Diabetes in pregnancy
1. How does normal pregnancy affect fuel metabolism?
Pregnancy is a complex metabolic state that involves dramatic alterations in the hormonal milieu (increases in estrogen, progesterone, prolactin, cortisol, human chorionic gonadotropin, placental growth hormone, and human placental lactogen), inflammatory cytokines (tumor necrosis factor-alpha [TNF-α], C-reactive protein), and adipokines (leptin and adiponectin) to alter maternal insulin resistance so that the mother can provide the necessary nutrients for the growing fetal-placental unit.
2. Summarize the changes in the first trimester of pregnancy.
Pregnancy is characterized by profound metabolic changes that promote adipose tissue accretion in early gestation; many women show increased insulin sensitivity between 10 and 20 weeks of pregnancy. Interestingly, a few studies have reported transient increases in insulin resistance prior to 10 weeks, although others describe more frequent episodes of hypoglycemia. Fasting insulin levels and glucose values are lower, and women are prone to nocturnal hypoglycemia and ketogenesis, especially if they suffer from nausea and vomiting during pregnancy. In addition to increased insulin sensitivity and fat storage, the first and early second trimesters are usually characterized by an earlier transition from carbohydrate to fat utilization in the fasting state. Pregnant women deplete their glycogen stores quickly and switch from carbohydrate to fat metabolism within 12 hours, often becoming ketonemic.
3. Summarize the changes in the second and third trimesters and immediate postpartum period.
The late second and third trimesters, in contrast, are consistently characterized by insulin resistance, with a nearly 50% decrease in insulin-mediated glucose disposal (assessed by the hyperinsulinemic euglycemic clamp technique) and a 200% to 300% increase in insulin secretion in late pregnancy. These changes shunt necessary fuels to meet the metabolic demands of the placenta and growing fetus, which requires 80% of its energy as glucose, while maintaining euglycemia in the mother. Women usually have lower fasting plasma glucose levels and fasting hypoinsulinemia because of continued shunting of carbohydrate to the fetal-placental unit in the unfed state, often resulting in the presence of urinary ketones. Because of the increasing placental-fetal glucose demands, glycogen stores are depleted rapidly, and pregnant women must transition from carbohydrate to fat metabolism earlier in the fasting state, a phenomenon called “accelerated starvation.” There is a dramatic insulin resistance in skeletal muscle and in the liver, resulting in increased hepatic gluconeogenesis to ensure adequate substrate delivery to the fetus. The ability of insulin to suppress whole-body lipolysis is also reduced during late pregnancy, causing free fatty acid (FFA) levels to increase. However, owing to the placental hormone–mediated increase in insulin resistance with maternal hyperinsulinemia in the fed state, pregnant women demonstrate minimally elevated postprandial glucose excursions. In an extensive review of glycemia patterns in normal pregnancy, mean fasting blood glucose (FBG) levels were 72 mg/dL, 1-hour postprandial values were 109 mg/dL, and 2-hour values were 99 mg/dL, with a 24-hour mean glucose level of 88 mg/dL, all much lower than current therapeutic targets. Immediately after delivery, insulin sensitivity returns, and the early postpartum period is often one of extreme insulin sensitivity, especially if mothers are breastfeeding; a subgroup of postpartum diabetic women requires almost no insulin for several days.
4. Do normal-weight women have different glycemic patterns from those of obese women in pregnancy?
In a study that examined continuous glucose profiles in women in both early (∼16 weeks) and late (∼28 weeks) gestation and used a controlled eucaloric diet with the same macronutrient composition in both groups, obese pregnant women demonstrated 24-hour glycemic profiles that were higher than normal-weight women both early and late in gestation. At 28 weeks of gestation, nearly all fasting and postprandial glycemic values were higher in obese women, as were fasting insulin, triglyceride, and FFA values. Mean 1-hour and 2-hour postprandial glucose levels were 102 and 96 mg/dL, compared with 115 and 107 mg/dL in the normal-weight and obese women, respectively. In late pregnancy, 95% of all glucose values were lower than 116 mg/dL, compared with 133 mg/dL in obese women. Interestingly, although pre-pregnancy body mass index (BMI), late gestation average daytime glucose level, and late gestation fasting insulin correlated with infant percentage body fat, maternal triglyceride and FFA values were stronger predictors of excess newborn fat.
5. Is glucose the only fuel altered in normal pregnancy?
No. Amino acids, triglycerides, cholesterol, and FFAs are also increased; the increase in FFAs may further accentuate the insulin resistance of pregnancy. A growing number of studies support that elevations of maternal triglycerides and FFAs are an important source of excess fuel to the fetus and are predictive of LGA (large-for-gestational-age; > 90th percentile for gestational age) status and increased newborn adiposity. At this time, there are no formal recommendations to target maternal triglycerides in pregnancy as a potential intervention to decrease the risk for newborn adiposity or macrosomia (birth weight > 4000 gm), but trials using high doses of fish oils are ongoing.
6. Explain the effect of the metabolic changes in pregnancy on diabetes management in the first trimester.
Optimally diabetes should be under tight control before conception. During the first trimester, nausea, increased insulin sensitivity, and accelerated starvation may put the mother at risk for severe hypoglycemia, and thus, insulin requirements are the least stable at this time. This risk is especially high at night because of prolonged fasting and continuous fetal-placental glucose utilization. Women with type 1 diabetes must have a bedtime snack and usually need to have the basal insulin decreased or the evening dose of neutral protamine Hagedorn (NPH) insulin lowered and moved from supper to bedtime administration to avoid early-morning hypoglycemia. Severe hypoglycemia occurs in 30% to 40% of pregnant women with type 1 diabetes in the first 20 weeks of pregnancy, most often between midnight and 8:00 am. Diabetic women who have gastroparesis or hyperemesis gravidarum are at the greatest risk for daytime hypoglycemia. During the first trimester, glycemic control just above the normal range (hemoglobin A1C [HbA1C] < 7.0%) may thus be safer than “normal” and may decrease the risk of both maternal and fetal hypoglycemia.
7. How do metabolic changes in pregnancy affect the management of diabetes in the second and third trimesters?
After 20 weeks, peripheral insulin resistance increases insulin requirements. It is not unusual for a pregnant woman to require two to three times as much insulin as she did before pregnancy. Fasting hyperglycemia and postprandial hyperglycemia are risk factors for LGA status or macrosomia. Therefore, tight glucose control in women with preexisting diabetes usually requires both basal insulin and rapid-acting insulin at each meal with frequent monitoring to allow appropriate insulin dosage adjustments.
8. What are the key preconception recommendations in counseling a diabetic woman who wants to become pregnant?
The most important recommendation in preconception counseling is the need for optimal glucose control before conception. Unplanned pregnancies occur in about two thirds of women with diabetes, making it critical that the primary care physician, endocrinologist, or obstetrician-gynecologist address preconception care in women of childbearing age. Providing effective contraception until optimal glycemic control is achieved is the most common error of omission by all health professionals who care for women with diabetes. In a retrospective study, only 25% of women of childbearing age with preexisting diabetes had preconception counseling of any kind. Four times as many fetal and neonatal deaths and congenital abnormalities occurred in a group of women who did not receive prenatal counseling than in those who did. In all series, preconception counseling significantly improved glycemic control, lowered rates of major malformations, and reduced rates of major adverse pregnancy outcomes, including very premature delivery, stillbirth, and neonatal death.
Women should begin folic acid supplementation with 0.8 to 1 mg daily before trying to conceive because the neural tube is formed by 4 weeks after conception. Maternal screening for abnormal thyroid function, retinopathy, and nephropathy should be carried out. Women with cardiovascular symptoms or additional risk factors should also be evaluated for underlying coronary artery disease with a stress study. Women with diabetes are at high risk for depression, anxiety, and eating disorders, all of which can affect glycemic control and fetal outcomes, and therefore, psychosocial screening is recommended. The risk of fetal exposure to untreated major depression is considered a greater cause of concern than the risk of fetal exposure to antidepressant medications of the selective serotonin reuptake inhibitor (SSRI) class. In addition, women with type 1 diabetes are at risk for B12 deficiency, celiac sprue, and vitamin D deficiency and should be screened if they have any suggestive symptoms or signs.
9. Why is maintenance of glucose control essential for the well-being of the fetus and pregnancy outcomes?
The maintenance of normal glucose control is the key to preventing complications, such as fetal malformations in the first trimester, macrosomia in the second and third trimesters, and neonatal metabolic abnormalities. Hyperglycemia modulates the expression of an apoptosis regulatory gene as early as the preimplantation blastocyst stage in the mouse, resulting in fetal wastage that can be prevented by treating with insulin. This finding may account for the high risk of first-trimester loss in pregnant women with poor glycemic control. In later pregnancy, there is a fourfold to fivefold higher rate of stillbirths and perinatal deaths in diabetic women than in the general population. Glycemic control as indicated by HbA1C was assessed in the Diabetes and Preeclampsia Intervention Trial; women in whom preeclampsia developed had significantly higher HbA1C values before and during pregnancy. In comparison with optimal control, an HbA1C of 8.0% or higher in early pregnancy was associated with an odds ratio of 3.7 for preeclampsia.
10. Describe the relationship among A1C, the teratogenic effects of hyperglycemia, and abnormal fetal growth.
Epidemiologic and prospective studies have shown that the HbA1C level in the 6 months before conception and during the first trimester correlates with the incidence of major malformations, such as neural tube and cardiac defects. The neural tube is completely formed by 4 weeks and the heart by 6 weeks after conception. This fact underscores the need for preconception counseling to achieve these goals because many women do not even know that they are pregnant this early. Overall, the risk of an adverse outcome is halved with each percentage reduction in HbA1C level achieved before pregnancy. It has been demonstrated that women with a normal HbA1C value at conception and during the first trimester have no increased risk, whereas women with an HbA1C value greater than 12% have up to a 25% risk of major fetal malformations. The International Diabetes Federation recommends a goal pre-pregnancy HbA1C of less than 7.0%, and other guideline committees recommend less than 6.5% if it can be safely achieved. Excess fetal growth has been associated with an abnormal HbA1C in the first trimester as well as in the second and third trimesters. Both fasting hyperglycemia and postprandial hyperglycemia are contributors to excess fetal growth and metabolic complications.
