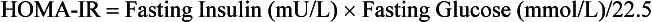Diabetes mellitus
Diabetes mellitus comprises a group of chronic metabolic disorders characterized by abnormalities in insulin secretion or action (or both) resulting in hyperglycemia. These conditions are associated with disordered carbohydrate, fat, and protein metabolism and can lead to long-term complications involving the nervous, cardiovascular, renal, and sensory organ systems. The types of diabetes are summarized in Table 1-1.
TABLE 1-1.
| Type 1 (T1DM) | Results from an absolute deficiency of insulin secretion due to beta-cell destruction (immune-mediated, 90% [type 1 A], or idiopathic [type 1B]). Patients require insulin and are prone to ketoacidosis. |
| Type 2 (T2DM) | Results from a combination of insulin resistance and insulin deficiency, which is often preceded by a period of abnormal carbohydrate metabolism (prediabetes). Patients are typically overweight, may not immediately require insulin, and are not usually prone to ketoacidosis. |
| Gestational (GDM) | Represents diabetes diagnosed during pregnancy and is based on specific screening protocols. |
| Other specific types | Diabetes caused by other conditions (chronic pancreatitis, pancreatectomy, acromegaly, hemochromatosis, hypercortisolism) or by medications (glucocorticoids, atypical antipsychotics, antiretrovirals), and monogenetic diabetes, also called maturity-onset diabetes of the young [MODY). |
2. What is the prevalence of diabetes?
According to 2011 statistics compiled by the U.S. Centers for Disease Control, 25.8 million children and adults, or 8.3% of the U.S. population, have diabetes. Of those, 18.8 million have been diagnosed, and 7.0 million Americans have diabetes but are unaware of it. Of individuals 20 years or older, 25.6 million (11.3%) have diabetes. In 2010, 1.9 million adults were newly diagnosed with diabetes. Additionally, an estimated 35% of adults were classified as prediabetic.
3. What is monogenic diabetes?
In contrast to type 2 diabetes, which is clearly polygenic, monogenic diabetes is hyperglycemia due to a single gene mutation. Monogenic diabetes is relatively rare, accounting for only 1% to 2% of all cases in Europe. It is loosely divided into neonatal diabetes (diabetes appearing within the first 6 months of life) and maturity-onset diabetes of the young (MODY; diagnosed outside the neonatal period and generally prior to 25 years of age). Mutations involving the beta-cell adenosine triphosphate–sensitive potassium channel (KATP channel) account for most cases of neonatal diabetes, and the disease in patients with these mutations responds to sulfonylureas, which block the persistently open, mutated KATP channels, thus allowing insulin secretion. MODY is associated with mutations involving glucokinase or genes coding transcription factors that are important in insulin signaling.
4. Who should be screened for diabetes?
Screening for type 1 diabetes is not feasible. Despite many studies, there is no effective means of preventing diabetes in patients who test positive for autoantibodies associated with type 1 diabetes without an abnormality in glucose tolerance, and there is no consensus as to what should be done about positive results.
The risk for development of type 2 diabetes increases with age, obesity, and sedentary lifestyle. There is an increased risk with a family history of diabetes, in certain ethnic groups, and in women with a history of gestational diabetes. Current recommendations are to screen the general population at 3-year intervals starting at age 45. Earlier or more frequent screening should be performed in adults with a body mass index (BMI) 25 kg/m2 or greater and additional risk factors (Table 1-2).
TABLE 1-2.
ADDITIONAL RISK FACTORS PROMPTING SCREENING FOR TYPE 2 DIABETES MELLITUS IN ADULTS
Physical inactivity
Diabetes in a first-degree relative
High-risk ethnicity: African American, Native American, Latino, Pacific Islander, Asian
History of gestational diabetes or of delivering a baby weighing > 9 lbs
Hypertension: blood pressure ≥ 140 mm Hg systolic/90 mm Hg diastolic or current hypertension therapy
Lipid disorders: High-density lipoprotein (HDL) cholesterol < 35 mg/dL or triglycerides > 250 mg/dL
Polycystic ovarian syndrome (PCOS)
History of abnormal glucose metabolism noted in prior testing: fasting glucose ≥ 100 mg/dL; hemoglobin A1C ≥ 5.7%; 2-hour oral glucose tolerance test (OGTT) result > 140 mg/dL
Clinical evidence of insulin resistance: acanthosis nigricans, pronounced obesity
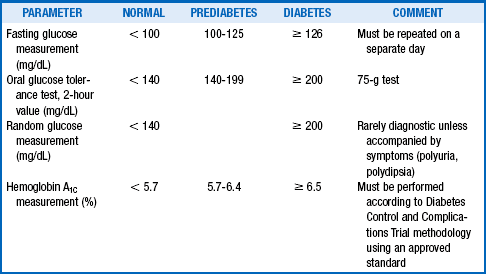
There are four different testing options for the diagnosis of diabetes: a fasting plasma glucose (FPG) test, a 75-g oral glucose tolerance test (OGTT), a hemoglobin A1C (HbA1C) measurement, and random plasma glucose measurement. Both the HbA1C measurement and FPG test are more convenient and less expensive and are, therefore, preferred. In a patient with a positive test result, the test should be repeated on a different day to confirm the diagnosis. Table 1-3 describes diagnostic criteria.
