CHAPTER 273 Diagnosis and Management of Diskogenic Lower Back Pain
Low back pain (LBP) is the most common health problem in men and women between the ages of 20 and 50 years; it results in approximately 13 million visits to physicians in the United States each year and costs $28 billion in annual loss of productivity.1–3 Although the exact origin of most LBP remains unknown, it is understood that degenerative damage to the intervertebral disk (IVD) plays a central role in the pathogenic mechanism leading to back pain.4 Despite advancements in our understanding of spinal biomechanics and technologic innovations in spinal instrumentation, the diagnosis and treatment of diskogenic LBP remain problematic. The development and implementation of effective treatment strategies for patients with diskogenic LBP require a thorough understanding of the structure and function of the normal IVD, as well as the cellular, biochemical, and biomechanical changes that occur with degeneration of the IVD. Once an understanding of IVD physiology and pathophysiology has been gained, current diagnostic tools and treatment strategies for patients with LBP secondary to degenerative disk disease (DDD) will be reviewed.
Physiology of Intervertebral Disks
Structure and Composition
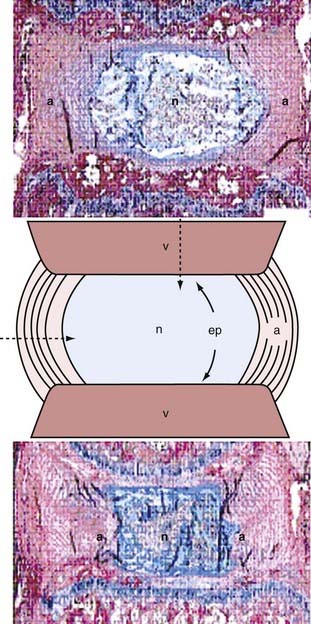
The IVD forms an avascular fibrocartilaginous joint between adjacent vertebral bodies. It consists of three major components: the gelatinous nucleus pulposus (NP), the annulus fibrosus (AF), and the superior and inferior end plates (Fig. 273-1). The NP is centrally located and composed of type II collagen within a dense matrix of proteoglycans and water. The end plates and annular matrix serve as selective permeability barriers that restrict transport to and from the nucleus based on charge and molecular weight. A fixed negative charge associated with nucleus proteoglycans generates an osmotic pressure gradient that creates the normal swelling pressure and volume of NP needed to support spinal forces. The AF has between 15 and 25 distinct type I collagen layers, or lamellae, attached to the vertebral rim and surrounding the NP.5 It serves both as a ligament to guide intervertebral movement and as a barrier to contain nuclear swelling and thereby facilitate disk pressurization.
Cell and Matrix Biology
The IVD contains few cells relative to its abundant extracellular matrix (ECM).6 In the IVDs of young individuals, the NP contains both notochordal and chondrocytic cells. The notochordal cells are remnants of spinal development7,8 and are uniquely designed for maintaining production of proteoglycans, but they disappear via apoptosis well before early adulthood, probably because of pressure from an upright stance,9 thereby leaving only the chondrocyte-like cells.10,11 The presence of these chondrocytic cells in the inner AF and end plates has led some researchers to suggest that these cells may migrate into the NP as part of the aging process.12–14 Chondrocytes in the mature NP are generally round or oval, whereas annular fibroblasts tend to be more elongated.15 Long cell processes that may be involved in sensing mechanical strain have also been identified in all areas of the IVD.16 Cells at the transition between the NP and AF have the capacity to behave both as chondrocytes and fibroblasts, perhaps fluctuating in response to local environmental cues. For example, loss of nuclear volume as a result of notochordal cell apoptosis causes redistribution of stress and inner annular chondroplasia, with denaturation of lamellar collagen leading to chondrocyte proliferation into the nuclear space.13 This process results in the age-related loss of nuclear/annular distinction.
IVD cells secrete and maintain a dense and heterogeneous ECM. The major constituent of the NP is proteoglycans, mostly large aggrecan molecules, and they make up 50% of the dry weight in normal disks.17 The NP also contains randomly organized collagen fibers, predominantly type II, and elastic fibers, which are oriented vertically near the superior and inferior end plates.18–20 Type I collagen is the major extracellular component of the AF; it accounts for 67% of the dry weight of the AF, with decreasing amounts from the outer portion of the AF toward the NP.18 The collagen fibers are aligned and oriented at 60 degrees relative to the vertical axis and alternate in adjacent lamellae.21 Although elastin represents only 2% of the dry weight of the IVD,22 recent findings suggest that it is an important contributor to normal disk function.23,24 Inside the lamella, long elastin fibers are oriented in parallel with collagen fibers.24 Interlamellar elastin fibers bridge adjacent layers, where “kinks” have been observed histologically to exit collagen bundles.23,25
Nutrition
Because the IVD is the largest avascular tissue in the body, transport of essential nutrients (oxygen and glucose) and substrates for matrix production (amino acids) by NP cells is essential for disk homeostasis.26,27 The IVD loses direct communication with the vertebral blood supply after the second decade of life27,28 and thereafter is dependent on diffusion of nutrients from surrounding capillaries29 at the periphery of the annulus and at the vertebral end plates.30 The majority of transport to and from NP cells occurs via the vertebral end plates.31 The availability of nutrients at the vertebral end plates is in turn dependent on capillary supply through mesenchymal stem cells within the subchondral bone.
