71 Diabetes Mellitus
Diabetes mellitus includes a variety of conditions that share in common hyperglycemia caused by a deficiency of insulin action. Diabetes can occur as a result of autoimmune destruction of insulin-producing pancreatic β-cells that causes absolute insulin deficiency (type 1 diabetes), insulin resistance in peripheral tissues with relative insulin deficiency (type 2 diabetes), genetic mutations in β-cell function (monogenic diabetes of the young [MODY] and neonatal diabetes), and other causes (Box 71-1). Although type 2 diabetes accounts for 90% to 95% of diabetes in the United States, type 1 diabetes is the most frequent form in children, occurring in about one in 1500 children by age 5 years and one in 350 children by age 18 years. Over the past 20 years, however, as a result of the obesity epidemic, type 2 diabetes has been increasing in prevalence in the pediatric population. This chapter focuses on type 1 diabetes, with relevant comparisons to type 2 diabetes in children.
Box 71-1
Diabetes Mellitus Types
Adapted from American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 34(suppl 1):62-69, 2011; and Botero D, Wolfsdorf JI: Diabetes mellitus in children and adolescents. Arch Med Res 2005;36:281-290.
Etiology and Pathogenesis
Type 1 Diabetes Mellitus
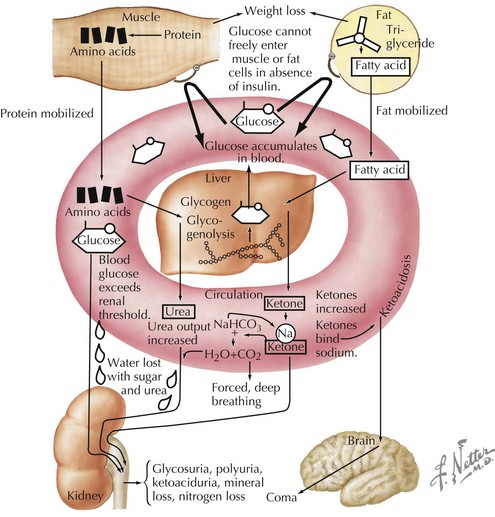
Insulin is an anabolic hormone that stimulates glucose uptake and hepatic glycogen synthesis and inhibits hepatic gluconeogenesis and glycogenolysis. It also stimulates lipogenesis, amino acid uptake, and protein synthesis (see Chapter 4, Figure 4-1). The absence of insulin triggers a series of biochemical events that emulate a starvation state even when food intake is adequate and that result in hyperglycemia and ketoacidosis (Figures 71-1 and 4-2). Glucose uptake by peripheral tissues is reduced, and hepatic glycogenolysis and gluconeogenesis are stimulated by insulin deficiency, which produces hyperglycemia. Lipolysis, proteolysis, and fatty acid oxidation lead to the accumulation of ketone bodies (β-hydroxybutyrate and acetoacetate), which eventually leads to metabolic acidosis.
As the serum glucose increases above 180 mg/dL, the renal threshold for glucose reabsorption, glycosuria results. Glycosuria causes an osmotic diuresis, resulting in polyuria and compensatory polydipsia. Over time, hyperosmolarity and dehydration develop, and decreased tissue perfusion can elicit a mild lactic acidosis. Patients without free access to fluid, such as infants and those with developmental disorders, are especially at risk. The osmotic diuresis also leads to the loss of crucial electrolytes, such as sodium, potassium, phosphorus, magnesium, and calcium. Metabolic acidosis and dehydration also stimulate counterregulatory hormones, such as growth hormone, cortisol, and epinephrine, further antagonizing insulin action. The end result is a serious metabolic disorder termed diabetic ketoacidosis (DKA; see Chapter 4).