11. How has the incidence of congenital abnormalities and macrosomia in the offspring of diabetic mothers changed over the past decade?
The incidence of congenital abnormalities in the offspring of diabetic mothers in the early era of insulin use was 33%. Since the mid-1990s, with the advent of home glucose monitoring and more rigid objectives, this percentage has fallen to less than 10%. The randomized prospective Diabetes Control and Complications Trial (DCCT) has shown that timely institution of intensive therapy for blood glucose prior to conception is associated with rates of spontaneous abortion and congenital malformations that are similar to those in the nondiabetic population. Although the rate of LGA infants has been recognized to be high in women with type 2 diabetes, this rate has also significantly increased in women with type 1 diabetes, likely owing to the growing number of women who are also overweight and insulin resistant.
12. What are the risks of severe hypoglycemic episodes in the mother and fetus?
The fetus has minimal ability for hepatic gluconeogenesis until close to delivery, so profound and sustained maternal hypoglycemia is likely to cause the same in the fetus. Further, the best predictors of severe hypoglycemia during pregnancy are hypoglycemic unawareness and the presence of at least one episode of severe hypoglycemia the year before pregnancy. Severe hypoglycemia is five times more common in pregnancy if present in the year prior to pregnancy and most often occurs in the first trimester, especially between 8 and 16 weeks. The risk is highest during fasting and sleep because the fetal-placental unit continues to extract glucose during these times. Whether severe and prolonged hypoglycemia could have long-term adverse neurologic effects in the offspring has not yet been well studied.
13. What are the risks if a woman conceives while taking an oral hypoglycemic agent?
Oral hypoglycemic agents, such as sulfonylureas and metformin, do not appear to be teratogenic. There are very few data on meglitinide use in pregnancy. A retrospective series of 332 women with type 2 diabetes treated with diet, insulin, or oral sulfonylureas during the first 8 weeks of gestation found no significant adverse effects. There are few data on the risk of thiazolidinediones in the first trimester; these agents should definitely be stopped before a woman actively tries to become pregnant. Alpha-glucosidase inhibitors, such as acarbose, have not been associated with any abnormal outcomes. Therapy with an incretin, such as an amylinomimetic, GLP-1-mimetic, or DPP-IV inhibitor, has not been well studied in pregnancy and therefore is not recommended, but it is unlikely that these agents would increase the risk of major malformations if conception occurred during their use. Women who are actively trying to become pregnant should be switched to insulin during the preconception period because it may take some time to determine the ideal insulin dose before the critical time of embryogenesis.
14. How is glyburide different from the other sulfonylureas?
Glyburide crosses the placenta less than all other sulfonylureas and appears to affect fetal insulin levels minimally. In the only large randomized prospective trial, however, it was not given until after 24 weeks of gestation to women with gestational diabetes mellitus (GDM). Since that time, many more studies have utilized glyburide in pregnancy, primarily after 24 weeks of gestation in women with GDM. The International Association of Diabetes in Pregnancy Study Group (IADPSG) approved the use of glyburide as an alternative treatment in a subset of women with GDM.
15. Can glyburide or metformin be continued in pregnancy?
It is recommended that oral hypoglycemic agents be avoided during pregnancy, with the possible exception of glyburide and metformin, which have been used to treat GDM in the late second and third trimesters. Although metformin does cross the placenta, there are a fair amount of data using metformin throughout the first trimester of pregnancy in women with polycystic ovarian syndrome (PCOS) without any apparent risk, and a few small studies suggest that it decreases the risk for development of GDM. There are no known adverse effects of glyburide use in the first trimester although the vast experience with glyburide, like that with metformin, is in women with GDM after 24 weeks of gestation. However, a woman taking a sulfonylurea or metformin who is discovered to be pregnant should not stop these agents until she can be effectively switched to insulin, because the risk of teratogenicity from hyperglycemia is much higher than any risk from these agents. Both metformin and glyburide are much less likely than insulin to be effective in pregnancy, especially for women with preexisting diabetes, because of the profound insulin resistance in the second and third trimesters of pregnancy.
16. How should hypertensive women who take angiotensin-converting enzyme inhibitors or angiotensin receptor blockers be counseled in the preconception period?
Women should be counseled that angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are contraindicated in the second and third trimesters of pregnancy because of the risk of fetal anuria. A report in 2006 from a Tennessee Medicaid population described increased cardiac and central nervous system malformations in fetuses exposed in the first trimester. However, a subsequent report of pregnant women in the Kaiser Permanente Northern California region from 1995 to 2008 did not confirm this association, instead showing ACE inhibitors in the first trimester to confer a risk similar to that from other antihypertensives. It is now recommended that women who are actively trying to conceive and who have no history of infertility should probably be switched to a safer agent before pregnancy (calcium channel blocker, methyldopa, or hydralazine). A woman who receives treatment with an ACE inhibitor or ARB for significant diabetic nephropathy and who is not actively trying to conceive should be told to perform home pregnancy tests if she misses a period and to immediately stop her ACE inhibitor or ARB if there is any suspicion of pregnancy. At that time, she can be switched safely to an alternative agent.
17. How does pregnancy affect the morbidity and mortality of coronary artery disease in diabetic women?
The morbidity and mortality rates of coronary artery disease are high in pregnant women with diabetes. Cardiac status should be assessed with functional testing before conception in women older than 35 years who have any additional cardiac risk factors, such as hyperlipidemia, hypertension, smoking, cardiac autonomic neuropathy, or a strong family history and in women with any suggestive symptoms. A resting electrocardiogram (ECG) should be considered for asymptomatic women age 35 or older. Pregnancy causes a 25% increase in cardiac output, a significant decrease in systemic vascular resistance (which can shunt blood away from the coronary arteries), and an increase in oxygen consumption, all of which reduce the ability of maternal coronary blood flow to meet the demands of the myocardium. Myocardial demands are even higher at labor and delivery, and activation of catecholamines can further promote myocardial ischemia.
18. How should women with diabetes be screened and treated for thyroid disease prior to pregnancy?
Women with type 1 diabetes have an increased risk of hypothyroidism due to Hashimoto’s thyroiditis, and it is recommended by both the Endocrine Society and the American Thyroid Association that they be screened. It is less clear that women with type 2 diabetes have an increased risk, but some consensus panels recommend screening for them as well. It is recommended that women with a thyroid-stimulating hormone (TSH) level higher than 2.5 to 3.0 mU/L in the first trimester and higher than 3.0 to 3.5 mU/L in the second and third trimesters be treated, especially if they are shown to have thyroid peroxidase (TPO) antibodies, although the long-term neurologic benefit to the offspring of such treatment has not yet been demonstrated. The American College of Obstetricians and Gynecologists (ACOG) has not made any formal recommendations to screen and treat subclinical hypothyroidism in pregnancy until results of the ongoing Maternal Fetal Medicine Units (MFMU) network trial are available. Screening for TPO antibodies is not recommended in euthyroid women with or without a history of pregnancy loss because of insufficient data that treatment with thyroid hormone is effective.
19. Should statins or fibrates be discontinued before conception?
Data about the safety of statins during human pregnancy are inadequate, but animal data on these agents are concerning. Although statins have been given a classification of “X” by the U.S. Food and Drug Administration (FDA), there are no data in human pregnancy to support their being a major teratogen in a woman who is taking them and does not realize she is pregnant. However, statins should be discontinued in women who are actively trying to conceive and should not be continued during pregnancy. There does not appear to be an increased risk of malformations in women who conceive while taking fibrates. In fact, if a woman has severe hypertriglyceridemia, which puts her at high risk for pancreatitis, it may be necessary to use a fibrate in the second or third trimester of pregnancy if a low-fat diet and use of fish oils are not effective or tolerated. Serum triglyceride levels double to triple in pregnancy and therefore treatment may be indicated, especially if they approach 1000 mg/dL after meals.
20. Summarize the effects of smoking during pregnancy.
Smoking continues to be the leading cause of low-birth-weight infants in patients with and without diabetes and puts the infant at increased risk for respiratory infections, reactive airway disease, and sudden infant death syndrome. Smoking cessation efforts must be intensified before conception. The nicotine patch is believed to be safer than continuing smoking in pregnancy and should be offered to women addicted to nicotine who are already pregnant and unable to quit without its use.
21. How does pregnancy affect diabetic nephropathy?
Proteinuria increases in pregnancy, and women with proteinuria often become nephrotic owing to the increased glomerular filtration of protein during pregnancy. In some patients, proteinuria can become massive and result in significant hypoalbuminemia, edema, and a hypercoagulable state, and ultimately, in fetal growth restriction. Although women with mild renal insufficiency are not at an appreciable risk for irreversible progression of nephropathy, those with more severe renal insufficiency (serum creatinine > 2.5 mg/dL or estimated glomerular filtration rate [GFR] < 30 mL/min) have a 30% to 50% risk of a permanent pregnancy-related decline in GFR and may require dialysis in pregnancy.
22. Does nephropathy increase the risk of preeclampsia?
Preeclampsia complicates approximately 20% of pregnancies in women with preexisting diabetes, and the risk is much higher in women with hypertension or renal disease. The risk for development of preeclampsia in women with nephropathy is greater than 50%. The preeclampsia may be severe, especially in women who are hypertensive and have decreased renal function, particularly those with a serum creatinine level higher than 1.4 mg/dL. Women with significant nephropathy are also at higher risk of having preterm and low-birth-weight infants. Therefore a woman with diabetic nephropathy should be counseled to have children when her diabetes is optimally controlled and preferably early in the course of the nephropathy. Some experts recommend a target blood pressure of less than 135/85 mm Hg in women with diabetic renal disease to reduce the risk of further end-organ damage and, possibly, preeclampsia. However, reducing the blood pressure too aggressively may worsen perfusion pressure in the woman whose placental bed has a high vascular resistance due to preeclampsia, and it may worsen instead of improve fetal growth.
23. How does renal transplantation affect the outcome in pregnant women?
Women who have undergone successful renal transplantation at least 1 to 2 years before pregnancy and who have good renal function, adequate blood pressure control, and a low requirement for antirejection medications have a much more favorable outcome than women with severe renal disease who have not received a transplant. Women with severe renal insufficiency who require dialysis in pregnancy have the highest risk of adverse pregnancy outcomes, including severe growth restriction, prematurity, preeclampsia, and stillbirth.