Innervation
Despite being almost entirely avascular, the normal adult IVD is innervated. The posterior part of the AF and the posterior longitudinal ligament are innervated by the sinuvertebral nerve, which is thought to be capable of nociception, whereas the anterior and lateral portions of the AF are supplied by branches of the autonomic nervous system.32 Nociceptors are normally found in the outer layers of the AF and, as recent evidence indicates, in the central portion of the vertebral end plates as well.33,34 These observations have been confirmed by pain provocation studies in human participants.35
Biomechanics
The IVD is the only joint that can deform along 6 degrees of freedom (three orthogonal translations and three rotations). Its main function is to support spinal compression and provide flexibility, and along with the facet joints, it defines the range of motion of the spine. The structural unit consisting of the IVD, adjacent vertebra, and facet joints experiences a wide range of loading modalities in vivo, including axial compression, shear, flexion and extension, lateral bending, and axial rotation. During activities of daily living, forces are shared between the disk, vertebra, and facets in a posture-dependent manner. Typically, the vertebral end plate is the vulnerable tissue during supraphysiologic compression, whereas the facets are vulnerable during supraphysiologic shear and the facets/annulus during supraphysiologic rotations.36,37
Degeneration of the Intervertebral Disk
Pathologic Features
Morphologic changes in the IVD commence with loss of the notochordal nucleus before the second decade of life and progress continuously with age.38 With this age-related remodeling, the normal lamellar architecture of the AF becomes progressively disorganized and the NP becomes less hydrated and more fibrotic (see Fig. 273-1).39 At the same time, the border between the AF and NP becomes less distinct.40 Structural disorganization of the end plate, including cracks and microfractures in the subchondral bone, are also evident.38 Macroscopically, three types of lesions can be distinguished in a degenerate AF: (1) circumferential tears, or delaminations, which are formed by mechanical separation of the layers along the circumference of the disk; (2) radial fissures, which progress outward from the NP and cut through layers; and (3) rim lesions, which are peripheral radial tears that occur near the end plates (see Fig. 273-3).41,42 These tears are accompanied by vascularized granulation tissue and growth of new nerves and blood vessels not present in the normal IVD.43,44 Microscopically, cellular proliferation occurs with the formation of cell clusters, especially in the NP.45 These observations are concurrent with necrotic and apoptotic changes at the cellular level.7 It is also believed that the inflammatory process is an important characteristic of DDD,46 and elevated levels of cytokines, matrix metalloproteinases (MMPs), and growth factors have been identified in the degenerate disk.47–49
Cell and Matrix Biology
The earliest biochemical change involves loss of proteoglycan in the NP, which results in decreased hydration and swelling pressure.39 Although absolute amounts of collagen in the disk change relatively little, the type and distribution of collagen are altered. For example, with degeneration, type II collagen is increasingly denatured.50 A recent study demonstrated that the total elastin content increases with degeneration as well.51 Degenerate disks have elevated levels of proteolytic and degradative enzymes, including collagenase, lysozyme, elastase, and several MMPs.52–54 In addition, reduced levels of enzyme inhibitors have been observed in the degenerate IVD.54,55 IVD cells continue to synthesize new ECM during the early stages of degeneration,56,57 yet this anabolic behavior becomes outpaced by catabolism in more degenerate disks. Incomplete breakdown of disk macromolecules leads to the accumulation of degenerative debris in the ECM,58 which can form a catabolic stimulus for disk cells.
Biomechanics
Altered biomechanics secondary to changes in the ECM are characteristic of the progression of disk degeneration. Loss of proteoglycan in the NP leads to a reduction in water content, swelling pressure, and subsequent disk height.59,60 Loss of nuclear volume and disk height causes relaxation of stress in ligamentous structures surrounding the disk and leads to spinal hypermobility. With progressive nuclear fibrosis and collagenous cross-linking, the IVD stiffens, which results in the reduced flexibility associated with late-stage degeneration. The degenerate NP no longer behaves hydrostatically; it loses fluid rapidly under load, and the resultant changes in viscoelasticity suggest a shift from a “fluid-like” NP to a more “solid-like” material with degeneration.61,62 This also reflects a shift in the load-bearing mechanism: from hydration and pressurization in the NP to greater elastic deformation and peak stress incurred in the AF.63,64 Loss of normal nuclear function and increased stress in the AF predispose the disk to annular tears and end plate damage, as well as to mechanical delamination.65
Etiology of Degeneration
Nutrition
Although many factors are involved in the pathogenesis of disk degeneration, reduction of disk cell nutrition is probably a contributor. Cell culture studies demonstrate that synthesis of matrix is drastically reduced when the oxygen concentration falls below 5% or the pH is below 6.8, but active MMPs are continually being produced regardless of the conditions.66,67 Cells do not survive in this type of environment for long; exposure to an environment with a pH below 6.3, observed in some symptomatic disks, will result in cell death.26,62,68 Further evidence for loss of nutrition in the degenerate disk comes from findings of increased lactate levels in patients reporting diskogenic pain.69 The mechanism leading to loss of disk nutrition is still unknown, although several areas are being investigated.62 Atherosclerosis, which would affect the blood supply to the vertebrae, is associated with significant increases in degeneration.70,71 Long-term lack of exercise may also have a negative impact on transport of nutrients into the disk, although a potential mechanism is still unclear.72 Smoking is a risk factor for LBP in humans, and studies in rats have shown a direct link between smoking and disk degeneration.73,74 Finally, calcification of the cartilaginous end plate, as occurs with aging, impedes delivery of nutrients to the disk.28,75 Because of its avascularity, the IVD depends on a strict pathway for delivery of nutrients. Regardless of the inciting stimulus, disruption of this nutrient pathway will lead to harmful cellular changes and alterations in the ECM that contribute to the pathogenesis of DDD.
Catabolic Activity
Matrix degradation appears to be a key factor in the progression of disk degeneration. In its simplest form, disk degeneration is caused by an imbalance between anabolic and catabolic factors.76 Increased levels of MMPs, lysozyme, elastase, and inflammatory mediators such as macrophages, mast cells, interleukin-6 (IL-6), IL-8, prostaglandin E2 (PGE2), and tumor necrosis factor-α have been identified in human degenerate IVDs.49,52–54,77–79 Animal models have implicated nitric oxide and IL-1 as mediators in the pathologic process as well, but studies of human disks have not confirmed this finding yet.46 The exact mechanism by which an elevated catabolic process occurs and how it results in degeneration are unclear; however, it is possible that an acute injury leads to the synthesis of inflammatory mediators by disk cells in an attempt to heal themselves. Degradation of matrix by these molecules can enhance hypermobility and induce further injury to the disk. Furthermore, degraded macromolecules trapped in the disk ECM may independently signal additional degenerative changes.58
Mechanical Injury
Recently, Adams and Roughley proposed a simplified definition of disk degeneration to be “an aberrant cell-mediated response to progressive structural failure” and suggested that excessive mechanical loading in a vulnerable disk precipitates degeneration.41 Direct mechanical damage, whether through cyclic fatigue loading, hypermobility, or increased shear stress, can be associated with degenerative progression and clinically significant LBP.80 This hypothesis is supported by experimental animal models13,81,82 and epidemiologic data linking heavy lifting, intense physical work, and obesity with increased rates of mechanical LBP.83–85 Supraphysiologic combinations of bending, rotation, and compression can cause the major structural defects that are associated with degeneration, such as annular tears.37 Recent studies have also shown that loads of higher magnitude induce a catabolic response in the IVD characterized by increased protease gene and protein expression and activity.12 There is some evidence that high mechanical loads can also induce apoptosis in end plate cells.86 Furthermore, vertebral end plate damage can initiate degenerative changes by decompressing the NP and thus placing greater loads on the AF.87–89 The increased axial loading of the AF causes radial bulging both outwardly and inwardtoward the NP.41,90 The resultant shear stresses in the AF, which also occur as a result of preexisting annular tears, may lead to further degeneration.65,91 Additional support for a mechanical cause of DDD comes from evidence that altered mechanics in both the lumbar and cervical spine after surgical fusion leads to pathologic changes in adjacent segments.92
Genetics
Inherited traits are reported to account for up to 74% of the variability in disk degeneration.41,93 In a classic series of twin studies, Battie and coworkers demonstrated similar degeneration patterns in monozygotic twins who were not concordant for environmental risk factors such as smoking and heavy physical work.94 These studies are consistent with others noting that risk for back pain runs in families. Several genetic markers have been linked to disk degeneration, including alleles for collagen type IX, aggrecan, MMP-3, and the vitamin D receptor.95–98 Most of these markers have been identified through a candidate gene approach. Given that these genes encode proteins associated with physical properties of the ECM, it may be that inherited traits modulate the balance between damage accumulation and healing by tipping the scales toward accumulation of damage in at-risk patients. Better understanding of the genetic determinants and signaling pathways involved in disk degeneration may be achieved only through alternative approaches, such as genetic mapping.62 Research in this area may aid the development of improved risk stratification, diagnostic criteria, and prevention strategies.