Clinical Presentation
Complicating the presentation is the fact that patients with new-onset diabetes often present during an intercurrent illness, which may confound the classic presentation of diabetes. In addition, a number of other conditions should be considered in the differential diagnosis of diabetes (Table 71-1).
| Symptom | Differential Diagnosis |
|---|---|
| Polyuria | Diabetes insipidus, urinary tract infection, psychogenic polydipsia |
| Polydipsia | Diabetes insipidus, psychogenic polydipsia |
| Glycosuria | Benign renal glycosuria |
| Weight loss | Anorexia nervosa, inflammatory bowel disease, celiac disease, infectious disease |
| Vomiting, abdominal pain | Gastroenteritis, inflammatory bowel disease, appendicitis, toxic ingestion, pancreatitis |
| Abnormal breathing | Pneumonia, asthma exacerbation |
| Hyperglycemia | Stress-induced hyperglycemia, medication-induced hyperglycemia |
Evaluation and Management
Initial Evaluation
Well-Appearing Children
Initial biochemical evaluation of a patient suspected to have diabetes but not appearing ill should include a basic metabolic panel, including serum levels of electrolytes and glucose and urinalysis; a serum HbA1c can also be helpful. An oral glucose tolerance test is almost never necessary to diagnose T1DM but may be necessary for the early diagnosis of T2DM if the fasting blood glucose is not elevated. The American Diabetes Association (ADA) has established definitions for diabetes and increased risk for diabetes (prediabetes) (Box 71-2). Impaired glucose tolerance and impaired fasting glucose are both considered “prediabetes.” These conditions are more relevant for T2DM and require close follow-up. In 2010, the ADA added Hba1c diagnostic criteria for diabetes and prediabetes as well.
Box 71-2
American Diabetes Association Criteria
Criteria for diagnosis of diabetes:
Criteria for diagnosis of prediabetes:
Adapted from American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 34(suppl):S62-S69, 2011; American Diabetes Association: Standards of medical care in diabetes—2011. Diabetes Care 34(suppl):S11-S61, 2011.
Ill Children
DKA is common in patients with known T1DM, with a risk of about 1% to 10% per patient per year. In addition, about 25% of patients with new-onset diabetes present with DKA. DKA is present when a patient has marked hyperglycemia (glucose >300 mg/dL), ketonemia or ketonuria, and acidosis (pH<7.3 and bicarbonate <15 mEq/L). The details of evaluation and management of DKA are discussed in Chapter 4.
Post–Diabetic Ketoacidosis and Home Management
Type 1 Diabetes
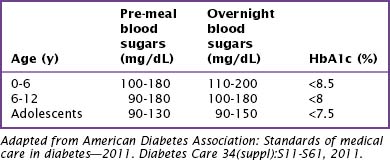
Home management of children with diabetes involves careful balancing of insulin requirements with carbohydrate intake, exercise, and activity. Realistic blood glucose goals for children vary with age (Table 71-2). The risk of hypoglycemia in children who have hypoglycemia unawareness or lack the maturity to respond to the symptoms of hypoglycemia are limiting factors in setting goals for intensive diabetes management in pediatric patients. Goals for intensive management should be set with the family, taking into consideration the abilities of the family and restrictions of age and social circumstances.
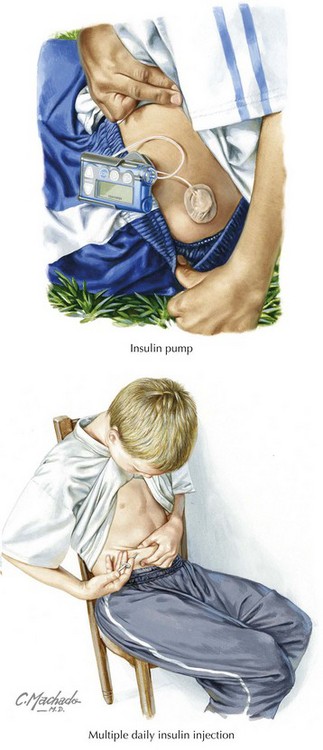
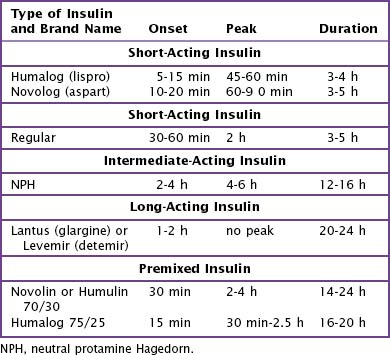
For their initial insulin regimen, children with diabetes are typically placed either on (1) a program of multiple daily injections (MDI) or (2) a basal and bolus program with injections or an insulin pump (see Figure 71-2). The MDI program generally consists of NPH (neutral protamine Hagedorn) insulin and a form of short-acting insulin (Table 71-3). In a basal and bolus program, either an insulin pump or a combination of long-acting insulin (Lantus or Levemir) and short-acting insulin is used. Which regimen is initiated is decided in consultation with the patient and family, taking into careful consideration the family’s schedule and lifestyle (Box 71-3).
