatic thoracic spinal fracture dislocation with minimal or no cord injury. Report of four cases and review of the literature. J Neurosurg. 2002;96:333.
19 Lauridsen KN, De Carvalho A, Andersen AH. Degree of vertebral wedging of the dorso-lumbar spine. Acta Radiol Diagn (Stockh). 1984;25:29.
20 Whitesides TEJr. Traumatic kyphosis of the thoracolumbar spine. Clin Orthop Relat Res. 1977;128:78.
21 White AA3rd, Panjabi MM. The basic kinematics of the human spine. A review of past and current knowledge. Spine. 1978;3:12.
22 Magerl F, Aebi M, Gertzbein SD, et al. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184.
23 White A, Panjabi M. Clinical Biomechanics of the Spine, 2nd ed. Philadelphia: JB Lippincott; 1990.
24 Nicoll EA. Fractures of the dorso-lumbar spine. J Bone Joint Surg Br. 1949;31:376.
25 Holdsworth F. Fractures, dislocations, and fracture-dislocations of the spine. J Bone Joint Surg Am. 1970;52:1534.
26 Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817.
27 Gertzbein SD. Spine update. Classification of thoracic and lumbar fractures. Spine. 1994;19:626.
28 Blauth M, Bastian L, Knop C, et al. Inter-observer reliability in the classification of thoraco-lumbar spinal injuries. Orthopade. 1999;28:662.
29 Kriek JJ, Govender S. AO-classification of thoracic and lumbar fractures—reproducibility utilizing radiographs and clinical information. Eur Spine J. 2006;15:1239.
30 Vaccaro AR, Lehman RAJr, Hurlbert RJ, et al. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine. 2005;30:2325.
31 Vaccaro AR, Zeiller SC, Hulbert RJ, et al. The thoracolumbar injury severity score: a proposed treatment algorithm. J Spinal Disord Tech. 2005;18:209.
32 Whang PG, Vaccaro AR, Poelstra KA, et al. The influence of fracture mechanism and morphology on the reliability and validity of two novel thoracolumbar injury classification systems. Spine. 2007;32:791.
33 Harrop JS, Vaccaro AR, Hurlbert RJ, et al. Intrarater and interrater reliability and validity in the assessment of the mechanism of injury and integrity of the posterior ligamentous complex: a novel injury severity scoring system for thoracolumbar injuries. Invited submission from the Joint Section Meeting On Disorders of the Spine and Peripheral Nerves, March 2005. J Neurosurg Spine. 2006;4:118.
34 Patel AA, Vaccaro AR, Albert TJ, et al. The adoption of a new classification system: time-dependent variation in interobserver reliability of the thoracolumbar injury severity score classification system. Spine. 2007;32:E105.
35 Vaccaro AR, Baron EM, Sanfilippo J, et al. Reliability of a novel classification system for thoracolumbar injuries: the Thoracolumbar Injury Severity Score. Spine. 2006;31:S62.
36 Eismont F, Garfin S, Abitol J. Thoracic and upper lumbar spine injuries, Vol 1. In: Browner BD, Jupiter JB, Levine AM, et al, editors. Skeletal Trauma. Philadelphia: WB Saunders; 1993:727-803.
37 Maiman DJ, Pintar FA. Anatomy and clinical biomechanics of the thoracic spine. Clin Neurosurg. 1992;38:296.
38 Roaf R. A study of the mechanics of spinal injuries. J Bone Joint Surg Br. 1960;42:810.
39 Sasson A, Mozes G. Complete fracture-dislocation of the thoracic spine without neurologic deficit. A case report. Spine. 1987;12:67.
40 Chance C. Note on a type of flexion fracture of the spine. Br J Radiol. 1948;21:452.
41 Rennie W, Mitchell N. Flexion distraction fractures of the thoracolumbar spine. J Bone Joint Surg Am. 1973;55:386.
42 Denis F, Burkus JK. Shear fracture-dislocations of the thoracic and lumbar spine associated with forceful hyperextension (lumberjack paraplegia). Spine. 1992;17:156.
43 Lee JY, Vaccaro AR, Schweitzer KMJr, et al. Assessment of injury to the thoracolumbar posterior ligamentous complex in the setting of normal-appearing plain radiography. Spine J. 2007;7:422.