Diskogenic Low Back Pain
With degeneration there is growth of new nerves fibers, with some extending into the inner AF and NP in patients with chronic LBP.43,99 Most of the newly formed nerve fibers in persons with LBP accompany granulation tissue and are associated with the nociceptive neurotransmitters substance P and vasoactive intestinal peptide.43 Additionally, increases in sensory nerves associated with substance P and calcitonin gene–related peptide (CGRP) were found in the vertebral bodies and end plates in patients with DDD.100 Substance P and CGRP are nociceptive neurotransmitters associated with nerve growth factor (NGF)-dependent neurons that bind to the NGF receptor tyrosine kinase A (TrKA). A recent study involving rat disks demonstrated that inflammatory-mediated pain was associated with significant growth of NGF-dependent neurons.101 It has been hypothesized that NGF may lower the IVD’s threshold for pain and that elevated TrKA expression is associated with the advent and maintenance of chronic pain.46
It has been widely held that symptomatic disk degeneration is associated with structural damage to the disk, as is the case with annular tears.41 Recent work has led to better understanding of the potential mechanisms that may lead to LBP. In analyzing IVDs from patients with diskogenic pain, researchers found vascularized granulation tissue forming alongside annular tears in the inner AF and NP.43 Growth factors such as basic fibroblast growth factor (bFGF) and transforming growth factor-β (TGF-β) were found to be localized to this region of the disk only in painful IVDs; these growth factors promote cellular proliferation and differentiation, ECM synthesis, and fibrosis.49 The cytokines IL-6, IL-8, and PGE2 are released from the granulation tissue and may sensitize the nociceptors to a lower threshold for pain.78 NGF is also increased in painful degenerate disks and leads to a lowered pain threshold.46 Thus, together with neoinnervation and nociceptor sensitization secondary to the inflammatory process, hypermobile mechanics as a result of altered matrix composition and tissue fibrosis may lead to diskogenic pain.
Diagnosis of Diskogenic Lower Back Pain
Identifying a Degenerated Intervertebral Disk
Plain Radiographs
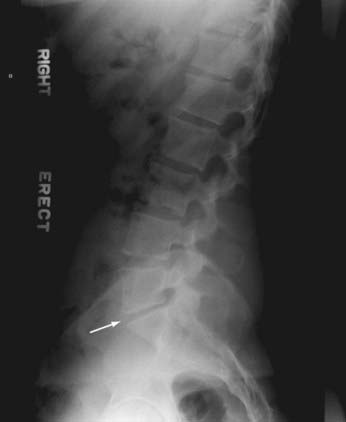
The hallmark of IVD degeneration on plain anteroposterior (AP) and lateral radiographs is disk space narrowing (Fig. 273-2). Other features of disk degeneration on plain radiographs include reactive end plate sclerosis, osteophytes, and “vacuum” disks (Knuttson’s phenomenon).102
Magnetic Resonance Imaging
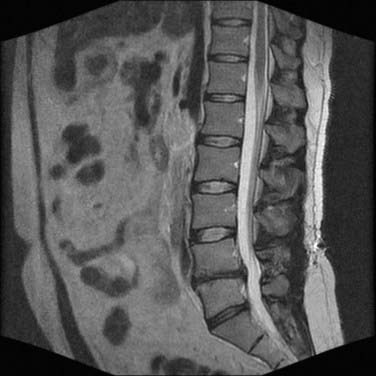
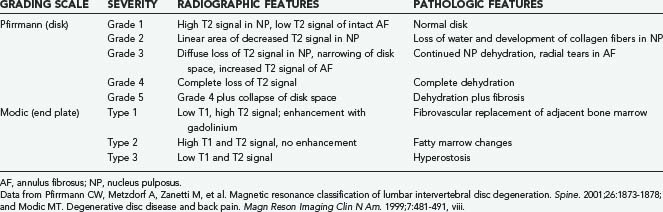
Magnetic resonance imaging (MRI) is by far the most useful tool for identifying IVD degeneration. Sagittal T2-weighted images are best for evaluating disk water content (Fig. 273-3) and Pfirrmann grading,103 whereas short tau inversion recovery (STIR) sequences are useful for visualization of bone marrow edema and Modic changes (Table 273-1).104 Loss of water content is evident as progressive collapse of disk height and loss of T2 signal within the NP.
Despite the utility of MRI in identifying a degenerated IVD, evidence of disk degeneration on MRI does not necessarily correlate with symptoms.105 Approximately 90% to 100% of asymptomatic subjects older than 50 years will have evidence of lumbar disk degeneration on MRI, whereas disk bulges and Modic changes are evident in 20% of people without symptoms.101 Braithwaite and colleagues concluded that Modic changes are a specific, but not sensitive sign of a painful lumbar disk in patients with DDD.106 Aprill and coworkers and Carragee and associates have described a high-intensity zone in the posterior median AF on T2-weighted images that is the result of an annular tear and has been found in 30% to 60% of patients undergoing MRI for back pain.107,108 Nevertheless, high-intensity zones have also been found in asymptomatic subjects and do not reliably indicate the presence of painful disk degeneration.109
Identifying a Painful Intervertebral Disk with Lumbar Diskography
Lumbar diskography is used as an adjunct to MRI to identify symptomatic disk levels in patients with presumed diskogenic LBP who may be candidates for surgical treatment.110–112 The basic principle of provocative diskography is to reproduce the patient’s LBP symptoms by pressurizing a disk with radiographic evidence of degeneration. An injection is deemed “positive” if it causes significant (>5/10) pain that is similar or exact in nature to the patient’s usual pain with a negative control disk during the same examination.