Box 71-3 Selecting an Initial Insulin Regimen
Consider Lantus or Levemir with meal time short-acting insulin (Novolog/Humalog) if:
Consider “split-mixed” dosing of NPH and short-acting insulin if:
Determination of the initial insulin total daily dose (TDD) depends on the patient’s clinical presentation at diagnosis. In addition, within the first few weeks after diagnosis with T1DM, many patients have a decrease in their insulin requirements because of residual pancreatic β-cell function, referred to as the “honeymoon period.” This period can last from a few months up to 2 years. However, most children who are no longer making insulin need 0.7 to 1.0 U/kg/d. Children who present in DKA tend to require larger insulin doses than those children who present with mild hyperglycemia without acidosis (Table 71-4).
| Condition of Child | Insulin Dose (U/kg/day*) |
|---|---|
| DKA | 0.8-1.0 |
| Ketonuria or no DKA | 0.5-0.7 |
| Incidental | 0.4-0.6 |
DKA, diabetic ketoacidosis.
* Adjust for age and puberty by adding up to 0.2 U/kg/d in pubertal children or subtracting 0.2 U/kg/d for preschool children.
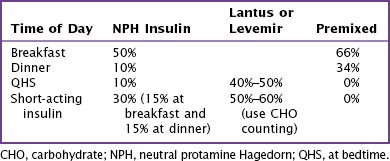
For children started on MDI or on a basal and bolus program of Lantus or Levemir, after the TDD has been established, the clinician must divide the TDD between long- and short-acting insulin (Table 71-5). Children who are started on an MDI program receive NPH before eating breakfast, dinner, and bedtime and short-acting insulin (Humalog or Novolog) before eating breakfast and dinner. Additional short-acting insulin can be given at lunch or bedtime to cover high blood glucose levels or to cover additional carbohydrates that are not planned into this coverage scheme (Table 71-6). Children on a basal and bolus program with Lantus or Levemir must receive short-acting insulin with every meal and snack to cover any hyperglycemia, as well as the carbohydrate load. Lantus or Levemir is typically given at dinner or bedtime and must be given as a separate injection and not mixed with the short-acting insulin. In younger children, the basal insulin may be split in half and given every 12 hours.
Table 71-6 Insulin : Carbohydrate Ratios and Correction Factors
| Age | Insulin : CHO* | Correction |
|---|---|---|
| 0-5 | 1 U/30 g | 1 U/100-150 mg/dL |
| 6-11 | 1 U/15 g | 1 U/75 mg/dL |
| 12+ | 1 U/8-10 g | 1 U/50 mg/dL |
CHO, carbohydrate.
* Insulin:CHO for children with no β-cell reserve (“non-honeymooning”). Children with some residual β-cell function require less insulin coverage for CHO.
A postmeal dosing scheme can be used to cover carbohydrates in young children who have unpredictable eating patterns. The blood glucose level should be checked before the meal to calculate the need for a correction dose. After the meal, the insulin dose based on the grams of carbohydrate eaten is added to the correction dose to determine the total dose of short-acting insulin (see Table 71-6). The delayed onset of insulin action in this dosing scheme is not ideal but may be used temporarily to manage food issues in young children.