24. Summarize the effects of pregnancy on diabetic retinopathy.
Retinopathy may progress during pregnancy either from the institution of tight glycemic control or from the increases in growth factors, blood volume, cardiac output, anemia, and the hypercoagulable state of pregnancy. Women with proliferative retinopathy are at the highest risk of progression; in one series, retinopathy worsened in more than 50% of the women. It is therefore imperative that retinopathy be optimally treated with laser therapy before pregnancy, although laser therapy can be instituted during pregnancy. It is less likely that only background retinopathy will progress significantly during pregnancy, but there are reports that as many as 20% of cases do progress. Baseline and follow-up retinal examinations are recommended for all diabetic pregnant women at risk for retinopathy.
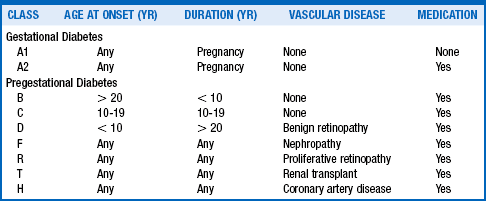
25. What is the White classification of diabetes in pregnancy?
Priscilla White at the Joslin Diabetes Center observed that the patient’s age at onset of diabetes, duration of diabetes, and severity of complications, including vascular disease, nephropathy, and retinopathy, significantly influenced maternal and perinatal outcomes. In 1949, she developed a classification scheme based on these parameters. The initial scheme was developed for women with type 1 diabetes; there is no separate classification for type 2 diabetes.
26. Why is the White classification used by obstetricians?
Its predictive value allows identification of patients at greatest risk for obstetric complications during pregnancy so that physicians can intensify management and fetal surveillance. In the updated classification (Table 5-1), women with pregestational diabetes are designated by the letters B, C, D, F, R, T, and H according to duration of diabetes and complications. Class A1 or A2 is used to classify women with GDM that is controlled with diet alone or with medications, respectively. It is unclear whether the White classification is better at predicting adverse pregnancy outcomes than categorizing the patients with respect to the absence or presence of vascular disease. However, it is still used by obstetricians to classify the duration and complications of women with diabetes.
27. What are the goals of glucose control for pregnant women with diabetes?
The goals of glucose control during pregnancy are rigorous. Optimally, the premeal glucose level should be less than 90 to 95 mg/dL, the 1-hour postprandial glucose level less than 130 to 140 mg/dL, and the 2-hour postprandial glucose level less than 120 mg/dL. More intensive goals have not been tested in an adequately powered randomized controlled trial (RCT) to determine whether they can achieve a reduction in macrosomia without increasing the risk of small-for-gestational-age (SGA) status. The results from the multicenter HAPO (Hyperglycemia and Adverse Pregnancy Outcomes) trial, which studied 25,000 pregnant women in nine countries, suggest that abnormal fetal growth occurs along a continuum and at lower glucose values than previously recognized. They calculated that a 1.75-fold risk of LGA infants occurs at an FBG level of 92 mg/dL or higher. Because macrosomia is related to both fasting and postprandial blood glucose excursions, pregnant diabetic women need to monitor premeal and postprandial glucose values regularly. Patients with type 1 and type 2 diabetes usually require three or four insulin injections per day or an insulin pump to achieve adequate control during pregnancy.
28. What is the role of the continuous glucose monitoring system in pregnancy?
A continuous glucose monitoring system (CGMS) can be helpful, especially in patients with type 1 diabetes who are having frequent hypoglycemic episodes and have hypoglycemic unawareness, allowing better delineation of glucose patterns so that basal and/or bolus insulin can be appropriately adjusted. A CGMS can also reveal postprandial hyperglycemia that might be otherwise unrecognized and that is strongly associated with excess fetal growth. In one series, a mother had to check her glucose levels a minimum of 10 times per day to give an indication of the glucose patterns obtained during CGMS. Another study showed that utilizing CGMSs in pregnant women with preexisting diabetes may not be an effective tool to decrease LGA rates. However, CGMSs have been shown to be very useful in women with type 1 diabetes who may have hypoglycemic unawareness, wide glycemic swings, or difficulty reaching an optimal HbA1C level despite checking their blood glucose values many times each day.
29. Discuss the role of the insulin pump during pregnancy.
Experience with the insulin pump in the treatment of type 1 diabetes in pregnancy is increasing. Most trials have found that continuous subcutaneous insulin infusion is equivalent to multiple daily injections using basal and bolus insulin. The pump may be advantageous in women with recurrent hypoglycemia, especially at night, because different basal rates can be programmed. However, there are reports of women who began using the pump in pregnancy in ketoacidosis developed from pump failure. Therefore it may be optimal to begin pump therapy before pregnancy, given the steep learning curve involved in its use and the continuous changes that must be made in dosing basal and bolus insulin because of the changing insulin resistance throughout pregnancy.
30. Discuss the role of glargine and detemir insulins during pregnancy.
Insulin detemir (Levemir) has been approved by the FDA for use in pregnancy and may result in less nocturnal hypoglycemia than NPH insulin. For women with severe hepatic insulin resistance, NPH insulin before bedtime may also be required to achieve sufficient fasting glucose control, although its peak can be variable. Experience with insulin glargine (Lantus) in pregnancy is fairly extensive, and although not yet approved by the FDA, such use has been approved by the European Medicine Agency. However, there are still some concerns about glargine’s potential mitogenic effects and higher affinity for the insulin-like growth factor-1 (IGF-1) receptor, especially in women with proliferative retinopathy. Insulin glargine does not cross the placenta, and no evidence indicates reproductive toxicity or embryotoxicity. Similar pregnancy outcomes have been reported in women taking glargine and women taking NPH. If a patient without proliferative retinopathy is doing well on insulin glargine, it is probably not necessary to switch her to another insulin during pregnancy. Either glargine or detemir insulin may be useful in women who experience recurrent hypoglycemia with NPH insulin therapy.
31. What is the role of short-acting insulin analogs in pregnancy?
Insulins lispro (Humalog) and aspart (NovoLog) have been used in pregnancy and have been shown to be safe and effective. Both reduce postprandial hyperglycemia and the risk of hypoglycemia compared with regular insulin in patients with type 1 diabetes. They may be especially helpful in women with type 1 diabetes and gastroparesis, because they can be dosed after eating, ensuring that food is not immediately vomited after a full bolus is given. Dosing these rapid-acting insulin analogs according to carbohydrate ratios and a premeal correction factor is usually necessary to achieve adequate control in women type 1 diabetes. Their use is also often more successful than use of regular insulin in women with type 2 diabetes, especially if regular insulin cannot effectively lower the 1- or 2-hour postprandial glucose levels into the target range without causing hypoglycemia 3 to 4 hours after injection.
32. How common is hypoglycemia in pregnant women with type 1 diabetes during and after pregnancy?
Maternal hypoglycemia is common and often severe in pregnant women with type 1 diabetes. In one series, hypoglycemia requiring assistance occurred in 71% of patients, with a peak incidence at 10 to 16 weeks of gestation. One third of the women had at least one episode resulting in seizures, loss of consciousness, or injury, any of which could potentially result in long-term neurological effects in the offspring. Current data suggest that the counterregulatory hormonal response to hypoglycemia is diminished in pregnancy. The physician must have a low threshold for bringing the expectant mother into the hospital to optimize education and glycemic control. Occasional monitoring in the middle of the night is recommended in the woman with type 1 diabetes because of the increased risk of nocturnal hypoglycemia, especially if she has hypoglycemia unawareness. Immediately after delivery, the mother’s need for insulin declines to approximately 60% of her pre-pregnancy dose as a result of the rapid cessation of the placental hormone influence; there is a concomitant higher risk of hypoglycemia at this time. Insulin doses typically rise over the next weeks nearer to pre-pregnancy doses unless the mother breastfeeds.
33. Discuss special concerns in pregnant women with type 2 diabetes as compared with type 1 diabetes.
Women with type 2 diabetes are at least as high a risk of pregnancy complications as women with type 1 diabetes, especially if they have hypertension, obesity, or are in poor glycemic control. Some series, in fact, show that pregnancy outcomes may be less favorable in women with type 2 than in those with type 1 diabetes, including a higher perinatal mortality rate. The reasons for this may include older age, a lower rate of preconception counseling, a higher incidence of poor glycemic control in the first trimester, the coexistence of the metabolic syndrome (hypertension and obesity), undiagnosed sleep apnea, and occult cardiopulmonary disease, all of which are significant risk factors for pregnancy complications. Failure to achieve optimal control in early pregnancy in women with any type of preexisting diabetes may have teratogenic effects or may lead to early fetal loss. Poor glycemic control later in pregnancy increases the risks of intrauterine fetal demise, LGA status, and metabolic complications in the newborn.
Stillbirths in women with type 1 or type 2 diabetes are fivefold more common and are unexplained in about 50% of cases. Fetal hypoxia and cardiac dysfunction, associated with cardiac enlargement and asymmetric septal hypertrophy, result from poor glycemic control and are probably the most important pathogenic factors. Fetal hyperglycemia and hyperinsulinemia result in excess fetal growth, increased fetal metabolism with increased fetal oxygen consumption, and relative tissue hypoxia. As in the case with type 1 diabetes, an early dating ultrasound is necessary to determine the gestational age of the fetus, and a formal anatomy scan should be performed at 18 to 20 weeks to evaluate for fetal anomalies. A fetal echocardiogram should be offered at 20 to 22 weeks if the HbA1C was elevated during the first trimester. Daily fetal movement monitoring should start at around 28 weeks, and women with type 1 or type 2 diabetes should be offered fetal surveillance beginning at about 32 weeks of gestation with twice-weekly non-stress tests (NSTs). A fetal ultrasound for growth should be considered at 28 to 32 weeks and before term. An earlier delivery should be offered to women who have either preexisting diabetes of longer duration or with vascular disease, especially if glucose control is suboptimal; at this time an amniocentesis may be done to confirm fetal lung maturity.