44 Mody MG, Nourbakhsh A, Stahl DL, et al. The prevalence of wrong level surgery among spine surgeons. Spine. 2008;33:194.
45 Kirshblum SC, Memmo P, Kim N, et al. Comparison of the revised 2000 American Spinal Injury Association classification standards with the 1996 guidelines. Am J Phys Med Rehabil. 2002;81:502.
46 Savic G, Bergstrom EM, Frankel HL, et al. Inter-rater reliability of motor and sensory examinations performed according to American Spinal Injury Association standards. Spinal Cord. 2007;45:444.
47 Hurlbert RJ, Hamilton MG. Methylprednisolone for acute spinal cord injury: 5-year practice reversal. Can J Neurol Sci. 2008;35:41.
48 Sayer FT, Kronvall E, Nilsson OG. Methylprednisolone treatment in acute spinal cord injury: the myth challenged through a structured analysis of published literature. Spine J. 2006;6:335.
49 Blood pressure management after acute spinal cord injury. Neurosurgery. 2002;50:S58.
50 Management of acute spinal cord injuries in an intensive care unit or other monitored setting. Neurosurgery. 2002;50:S51.
51 Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179.
52 Folman Y, Gepstein R. Late outcome of nonoperative management of thoracolumbar vertebral wedge fractures. J Orthop Trauma. 2003;17:190.
53 Moller A, Hasserius R, Besjakov J, et al. Vertebral fractures in late adolescence: a 27- to 47-year follow-up. Eur Spine J. 2006;15:1247.
54 Moller A, Hasserius R, Redlund-Johnell I, et al. Nonoperatively treated burst fractures of the thoracic and lumbar spine in adults: a 23- to 41-year follow-up. Spine J. 2007;7:701.
55 Reinhold M, Knop C, Lange U, et al. Non-operative treatment of thoracolumbar spinal fractures. Long-term clinical results over 16 years. Unfallchirurg. 2003;106:566.
56 Weinstein JN, Collalto P, Lehmann TR. Thoracolumbar “burst” fractures treated conservatively: a long-term follow-up. Spine. 1988;13:33.
57 Willen J, Anderson J, Toomoka K, et al. The natural history of burst fractures at the thoracolumbar junction. J Spinal Disord. 1990;3:39.
58 Denis F, Armstrong GW, Searls K, et al. Acute thoracolumbar burst fractures in the absence of neurologic deficit. A comparison between operative and nonoperative treatment. Clin Orthop Relat Res. 1984;189:142.
59 Gertzbein SD. Neurologic deterioration in patients with thoracic and lumbar fractures after admission to the hospital. Spine. 1994;19:1723.
60 Dunn HK. Anterior spine stabilization and decompression for thoracolumbar injuries. Orthop Clin North Am. 1986;17:113.
61 Edwards CC, Levine AM. Early rod-sleeve stabilization of the injured thoracic and lumbar spine. Orthop Clin North Am. 1986;17:121.
62 Gertzbein SD, Court-Brown CM, Marks P, et al. The neurological outcome following surgery for spinal fractures. Spine. 1988;13:641.
63 Kostuik JP. Anterior fixation for fractures of the thoracic and lumbar spine with or without neurologic involvement. Clin Orthop Relat Res. 1984;189:103.
64 Bohlman HH, Freehafer A, Dejak J. The results of treatment of acute injuries of the upper thoracic spine with paralysis. J Bone Joint Surg Am. 1985;67:360.
65 Siebenga J, Leferink VJ, Segers MJ, et al. Treatment of traumatic thoracolumbar spine fractures: a multicenter prospective randomized study of operative versus nonsurgical treatment. Spine. 2006;31:2881.
66 Wood K, Buttermann G, Mehbod A, et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg Am. 2003;85:773.
67 Jacobs RR, Casey MP. Surgical management of thoracolumbar spinal injuries. General principles and controversial considerations. Clin Orthop Relat Res. 1984;189:22.
68 Shuman WP, Rogers JV, Sickler ME, et al. Thoracolumbar burst fractures: CT dimensions of the spinal canal relative to postsurgical improvement. AJR Am J Roentgenol. 1985;145:337.
