Chapter 36 Diabetes mellitus, insulin, oral antidiabetes agents, obesity
History of insulin therapy in diabetes
Diabetes was known to ancient Greek medicine with the description of ‘a melting of the flesh and limbs into urine … the patients never stop making water but the flow is incessant … their mouth becomes parched and their body dry’.1
Many doctors, after they have developed a disease, take up the speciality in it … But that was not so with me. I was studying for surgery when diabetes took me up. The great book of Joslin said that by starving you might live four years with luck. [He went to Italy and, whilst his health was declining there, he received a letter from a biochemist friend which said] there was something called ‘insulin’ appearing with a good name in Canada, what about going there and getting it. I said ‘No thank you; I’ve tried too many quackeries for diabetes; I’ll wait and see’. Then I got peripheral neuritis … So when [the friend] cabled me and said, ‘I’ve got insulin – it works – come back quick’, I responded, arrived at King’s College Hospital, London, and went to the laboratory as soon as it opened … It was all experimental for [neither of us] knew a thing about it … So we decided to have 20 units a nice round figure. I had a nice breakfast. I had bacon and eggs and toast made on the Bunsen. I hadn’t eaten bread for months and months … by 3 o’clock in the afternoon my urine was quite sugar free. That hadn’t happened for many months. So we gave a cheer for Banting and Best.2
But at 4 pm I had a terrible shaky feeling and a terrible sweat and hunger pain. That was my first experience of hypoglycaemia. We remembered that Banting and Best had described an overdose of insulin in dogs. So I had some sugar and a biscuit and soon got quite well, thank you.3
Diabetes mellitus is classified broadly as:
• Type 1 (formerly, insulin dependent diabetes mellitus, IDDM) which typically occurs in younger people who cannot secrete insulin.
• Type 2 (formerly, non-insulin dependent diabetes mellitus, NIDDM), which usually occurs in older people who are typically (although not always) obese. Type 2 diabetes is best thought of as a group of conditions characterised by a variable combination of reduced insulin secretion and resistance to insulin’s blood glucose lowering action.
• Other causes: including gestational diabetes, disease processes affecting the liver or pancreas such as cystic fibrosis causing a ‘secondary’ diabetes, monogenic forms (maturity onset diabetes of the young, MODY).
Sources of insulin
• Bovine insulin differs from human insulin by three amino acids.
• Porcine insulin differs from human by only one amino acid.
• Human insulin is made either by enzyme modification of porcine insulin, or by using recombinant DNA to synthesise the pro-insulin precursor molecule for insulin. This is done by artificially introducing the DNA into either Escherichia coli or yeast.4
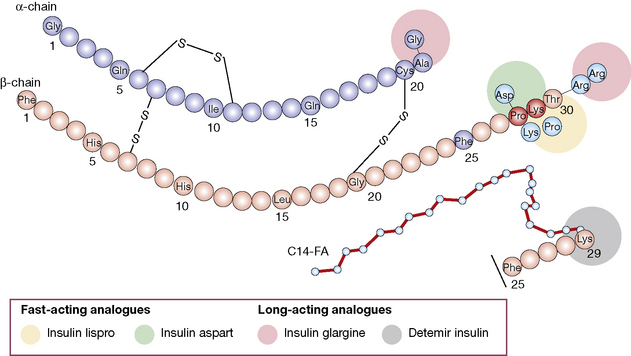
• Insulin analogues are now widely used and have modifications introduced to the A and/or B chains, which result in more rapid onset and offset of action (rapidly acting analogues), or slower offset (long acting analogues) than naturally occurring insulin.
Actions of insulin
• Reduction in blood glucose is due to increased glucose uptake in peripheral tissues (which oxidise glucose or convert into glycogen or fat), and a reduction in hepatic output of glucose (diminished breakdown/increased synthesis of glycogen and diminished gluconeogenesis).
• Other metabolic effects. Although, therapeutically, insulin is thought of as a blood glucose lowering hormone, it has a number of other cellular actions. Insulin is an anabolic hormone, enhancing protein synthesis (which has resulted in cases of misuse by bodybuilders). It also inhibits both breakdown of fats (lipolysis) and ketogenesis. Insulin also has actions on electrolytes, stimulating potassium uptake into cells and renal sodium retention (anti-natriuretic effect). Within brain, insulin may have actions to stimulate memory and act as a nutritional signal to help control appetite/food intake.