34. What is the risk of diabetic ketoacidosis in pregnancy?
Pregnancy predisposes to accelerated starvation, which can result in ketonuria after an overnight fast. Diabetic ketoacidosis (DKA) may occur at lower glucose levels (often referred to as “euglycemic DKA”) because of the increased glomerular glucose filtration, continuous glucose utilization by the fetal-placental unit, and greater volume of distribution of glucose due to a 30% to 40% expansion of plasma volume. Women also have a lower buffering capacity because of progesterone-induced respiratory alkalosis, which results in a compensatory metabolic acidosis. An early switch from carbohydrate metabolism to lipolysis occurs in pregnant women who have depleted their glycogen stores after a 12-hour fast, resulting in a starvation ketoacidosis.
35. How should the risk of DKA be managed?
Any pregnant women with type 1 diabetes who is unable to keep down food or fluids or has persistent severe hyperglycemia should check for urinary ketones at home. If the urinary ketones cannot be cleared quickly, a blood chemistry panel should be ordered to rule out an anion gap, even if the maternal glucose value is less than 200 mg/dL. Often the only precipitant for DKA in pregnancy is nausea and vomiting, but the possibility of an infection, particularly urinary tract infections, should be aggressively investigated. Women with type 2 diabetes and even women with gestational diabetes can also experience DKA, especially in the context of prolonged fasting, infections, use of beta-agonists for preterm labor, or steroids to promote fetal lung maturity.
36. How does maternal DKA affect the fetus?
In a study of 20 consecutive cases of DKA, only 65% of the fetuses were alive on admission to the hospital. Risk factors for fetal loss included DKA manifesting later in pregnancy (32 weeks vs. 24 weeks), high insulin requirements, and longer duration of DKA. Electrolyte disturbances and fetal hypoxemia are additional risk factors for fetal death. The fetal heart rate must therefore be monitored continuously until the acidosis has resolved.
37. What must the physician remember about DKA in pregnant women?
Pregnant women unable to take oral nutrients require an additional 100 to 150 g/day of intravenous glucose to meet the metabolic demands of the fetal-placental unit. Without adequate carbohydrate (often a 10% dextrose solution is necessary), fat will be burned for fuel, and the patient in DKA will remain ketotic.
38. What is gestational diabetes mellitus and why is there no consensus on its diagnosis between the ACOG and the ADA?
The previous definition of GDM as a glucose-intolerant state with onset or first recognition during pregnancy has been challenged by the IADPSG and the American Diabetes Association (ADA). They recognized that many women with undiagnosed preexisting (overt) diabetes were being referred to as having GDM even though the degree of their hyperglycemia or its early manifestation (before 24 weeks of gestation) clearly indicated that these women had diabetes that was simply not identified until GDM screening was performed in pregnancy. Given that such women have a much higher risk of maternal and fetal complications, including major malformations if their HbA1C levels are 6.5% or higher, the IADPSG and ADA now recommend that GDM be diagnosed only if the glucose intolerance was identified during pregnancy AND the woman did not qualify for preexisting (overt) diabetes. The IADPSG and ADA recommend that women diagnosed for the first time in pregnancy should be considered as having overt diabetes (and not GDM) if any of the following criteria is fulfilled—HbA1C ≥ 6.5%, FBG level ≥ 126 mg/dL, or random glucose level ≥ 200 mg/dL—which are the same criteria for diagnosis of diabetes outside of pregnancy (Table 5-2).
TABLE 5-2.
CRITERIA FOR DIAGNOSIS OF OVERT DIABETES*
| Fasting glucose measurement | ≥ 125 mg/dL |
| Hemoglobin A1C measurement | ≥ 6.5% |
| Random glucose | ≥ 200 mg/dL |
The ADA also adopted the IADPSG recommendations to diagnose GDM at lower blood glucose thresholds than what has been used by the ACOG, on the basis of findings from the HAPO (Hyperglycemia and Adverse Pregnancy Outcomes) trial. Further, because the HAPO trial showed an increased risk of LGA status using a single abnormal threshold glucose value on a 75-g 2-hour oral glucose tolerance test (OGTT), the ADA now recommends that this test be used to diagnose GDM (Table 5-3) rather than two abnormal values on the 100-g 3-hour OGTT traditionally used by ACOG (Table 5-4). However, adopting the new ADA criteria will result in a tripling of the prevalence of GDM—to an estimated 18% of the pregnant population—in comparison with the 5% to 6% currently estimated prevalence according to the ACOG criteria (see Table 5-4). This prevalence increase could be even higher in some ethnic groups (Hispanic Americans, Native Americans, Pacific Islanders, and Asian Americans). Asian women have a higher risk of development of GDM at a lower BMI. Interestingly, women of African ancestry have a high prevalence of obesity but lower GDM rates than other groups. However, postpartum they have a higher rate of development of diabetes after GDM. Even prior to the changes in the ADA guidelines on the diagnosis of GDM, the prevalence of GDM had doubled in the past 10 to 15 years because of the obesity epidemic.
TABLE 5-3.
AMERICAN DIABETIC ASSOCIATION CRITERIA FOR A POSITIVE 75-G ORAL GLUCOSE TOLERANCE TEST RESULT*
| Fasting glucose | ≥ 92 mg/dL |
| 1-hour glucose | ≥ 180 mg/dL |
| 2-hour glucose | 153 mg/dL |
TABLE 5-4.
AMERICAN COLLEGE OF OBSTETRICIANS AND GYNECOLOGISTS CRITERIA FOR A POSITIVE 100-G ORAL GLUCOSE TOLERANCE TEST RESULT*
| Fasting glucose | ≥ 95 mg/dL |
| 1-hour glucose | ≥ 180 mg/dL |
| 2-hour glucose | ≥ 155 mg/dL |
| 3-hour glucose | ≥ 140 mg/dL |
Currently there is no consensus about the adoption of the ADA criteria over the ACOG criteria. Critics of the ADA criteria argue that adopting it will triple the prevalence of GDM, potentially outstripping the resources to treat it. They also argue that it is not clear how much the increased risk of LGA status at lower glucose thresholds observed in the HAPO trial on which it was based was due to maternal obesity or mild hyperglycemia. Further, there are no randomized controlled trials pitting the diagnostic criteria against each other and showing that implementation of and treatment based on the new ADA criteria will result in lower rates of LGA infants or other adverse pregnancy outcomes.
39. What are the different diagnostic criteria for GDM according to the ACOG and the ADA?
The lack of a consensus for the diagnosis of GDM in the United States between the ACOG and the ADA is unfortunately forcing practitioners to choose to use one criterion over the other, resulting in a complete lack of standardization. A National Institutes of Health (NIH) consensus conference has selected a committee of members who have not published in the area of GDM in an effort to avoid bias, and it is hoped that this conference can resolve this lack of consensus in the near future. The Carpenter and Coustan criteria continue to be used by the ACOG; they require two abnormal glucose values out of four values on a 100-g 3-hour OGTT. However, the ADA adopted the IADPSG recommendations based on the HAPO trial, which showed that an FBG 92 mg/dL or higher, a 1-hour glucose level 180 mg/dL or higher, OR a 2-hour glucose level 153 mg/dL or higher (only one abnormal value required) on a 75-g 2-hour OGTT resulted in a 1.75-fold higher risk of an LGA infant and should be the basis for the diagnosis (see Table 5-2). The HAPO trial, performed in 25,000 women in nine countries, suggested that fetal overgrowth occurs at lower glucose targets than previously used and that the diagnostic glucose targets should be lowered. The values listed in Table 5-2 were statistically chosen because they resulted in a 1.75-fold increase in LGA status. Because the increased risk of LGA status occurred along a continuum, there was no clear threshold to adopt on the basis of the trial results. Further, obesity itself increased the risk of LGA status, and there is argument as to how much of the higher LGA risk was due to obesity and how much to mild hyperglycemia. Opponents argue that adopting a 1.75-fold higher risk about the mean versus a twofold or threefold increased risk was also somewhat arbitrary and will result in a tripling of the prevalence of GDM without compelling data from an RCT that adopting the new criteria would result in a benefit.
Nearly 95% of all women in the HAPO trial who met criteria for GDM using the 75-g 2-hour OGTT were diagnosed on the basis of the FBG and 1-hour glucose values, raising the question of whether the 2-hour value is worth the extra time and cost. Some countries are considering basing the GDM diagnostic criteria only on the fasting and 1-hour values to reduce subject burden and possibly the cost. There is also debate about early screening of high-risk women for GDM (see later) because the ADA has abandoned the use of the 50-g glucose challenge (1-hour Glucola test), which continues to be used by the ACOG, to screen women for GDM.
40. Explain the differences in early screening between the ACOG and ADA guidelines.
According to the ACOG, women who have NO risk factors do not require any screening or diagnostic testing at 24 to 28 weeks of gestation, but this category is limited to women meeting all of the following criteria: age under 25 years, normal weight before pregnancy, member of an ethnic group with a low prevalence of GDM, no known diabetes in first-degree relatives, no history of abnormal glucose tolerance, and no history of poor obstetric outcome or macrosomic infant (> 9 lb). Most obstetricians advocate for universal screening because few women meet all of these criteria. The ADA recommends diagnostic testing at 24 to 28 weeks for everyone with the 75-g 2-hour OGTT (and no screening using a 1-hour 50-g glucose challenge).
41. What are the differences in recommendations for testing high-risk women early in pregnancy?
High-risk status requires glucose testing as soon as pregnancy is diagnosed and again at 24 to 28 weeks if the early test result is normal. Women meeting any of the following criteria should be tested early: obesity, personal history of GDM (recurrence rate 30%-50%), previous macrosomic infant (> 9 lb), glycosuria, family history of diabetes in a first-degree relative, and polycystic ovary syndrome (PCOS). The ACOG recommends that these high-risk women be screened on their first prenatal visit with a 50-g oral glucose load and that, if the 1-hour glucose value exceeds 130 to 140 mg/dL, a diagnostic 3-hour 100-g OGTT be performed. The new ADA criteria do not use a 50-g OGTT test for screening. Instead, they advocate that high-risk women be tested on the first prenatal visit with either measurement of HbA1C or performance of an FBG or a 75-g 2-hour OGTT, primarily to rule out overt diabetes. In any woman who meets criteria for overt diabetes (HbA1C ≥ 6.5%; FBG ≥ 126 mg/dL or random glucose ≥ 200 mg/dL), a diagnosis of preexisting diabetes rather than GDM should be made. If the fasting glucose value is 92 mg/dL or higher, a diagnosis of GDM can be made. A 75-g 2-hour OGTT to determine whether the 1-hour or 2-hour glucose value exceeds or equals 180 mg/dL or 153 mg/dL, respectively, is optional and not mandated in all high-risk women early in pregnancy.