Although diskography has proved useful in the diagnosis of a painful IVD, some authors have reported a false-positive rate as high as 37%, with higher rates in patients with depression, hypochondriasis, and chronic pain syndromes.113 Other studies have found diskography to be more accurate, with Walsh and associates reporting it to have a specificity of 100%.114 Others have also found varying results with regard to the utility of diskography as a tool to predict outcomes after surgical intervention for diskogenic LBP. In the only prospective study to date, Colhoun and colleagues analyzed 162 patients with symptomatic DDD who underwent lumbar arthrodesis.110 The authors noted a favorable outcome in 89% of patients with a positive diskogram, but the outcome was favorable in only 52% of patients with a negative diskogram. Some retrospective studies have shown similar benefit in using provocative diskography to predict outcomes,115 whereas others have found equivocal or no benefit with preoperative diskography.116,117 The heterogeneous patient population and lack of standardized criteria for what constitutes a positive diskogram, as well as variations in diskography technique, make definitive conclusions from the existing literature on provocative diskography difficult to make. In our opinion, diskography is most useful in two settings: (1) a patient with DDD at multiple levels, in whom diskography can be used to identify symptomatic levels and reduce fusion length, and (2) a patient with obvious DDD at one level but equivocal MRI findings at an adjacent level, in which case the results of diskography at the adjacent level would be useful in deciding whether to include that level in the surgical fusion. It should be noted that psychological, financial, and social factors have all been found to influence the reported pain on disk injections, and careful patient selection should always be ensured even after a “positive” diskogram is obtained.118–120
Nonoperative Management of Diskogenic Lower Back Pain
Nonoperative management of diskogenic LBP, including pain medication, bracing, and physical therapy, should always be the initial therapeutic strategy. Smith and coworkers found that 68% of patients with diskogram-positive LBP had improved with conservative management at a minimum follow-up of 3 years.121 Surgical management should be considered only after nonoperative treatment strategies have been exhausted.
Surgical Management of Diskogenic Lower Back Pain
Lumbar Fusion
The most powerful study to date on the efficacy of lumbar fusion for the treatment of diskogenic LBP comes from the 2001 Swedish multicenter randomized clinical trial of lumbar fusion versus nonsurgical treatment.122 In this study, Fritzell and colleagues prospectively studied a group of 294 patients with axial LBP randomized to conservative treatment or one of three different fusion groups: (1) noninstrumented posterolateral fusion, (2) instrumented posterolateral fusion, or (3) instrumented posterolateral fusion with an interbody graft. Overall objective clinical results from independent observers, subjective patient-based results, and improvements in pain (visual analogue scale [VAS]) and disability (Oswestry Disability Index [ODI]) were significantly better in the surgical group than in the nonsurgical group. Results were similar when comparing the three fusion strategies, although instrumented fusions tended to heal more quickly.
Despite Fritzell and colleagues’ landmark study, there has not been a consistent correlation between fusion status and clinical success in the treatment of diskogenic LBP. Several authors have found that although successful fusion occurred in 85% to 93% of patients, a successful clinical outcome was achieved in just 58% to 67%.123–126 It is generally thought that complete elimination of motion is crucial for successful treatment of DDD. In biomechanical studies, posterolateral fusion alone has been shown to be less stiff than circumferential fusion,127 and interbody fixation has been shown to be the most effective means of eliminating motion between two vertebrae.128 The addition of an interbody graft, whether via transforaminal lumbar interbody fusion (TLIF), posterior lumbar interbody fusion (PLIF), or anterior lumbar interbody fusion (ALIF), has been shown to increase clinical success rates in comparison to posterolateral fusion alone.129–131 Madan and Boeree compared PLIF with posterolateral fusion alone and found better patient satisfaction and fusion rates after PLIF, as well as better results in eliminating neurological symptoms.132 In a retrospective analysis of 42 patients with lumbar DDD and a minimum 4-year follow-up, Chastain and colleagues found significant improvements in both VAS (before, 6.3; after, 3.9) and ODI scores (before, 40.5%; after, 30.0%) after TLIF, although they concluded that radiographic evidence of solid fusion did not correlate with successful clinical outcomes.133
With regard to analyzing differences in outcomes and complications among the three circumferential fusion techniques (PLIF, TLIF, ALIF), Villavicencio and associates compared complications after TLIF with those after AP fixation and found that AP fusion is associated with a greater than two times higher complication rate, increased blood loss, and longer operative and hospitalization times.134 Freudenberger and colleagues retrospectively analyzed 59 patients with LBP as a result of one- or two-level lumbar DDD that was treated by either PLIF or stand-alone ALIF with anterior tension band plating (ALIF-ATB) and found similar fusion and functional outcomes but shorter operative times and blood loss after ALIF-ATB.135 Madan and Boeree also found similar clinical outcomes after either instrumented standalone ALIF or PLIF for patients with lumbar DDD.136 Hackenberg and colleagues found significant improvements in both ODI and VAS scores after TLIF at an average follow-up of 46 months, along with a fusion rate of 89%.137 The authors concluded that radiographic and clinical outcomes after TLIF were as good as those reported in the literature for either PLIF or ALIF in the treatment of degenerative lumbar disease while avoiding the morbidity of an anterior approach (ALIF) and reducing the approach-related posterior manipulation of the spinal canal (PLIF). Moreover, TLIF allows direct decompression of neural structures, whereas stand-alone ALIF provides only indirect decompression.134
Total Disk Replacement
Fusion of the lumbar spine is associated with several drawbacks, including a risk for pseudoarthrosis, degeneration of adjacent segments, and limitations in mobility after surgery. The idea of total disk replacement, or disk arthroplasty, was developed out of a desire to avoid these drawbacks while at the same time restoring the segmental motion of the lumbar spine to physiologic parameters. There are far fewer studies on the efficacy of total disk replacement in the treatment of LBP. Mayer and coworkers analyzed 23 patients treated with the ProDisc Lumbar Disc Replacement (Synthes Spine) for diskogenic LBP and reported significant reductions in ODI and VAS scores at a 1-year follow-up.138 Additionally, 74% of patients were “completely satisfied” or “satisfied” with their result. A prospective randomized study by Blumenthal and colleagues in which the Charité (DePuy Spine) disk replacement was examined found clinical outcomes comparable to those of ALIF, but with shorter hospitalization, a lower rate of reoperation, and a higher rate of patient satisfaction in the Charité goup.139 In general, total disk replacement seems to be comparable to lumbar arthrodesis; however, longer term studies are needed.