Uses
• Diabetes mellitus is the main indication.
• Insulin promotes the passage of potassium into cells by stimulating cell surface Na/K ATPase action, and this effect is utilised to correct hyperkalaemia (see p. 458).
• Insulin-induced hypoglycaemia can also be used as a stress test of anterior pituitary function (growth hormone and corticotropin and thus cortisol are released).
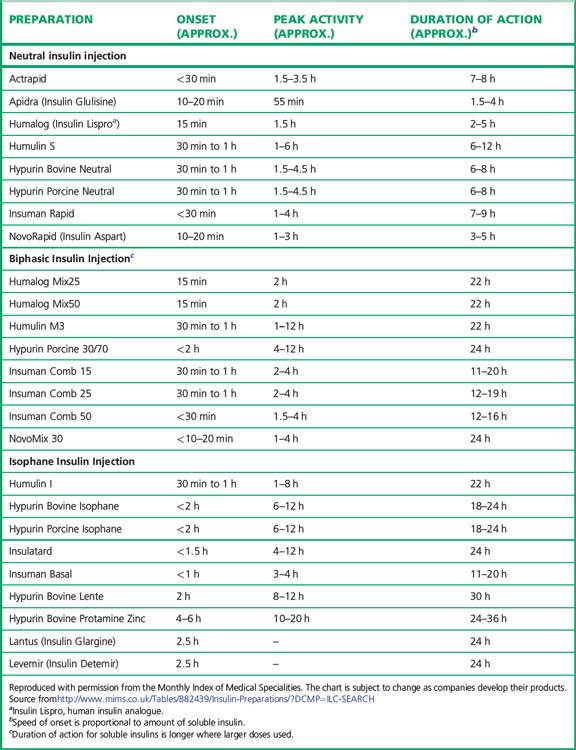
Preparations of insulin (Table 36.1)
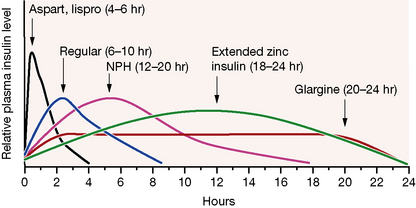
Broadly speaking, four different types of insulin with differing time-courses of action are available for treating diabetes (illustrated in Fig. 36.1):
1. Short duration of action (and rapid onset). Soluble insulin (also called neutral or regular insulin). The most recent additions to this class of insulin, lispro, aspart and glulisine, are modified human insulins with changes in the B chain resulting in more rapid absorption after subcutaneous injection and thus a faster onset and shorter duration of action.
2. Intermediate duration of action (and slower onset). Preparations in which the insulin has been modified physically by combination with protamine or zinc to give an amorphous or crystalline suspension; this is given subcutaneously and slowly dissociates to release insulin in its soluble form. Isophane (NPH) insulin, a suspension with protamine, is still widely used. Insulin Zinc Suspensions (amorphous or a mixture of amorphous and crystalline) are now rarely used.
3. Longer duration of action. Newer analogues glargine and detemir (Fig. 36.2) have become widely used, especially in type 1 diabetes. Small changes in the amino acid structure of glargine result in a significant slowing of absorption from subcutaneous depots. In contrast, detemir owes its protracted action to fatty acylation. After absorption, detemir is thus bound to circulating albumin which delays its action.
4. A biphasic mixture of soluble or short acting analogue insulin with isophane insulin.
Choice of insulin regimen
1. ‘Basal bolus’ therapy: multiple injections of short acting insulin are given during the day to mimic prandial secretion of insulin by the pancreas, combined with once or twice daily intermediate or long acting insulin to provide the background insulin. This approach aims to mimic the non-diabetic pattern of insulin release. The total insulin dose is usually apportioned to be 40–60% background and 40–60% prandial.