69 Edwards C, Levine A, Weigel M. Determinants of neurologic recovery following post-traumatic incomplete paraplegia. Orthop Trans. 1987;11:453.
70 Soreff J, Axdorph G, Bylund P, et al. Treatment of patients with unstable fractures of the thoracic and lumbar spine: a follow-up study of surgical and conservative treatment. Acta Orthop Scand. 1982;53:369.
71 McAfee PC, Bohlman HH, Yuan HA. Anterior decompression of traumatic thoracolumbar fractures with incomplete neurological deficit using a retroperitoneal approach. J Bone Joint Surg Am. 1985;67:89.
72 Abel R, Gerner HJ, Smit C, et al. Residual deformity of the spinal canal in patients with traumatic paraplegia and secondary changes of the spinal cord. Spinal Cord. 1999;37:14.
73 Vaccaro AR, Silber JS. Post-traumatic spinal deformity. Spine. 2001;26:S111.
74 Cantor JB, Lebwohl NH, Garvey T, et al. Nonoperative management of stable thoracolumbar burst fractures with early ambulation and bracing. Spine. 1993;18:971.
75 Tropiano P, Huang RC, Louis CA, et al. Functional and radiographic outcome of thoracolumbar and lumbar burst fractures managed by closed orthopaedic reduction and casting. Spine. 2003;28:2459.
76 Watson-Jones R. The results of postural reduction of fractures of the spine. J. Bone Joint Surg. 1938;20:567.
77 Capen DA, Gordon ML, Zigler JE, et al. Nonoperative management of upper thoracic spine fractures. Orthop Rev. 1994;23:818.
78 Dickson JH, Harrington PR, Erwin WD. Results of reduction and stabilization of the severely fractured thoracic and lumbar spine. J Bone Joint Surg Am. 1978;60:799.
79 Flesch JR, Leider LL, Erickson DL, et al. Harrington instrumentation and spine fusion for unstable fractures and fracture-dislocations of the thoracic and lumbar spine. J Bone Joint Surg Am. 1977;59:143.
80 Jacobs RR, Asher MA, Snider RK. Thoracolumbar spinal injuries. A comparative study of recumbent and operative treatment in 100 patients. Spine. 1980;5:463.
81 McAfee PC, Yuan HA, Fredrickson BE, et al. The value of computed tomography in thoracolumbar fractures. An analysis of one hundred consecutive cases and a new classification. J Bone Joint Surg Am. 1983;65:461.
82 Mirza SK, Mirza AJ, Chapman JR, et al. Classifications of thoracic and lumbar fractures: rationale and supporting data. J Am Acad Orthop Surg. 2002;10:364.
83 Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg Am. 1995;77:1042.
84 Dimar JR2nd, Glassman SD, Raque GH, et al. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine. 1999;24:1623.
85 Kobrine AI, Evans DE, Rizzoli HV. Experimental acute balloon compression of the spinal cord. Factors affecting disappearance and return of the spinal evoked response. J Neurosurg. 1979;51:841.
86 Calancie B, Klose KJ, Baier S, et al. Isoflurane-induced attenuation of motor evoked potentials caused by electrical motor cortex stimulation during surgery. J Neurosurg. 1991;74:897.
87 Dorr LD, Harvey JPJr, Nickel VL. Clinical review of the early stability of spine injuries. Spine. 1982;7:545.
88 Hodgson AR, Stock FE. Anterior spinal fusion: a preliminary communication on the radical treatment of Pott’s disease and Pott’s paraplegia. Br J Surg. 1956;44:266.
89 Chou SN, Seljeskog EL. Chapter 25. Alternative surgical approaches to the thoracic spine. Clin Neurosurg. 1973;20:306.
90 Paul RL, Michael RH, Dunn JE, et al. Anterior transthoracic surgical decompression of acute spinal cord injuries. J Neurosurg. 1975;43:299.
91 Bohlman HH. Treatment of fractures and dislocations of the thoracic and lumbar spine. J Bone Joint Surg Am. 1985;67:165.
92 Gurr KR, McAfee PC, Shih CM. Biomechanical analysis of anterior and posterior instrumentation systems after corpectomy. A calf-spine model. J Bone Joint Surg Am. 1988;70:1182.