Future Treatment of Diskogenic Lower Back Pain
NP replacement with an injectable hydrogel or tissue-engineered construct is one nonfusion technique currently being explored. Because fusion can lead to disease in adjacent segments, the objective of NP replacement is to restore normal disk mechanics by effectively transferring loads to the AF. The implant material must be nontoxic and noncarcinogenic and withstand a large amount of fatigue loading. To date, only the Prosthetic Disc Nucleus (PDN; Raymedica, Bloomington, MN) has received approval by the Food and Drug Administration for use in the United States. Approximately 500 patients have had the PDN device implanted into the lumbar spine, with a success rate close to 80%.140 Four-year follow-up has shown a significant reduction in ODI scores and improvement in symptoms of DDD. The main complications associated with the PDN device include end plate damage with subsidence or extrusion.141 Several other materials are under investigation in animal studies. The DASCOR disk arthroplasty device (Disc Dynamics, Eden Prairie, MN) is currently in postmarket studies in Europe and undergoing a pilot study in the United States.
With better understanding of the molecular mechanisms that lead to DDD, greater emphasis has been placed on potential biologic treatments. There is compelling in vitro evidence for the feasibility of delivery of growth factors to reverse degenerative changes in the IVD. Animal and culture studies have shown that autologous delivery of TGF-β, epidermal growth factor, bFGF, insulin-like growth factor type I, recombinant human bone morphogenic protein 2 (rhBMP-2), rhBMP-7 (also known as osteogenic protein 1 [OP-1]), recombinant human growth and differentiation factor 5 (rhGDF-5), platelet-rich plasma, and an IL-1 receptor antagonist induce anabolic changes in IVD cells that result in increased synthesis of matrix and reduced levels of DDD biomarkers.142,143 Work by Takegami and colleagues has shown that matrix degraded by IL-1 or chemonucleolysis could be regenerated by delivery of OP-1 to rabbit IVD cells.144,145 Recent in vivo work supports the potential use of growth factors as well. A single injection of OP-1 into rabbit IVDs after needle puncture–induced degeneration led to restoration of disk height and increased signaling on T2-weighted MRI that appeared at 6 weeks and remained throughout the 24-week study period. Proteoglycan synthesis was greater and the histologic degenerative grade was lower than in the control injection group.146 Similar results in a rabbit model have been found with the use of rhGDF-5.147
One of the shortcomings of growth factor delivery as therapy for DDD is its apparent short duration. One novel approach that would meet this challenge is gene therapy. Two concerns need to be addressed before this technology can become a reality, which genes to administer and the most appropriate delivery method. Better understanding of the molecular mechanisms underlying DDD, development of safer and more reliable vectors, and improved regulation of transgenes continue to make this an exciting area of research with great clinical potential.148
Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine. 2005;30:1565-1575.
Boos N, Semmer N, Elfering A, et al. Natural history of individuals with asymptomatic disc abnormalities in magnetic resonance imaging: predictors of low back pain-related medical consultation and work incapacity. Spine. 2000;25:1484-1492.
Braithwaite I, White J, Saifuddin A, et al. Vertebral end-plate (Modic) changes on lumbar spine MRI: correlation with pain reproduction at lumbar discography. Eur Spine J. 1998;7:363-368.
Carragee EJ, Paragioudakis SJ, Khurana S. 2000 Volvo Award winner in clinical studies: lumbar high-intensity zone and discography in subjects without low back problems. Spine. 2000;25:2987-2992.
Elfering A, Semmer N, Birkhofer D, et al. Risk factors for lumbar disc degeneration: a 5-year prospective MRI study in asymptomatic individuals. Spine. 2002;27:125-134.
Fritzell P, Hagg O, Wessberg P, et al. 2001 Volvo Award Winner in Clinical Studies: lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26:2521-2532.
Masuda K, Oegema TRJr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine. 2004;29:2757-2769.
Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120-130.
Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700-2709.
1 Andersson GB. Epidemiology of low back pain. Acta Orthop Scand Suppl. 1998;281:28-31.
2 Maetzel A, Li L. The economic burden of low back pain: a review of studies published between 1996 and 2001. Best Pract Res Clin Rheumatol. 2002;16:23-30.
3 Spivak JM. Vertebroplasty: weighing the benefits and the risks. Am Fam Physician. 2002;66:565.
4 Benneker LM, Heini PF, Alini M, et al. 2004 Young Investigator Award Winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine. 2005;30:167-173.
5 Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine. 1990;15:402-410.
6 Maroudas A, Stockwell RA, Nachemson A, et al. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113-130.
7 Trout JJ, Buckwalter JA, Moore KC. Ultrastructure of the human intervertebral disc: II. Cells of the nucleus pulposus. Anat Rec. 1982;204:307-314.
8 Trout JJ, Buckwalter JA, Moore KC, et al. Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell. 1982;14:359-369.
9 Lotz JC, Hsieh AH, Walsh AL, et al. Mechanobiology of the intervertebral disc. Biochem Soc Trans. 2002;30:853-858.
10 Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res. 1999;246:129-137.
11 Pazzaglia UE, Salisbury JR, Byers PD. Development and involution of the notochord in the human spine. J R Soc Med. 1989;82:413-415.
12 Setton LA, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am. 2006;88(suppl 2):52-57.
13 Lotz JC, Colliou OK, Chin JR, et al. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine. 1998;23:2493-2506.
14 Walsh AJ, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech. 2004;37:329-337.
15 Chelberg MK, Banks GM, Geiger DF, et al. Identification of heterogeneous cell populations in normal human intervertebral disc. J Anat. 1995;186:43-53.
16 Errington RJ, Puustjarvi K, White IR, et al. Characterisation of cytoplasm-filled processes in cells of the intervertebral disc. J Anat. 1998;192:369-378.
17 Buckwalter JA, Mow VC, Boden SD, et al. Intervertebral disc structure, composition, and mechanical function. In: Buckwalter JA, Mow VC, editors. Orthopaedic Basic Science. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2000:547-556.