6. What are the genetics of type 1 diabetes?
The exact role of genetics versus environment in the development of type 1 diabetes is unknown. Monozygotic twins have a 20% to 50% concordance for type 1 diabetes. The cumulative risk for siblings of diabetic patients is 6% to 10%, versus 0.6% for the general population. Regarding the effect of parental genes, the offspring of women with type 1 diabetes have a lower risk of disease (2.1%) than those of men with type 1 diabetes (6.1%). The reason for this disparity is unknown. The susceptibility for type 1 diabetes is associated with the genetic expression of certain proteins coded by the human leukocyte antigen (HLA) region of the major histocompatibility complex. These proteins are present on the surfaces of lymphocytes and macrophages and are considered essential for triggering the autoimmune destruction of beta cells. Although all of the genetic markers (HLA and others) for type 1 diabetes are not known, future progress in this field will allow population screening for genetic susceptibility. Type 1 diabetes is a major element of autoimmune polyglandular syndrome type 2 (APS-2; see Chapter 52).
7. What are the genetics of type 2 diabetes?
As with type 1 diabetes, the exact interaction of genetics and environment in the development of type 2 diabetes is unclear. However, the familial clustering of type 2 diabetes suggests a strong genetic component. Monozygotic twins have a 60% to 90% concordance for type 2 diabetes. The cumulative risk for type 2 diabetes in siblings of diabetic patients is 10% to 33%, versus 5% for the general population. Offspring of women with type 2 diabetes have a twofold to threefold greater risk of diabetes than offspring of men with the disease. The exact mode of inheritance for type 2 diabetes is not known but is thought to be polygenic. Specific mutations that are associated with risk for type 2 diabetes have been identified, but many of these genes are widely found in the population at large. Because type 2 diabetes is so commonly associated with obesity, many investigators suspect that genes that predispose to obesity are associated with type 2 diabetes as well. There appears to be a strong interplay between genetic and environmental influences in the cause of type 2 diabetes. One illustration is the demonstration of higher fasting insulin levels for every weight category in the offspring of two parents with type 2 diabetes than in control subjects. High insulin levels are a marker for insulin resistance and are predictive of progression to type 2 diabetes.
8. Describe the pathogenesis of type 1 diabetes.
Type 1 diabetes results from host T-cell destruction of beta cells within the pancreas, which causes absolute insulin deficiency. Markers of this autoimmune process include antibodies to islet cells, insulin, and glutamic acid decarboxylase. The autoimmune destruction is believed to be related to genetic predispositions (HLA-DR/DQ alleles) in combination with poorly defined environmental factors. Patients with type 1 diabetes are prone to other autoimmune disorders (Grave’s disease and Hashimoto’s thyroiditis, celiac sprue, etc.).
9. Describe the pathogenesis of type 2 diabetes.
The pathogenesis of type 2 diabetes is multifactorial, although specific etiologies are unknown. Autoimmune beta-cell destruction does not occur in this form of diabetes, which accounts for 90% to 95% of all cases of diabetes. Instead, type 2 diabetes is characterized by both a defect in insulin action (known as insulin resistance) and a relative insulin deficiency. Years of hyperglycemia often precede the diagnosis, which typically occurs only after non-autoimmune beta-cell failure has begun. Loss of first-phase insulin secretion is the initial defect, with resulting postprandial glucose elevations. Eventually beta-cell death accelerates, and glucose levels rise. It is estimated that by the time of diagnosis of diabetes, patients have lost nearly 50% of their beta-cell mass.