The recommendations given by the ADA to diagnose overt diabetes in early pregnancy have resulted in opponents underscoring that some high-risk women with only impaired glucose tolerance (IGT) (by OGTT) will be missed early using the new criteria because a practitioner can choose whether to obtain an HbA1C measurement, FBG test, or 75-g 2-hour OGTT early in pregnancy. Some practitioners recommend that an HbA1C value of 5.7% or greater be used to diagnose GDM early because prediabetes outside pregnancy is diagnosed with this value. However, an HbA1C of 5.7% or greater was not given as an optional criterion by either the IADPSG or the ADA to diagnose GDM. Further, many studies outside of pregnancy have demonstrated that the HbA1C measurement is the least sensitive test to diagnose either prediabetes or diabetes, especially because anemia is common in pregnancy and the HbA1C result will be falsely low in states involving high red blood cell turnover.
Also, it has been demonstrated that the FBG value is less sensitive than the post–glucose load value on a 75-g 2-hour OGTT for diagnosing prediabetes or diabetes. One article has underscored the profound difference among different ethnic populations studied in the HAPO trial in regard to the sensitivities of an FBG value and a 1-hour or 2-hour 75-g glucose value in diagnosing GDM. Especially in the Asian population, an FBG value is unlikely to identify a woman as having GDM because most Asian women have only IGT after a glucose load. In Hong Kong, of all the women in the HAPO trial who were diagnosed as having GDM using the new criteria, only 26% had an abnormal FBG value, and the remainder were diagnosed by either an abnormal value on a 1-hour (45%) or 2-hour (29%) OGTT. This finding raises the question as to whether early diagnostic testing recommended by the ADA for high-risk women will miss many with only IGT, because administering the 75-g 2-hour OGTT is optional according to ADA criteria (women can be screened with either HbA1C measurement or FBG test). Both the ACOG and the ADA agree that if initial test results are normal (using their different recommendations), repeat testing should be done at 24 to 28 weeks of gestation using either the 100-g 3-hour OGTT (ACOG) or the 75-g 2-hour OGTT (ADA).
42. Describe the 50-g glucose challenge used by ACOG.
The 50-g glucose challenge is the accepted screen by the ACOG for the presence of GDM, but a positive result must be followed by a diagnostic 100-g 3-hour OGTT. A positive screen result is in the range of 130 to140 mg/dL or above. The sensitivity and specificity of the test depend on what threshold value is chosen, and the cutoff may be selected according to the prevalence of GDM in the population being screened. The test does not have to be performed during a fasting state, but a serum sample must be drawn exactly 1 hour after administration of the oral glucose. Owing to the poor reproducibility of this test from one day to the next and the lack of any sensitivity cutoffs based on a diagnostic 75-g 2-hour OGTT result rather than the 100-g 3-hour OGTT, the ADA has abandoned the use of the 50-g glucose test as a screening tool.
43. Describe the 100-g 3-hour and the 75-g 2-hour OGTTs.
Both tests should be performed after 3 days of an unrestricted carbohydrate diet and while the patient is fasting. For the 100-g 3-hour OGTT, two abnormal blood glucose values are required, and for the 75-g 2-hour OGTT, only one abnormal value is required (see Tables 5-e3 and 5-4). If the 100-g 3-hour OGTT test is performed and only one value is abnormal, a second 100-g 3-hour test should be performed 1 month later because a single elevated glucose value increases the risk of LGA status and one third of patients with such a result will ultimately meet the diagnostic criteria for GDM (see Table 5-4). Implementing the diagnostic criteria for the 75-g 2-hour OGTT instead of the 100-g 3-hour OGTT is estimated to increase the prevalence of GDM by threefold (from ∼5%-6% to ∼18%), and it is not yet clear whether such implementation will ultimately decrease risk of LGA infants. Obviously, the lack of consensus regarding which criterion to use from one institution to the next is extremely confusing for patient management as well as for clinical research trials for which either diagnostic criteria could be used.
44. Summarize the risks to the mother with GDM.
The immediate risks to the mother with GDM are increased incidences of cesarean delivery (∼30%), preeclampsia (10%-30%), and polyhydramnios (∼10%-20%), which can result in preterm labor. The long-term risks to the mother are related to recurrent GDM pregnancies (30%-50% recurrence) and the substantial risk for development of type 2 diabetes mellitus.
45. What factors increase the risk of subsequent development of type 2 diabetes?
Women with GDM have an extremely high risk (33%-50%) for development of type 2 diabetes in the subsequent 5 to 10 years. Risk factors include fasting hyperglycemia, an insulin requirement, GDM diagnosed before 24 weeks of gestation (preexisting glucose intolerance), obesity, membership in an ethnic group with a high prevalence of type 2 diabetes, and IGT at 6 weeks postpartum. Women with GDM who have multiple subsequent pregnancies also have a higher risk for development of type 2 diabetes.
46. What interventions may reduce the risk for development of type 2 diabetes?
Counseling with regard to diet, exercise, and weight loss is essential and is likely to improve insulin sensitivity, according to the findings of the Diabetes Prevention Program (DPP) trial. A subgroup analysis examining women in the DPP with a history of GDM showed that they had a much higher risk for development of type 2 diabetes (17% per year) than women with IGT but without a history of GDM. This risk could be halved to approximately 8% per year with diet and exercise or metformin. Such dietary modifications should be adopted by the family, because the infant of a woman with GDM is also at risk for obesity and the metabolic syndrome. One trial also demonstrated that the use of a thiazolidinedione, versus placebo, postpartum decreased the rate of development of type 2 diabetes in 30 months from 12.1% to 5.4% in the 133 randomly assigned women, apparently by decreasing insulin secretion and preserving beta-cell function. At this time, it is recommended that intensified efforts through diet and exercise be made to help a woman with GDM return to her pre-pregnancy weight and to lose additional weight if her BMI is still elevated. If diet and exercise are unsuccessful or do not normalize glucose tolerance, metformin should be considered, especially in women with both impaired fasting glucose (IFG) and IGT.
47. What is the incidence of complications in the infant of a mother with GDM?
Even with the advent of screening and aggressive GDM management, the incidence of neonatal complications for women with GDM ranges from 12% to 28%.
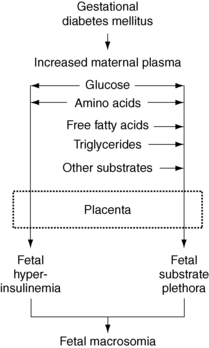
48. Summarize the basic mechanism behind fetal complications related to GDM.
Excessive transfer of glucose, amino acids, FFAs, and triglycerides from mother to fetus provides an overabundance of nutrients, resulting in excess fetal fat accretion. Maternal hyperglycemia induces fetal hyperglycemia, which results in fetal pancreatic islet hypertrophy and beta-cell hyperplasia with consequent fetal hyperinsulinemia. Fetal insulin is a potent growth hormone.
49. What is the most common complication of GDM?
The placenta plays a key role in the regulation of nutrient transport in GDM, contributing to the most common complication, which is macrosomia or LGA status (Fig. 5-1). Although transplacental glucose flux is flow limited, transport of essential and nonessential fatty acids is regulated. The expression of genes involved with inflammation and lipid transport and metabolism are significantly altered in the placentas of women with GDM, favoring excess fetal fat accretion. Increased fat availability to the fetus leads to adiposity and visceromegaly (especially heart, liver, and pancreas), which put the mother at increased risk of requiring a cesarean section and the infant at risk for shoulder dystocia. The excessive supply of nutrients also causes an increase in fetal abdominal girth disproportionate to other body measurements (body-to-head disproportion), resulting in a difficult delivery.
50. What other complications may result from GDM or preexisting diabetes?
 Shoulder dystocia with Erb’s palsy and clavicular fractures, fetal distress, low Apgar scores, and birth asphyxia when GDM is unrecognized.
Shoulder dystocia with Erb’s palsy and clavicular fractures, fetal distress, low Apgar scores, and birth asphyxia when GDM is unrecognized.
 If mothers have poor glycemic control, respiratory distress syndrome may occur in up to 30% of infants because of decreased lung surfactant synthesis.
If mothers have poor glycemic control, respiratory distress syndrome may occur in up to 30% of infants because of decreased lung surfactant synthesis.
 Cardiac septal hypertrophy may be seen in 35% to 40%.
Cardiac septal hypertrophy may be seen in 35% to 40%.
 With extremely poor glucose control, especially in women with type 1 or type 2 diabetes, there is also an increased risk of fetal mortality as a result of fetal acidemia and hypoxia.
With extremely poor glucose control, especially in women with type 1 or type 2 diabetes, there is also an increased risk of fetal mortality as a result of fetal acidemia and hypoxia.
 Common metabolic abnormalities in the infant of a mother with diabetes include neonatal hypoglycemia from sustained hyperinsulinemia as well as hypocalcemia, polycythemia, and hyperbilirubinemia.
Common metabolic abnormalities in the infant of a mother with diabetes include neonatal hypoglycemia from sustained hyperinsulinemia as well as hypocalcemia, polycythemia, and hyperbilirubinemia.
 Excess FFAs and triglycerides delivered to the fetus may also contribute to excess fetal growth and is now the subject of increased research to discern whether these substrates may need to be targeted for lowering.
Excess FFAs and triglycerides delivered to the fetus may also contribute to excess fetal growth and is now the subject of increased research to discern whether these substrates may need to be targeted for lowering.
51. Why can macrosomia still occur despite adequate maternal glycemic control? Role of triglycerides and free fatty acids.