18 Cassinelli EH, Hall RA, Kang JD. Biochemistry of intervertebral disc degeneration and the potential for gene therapy applications. Spine J. 2001;1:205-214.
19 Johnson EF, Chetty K, Moore IM, et al. The distribution and arrangement of elastic fibres in the intervertebral disc of the adult human. J Anat. 1982;135:301-309.
20 Yu J, Winlove PC, Roberts S, et al. Elastic fibre organization in the intervertebral discs of the bovine tail. J Anat. 2002;201:465-475.
21 Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connect Tissue Res. 1989;23:75-88.
22 Olczyk K. Age-related changes of elastin content in human intervertebral discs. Folia Histochem Cytobiol. 1994;32:41-44.
23 Smith LJ, Fazzalari NL. Regional variations in the density and arrangement of elastic fibres in the anulus fibrosus of the human lumbar disc. J Anat. 2006;209:359-367.
24 Yu J, Fairbank JC, Roberts S, et al. The elastic fiber network of the anulus fibrosus of the normal and scoliotic human intervertebral disc. Spine. 2005;30:1815-1820.
25 Pezowicz CA, Robertson PA, Broom ND. The structural basis of interlamellar cohesion in the intervertebral disc wall. J Anat. 2006;208:317-330.
26 Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700-2709.
27 Nachemson A, Lewin T, Maroudas A, et al. In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthop Scand. 1970;41:589-607.
28 Bernick S, Cailliet R. Vertebral end-plate changes with aging of human vertebrae. Spine. 1982;7:97-102.
29 Bartels EM, Fairbank JC, Winlove CP, et al. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine. 1998;23:1-7.
30 Brodin H. Paths of nutrition in articular cartilage and intervertebral discs. Acta Orthop Scand. 1955;24:177-183.
31 Ogata K, Whiteside LA. 1980 Volvo award winner in basic science. Nutritional pathways of the intervertebral disc. An experimental study using hydrogen washout technique. Spine. 1981;6:211-216.
32 Bogduk N. The innervation of the lumbar spine. Spine. 1983;8:286-293.
33 Palmgren T, Gronblad M, Virri J, et al. An immunohistochemical study of nerve structures in the anulus fibrosus of human normal lumbar intervertebral discs. Spine. 1999;24:2075-2079.
34 Fagan A, Moore R, Vernon Roberts B, et al. ISSLS prize winner: the innervation of the intervertebral disc: a quantitative analysis. Spine. 2003;28:2570-2576.
35 Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22:181-187.
36 Adams MA. Biomechanics of back pain. Acupunct Med. 2004;22:178-188.
37 Adams MA, Bogduk N, Burton K, et al. The Biomechanics of Back Pain. Edinburgh: Churchill Livingstone; 2002.
38 Boos N, Weissbach S, Rohrbach H, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631-2644.
39 Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307-1314.
40 Thompson JP, Pearce RH, Schechter MT, et al. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411-415.
41 Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151-2161.
42 Iatridis JC, Gwynn I. Mechanisms for mechanical damage in the intervertebral disc annulus fibrosus. J Biomech. 2004;37:1165-1175.
43 Peng B, Wu W, Hou S, et al. The pathogenesis of discogenic low back pain. J Bone Joint Surg Br. 2005;87:62-67.
44 Roberts S, Eisenstein SM, Menage J, et al. Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides. Spine. 1995;20:2645-2651.
45 Johnson WE, Eisenstein SM, Roberts S. Cell cluster formation in degenerate lumbar intervertebral discs is associated with increased disc cell proliferation. Connect Tissue Res. 2001;42:197-207.
46 Lotz JC, Ulrich JA. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. J Bone Joint Surg Am. 2006;88(suppl 2)):76-82.
47 Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47-54.
48 Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732-45.
49 Peng B, Hao J, Hou S, et al. Possible pathogenesis of painful intervertebral disc degeneration. Spine. 2006;31:560-566.
50 Hollander AP, Heathfield TF, Liu JJ, et al. Enhanced denaturation of the alpha (II) chains of type-II collagen in normal adult human intervertebral discs compared with femoral articular cartilage. J Orthop Res. 1996;14:61-66.
51 Cloyd JM, Elliott DM. Elastin content correlates with disc degeneration in the annulus fibrosus and nucleus pulposus. Spine. 2007;32:1826-1831.
52 Fujita K, Nakagawa T, Hirabayashi K, et al. Neutral proteinases in human intervertebral disc. Role in degeneration and probable origin. Spine. 1993;18:1766-1773.
53 Melrose J, Ghosh P, Taylor TK. Lysozyme, a major low-molecular-weight cationic protein of the intervertebral disc, which increases with ageing and degeneration. Gerontology. 1989;35:173-180.
54 Roberts S, Caterson B, Menage J, et al. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005-3013.
55 Kanemoto M, Hukuda S, Komiya Y, et al. Immunohistochemical study of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 human intervertebral discs. Spine. 1996;21:1-8.
56 Duance VC, Crean JK, Sims TJ, et al. Changes in collagen cross-linking in degenerative disc disease and scoliosis. Spine. 1998;23:2545-2551.
57 Johnstone B, Bayliss MT. The large proteoglycans of the human intervertebral disc. Changes in their biosynthesis and structure with age, topography, and pathology. Spine. 1995;20:674-684.
58 Anderson DG, Tannoury C. Molecular pathogenic factors in symptomatic disc degeneration. Spine J. 2005;5:260S-266S.
59 Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673:443-453.
60 Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine. 1988;13:179-187.
61 Iatridis JC, Setton LA, Weidenbaum M, et al. Alterations in the mechanical behavior of the human lumbar nucleus pulposus with degeneration and aging. J Orthop Res. 1997;15:318-322.
62 Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120-130.
63 Iatridis JC, Setton LA, Foster RJ, et al. Degeneration affects the anisotropic and nonlinear behaviors of human anulus fibrosus in compression. J Biomech. 1998;31:535-544.
64 Adams MA, McNally DS, Dolan P. tress’ distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br. 1996;78:965-972.
65 Goel VK, Monroe BT, Gilbertson LG, et al. Interlaminar shear stresses and laminae separation in a disc. Finite element analysis of the L3-L4 motion segment subjected to axial compressive loads. Spine. 1995;20:689-698.
66 Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17:829-835.
67 Razaq S, Wilkins RJ, Urban JP. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur Spine J. 2003;12:341-349.
68 Kitano T, Zerwekh JE, Usui Y, et al. Biochemical changes associated with the symptomatic human intervertebral disk. Clin Orthop Relat Res. 1993;293:372-377.