With loss of beta-cell mass, insulin secretion is no longer sufficient to compensate for insulin resistance, defined as a subnormal response to a given insulin concentration. Elevated fasting or postprandial insulin values are the hallmark of insulin resistance, which is often associated with obesity; weight reduction may improve insulin sensitivity.
10. Can diabetes be prevented?
Several studies involving individuals at high risk for development of type 2 diabetes have documented potential beneficial effects of thiazolidinediones (Troglitazone in Prevention of Diabetes [TRIPOD] and Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication [DREAM] studies), metformin (Diabetes Prevention Program [DPP]), alpha-glucosidase inhibitors (Study to Prevent Non–Insulin-Dependent Diabetes Mellitus [STOP-NIDDM] study), intestinal lipase inhibitors (XENical in the Prevention of Diabetes in Obese Subjects [XENDOS] study), and even insulin (Outcome Reduction with Initial Glargine Intervention [ORIGIN] trial) in reducing the rate of progression to overt diabetes. Individuals in the lifestyle modification (7% weight loss and moderate exercise for 150 minutes/week) arm of the DPP showed excellent results, with a 60% lower risk for development of diabetes than those receiving metformin (30%). However, the American Diabetes Association (ADA) recommends pharmacotherapy only in patients who are at high risk for progression to diabetes because of multiple risk factors or an HbA1C level higher than 6% despite lifestyle modifications.
The lower prevalence of type 1 diabetes has made determining who is at risk more difficult. Identifying people in the prediabetic phase of type 1 diabetes requires serial measurements of beta-cell function and close monitoring of immunologic markers, making selection of an appropriate cohort difficult. Two studies, the Diabetes Prevention Trial–Type 1 (DPT-1) and the European Nicotinamide Diabetes Intervention Trial (ENDIT), overcame this issue and examined the use of insulin and nicotinamide, respectively, in high-risk relatives of patients with type 1 diabetes. However, neither study demonstrated effective prevention of progression to type 1 diabetes.
11. What techniques are available to assess insulin resistance?
Lack of standardization of insulin assays prevents use of a specific insulin concentration to define insulin resistance. The gold standards for defining insulin resistance are the intravenous glucose tolerance test, insulin suppression test, and euglycemic insulin clamp. However, these research tools are impractical in a clinical setting. A more clinically applicable tool is the homeostasis model assessment of insulin resistance (HOMA-IR), defined as the product of fasting insulin (in mU/L) and fasting plasma glucose concentrations (in mmol/L) divided by a constant (22.5), as in the following equation:
12. Describe metabolic syndrome.
Metabolic syndrome is defined as the presence of three of the five following criteria:
 Increased waist circumference (> 40 inches in men, > 35 inches in women)
Increased waist circumference (> 40 inches in men, > 35 inches in women)
 Plasma triglycerides ≥ 150 mg/dL
Plasma triglycerides ≥ 150 mg/dL
 Plasma high-density lipoprotein cholesterol < 40 mg/dL in men, < 50 mg/dL in women
Plasma high-density lipoprotein cholesterol < 40 mg/dL in men, < 50 mg/dL in women
In 2004, the American Heart Association modified this definition to include the use of medications for hypertension or to the criteria for blood pressure and hyperglycemia to the fasting plasma glucose levels.
13. What causes beta-cell failure in type 2 diabetes?
It is estimated that at the time of diagnosis, patients with type 2 diabetes have lost nearly 50% of their insulin-producing cells. The system of programmed beta-cell death (apoptosis) occurs progressively over the course of type 2 diabetes and has many potential triggers, although two specific possibilities have been characterized. Elevations of glucose and free fatty acids, collectively called glucolipotoxicity, and chronic increases in certain cytokines, notably tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), have been documented to activate “death” genes (caspases) in beta cells. Both of these conditions have been amply described in subjects with either prediabetes or overt diabetes and clearly contribute to the genesis of type 2 diabetes by reducing the number of functioning beta cells.