Fetal hyperinsulinemia may cause exaggerated fetal siphoning of glucose from the mother, which blunts the maternal postload glucose peaks, resulting in what appears to be optimal glycemic control in the mother. Further, triglycerides and FFAs are now recognized as important nutrients for fetal fat accretion and the placenta has a lipoprotein lipase (LPL) that hydrolyzes maternal triglycerides to FFAs, which can be transported across the placenta. Triglyceride levels are higher in women with GDM and have been shown to be stronger predictors than glucose levels of excess neonatal fat in the offspring of who are obese or have GDM with adequate glycemic control. Upregulation of many genes involved with lipid transport and storage as well as inflammation and oxidative stress have been shown to characterize the placentas in women with GDM and may affect nutrient transport and fetal fat accretion.
52. Describe the fetal-based management strategy and fetal surveillance in GDM.
A number of RCTs have demonstrated that using fetal overgrowth as an indicator of optimal diabetes management is beneficial. Because measurements of maternal glucose may be deceptive and other nutrients such as excess lipids may also contribute to fetal fat accretion, assessing fetal growth as a gauge for the adequacy of treatment is now recommended. Amniotic fluid insulin levels, a marker of fetal hyperinsulinemia (because maternal insulin does not cross the placenta in appreciable quantities) correlates strongly with the fetal abdominal circumference at 28 to 32 weeks of gestation. These RCTs support intensifying maternal medical therapy when fetuses have an abdominal circumference above the 70th percentile because the latter feature is associated with increased abdominal fat accretion. The trials that were successful in reducing LGA status used insulin as a treatment, but it is unclear how much of the benefit of maternal insulin therapy was due to reducing very mild maternal hyperglycemia or suppressing FFAs. It is recommended that women with GDM who require medical therapy or have suboptimal glycemic control undergo fetal surveillance beginning at 32 to 34 weeks of gestation. Delivery may be considered at around 39 weeks if the woman has good dating criteria and favorable cervical status, but delivery at 40 weeks is an option if glycemic control continues to be optimal and fetal test results are reassuring. An estimated fetal weight of greater than 4500 g carries such a high risk of shoulder dystocia that an elective cesarean delivery is usually recommended in women with GDM or preexisting diabetes.
53. Discuss the long-term sequelae of GDM or preexisting diabetes in offspring of affected mothers.
The long-term sequelae of diabetes for offspring are concerning. Proliferation of fetal adipocytes and pancreatic beta cells may be responsible for “fetal programming” of the later development of obesity and the metabolic syndrome. Reports of an increased risk of adolescent obesity and type 2 diabetes in infants of mothers with GDM or preexisting diabetes are compelling. The incidence of childhood type 2 diabetes was approximately tenfold higher in Pima Indian offspring born to mothers with diabetes than in offspring whose mothers did not have diabetes until after pregnancy. Furthermore, despite similar incidences of obesity at 20 years of age in the two offspring groups, the incidence of type 2 diabetes was nearly 70% at age 25 to 29 years in the offspring of diabetic mothers, compared with approximately 10% in the offspring of prediabetic mothers (those in whom diabetes did not develop until after the pregnancy).
54. How does in utero hyperglycemia or other metabolic risk factors affect the long-term sequelae of infants born to diabetic mothers?
In utero hyperglycemia appears to be an independent risk factor for the development of childhood glucose intolerance. However, being born LGA or being born to an obese mother also increases the risk of childhood obesity and metabolic syndrome. Interestingly, women with severe obesity, many with a history of GDM, who undergo bariatric surgery before their next pregnancies have been shown to have far fewer maternal and fetal complications, and the offspring risk for childhood obesity is reduced. A maternal high-fat diet has also been associated with an increased risk of offspring metabolic disorders. Elevated insulin values in amniotic fluid (owing to fetal hyperinsulinemia) predicted teenage obesity in one study independently of fetal weight, and approximately 30% of these offspring had IGT by 17 years of age. Fetal programming or epigenetic influences appears to occur in this intrauterine environment of nutrient excess and may contribute to the growing incidence of type 2 diabetes as children with IGT become mothers themselves, perpetuating the cycle.
55. What causes women to get GDM?
GDM is caused by abnormalities in at least three aspects of fuel metabolism: insulin resistance in fat and muscle, increased hepatic glucose production, and impaired insulin secretion. Although insulin levels may be high, the increased insulin resistance of pregnancy still results in inadequate compensation because impaired beta-cell function leads to insufficient insulin secretion to maintain euglycemia in the presence of insulin resistance. The insulin resistance is thought to be due primarily to the effects of increased production of human placental lactogen, placental growth hormone, tumor necrosis factor-α, and inflammatory cytokines. Women in whom GDM develops have lower pregravid insulin sensitivity than matched control groups, and some abnormalities may persist after delivery. The majority of women in whom GDM develops are overweight and many have characteristics of the metabolic syndrome before pregnancy. Thin or normal-weight women in whom GDM develops are in the minority and may display a maturity-onset diabetes of the young (MODY) gene or, more commonly, may be at risk for development of latent autoimmune diabetes of adulthood (LADA). Many of these unusual patients are found to be positive for glutamic acid decarboxylase (GAD) antibody or islet cell antibodies and have lowish C-peptide levels, putting them at increased risk for manifesting type 1 diabetes later.
56. What causes increased hepatic glucose production?
Increased hepatic glucose production results from inadequate insulin suppression of excessive hepatic gluconeogenesis. There are some women with GDM who primarily have fasting hyperglycemia, and it is thought that they have greater hepatic insulin resistance than peripheral (muscle and fat) insulin resistance. Beta-cell sensing of glucose is also abnormal and is manifested as an inadequate insulin response for a given degree of hyperglycemia.
57. Summarize the role of impaired insulin secretion in GDM.
Impaired insulin secretion renders the woman unable to meet the requirement for greater insulin production necessitated by the insulin resistance and increased hepatic glucose production. These same pathophysiologic disorders, which are in large part genetically determined, make the patient with GDM more likely to have type 2 diabetes mellitus later in life, when weight gain and aging often contribute further to insulin resistance and impaired insulin secretion. Pregnancy can be thought of as a “stress test” for the development of type 2 diabetes, because the marked insulin resistance of pregnancy necessitates a twofold to threefold increase in insulin secretion that the beta cell may not be able to achieve, resulting in a clinically evident abnormality in glucose metabolism. It has been demonstrated that this beta-cell defect persists postpartum, and the severity of the defect is predictive of the risk for development of type 2 diabetes.
58. What is the best therapy for women with GDM, and how much weight should they gain?
Women with GDM should be taught home blood glucose monitoring to ensure that glycemic goals are met throughout the duration of pregnancy. The best therapy for GDM depends entirely on the extent of the glucose intolerance and on the mother’s response. In at least half of cases, diet alone maintains postprandial blood glucose values within the target range but is more likely to fail if fasting hyperglycemia also exists. Women should not exceed the Institute of Medicine (IOM) recommendations for weight gain in pregnancy (BMI < 18.5 kg/m2, 28-40 lb; BMI 18.5-24.9 kg/m2, 25-35 lb; BMI 25-29.9 kg/m2, 15-25 lb; BMI ≥ 30 kg/m2, 11-20 lb) because both a higher maternal BMI and gestational weight gain independently increase the risk of an LGA infant. Further, many experts recommend targeting the lower end of the weight gain ranges. It has been shown that women with more severe obesity (BMI > 35 kg/m2) should gain even less weight and that many severely obese women do not need to gain any weight to deliver a normally grown infant. Postprandial glucose levels have been strongly associated with the risk of LGA infants, and therefore restriction of simple carbohydrates may be helpful to blunt postprandial glucose excursions. However, saturated fats should also be limited because they increase insulin resistance and independently contribute to excess triglycerides and FFAs for fetal fat accretion. Women with a BMI greater than 30 kg/m2 may benefit from a 30% to 33% caloric restriction to approximately 20-25 kcal/kg or approximately 1800 kcal per day, which has been shown to reduce hyperglycemia and serum triglycerides with no increase in ketonuria. Weight loss during pregnancy is not advocated at this time.
59. Discuss the role of oral sulfonylureas in GDM.
The only oral hypoglycemic drugs approved for use in women with GDM are glyburide and acarbose; the latter is usually problematic because of gastrointestinal side effects. None of the other insulin secretagogues is approved, nor is metformin or thiazolidinediones. In a landmark multicenter trial, 400 women with GDM were randomly assigned to receive either insulin or glyburide after 24 weeks of gestation. Maternal glycemic control, macrosomia, neonatal hypoglycemia, and neonatal outcomes were no different between the groups. Most important, the cord serum insulin concentrations were similar in the two groups, and glyburide was not detected in the cord serum of any infant tested. However, subsequent studies suggested that a very small amount of glyburide may cross the placenta. Overall, glyburide therapy will fail in approximately 20% of women with GDM and they will require insulin treatment to achieve adequate glycemic control. Risk factors associated with glyburide failure include diagnosis of GDM before 24 weeks, fasting hyperglycemia, recurrent pregnancies, and more severe hyperglycemia.
60. Discuss the role of metformin in pregnancy.
The MiG (Metformin in Gestation) trial was an RCT of 751 women with GDM randomly assigned to receive metformin or insulin. Because of concerns about the possible risk of fetal lactic acidosis, women with a contraindication to metformin, fetal anomalies, gestational hypertension, preeclampsia, fetal growth restriction, or ruptured membranes were excluded from the study. Women with preexisting diabetes were also excluded. Metformin did not appear to increase the rates of any adverse outcomes, although it was associated with a slight increase in preterm birth rate and 46% of women in the metformin group required supplemental insulin. The 2-year-old offspring data have been reported in a follow-up trial (Metformin in Gestation—The Offspring Follow Up [MiG-TOFU]). There was some evidence of increased subcutaneous fat in the offspring exposed to metformin in utero but total fat as measured by dual-energy x-ray absorptiometry (DXA) scan did not appear to be different in the two groups, even though only a minority of the offspring underwent DXA. Although the researchers of the trial speculated that metformin might be of value in decreasing visceral fat in the offspring, no good visceral fat measures were made because DXA is insensitive in measuring visceral fat and magnetic resonance imaging was not performed. Metformin should not be used in women at risk for placental insufficiency because it crosses the placenta and it has not been approved in pregnancy for the routine treatment of GDM. It is recommended that its use be limited to prospective trials, although many practitioners are using it because of its lower risk of hypoglycemia, its ease of use, and the challenges of treating a rapidly growing GDM population.