When choosing the short acting insulin in a basal bolus regimen, soluble insulin is given 30 min before meals. Short acting analogues may be given immediately before, during or even after the meal, although recent data suggest that even these insulins may be more effective if given 15 min prior to eating. The more rapid waning of action profile also means that the risk of hypoglycaemic reactions before the next meal may be lower with the analogues. For choice of background insulin, long acting analogues may give less risk of nocturnal hypoglycaemia than NPH insulin (see Fig. 36.1) although NPH insulins offer greater flexibility if patients need to change background insulin from day to day (as with some sportsmen or pregnant women, for example).
2. Twice daily therapy involves two injections of biphasic insulin. Although less ‘physiological’ than basal bolus, it is simpler, with fewer insulin injections. The available mixtures are listed in Table 36.1. The most commonly used is 30:70 (soluble: NPH). Typically half to two-thirds of the daily dose may be given in the morning before breakfast and half to one-third before the evening meal. A combination of biphasic insulin with breakfast and fast acting insulin with evening meal and bedtime background insulin may be useful in some children with type 1 diabetes to avoid having to inject insulin at school.
3. Background or prandial insulin alone may be sufficient in type 2 diabetes when patients progress from oral therapy on to insulin. In this situation, oral therapy is usually continued in combination with insulin.
Oral antidiabetes drugs
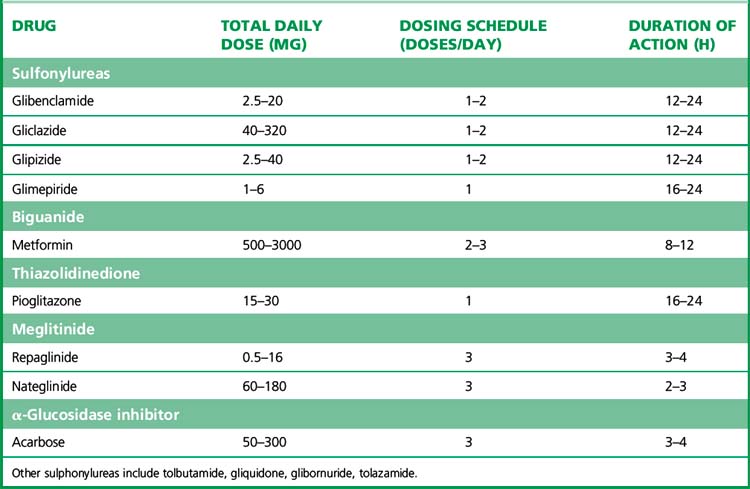
(i) Insulin secretagogues
Several sulfonylureas are available (see also Table 36.2). Choice is determined by the duration of action as well as the patient’s age and renal function, and unwanted effects. The long acting sulfonylureas, e.g. glibenclamide, are associated with a greater risk of hypoglycaemia; for this reason they should be avoided in the elderly, for whom shorter acting alternatives, such as gliclazide, are preferred. In patients with impaired renal function, gliclazide, glipizide and tolbutamide are preferred as they are not excreted by the kidney. Gliclazide is a commonly used second-generation agent. If the dose exceeds 80 mg, the drug should be taken twice daily before meals, or once daily if prescribed as a modified-release preparation. Glimepiride is designed to be used once daily and provokes less hypoglycaemia than glibenclamide.
Meglitinides
such as repaglinide (t½ 1 h) are short-acting oral hypoglycaemic agents that have not been widely used in clinical practice. Like sulfonylureas, they act by blockade of ATP-dependent potassium channels (Table 36.2). The shorter action profile of meglitinides compared with sulfonylureas should in theory reduce risk of hypoglycaemia.
(ii) Insulin sensitisers
Biguanides
(see also Table 36.2) have been available since 1957. Metformin is now the only biguanide in use, and is a major agent in the management of type 2 diabetes. The most important physiological effect appears to be an increase in hepatic insulin sensitivity/reduction of hepatic glucose production. Recent studies have suggested that the intracellular target of metformin in the liver is the enzyme adenosine monophosphate-activated protein kinase (AMPK) system. AMPK is a conserved regulator of the cellular response to low energy, being activated when intra-cellular ATP levels decrease and AMP concentrations increase.5
Interactions with non-diabetes drugs
Interaction may occur with alcohol (hypoglycaemia with any antidiabetes drug).