69 Keshari KR, Lotz JC, Link TM, et al. Lactic acid and proteoglycans as metabolic markers for discogenic back pain. Spine. 2008;33:312-317.
70 Kauppila LI, McAlindon T, Evans S, et al. Disc degeneration/back pain and calcification of the abdominal aorta. A 25-year follow-up study in Framingham. Spine. 1997;22:1642-1647.
71 Kauppila LI. Prevalence of stenotic changes in arteries supplying the lumbar spine. A postmortem angiographic study on 140 subjects. Ann Rheum Dis. 1997;56:591-595.
72 Holm S, Nachemson A. Variations in the nutrition of the canine intervertebral disc induced by motion. Spine. 1983;8:866-874.
73 Akmal M, Kesani A, Anand B, et al. Effect of nicotine on spinal disc cells: a cellular mechanism for disc degeneration. Spine. 2004;29:568-575.
74 Oda H, Matsuzaki H, Tokuhashi Y, et al. Degeneration of intervertebral discs due to smoking: experimental assessment in a rat-smoking model. J Orthop Sci. 2004;9:135-141.
75 Roberts S, Urban JP, Evans H, et al. Transport properties of the human cartilage endplate in relation to its composition and calcification. Spine. 1996;21:415-420.
76 An HS, Anderson PA, Haughton VM, et al. Introduction: disc degeneration: summary. Spine. 2004;29:2677-2678.
77 Burke JG, G Watson RW, Conhyea D, et al. Human nucleus pulposus can respond to a pro-inflammatory stimulus. Spine. 2003;28:2685-2693.
78 Burke JG, Watson RW, McCormack D, et al. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196-201.
79 Weiler C, Nerlich AG, Bachmeier BE, et al. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine. 2005;30:44-53.
80 Allan DB, Waddell G. An historical perspective on low back pain and disability. Acta Orthop Scand Suppl. 1989;234:1-23.
81 Kroeber MW, Unglaub F, Wang H, et al. New in vivo animal model to create intervertebral disc degeneration and to investigate the effects of therapeutic strategies to stimulate disc regeneration. Spine. 2002;27:2684-2690.
82 Rousseau MA, Ulrich JA, Bass EC, et al. Stab incision for inducing intervertebral disc degeneration in the rat. Spine. 2007;32:17-24.
83 Boos N, Semmer N, Elfering A, et al. Natural history of individuals with asymptomatic disc abnormalities in magnetic resonance imaging: predictors of low back pain-related medical consultation and work incapacity. Spine. 2000;25:1484-1492.
84 Deyo RA, Bass JE. Lifestyle and low-back pain. The influence of smoking and obesity. Spine. 1989;14:501-506.
85 Heliovaara M. Risk factors for low back pain and sciatica. Ann Med. 1989;21:257-264.
86 Ariga K, Yonenobu K, Nakase T, et al. Mechanical stress–induced apoptosis of endplate chondrocytes in organ-cultured mouse intervertebral discs: an ex vivo study. Spine. 2003;28:1528-1533.
87 Holm S, Holm AK, Ekstrom L, et al. Experimental disc degeneration due to endplate injury. J Spinal Disord Tech. 2004;17:64-71.
88 Sato K, Kikuchi S, Yonezawa T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine. 1999;24:2468-2474.
89 Przybyla A, Pollintine P, Bedzinski R, et al. Outer annulus tears have less effect than endplate fracture on stress distributions inside intervertebral discs: relevance to disc degeneration. Clin Biomech (Bristol, Avon). 2006;21:1013-1019.
90 Brinckmann P, Grootenboer H. Change of disc height, radial disc bulge, and intradiscal pressure from discectomy. An in vitro investigation on human lumbar discs. Spine. 1991;16:641-646.
91 Meakin JR, Redpath TW, Hukins DW. The effect of partial removal of the nucleus pulposus from the intervertebral disc on the response of the human annulus fibrosus to compression. Clin Biomech (Bristol, Avon). 2001;16:121-128.
92 Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4:190S-194S.
93 Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366-372.
94 Battie MC, Videman T, Gibbons LE, et al. 1995 Volvo Award in clinical sciences. Determinants of lumbar disc degeneration. A study relating lifetime exposures and magnetic resonance imaging findings in identical twins. Spine. 1995;20:2601-2612.
95 Paassilta P, Lohiniva J, Goring HH, et al. Identification of a novel common genetic risk factor for lumbar disk disease. JAMA. 2001;285:1843-1849.
96 Kawaguchi Y, Osada R, Kanamori M, et al. Association between an aggrecan gene polymorphism and lumbar disc degeneration. Spine. 1999;24:2456-2460.
97 Videman T, Gibbons LE, Battie MC, et al. The relative roles of intragenic polymorphisms of the vitamin D receptor gene in lumbar spine degeneration and bone density. Spine. 2001;26:E7-E12.
98 Takahashi M, Haro H, Wakabayashi Y, et al. The association of degeneration of the intervertebral disc with 5a/6a polymorphism in the promoter of the human matrix metalloproteinase-3 gene. J Bone Joint Surg Br. 2001;83:491-495.
99 Freemont AJ, Peacock TE, Goupille P, et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178-181.
100 Brown MF, Hukkanen MV, McCarthy ID, et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79:147-153.
101 Aoki Y, Ohtori S, Takahashi K, et al. Innervation of the lumbar intervertebral disc by nerve growth factor–dependent neurons related to inflammatory pain. Spine. 2004;29:1077-1081.
102 Dietemann J-L. Imaging of degenerative disc disease. In: Gunzburg R, Szpalski M, Andersson GB, editors. Degenerative Disc Disease. Philadelphia: Lippincott Williams & Wilkins, 2004.
103 Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873-1878.
104 Modic MT. Degenerative disc disease and back pain. Magn Reson Imaging Clin N Am. 1999;7:481-491. viii
105 Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403-408.
106 Braithwaite I, White J, Saifuddin A, et al. Vertebral end-plate (Modic) changes on lumbar spine MRI: correlation with pain reproduction at lumbar discography. Eur Spine J. 1998;7:363-368.
107 Aprill C, Bogduk N. High-intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65:361-369.
108 Carragee EJ, Paragioudakis SJ, Khurana S. 2000 Volvo Award winner in clinical studies: lumbar high-intensity zone and discography in subjects without low back problems. Spine. 2000;25:2987-2992.
109 Elfering A, Semmer N, Birkhofer D, et al. Risk factors for lumbar disc degeneration: a 5-year prospective MRI study in asymptomatic individuals. Spine. 2002;27:125-134.