61. When should insulin be used to treat GDM?
Women who have fasting blood glucose levels greater than 95 mg/dL, 1-hour postprandial glucose levels greater than 140 mg/dL, or 2-hour postprandial glucose levels greater than 120 mg/dL should be started on insulin therapy. Those who are unwilling to start insulin and who exhibit mild hyperglycemia without substantial increases in fasting blood glucose may be candidates for glyburide therapy or possibly metformin, although the latter is not yet approved by the FDA for use in GDM. A woman with a fetus that is LGA, as demonstrated by ultrasound, is also a candidate for medical management. Often GDM can be treated with twice-daily injections of NPH and regular insulin, but occasionally, postprandial glycemic excursions are so excessive that mealtime injections of a short-acting insulin analog are necessary. Serious hypoglycemia tends to be an infrequent occurrence in such patients because of their underlying insulin resistance and symptomatic awareness of hypoglycemia.
62. What is the role of exercise in patients with GDM or preexisting diabetes?
Both the ACOG and the ADA advise that pregnant women adopt the national guidelines of exercising 30 minutes daily as long as there is not an obstetric contraindication. Moderate exercise is well tolerated in pregnancy. Exercise also improves insulin sensitivity in women with type 2 diabetes and may limit excess weight gain. Fetal safety of such exercise has been established, especially if the maternal heart rate is kept under 140 to 160 beats/minute at durations of 30 minutes and if the mother is well hydrated and does not get overheated. Two of three small trials in GDM pregnancies have shown that exercise three times a week can achieve glycemic control and infant birth weights that are similar to those seen in women who are treated with insulin. Unfortunately, exercise has been shown in one meta-analysis not to effectively decrease LGA and to be only marginally effective in limiting excess gestational weight gain. However, if women increase their physical activity by walking after they eat, postprandial glucose rises can be significantly blunted. Additionally, establishing a regular routine of modest exercise during pregnancy may also have long-lasting benefits for the mother with GDM, who clearly has an appreciable risk for development of type 2 diabetes in the future.
63. When is a controlled exercise program contraindicated?
Women at risk for preterm labor, vaginal bleeding, or conditions predisposing to growth restriction are not candidates for a controlled exercise program. Women with poorly controlled hypertension and preeclampsia are usually advised to adhere to bed rest. Some women, especially women with a low BMI and long-standing type 1 diabetes with vascular disease, may be at risk for placental insufficiency or growth restriction and may not be candidates for exercise.
64. What important postpartum management issues should be addressed in women with pregestational or gestational diabetes?
Critical issues in the postpartum period include maintenance of glycemic control, diet, exercise, weight loss, blood pressure and renal protection management, breastfeeding, and contraception. Women with type 1 diabetes should be checked for TPO antibodies which, if present, confer up to a 50% risk for development of postpartum thyroiditis. Breastfeeding has been shown to be advantageous to mothers with type 2 diabetes and GDM by facilitating weight loss. Inability to lose the weight gained in pregnancy is one of the strongest risk factors for progressing to type 2 diabetes in women with GDM. The majority of women with preexisting diabetes has dramatic decreases in their insulin requirements immediately postpartum and are likely to require significantly less than their pre-pregnancy doses, especially those who breastfeed. Glycemic goals need to be relaxed, especially given erratic eating and sleeping schedules and the improved insulin sensitivity. Women with preexisting diabetes, even those who have been extremely compliant and have had optimal glycemic control during pregnancy, experience a dramatic worsening of glucose control after delivery. Furthermore, many stop seeking medical care for their diabetes. The postpartum period is relatively neglected as both the new mother and her physician relax their vigilance. However, this period offers a unique opportunity to institute health habits that can have highly beneficial effects on the quality of life of both the mother and her infant. The importance of effective contraception cannot be overstated, because 50% of pregnancies are unplanned and each subsequent pregnancy in a woman with GDM increases her risk for development of type 2 diabetes.
65. Explain the value of diet and exercise in the postpartum period.
A weight loss program consisting of diet and exercise should be instituted for women with GDM to improve insulin sensitivity and prevent the development of type 2 diabetes. It has been shown that most women with GDM or type 2 diabetes do not lose their pregnancy weight gain and often enter a subsequent pregnancy at an even higher weight. Diet and exercise reduced the development of type 2 diabetes by approximately 50% in the subgroup of women with GDM in the DPP trial, and therefore every effort must be made to intervene in this exceedingly high-risk population. Overweight women with type 2 diabetes are likely to be able to change from insulin to oral medications if they can also reduce their weight.
66. Discuss the importance of glucose monitoring and subsequent testing during the postpartum period.
Vigilant home blood glucose monitoring should be continued in the postpartum period by women with pregestational diabetes because insulin requirements drop almost immediately and often dramatically at this time, increasing the risk of hypoglycemia. For women with GDM who required insulin, occasional home blood glucose monitoring may be useful because about 10% of such patients have been shown to meet the criteria for diabetes on postpartum testing. In all women with a history of GDM, glycemic status should be reassessed 6 to 12 weeks after delivery. At a minimum, an FBG test should be performed to determine whether the woman has persistent diabetes (FBG > 126 mg/dL) or IFG (FBG 100-125 mg/dL). A 75-g 2-hour OGTT is recommended by the ADA and IADPSG because a 2-hour glucose value of 200 mg/dL or higher establishes a diagnosis of diabetes and a 2-hour value of 140-199 mg/dL makes the diagnosis of IGT. The majority of women with persistent IGT is missed if only an FBG measurement is checked. An HbA1C measurement is not sensitive in diagnosing prediabetes at 6 weeks postpartum because it is often low owing to iron deficiency anemia or postpartum blood loss and because it reflects glycemia over a 3-month period (which would include the last 6 weeks of pregnancy).
67. Why is a diagnosis of impaired glucose tolerance or prediabetes of critical importance?
The importance of diagnosing impaired glucose intolerance or prediabetes lies in its value in predicting the future development of type 2 diabetes. In one series, a diagnosis of IGT was the most potent predictor of the development of type 2 diabetes in Latino women with a history of GDM; diabetes developed in 80% of such women in the subsequent 5 to 7 years. Intensified efforts promoting diet, exercise, and weight loss, and possibly metformin if lifestyle changes fail, should be instituted in this extraordinarily high-risk group of women.
68. Summarize the role of ACE inhibitors or other antihypertensives in the postpartum period.
Postpartum women who are candidates for an ACE inhibitor can be started on enalapril or captopril, which have not been shown to appear in breast milk in appreciable concentrations. There are few data on ARBs, but calcium channel blockers are also compatible with breastfeeding. Diuretics should be avoided because of their possible suppressive effects on breast milk production, and beta-blockers such as atenolol with a low degree of protein binding can reach breast milk and so should be avoided.
69. Should women with diabetes breastfeed their infants?
Women should be encouraged to breastfeed unless difficulties in glycemic control arise. Women with type 1 diabetes who breastfeed have even lower insulin requirements than those who do not, necessitating a reduction of insulin dosing to 50-60% of pre-pregnancy requirements. Due to fluctuating glucose levels during breastfeeding, which often occurs at night in the fasted state, women are at high risk for severe maternal hypoglycemia and therefore glucose goals should be relaxed. For women with insulin-resistant type 1 diabetes, type 2 diabetes, or GDM, breastfeeding promotes loss of maternal weight gain and also decreases the risk of childhood obesity, especially if continued for at least 6 months. There are reports suggesting that modest doses of glyburide and metformin can be used in breastfeeding mothers, but the sample sizes of these studies are small; the pediatrician should be notified, especially if glyburide is used. Women with a history of GDM who breastfeed appear to have a lower incidence for development of type 2 diabetes, partly because of enhanced weight loss since breastfeeding requires approximately 300-400 kcal per day. The mother must also ensure that her calcium intake is at least 1500 mg/day.
70. How common is postpartum thyroiditis in women with type 1 diabetes? When does it appear?
Women with type 1 diabetes have been reported to have a 20% to 25% incidence of postpartum thyroiditis, the risk being highest in women who test positive for TPO antibodies. Hyperthyroidism can occur in the 2- to 4-month postpartum period, and hypothyroidism may present in the subsequent 4 to 8 months. Given the significance of this disorder, measurement of thyroid-stimulating hormone is recommended in patients with type 1 diabetes and TPO antibodies at 3 months and at 6 months after delivery or with any suggestive symptoms.
71. Summarize the long-term follow-up of nondiabetic women with a history of GDM.
Women with a history of GDM should undergo a 75-g 2-hour OGTT at approximately 6 to 12 weeks postpartum so they can be determined to be normal or to have prediabetes (IFG, IGT) or diabetes. The ADA recommends retesting every 1 to 3 years using an FBG HbA1C measurement or 75-g 2-hour OGTT; but it has recently been shown that an HbA1C value of 5.7% or higher has been shown to have poorer sensitivity than an abnormal FBG value or 75-g 2-hour OGTT result at 6 weeks to 1 year. The 75-g 2-hour OGTT remains the most sensitive method for detecting any glucose intolerance, especially in some ethnic groups, such as Asian American women, who typically have normal FBG values. There are definite racial-ethnic differences in hemoglobin glycation. A number of women with GDM will go from having GDM to having type 1 or type 2 diabetes between pregnancies. If such a woman enters the next pregnancy with an HbA1C value of 6.5% or higher, her fetus will carry an increased risk of a major malformations, underscoring the importance of repeat testing and effective contraception.
72. Which contraceptive agents can be used by women with diabetes or a history of GDM?
It should be documented at every visit that women are using or have been offered an effective birth control method. The vast majority of contraceptive methods are relatively safe in women with diabetes, including combined oral contraceptives, except for women who have poorly controlled hypertension or hypertriglyceridemia or are at risk for thromboembolic disease. Triglycerides should be measured after the initiation of oral contraceptives in all women with diabetes or a history of GDM because of the significant incidence of hypertriglyceridemia and the associated risk of pancreatitis with oral estrogen use.