Salicylates and fibrates can increase insulin sensitivity, resulting in lower blood glucose.
The use of glucagon as rescue therapy for hypoglycaemia is described above. Adrenaline/epinephrine raises the blood sugar concentration by mobilising liver and muscle glycogen (a β2-adrenoceptor effect), and suppressing secretion of insulin (an α-adrenoceptor effect). Hyperglycaemia may occur in patients with phaeochromocytoma, and is usually reversed by α-adrenoceptor blockade (see p. 383).
Drug-induced diabetes
Diazoxide
(see p. 401) is chemically similar to thiazide diuretics, but stimulates the ATP-dependent K+ channel that is blocked by the sulfonylureas. Although formerly used as an antihypertensive agent, its current use in therapeutics is confined to the rare indication of treating hypoglycaemia due to islet cell tumour (insulinoma). Adrenocortical steroids are also diabetogenic (see above).
Surgery in diabetic patients
Type 1 diabetes
Elective major surgery
• Ideally admit to hospital the day before surgery and arrange surgery for the morning.
• Evening before surgery: give patient’s usual insulin (consider 20% reduction in long-acting insulin if prone to hypoglycaemia).
• Day of operation: omit morning s.c. dose; set up i.v. infusion: glucose 5% + KCl 20 mmol/L, infuse at 100 mL/h; insulin should be infused by pump at an approximate rate of 2 units/h and adjusted according to a sliding scale.
• Modify regimen during and after surgery according to monitoring; insulin doses should be adjusted according to similar scale as that in Table 36.3.
• Stop i.v. infusion 1 h after first post-surgical s.c. soluble insulin (given when eating again).
• Insulin requirements may be high, 10–15 units/h, in cases of major surgery, serious infection, corticosteroid use, obesity.
Table 36.3 Sliding scale of insulin doses according to blood glucose concentrations (not for ketoacidosis)
| Blood glucose (mmol/L) | Infusion rate (mL/h = units/h for 50-mL syringe containing 50 units insulin) |
|---|---|
| ≥ 22.0 | 10.0 (and check pump and connections) |
| 19–21.9 | 8.0 |
| 16–18.9 | 6.0 |
| 12–15.9 | 4.0 |
| 8–11.9 | 2.0 |
| 4–7.9 | 1.0 |
| < 3.9 | 0.5 (and increase glucose infusion) |
Diabetic ketoacidosis
Intravenous fluid
Note that fluid replacement itself causes a fall in blood glucose concentration both by dilution and also by restoring blood volume to perfuse skeletal muscle, a major insulin target tissue.
Hyperosmolar diabetic coma
occurs chiefly in type 2 diabetics who fail to compensate for their continuing osmotic glucose diuresis. It is characterised by severe dehydration, a very high blood sugar level (> 33 mmol/L), and lack of ketosis and acidosis. Treatment is with isotonic (0.9%) saline, at half the rate recommended for ketoacidotic coma, and with less potassium than in severe ketoacidosis. Insulin requirements are less than in ketoacidosis, where the acidosis causes resistance to the actions of insulin, and should generally be half those shown in Table 36.3. Patients may be profoundly dehydrated and liable to thrombosis so that prophylactic low molecular weight heparin should be considered.
Preventing complications other than by glucose lowering
Aggressive treatment of hypertension and hyperlipidaemia in addition to glycaemia is particularly important in patients with diabetes. For example, the landmark UK Prospective Diabetes Study (UKPDS) of type 2 diabetes confirmed that good glycaemic control and aggressive blood pressure reduction independently improve outcome.6,7 For every 1% reduction in haemoglobin A1c (HbA1c) there was a 21% reduction in diabetes-related deaths and a 37% reduction in microvascular disease. Of highest importance was the finding that effective blood pressure control – regardless of the type of antihypertensive drug – was more influential than glycaemic control in preventing macrovascular complications. Reduction of blood pressure in 758 patients to a mean of 144/82 mmHg achieved a 32% reduction in deaths related to diabetes and a 37% reduction in microvascular endpoints, compared with findings in 390 patients treated to a blood pressure of 154/87 mmHg.