14. What are the standards of care for the management of diabetes mellitus?
Both the ADA and the American Association of Clinical Endocrinologists (AACE) have published evidence-based minimum standards of diabetes care. Both recommend that patients have a complete history and physical examination at the initial visit. Laboratory testing should include a fasting lipid profile and measurement of HbA1C. Surveillance for complications should include an annual physical examination, ophthalmologic examination, and a screen for microalbuminuria. Overall glycemic control (HbA1C) should be assessed at least semiannually in all patients and quarterly in insulin-treated patients and in patients with poorly controlled type 2 diabetes. Published targets include an HbA1C value under 7.0% (ADA) or 6.5% (AACE), low-density lipoprotein (LDL) cholesterol less than 100 mg/dL (< 70 mg/dL in high-risk patients), and blood pressure lower than 130/80 mm Hg.
15. Describe the current management approach to type 1 diabetes and the role of intensive therapy modeled by the Diabetes Control and Complications Trial (DCCT).
Diabetes is a self-management illness and requires that the patient be educated in glucose self-monitoring, nutrition therapy, exercise, and the proper use of medications. Similarly, the patient must be taught how to recognize and treat hypoglycemia. Because patients with type 1 diabetes are completely insulin deficient, the medical regimen is straightforward and centered on insulin replacement. The most physiologic replacement regimen, known as the basal-bolus technique, can be accomplished either with the use of a long-acting (basal) insulin in combination with a rapid-acting (bolus) insulin or a continuous subcutaneous infusion using an insulin pump.
The DCCT showed a 34% to 76% reduction in clinically significant diabetic microvascular complications (retinopathy, neuropathy, and nephropathy) in type 1 diabetes subjects randomly assigned to intensive insulin therapy in comparison with subjects assigned to standard diabetes management. After an average 17 years of follow-up, the intensively treated cohort also enjoyed an approximate 50% reduction in cardiovascular risk. The only major adverse effect of intensified control was a threefold higher risk of severe hypoglycemia. An intensive therapy regimen requires blood glucose monitoring four to eight times daily with multiple daily insulin injections or an insulin pump and is best managed by a team comprising a physician, certified diabetes educator, nurse, and dietitian.
16. Is intensive diabetes therapy cost-effective?
The potential reduction in cost for treating diabetic complications (laser photocoagulation, dialysis, kidney transplants, hospitalizations, and rehabilitation following amputations) has been shown to justify the cost of personnel and supplies for intensive therapy. The risk-to-benefit ratio for intensive therapy may be less favorable for prepubertal children, patients with advanced complications, and patients with coronary or cerebrovascular disease.
17. What is the United Kingdom Prospective Diabetes Study (UKPDS)?
The UKPDS is the largest and longest prospective study on type 2 diabetes ever conducted. Investigators recruited 5102 patients with newly diagnosed type 2 diabetes in 23 centers within the United Kingdom between 1977 and 1991. Patients were followed up for an average of 10 years to determine the impact of intensive therapy using pharmacologic agents in comparison with dietary therapy alone. The study also tested the relative efficacy of intensive (tight) blood pressure control and “less tight blood pressure control.” The results of the study showed a significant reduction in microvascular complications in patients randomly assigned to the intensive therapy arm. Tight blood pressure control was associated with a reduction in both microvascular and macrovascular events. When the entire cohort of patients was studied together, the mean HbA1C level for the duration of the study was a strong positive predictor of all diabetes-related end points, including death, amputation, myocardial infarction, and stroke. The benefits of early glucose and blood pressure control in reducing the both the microvascular and macrovascular complications and all-cause mortality persisted 10 years after the end of the original trial.
18. What is the current management approach to type 2 diabetes?
Because type 2 diabetes is a heterogeneous disorder and patients may have other comorbid illnesses, treatment and therapeutic targets must be individualized. A patient-centered approach has been advocated. The foundation of therapy is diet, exercise, and patient education; unless there are contraindications to its use, metformin should be started at the time of diagnosis. The next steps may involve additional oral agents or injectable medications, and all further treatment decisions must consider the individual characteristics of the patient as well as comorbidities. Ultimately, the majority of patients requires supplemental insulin. Emphasis should be placed on addressing cardiovascular risk reduction (blood pressure, lipids) at each encounter with the patient.