73. Summarize the role of low-dose combined oral contraceptives in women with diabetes or a history of GDM.
Low-dose combined oral contraceptives have been shown to be effective and to have minimal metabolic effects in women with diabetes. The ORTHO EVRA patch and the estrogen-progestin contraception ring (NuvaRing) are also options, but the patch has been associated with a higher risk of thromboembolism. In a retrospective cohort of 904 women with GDM, combined oral contraceptives did not influence the development of type 2 diabetes.
74. What other contraceptive options are appropriate in women with diabetes or a history of GDM?
Progestational agents, such as Depo-Provera (medroxyprogesterone acetate) and norethindrone, are less favorable alternatives because Depo-Provera may affect carbohydrate tolerance and norethindrone alone (Minipill) is less efficacious. Depo-Provera has been associated with an increased risk of type 2 diabetes in nursing mothers with a history of GDM, primarily because of excess weight gain. The etonogestrel implant (Implanon) has not been shown to worsen carbohydrate intolerance and may be a good choice for women who want contraception for 3 years. There is no increase in pelvic inflammatory disease with the use of intrauterine devices (IUDs) in women with well-controlled type 1 or type 2 diabetes after the postinsertion period. Therefore an IUD (containing either copper or progestin) is a very attractive choice in women who do not desire pregnancies in the near future. Nearly any contraceptive method is superior to an unwanted pregnancy, given the risks to the mother with pregestational diabetes and the increased risk for development of type 2 diabetes in mothers with a history of GDM.
ACOG Committee Opinion. Postpartum screening for abnormal glucose tolerance in women who had gestational diabetes mellitus; 2009. June, Number 435
, ACOG Committee Opinion. Screening and diagnosis of gestational diabetes mellitus, 2011;1–3. Sept, Number 504
, American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011;34:S62–S69.
Ballas, J, Moore, TR, Ramos, GA. Management of diabetes in pregnancy. Curr Diab Rep. 2012;12:33–42.
Baptiste-Roberts, K, Barone, BB, Gary, TL, et al, Risk factors for type 2 diabetes among women with gestational diabetes. a systematic review. Am J Med 2009;122:207–214.
Barbour, LA, McCundy, CE, Hernandez, TL, et al. Cellular mechanisms in gestational diabetes. Diabetes Care. 2007;30(Suppl 2):S112–S119.
Barbour, LA, Weight gain in pregnancy. is less truly more for mother and infant. Obstetric Medicine 2012;5:58–64.
Buchanan, TA, Xiang, AH, Page, KA, Gestational diabetes mellitus. risks and management during and after pregnancy. Nat Rev Endocrinol 2012;8:639–649.
Buchanan, TA, Xiang, A, Kjos, SL, Watanabe, R. What is gestational diabetes. Diabetes Care. 2007;30(Suppl 2):S105–S111.
Castorino, K, Paband, R, Zisser, H, Jovanovic, L, Insulin pumps in pregnancy. using technology to achieve normoglycemia in women with diabetes. Curr Diab Rep 2011;12:53–59.
Catalano, PM, Thomas, A, Huston-Presley, L, Amini, SB. Phenotype of infants of mothers with gestational diabetes. Diabetes Care. 2007;30(Suppl 2):S156–S160.
Clausen, TD, Mathiesen, E, Ekbom, P, et al. Poor pregnancy outcome in women with type 2 diabetes. Diabetes Care. 2005;28:323–328.
Cooper, WO, Hernandez-Diaz, S, Arbogast, PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354:2443–2451.
Cormier, CM, Martinez, CA, Refuerzo, JS, et al, White’s classification of diabetes in pregnancy in the 21st century. is it still valid. Am J Perinatol 2012;27:349–352.
Crowther, CA, Hiller, JE, Moses, JR, et al, for the Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477–2486.
Crume, TL, Ogden, LG, Mayer-Davis, EJ, et al, The impact of neonatal breast-feeding on growth trajectories of youth exposed and unexposed to diabetes in utero. the EPOCH study. Internat J Obesity 2012;36:529–534.
Dabelea, D, Crume, T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes. 2011;60:1849–1855.
England, LJ, Dieta, PM, Njoroge, T, et al, Preventing type 2 diabetes. public health implications for women with a history of gestational diabetes. Am J Obstet Gynecol 2009;200:365.
Garcia-Patterson, A, Gich, I, Amini, SB, et al, Insulin requirements throughout pregnancy in women with type 1 diabetes mellitus. three changes of direction. Diabetologia 2010;53:446–451.
Gauster, M, Desoye, G, Totsch, M, Hiden, U. The placenta and gestational diabetes mellitus. Curr Diab Rep. 2012;12:16–23.
Glatstein, M, Djokanovic, N, Garcia-Bournissen, F, et al. Use of hypoglycemic drugs during lactation. Canadian Family Physician. 2009;55:371–373.
Harmon, KA, Gerard, L, Jensen, DR, et al. Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet. Diabetes Care. 2011;34:1–7.
Hernandez, TL, Friedman, JE, Van Pelt, RE, Barbour, LA, Patterns of glycemia in normal pregnancy. should the current therapeutic targets be challenged. Diabetes Care 2011;34:1660–1668.
Holmes, VA, Young, IS, Patterson, CC. Optimal glycemic control, pre-eclampsia, and gestational hypertension in women with type 1 diabetes in the diabetes and pre-eclampsia intervention trial. Diabetes Care. 2011;34:1683–1688.
, IADPSG Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2012;33:676–682.
Kahn, B, Davies, JK, Lynch, AM, et al. Predictors of glyburide failure in women with gestational diabetes. Obstet Gynecol. 2006;107:1306–1309.
Kim, C, Herman, WH, Cheung, NW, et al. Comparison of hemoglobin A1C with fasting glucose and 2-h postchallenge glucose for risk stratification among women with recent gestational diabetes. Diabetes Care. 2011;34:1949–1951.
Kitzmiller, JL, Block, JM, Brown, FM, et al. Managing preexisting diabetes for pregnancy. Diabetes Care. 2008;31:1060–1079.
Kjos, SL, Schaeffer-Graf, UM. Modified therapy for gestational diabetes using high-risk and low-risk fetal abdominal circumference growth to select strict versus relaxed maternal glycemic targets. Diabetes Care. 2007;30(Suppl 2):S200–S205.
Kjos, SL, After pregnancy complicated by diabetes. postpartum care and education. Obstet Gynecol Clinics North Am 2007;34:335–349.
Landon, MB, Gabbe, SG. Gestational diabetes mellitus. Obstet Gynecol. 2011;118:1379–1393.
Langer, O, Conway, DL, Berkus, MD, et al. A comparison of glyburide and insulin in women with gestational diabetes. N Engl J Med. 2000;343:1134–1138.
Li, D-K, Yang, C, Andrade, S, et al, Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring. a retrospective cohort study. BMJ 2011;343:1–10.
Malcolm, N, Through the looking glass. gestational diabetes as a predictor of maternal and offspring long-term health. Diab Metab Res Rev 2012;28:307–311.
Mathiesen, E, Kinsley, B, Amiel, SA, et al, The Insulin Aspart Pregnancy Study Group. Maternal glycemic control and hypoglycemia in type 1 diabetes pregnancya randomized trial of insulin aspart versus human insulin in 322 pregnant women. Diabetes Care 2007;30:771–776.
Mathiesen, ER, Ringholm, L, Damm, P. Stillbirth in diabetic pregnancies. Best Practice Res Clin Obstet Gynaecol. 2011;25:105–111.
Metzger, BE, Buchanan, TA, Coustan, DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–S260.
Murphy, HR, Kumareswaran, K, Elleri, D, et al. Safety and efficacy of 24-h closed-loop insulin delivery in well-controlled pregnant women with type 1 diabetes. Diabetes Care. 2011;34:2527–2529.
Nielsen, GL, Moller, M, Sorenson, HT, HbA1C in early diabetic pregnancy and pregnancy outcomes. a Danish population-based cohort study of 573 pregnancies in women with type 1 diabetes. Diabetes Care 2006;29:2612–2616.
Price, N, Bartlett, C, Gillmer, M, Use of insulin glargine during pregnancy. a case-control study. BJOG 2007;114:453–457.
Ratner RE, Christophi CA, Metzger BE, et al: Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions, J Clin Endocrinol Metab 93:4774–4779.
Reece, EA, Leguizamon, G, Wiznitzer, A, Gestational diabetes. the need for common ground. Lancet 2009;373:1789–1797.
Ringholm, L, Mathiesen, ER, Kelstrup, L, Damm, P. Managing type 1 diabetes mellitus in pregnancy—from planning to breastfeeding. Nat Rev Endocrinol. 2012;8:659–667.
Roland, JM, Murphy, HR, Northcote-Wright, J, Temple, RC, The pregnancies of women with type 2 diabetes. poor outcomes but opportunities for improvement. Diabetes Med 2005;22:1774–1777.
Rowan, JA, Hague, WM, Gao, W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003–2015.
Rowan, JA, Rush, EC, Obolonkin, V, et al, Metformin in gestational diabetes. the offspring follow-up (MiG TOFU). Diabetes Care 2011;34:2279–2284.
Ryan, EA. Diagnosing gestational diabetes. Diabetologia. 2011;54:480–486.
Sacks, DA, Hadden, DR, Maresh, M, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel—recommended criteria. Diabetes Care. 2012;35:626–628.
Schaefer-Graf, UM, Graf, K, Kulbacka, I, et al. Maternal lipid as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care. 2008;31:1858–1863.
Schaefer-Graf, UM, Hartman, R, Pawliczak, J. Association of breast-feeding and early childhood overweight in children from mothers with gestational diabetes mellitus. Diabetes Care. 2006;29:1105–1107.
Seely, EW, Ecker, J. Chronic hypertension in pregnancy. N Engl J Med. 2011;365:436–439.
Temple, RC, Aldridge, VJ, Murphy, HR. Prepregnancy care and pregnancy outcomes in women with type 1 diabetes. Diabetes Care. 2007;29:1744–1749.
, The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002.
Xiang, AH, Peters, RK, Kjos, S, et al. Effect of pioglitazone on pancreatic b-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. 2006;55:517–522.