93 Spencer DL, DeWald RL. Simultaneous anterior and posterior surgical approach to the thoracic and lumbar spine. Spine. 1979;4:29.
94 Atlas SW, Regenbogen V, Rogers LF, et al. The radiographic characterization of burst fractures of the spine. AJR Am J Roentgenol. 1986;147:575.
95 Dai LY, Jiang SD, Wang XY, et al. A review of the management of thoracolumbar burst fractures. Surg Neurol. 2007;67:221.
96 Petersilge CA, Pathria MN, Emery SE, et al. Thoracolumbar burst fractures: evaluation with MR imaging. Radiology. 1995;194:49.
97 James KS, Wenger KH, Schlegel JD, et al. Biomechanical evaluation of the stability of thoracolumbar burst fractures. Spine. 1994;19:1731.
98 American Spinal Injury Association. Standards for Neurological and Functional Classification of Spinal Cord Injury. Chicago, IL: American Spinal Injury Association; 1992.
99 DeWald RL. Burst fractures of the thoracic and lumbar spine. Clin Orthop Relat Res. 1984;189:150.
100 McCormack T, Karaikovic E, Gaines RW. The load sharing classification of spine fractures. Spine. 1994;19:1741.
101 Parker JW, Lane JR, Karaikovic EE, et al. Successful short-segment instrumentation and fusion for thoracolumbar spine fractures: a consecutive  -year series. Spine. 2000;25:1157.
-year series. Spine. 2000;25:1157.
102 Reid AB, Letts RM, Black GB. Pediatric Chance fractures: association with intra-abdominal injuries and seatbelt use. J Trauma. 1990;30:384.
103 Manaster BJ, Osborn AG. CT patterns of facet fracture dislocations in the thoracolumbar region. AJR Am J Roentgenol. 1987;148:335.
104 Heiden JS, Weiss MH, Rosenberg AW, et al. Penetrating gunshot wounds of the cervical spine in civilians. Review of 38 cases. J Neurosurg. 1975;42:575.
105 Stauffer ES, Wood RW, Kelly EG. Gunshot wounds of the spine: the effects of laminectomy. J Bone Joint Surg Am. 1979;61:389.
106 Waters RL, Adkins RH. The effects of removal of bullet fragments retained in the spinal canal. A collaborative study by the National Spinal Cord Injury Model Systems. Spine. 1991;16:934.
107 Yashon D, Jane JA, White RJ. Prognosis and management of spinal cord and cauda equina bullet injuries in sixty-five civilians. J Neurosurg. 1970;32:163.
108 Logue V. Thoracic intervertebral disc prolapse with spinal cord compression. J Neurol Neurosurg Psychiatry. 1952;15:227.
109 Lesoin F, Leys D, Rousseaux M, et al. Thoracic disk herniation and Scheuermann’s disease. Eur Neurol. 1987;26:145.
110 Maiman DJ, Larson SJ, Luck E, et al. Lateral extracavitary approach to the spine for thoracic disc herniation: report of 23 cases. Neurosurgery. 1984;14:178.
111 Patterson RHJr, Arbit E. A surgical approach through the pedicle to protruded thoracic discs. J Neurosurg. 1978;48:768.
112 Perot PLJr, Munro DD. Transthoracic removal of midline thoracic disc protrusions causing spinal cord compression. J Neurosurg. 1969;31:452.
113 Bohlman HH, Kirkpatrick JS, Delamarter RB, et al. Anterior decompression for late pain and paralysis after fractures of the thoracolumbar spine. Clin Orthop Relat Res. 1994;300:24.
114 Chozick B, Toselli R. Complications of spinal instrumentation. In: Benzel EC, editor. Spinal Instrumentation. Chicago, IL: American Association of Neurological Surgeons; 1994:257-274.
115 Levine AM, Edwards CC. Complications in the treatment of acute spinal injury. Orthop Clin North Am. 1986;17:183.
116 Wenger D, Mubarak S. Managing complications of posterior spinal instrumentation and fusion. In: Garfin S, editor. Complications of Spine Surgery. Baltimore: Williams & Wilkins; 1989:127-1439.