Patients with evidence of diabetic nephropathy should receive either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor antagonist; the evidence for the superiority of the latter in reducing progression to renal failure compared with other antihypertensive agents is particularly strong.8 Addition of an ACE inhibitor to other drugs may also improve overall outcome in patients with diabetes.9 In addition, diabetic nephropathy is independently associated with an increased risk of macrovascular disease so that aggressive lipid and blood pressure lowering therapy, as described above, should be employed.
• Diabetes mellitus is important in global terms because of its chronicity, and high incidence and frequency of major complications. It is generally divided into two kinds: type 1 (previously, insulin dependent diabetes mellitus) and type 2 (previously, non-insulin dependent diabetes, essentially an umbrella term for a group of conditions which are non-type 1).
• Type 1 diabetes is commoner in those with onset before age 30 whereas type 2 diabetes prevails in older patients. Increasingly, insulin therapy is required in type 2 diabetes when glycaemic control is not optimised by oral drugs.
• Insulin is usually self-administered subcutaneously to stable patients, with a variety of regimens which can be tailored to the needs of a particular patient. Modern practice in type 1 diabetes is to educate patients in flexible insulin dosing which is adjusted for differing meals, activity levels, etc.
• In the treatment of diabetic ketoacidosis, in the perioperative patient, and during inpatient management of the critically ill patient with diabetes, insulin is best given by intravenous infusion of the soluble form.
• Diet plays a major role in the treatment of type 2 diabetes, particularly where associated with obesity.
• If a drug is required in type 2 diabetes, metformin (a biguanide) is now widely used as first-line therapy, especially for the obese. Other monotherapy options include a sulfonylurea in the non-obese or a thiazolidinedione in patients intolerant of, or uncontrolled by, metformin or sulfonylurea. Many patients with type 2 diabetes will need treatment escalation with time to multiple combination therapy and/or insulin.
• Aggressive blood glucose lowering treatment of type 1 and type 2 diabetes reduces risk of microvascular complications. Close attention to associated risk factors, especially hyperlipidaemia and hypertension, is important in reducing risk of macrovascular disease.
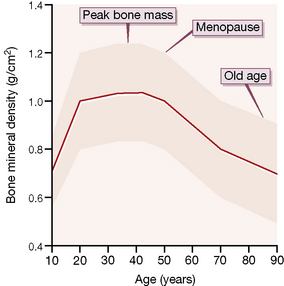
Obesity and appetite control
Individuals whose body mass index10 (BMI) lies between 25 and 30 kg/m2 are considered overweight and those in whom it exceeds 30 kg/m2 are defined as obese. Management of the condition involves a variety of approaches from nutritional advice to lifestyle alteration, drugs and, where available and appropriate, bariatric surgery. In the UK, an evidence-based algorithm coordinates these.11 The present account concentrates on pharmacological interventions.
Obesity and diabetes
As for patients without diabetes, diet and exercise are critical factors but diabetes treatment may need to be adjusted for this (for example insulin reductions to avoid hypoglycaemia with increased exercise or reduced carbohydrate intake). Metformin, either alone or as adjuvant therapy, especially with insulin as an ‘insulin-sparing’ agent is useful. GLP-1 analogue therapy offers an alternative to insulin therapy. Ultimately, as for patients without diabetes, orlistat and/or bariatric surgery12 may be appropriate.
American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2011;34(Suppl. 1):S11.
Bergenstal R.M., Tamborlane W.V., Ahmann A., et al. STAR 3 Study Group Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N. Engl. J. Med.. 2010;363:311–320.
Chan J.L., Mantzoros C.S. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet. 2005;366:74–85.
Cushman W.C., Evans G.W., Byington R.P., et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N. Engl. J. Med.. 2010;362:1575–1585.
Daneman D. Type 1 diabetes. Lancet. 2006;367:847–858.
Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med.. 2005;353:2643–2653.
Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration. Arch. Intern. Med.. 2009;169:1307–1316.
Dornhorst A. Insulinotropic meglitinide analogues. Lancet. 2001;358:1709–1716.
Drucker D.J., Nauck M.A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705.
Eckel R.H., Grundy S.M., Zimmet P.Z. The metabolic syndrome. Lancet. 2005;365:1415–1428.