19. What are the clinical implications of the ACCORD trial?
The Action to Control Cardiovascular Risk in Type 2 Diabetes (ACCORD) trial was undertaken to address whether intensive versus standard glucose control (HbA1C target < 6% vs. 7.0%-7.9%), intensive versus standard blood pressure control (systolic blood pressure < 120 mm Hg vs. < 140 mm Hg), and fenofibrate versus placebo (both treatment arms were allowed statins) further reduced cardiovascular outcomes in patients with long-standing type 2 diabetes. In all study arms there was no reduction in the primary outcome, which was a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. Unexpectedly, both total mortality (hazard ratio 1.22) and cardiovascular death (hazard ratio 1.35) were increased in the intensive glucose control arm. Benefits were seen, however, in some secondary cardiovascular and microvascular outcomes. The clinical translation of the ACCORD study is somewhat murky, but it is apparent that intensive glucose management with the intention of normalizing HbA1C (HbA1C < 6%) is likely not warranted. Another lesson learned from ACCORD is that the management of people with type 2 diabetes must be individualized.
Insulin analogs are recombinant proteins that are based on the structure of human insulin but that have undergone selected amino acid substitutions, deletions, or additions. These amino acid alterations are designed to either enhance or protract the subcutaneous absorption of the molecule without altering its biologic properties. Native human insulin (regular) exists as a molecular hexamer that must be progressively broken down into dimers and then monomers before absorption. Amino acid substitutions in the carboxy-terminal region of the beta chain of insulin tend to destabilize hexamer formation and speed the rate of absorption. Examples of these analogs are the insulins lispro (Humalog), aspart (NovoLog), and glulisine (Apidra). These insulins are excellent for premeal use, and because they also have a shorter duration of action than native human insulin (regular), they provide better mealtime coverage with a lower risk of postmeal hypoglycemia.
Conversely, basal insulin should have both a peakless action profile and a prolonged duration of action. In the case of insulin glargine (Lantus), these features are achieved by amino acid additions that shift the isoelectric point to promote hexamer formation. After injection, glargine is buffered to a physiologic pH and forms a microprecipitate that is then slowly absorbed. The protraction of insulin detemir (Levemir) is due to fatty acylation of the insulin molecule, which results in albumin binding. Degludec, a fatty acylated insulin, is currently undergoing review by the U.S. Food and Drug Administration (FDA).
Amylin is a beta-cell hormone that is co-secreted with but structurally distinct from insulin. Under normal circumstances, amylin acts to reduce postprandial glucose excursions by reducing the gastric emptying rate and suppressing glucagon production, thereby reducing postprandial hepatic glucose production. It is also believed to inhibit the stomach hormone ghrelin, resulting in appetite suppression. In addition to an absolute insulin deficiency, patients with type 1 diabetes also have a complete deficiency of amylin, and patients with type 2 diabetes taking insulin have clearly reduced amylin responses to meals. Mealtime replacement of amylin in subjects who required insulin was shown to reduce HbA1C levels modestly while promoting weight loss. Currently available as the synthetic analog pramlintide, amylin is approved for use in type 1 and type 2 diabetics as an injection before meals.
The incretin effect refers to the enhanced insulin secretory response observed after an oral glucose load in comparison with an intravenous or parenteral glucose load. After eating, the cells of the distal small intestine release incretins such as glucagon-like peptide-1 (GLP-1) into the blood. GLP-1 secretion is under neurogenic control. It acts to increase glucose-dependent insulin secretion, suppress glucagon release, delay gastric emptying, enhance satiety through a direct effect on the central nervous system, reduce beta-cell apoptosis, and possibly stimulate beta-cell neogenesis. GLP-1 is quickly inactivated by an enzyme, dipeptidyl peptidase IV (DPP-IV); as a result, the therapeutic potential for GLP-1 is limited by its extremely short half-life.
23. How are incretins used to treat type 2 diabetes?
There are currently two types of incretin-based therapies available, GLP-1 analogs, which are not substrates for DPP-IV, and DPP-IV inhibitors, which protract the half-life of endogenous GLP-1. The two GLP-1 analogs available are exenatide and liraglutide. Both are given by injection and are associated with moderate weight loss in addition to modest HbA1C lowering (0.6%-1.2%). The three DPP-IV inhibitors are available in the United States, sitagliptin, saxagliptin, and linagliptin. In comparison with the GLP-1 analogs, DPP-IV inhibitors are associated with lower weight loss and less HbA1C lowering is; however, they are administered orally. Both types of incretins can be used as monotherapy or in combination with other available antihyperglycemia agents.