Dietary management for most children involves carbohydrate counting, which has replaced the older exchange system. The goal of dietary management is to provide a balanced diet while covering carbohydrate loads with insulin. A thorough dietary assessment should be conducted to determine overall caloric and nutrient requirements. A good rule of thumb to determine the amount of calories a child needs is 1000 calories + (100 × Child’s age in years [from 3-13 years]), with additional calories added for children with significant weight loss. Placing newly diagnosed children on a standard meal plan with a constant carbohydrate load allows the clinician to work with the family to determine individual insulin-to-carbohydrate ratios (Table 71-7). These ratios allow the family much more flexibility with meals and snacks, particularly when the child is on a basal and bolus program. Efforts should be made to customize the meal plan to the child’s usual eating patterns before diagnosis. Tailoring the overall plan to lifestyle enhances the likelihood of long-term adherence.
Table 71-7 Carbohydrate Counts by Age Groups
| Age (y) | Meals (g) | Snacks (g) |
|---|---|---|
| 1-3 | 30 | 15 |
| 4-5 | 30-45 | 15-20 |
| 6-8 | 45 | 15-20 |
| 9-11 | 60 | 15-20 |
| 12-14 | 75 | 30 |
| 15+ Girls | 60 | 30 |
| 15+ Boys | 90 | 45 |
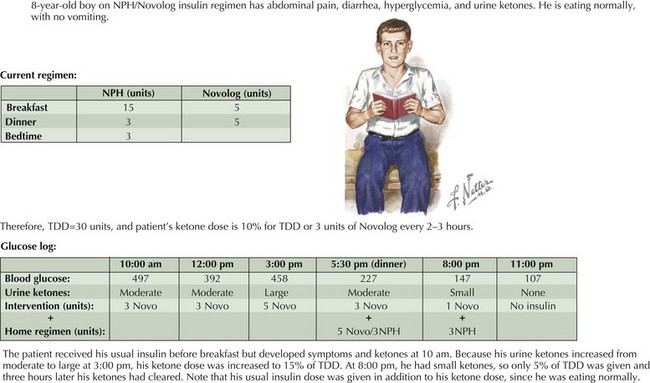
Educating families about ketone management (“sick day rules”) is crucial to prevent progression to DKA and consequent hospitalization. Urine should be tested routinely for ketones if the blood glucose is 240 mg/dL or above or if the patient is sick (irrespective of the patient’s blood glucose level). When ketones are present, additional short-acting insulin (10% of TDD) should be given every 2 to 3 hours. To prevent dehydration, patients should drink 1 oz of fluid for every year of age. If the patient’s blood glucose is less than 200 mg/dL and additional insulin is given because of ketones, the patient should be encouraged to drink carbohydrate-containing fluids to prevent hypoglycemia. The most common reason for ketonuria after the initial diagnosis is an intercurrent illness. Other factors contributing to ketonuria are insulin omission and an insulin dose that is inadequate for the child’s overall requirements (Figure 71-3).
Complications and Comorbidities
Children with T2DM are often obese and are therefore at risk for the complications of obesity as well as diabetes (see Chapter 15). Polycystic ovarian syndrome (PCOS) and obstructive sleep apnea are important comorbidities of obesity and T2DM. PCOS should be considered in girls with irregular menses, hirsutism, or acne. Children with daytime somnolence, headaches, and snoring should be evaluated with a sleep study.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(suppl):S62-S69.
American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2011;34(suppl):S13-S61.
Botero D, Wolfsdorf JI. Diabetes mellitus in children and adolescents. Arch Med Res. 2005;36:281-290.
Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetics on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
Diabetes Prevention Trial-Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685-1691.
Haller MJ, Atkinson MA, Schatz D. Type 1 diabetes mellitus: etiology, presentation and management. Pediatr Clin North Am. 2005;53:1553-1578.
2003 Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26:3160-3167.