Gerstein H.C., Miller M.E., Genuth S., et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N. Engl. J. Med.. 2011;364:818–828.
Ginsberg H.N., Elam M.B., Lovato L.C., et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med.. 2010;362:1563–1574.
Haslam D.W., James W.P.T. Obesity. Lancet. 2005;366:1197–1209.
Hirsch I.B. Insulin analogues. N. Engl. J. Med.. 2005;352:174–183.
Jellinger P.S. Focus on incretin-based therapies: targeting the core defects of type 2 diabetes. Postgrad. Med.. 2011;123:53–65.
Marshall S.M., Flyvbjerg A. Prevention and early detection of vascular complications of diabetes. Br. Med. J.. 2006;333:475–480.
Nathan D.M., Buse J.B., Davidson M.B., et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193.
Sherifali D., Nerenberg K., Pullenayegum E. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis.. 2010;33:1859–1864.
Stumvoll M., Goldstein B.J., van Haeften T.W., et al. Type 2 diabetes: principles of pathogenesis and treatment. Lancet. 2005;365:1333–1346.
Wright J.R. From ugly fish to conquer death: J J R Macleod’s fish insulin research, 1922–24. Lancet. 2002;359:1238–1242.
1 The Extant Works of Aretaeus, trans. Francis Adams (London 1856) p. 338 (quoted by Ackerknecht E H 1982 A short history of medicine. Johns Hopkins, Baltimore, pp. 71–72).
2 F G Banting and C H Best of Toronto, Canada (see also Journal of Laboratory and Clinical Medicine 1922; 7:251).
3 Abbreviated from Lawrence R D 1961 King’s College Hospital Gazette 40:220. Transcript from a recorded after dinner talk to students’ Historical Society.
4 The three forms of human insulin have the same amino acid sequence, but are separately designated as insulin emp (Enzyme Modified Porcine), prb (Pro-insulin Recombinant in Bacteria) and pyr (Precursor insulin Yeast Recombinant). Although one of the incentives for introducing human insulin was avoidance of insulin antibody production, the allergies to older insulins were caused largely by impurities in the preparations, and are avoided equally well by using the highly purified, monocomponent porcine and bovine insulins.
5 The discovery of the AMPK response, and of other players in the pathway, has enabled experiments to be performed in which the hepatic response to metformin is selectively knocked out. In the mouse, at least, these experiments show that actions of metformin at other sites are of little importance.
6 UK Prospective Diabetes Study (UKPDS) Group 1998 Effect of intensive blood-glucose control with metformin on complications in overweight patients with Type 2 diabetes (UKPDS 34). Lancet 352:854–865.
7 UK Prospective Diabetes Study (UKPDS) Group 1998 Tight blood pressure control and risk of macrovascular and microvascular complications in Type 2 diabetes. British Medical Journal 317:703–713.
8 Three trials compared an angiotensin blocker with other blood pressure lowering drugs and found a 20% reduction in the proportion of patients in whom proteinuria worsened or serum creatinine concentration doubled during follow-up: (1) Parving H H, Lehnert H, Brochner-Mortensen J et al 2001 The effect of irbesartan on the development of diabetic nephropathy in patients with Type 2 diabetes. New England Journal of Medicine 345:870–878; (2) Brenner B M, Cooper M E, de Zeeuw D et al 2001 Effects of losartan on renal and cardiovascular outcomes in patients with Type 2 diabetes and nephropathy. New England Journal of Medicine 345:861–869; (3) Lewis E J, Hunsicker L G, Clarke W R et al 2001 Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to Type 2 diabetes. New England Journal of Medicine 345:851–860.
9 The HOPE study included patients with diabetes as one of its high-risk group of cardiovascular patients, in whom ramipril reduced further coronary heart disease endpoints by about 30%. Yusuf S, Sleight P, Pogue J et al 2000 Effects of an angiotensin converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. New England Journal of Medicine 342:145–153.
10 The weight in kilograms divided by the square of the height in metres (kg/m2).
11 http://www.nice.org.uk/nicemedia/live/11000/30365/30365.pdf
12 Weight loss surgery: the procedures include reducing the size of the stomach by resection, gastric banding and gastric bypass.