24. What are the classes of oral diabetes medications? How do they work?
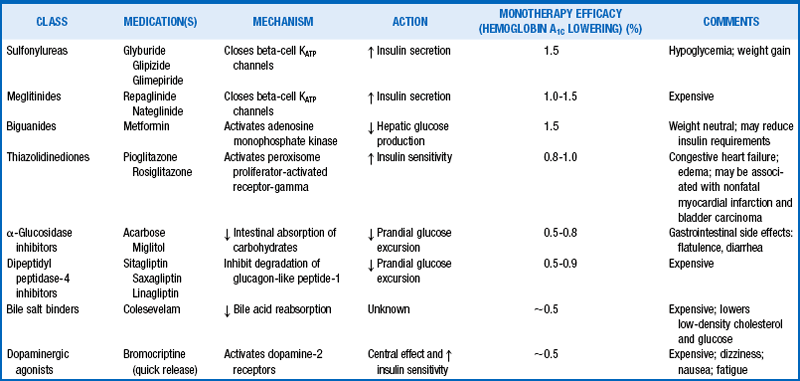
In addition to the DPP-IV inhibitors mentioned earlier, several classes of diabetes medications are available for optimizing glycemic control in people with type 2 diabetes (Table 1-4). Sulfonylureas (glyburide, glipizide, and glimepiride) and meglitinides (repaglinide and nateglinide) enhance the secretion of endogenous insulin through membrane-associated receptors. Metformin, the only biguanide available, reduces hepatic gluconeogenesis, thereby indirectly increasing peripheral insulin sensitivity. The alpha-glucosidase inhibitors (miglitol and acarbose) slow the absorption of dietary carbohydrates by inhibiting the intestinal brush-border enzymes (Table 1-4) that break down polysaccharides into absorbable monosaccharides. The thiazolidinediones (pioglitazone and rosiglitazone) act by binding nuclear peroxisome proliferator–activated receptor-gamma (PPAR-γ) to increase insulin sensitivity and directly enhance insulin action in muscle and fat cells. Rosiglitazone has been linked to nonfatal myocardial infarction, and observational studies have associated pioglitazone with a risk of bladder cancer.
, American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care. 2012;35(Suppl 1):S11–S63.
Ashcroft, FM, Rorsman, P, Diabetes mellitus and the β cell. the last ten years. Cell 2012;148:1160–1171.
Bolen, S, Feldman, L, Vassy, J, et al, Systematic review. comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Int Med 2007;147:386–399.
Davidson, JA, Incorporating incretin-based therapies into clinical practice. differences between glucagon-like peptide receptor 1 agonists and dipeptyl peptidase 4 inhibitors. (Suppl). Mayo Clin Proc 2010;85:S27–S47.
Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986.
, Diabetes Prevention Trial—Type 1 Diabetes Study group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002;346:1685–1691.
Edelman, SV, Weyer, C, Unresolved challenges with insulin therapy in type 1 diabetes. potential benefit of replacing amylin, a second b-cell hormone. Diabetes Technol Therapeut 2002;4:175–189.
Egan, JM, Bulotta, A, Hui, H, Perfetti, R. GLP-1 receptor agonists are growth and differentiation factors for pancreatic islet beta cells. Diabetes Metab Res Rev. 2003;19:114–123.
, Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20:1183–1196.
Gale, EA, European Nicotinamide Diabetes Intervention Trial (ENDIT). a randomized controlled trial of intervention before the onset of type 1 diabetes. Lancet 2004;363:925–931.
Genuth, S, Ismail-Beigi, F. Clinical implications of the ACCORD trial. J Clin Endocrinol Metab. 2012;97:41–48.
Gerstein, HC, Miller, ME, Byington, RP, et al. Action to control cardiovascular risk in diabetes study group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559.
Holman, RR, Farmer, AJ, Davies, MJ, et al. 4-T study group. Three year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736–1747.
Holman, RR, Paul, SK, Bethal, MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589.
Inzucchi, SE, Bergenstal, RM, Buse, JB, et al, Management of hyperglycemia in type 2 diabetes. a patient centered approach. Diabetes Care 2012;35:1364–1379.
Knowler, WC, Barrett-Conner, E, Fowler, SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
Owens, DR, Luzio, SD, Sert-Langeron, C, Riddle, MC, Effects of initiation and titration of a single pre-prandial dose of insulin glulisine in type 2 diabetes. a 6-month “proof-of-concept” study. Diabetes Obes Metab 2011;13:1020–1027.
, The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653.
, UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:857–858.
Vajo, Z, Fawcett, J, Duckworth, WC, Recombinant DNA technology in the treatment of diabetes. insulin analogs. Endocr Rev 2001;22:706–717.