110 Colhoun E, McCall IW, Williams L, et al. Provocation discography as a guide to planning operations on the spine. J Bone Joint Surg Br. 1988;70:267-271.
111 Fluke MM. The treatment of lumbar spine pain syndromes diagnosed by discography: lumbar arthrodesis. Spine. 1995;20:501-504.
112 Murtagh FR, Arrington JA. Computer tomographically guided discography as a determinant of normal disc level before fusion. Spine. 1992;17:826-830.
113 Holt EPJr. The question of lumbar discography. J Bone Joint Surg Am. 1968;50:720-726.
114 Walsh TR, Weinstein JN, Spratt KF, et al. Lumbar discography in normal subjects. A controlled, prospective study. J Bone Joint Surg Am. 1990;72:1081-1088.
115 Derby R, Howard MW, Grant JM, et al. The ability of pressure-controlled discography to predict surgical and nonsurgical outcomes. Spine. 1999;24:364-371.
116 Madan S, Gundanna M, Harley JM, et al. Does provocative discography screening of discogenic back pain improve surgical outcome? J Spinal Disord Tech. 2002;15:245-251.
117 Cohen SP, Larkin TM, Barna SA, et al. Lumbar discography: a comprehensive review of outcome studies, diagnostic accuracy, and principles. Reg Anesth Pain Med. 2005;30:163-183.
118 Carragee EJ. Is lumbar discography a determinate of discogenic low back pain: provocative discography reconsidered. Curr Rev Pain. 2000;4:301-308.
119 Carragee EJ, Tanner CM, Khurana S, et al. The rates of false-positive lumbar discography in select patients without low back symptoms. Spine. 2000;25:1373-1380.
120 Block AR, Vanharanta H, Ohnmeiss DD, et al. Discographic pain report. Influence of psychological factors. Spine. 1996;21:334-338.
121 Smith SE, Darden BV, Rhyne AL, et al. Outcome of unoperated discogram-positive low back pain. Spine. 1995;20:1997-2000.
122 Fritzell P, Hagg O, Wessberg P, et al. 2001 Volvo Award Winner in Clinical Studies: lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26:2521-2532.
123 Zucherman J, Hsu K, Picetti G3rd, et al. Clinical efficacy of spinal instrumentation in lumbar degenerative disc disease. Spine. 1992;17:834-837.
124 Jackson RK, Boston DA, Edge AJ. Lateral mass fusion. A prospective study of a consecutive series with long-term follow-up. Spine. 1985;10:828-832.
125 Wetzel FT, LaRocca SH, Lowery GL, et al. The treatment of lumbar spinal pain syndromes diagnosed by discography. Lumbar arthrodesis. Spine. 1994;19:792-800.
126 Zdeblick TA. A prospective, randomized study of lumbar fusion. Preliminary results. Spine. 1993;18:983-991.
127 Le Huec JC, Liu M, Skalli W, et al. Lumbar lateral interbody cage with plate augmentation: in vitro biomechanical analysis. Eur Spine J. 2002;11:130-136.
128 Zdeblick TA, Smith GR, Warden KE, et al. Two-point fixation of the lumbar spine. Differential stability in rotation. Spine. 1991;16:S298-S301.
129 Lee CK, Vessa P, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements. The results of disc excision and posterior lumbar interbody fusion. Spine. 1995;20:356-361.
130 Loguidice VA, Johnson RG, Guyer RD, et al. Anterior lumbar interbody fusion. Spine. 1988;13:366-369.
131 Blumenthal SL, Baker J, Dossett A, et al. The role of anterior lumbar fusion for internal disc disruption. Spine. 1988;13:566-569.
132 Madan S, Boeree NR. Outcome of posterior lumbar interbody fusion versus posterolateral fusion for spondylolytic spondylolisthesis. Spine. 2002;27:1536-1542.
133 Chastain CA, Eck JC, Hodges SD, et al. Transforaminal lumbar interbody fusion: a retrospective study of long-term pain relief and fusion outcomes. Orthopedics. 2007;30:389-392.
134 Villavicencio AT, Burneikiene S, Bulsara KR, et al. Perioperative complications in transforaminal lumbar interbody fusion versus anterior-posterior reconstruction for lumbar disc degeneration and instability. J Spinal Disord Tech. 2006;19:92-97.
135 Freudenberger C, Lindley EM, Beard DW, et al. Posterior versus anterior lumbar interbody fusion with anterior tension band plating: retrospective analysis. Orthopedics. 2009;32:492.
136 Madan SS, Boeree NR. Comparison of instrumented anterior interbody fusion with instrumented circumferential lumbar fusion. Eur Spine J. 2003;12:567-575.
137 Hackenberg L, Halm H, Bullmann V, et al. Transforaminal lumbar interbody fusion: a safe technique with satisfactory three to five year results. Eur Spine J. 2005;14:551-558.
138 Mayer HM, Wiechert K, Korge A. Total disc replacement for low back pain of discogenic origin. In: Gunzburg R, Szpalski M, Anderson SE, editors. Degenerative Disc Disease. Philadelphia: Lippincott Williams & Wilkins, 2004.
139 Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine. 2005;30:1565-1575.
140 Klara PM, Ray CD. Artificial nucleus replacement: clinical experience. Spine. 2002;27:1374-1377.
141 Di Martino A, Vaccaro AR, Lee JY, et al. Nucleus pulposus replacement: basic science and indications for clinical use. Spine. 2005;30:S16-S22.
142 Masuda K, An HS. Prevention of disc degeneration with growth factors. Eur Spine J. 2006;15(suppl 3):S422-S432.
143 Masuda K, Oegema TRJr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine. 2004;29:2757-2769.
144 Takegami K, An HS, Kumano F, et al. Osteogenic protein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chondroitinase ABC-induced in vitro chemonucleolysis. Spine J. 2005;5:231-238.
145 Takegami K, Thonar EJ, An HS, et al. Osteogenic protein-1 enhances matrix replenishment by intervertebral disc cells previously exposed to interleukin-1. Spine. 2002;27:1318-1325.
146 Masuda K, Imai Y, Okuma M, et al. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine. 2006;31:742-754.
147 Chujo T, An HS, Akeda K, et al. Effects of growth differentiation factor-5 on the intervertebral disc—in vitro bovine study and in vivo rabbit disc degeneration model study. Spine. 2006;31:2909-2917.
148 Vadala G, Sowa GA, Kang JD. Gene therapy for disc degeneration. Expert Opin Biol Ther. 2007;7:185-196.