Developmental Dysplasia of the Hip
Developmental dysplasia of the hip (DDH), formerly referred to as congenital dislocation of the hip, was first described thousands of years ago. Hippocrates is credited with ascribing intrauterine pressure as a possible etiology for this entity.1 This chapter covers the etiology, multimodality diagnostic imaging, and its implications in the treatment of DDH.
Etiology: DDH is a spectrum that may vary from a normal congruent hip with normal appearing acetabula with ligamentous laxity, to a structurally abnormal hip with joint incongruity related to primary acetabular dysplasia. Usually, DDH is a result of a combination of both structural and ligamentous laxity leading to an abnormal incongruent hip. Ultimately, the precise underlying etiology of DDH is not known. Purported in utero developmental factors and fetal crowding may result in DDH. During week 7 of gestation, hip joint cleavage occurs.2 The lower extremity rotates medially during week 12 of gestation and hip muscles develop during week 18. However, it has been suggested that only 2% of cases result from intrauterine factors at this early phase of development and that the remaining 98% of cases are caused by changes to a normal hip during the last 4 weeks of pregnancy or in the immediate postnatal period.2 Postnatal factors are also hypothesized to have a role in DDH, including tight swaddling or the use of cradle boards that force the legs into extension and adduction.
Epidemiology: Being the first born, breech birth, oligohydramnios, being large for gestational age, skull-molding deformities, female gender, and positive family history have all been associated with DDH. DDH is more common on the left.2 The theory of many of these factors relating to the cause of DDH reflects constricted in utero space. The uterus of a primiparous woman has less give. The hips are subjected to increased pressure and hyperflexion in breech presentations. With frank breech presentations, the risk is even greater. Decreased amniotic fluid or a large gestation also results in increased constriction. The most common fetal presentation is cephalic, with the fetal spine aligned to the mother’s left. This causes the left hip to be wedged against the maternal spine, which limits abduction and leads to a higher incidence of DDH on the left. Females are thought to be more affected because they are more sensitive to the circulating maternal hormone relaxin, resulting in ligamentous laxity.2
DDH is associated with myelodysplasia, arthrogryposis, multiple syndromes (such as Mobius and Poland syndromes), and chromosomal abnormalities. DDH is associated with talipes equinovarus or clubfoot, torticollis (fibromatosis colli, with a reported incidence of 5.5%), and congenital knee dislocation.3,4
The prevalence of DDH is reported up to 28.5 per 1000, depending on the population studied.5 Variation in rates may result from expertise of the examiners and inclusion of children with isolated ligamentous laxity. The incidence of DDH increases with family history: 6% chance with an affected sibling, 12% chance with one affected parent, 36% chance with both parents affected.2 During the first 2 weeks of life, physiologic instability exists, but it dissipates with increasing muscle tone and resolving ligamentous laxity as maternal hormones decrease.
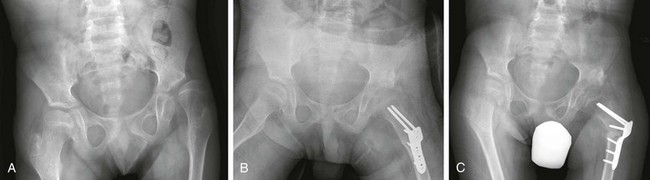
Natural History: If undiagnosed or left untreated, DDH may result in permanent acetabular dysplasia in children of walking age, typically having a shallow acetabular roof (sourcil) with anterior undercoverage related to increased acetabular version. Similarly, the femoral head may become secondarily dysplastic and lose its normal sphericity related to eccentric loads because of an incongruent hip, with resultant spherical growth plate disturbance and modeling changes.6 The femoral head must be seated within the acetabulum for normal development to occur. If the femoral head is dislocated, the transverse acetabular ligament and inferior capsular fibers become interpositioned, inhibiting hip congruity. Pulvinar (fibrofatty tissue) may also interpose between the femoral head and acetabulum, interfering with hip congruity. Hypertrophy of the fibrocartilaginous labrum often compensates for a shallow bony acetabular roof, and this has historically been referred to as a limbus.2 Long-term sequelae include incomplete coverage of the femoral head, excessive femoral anteversion, coxa valga, and coxa magna with muscle tightness (with the greatest impact on the adductors)7 (Fig. 136-1). As a result of these morphologic features, individuals with untreated DDH may develop premature degenerative changes of the hip joint and a permanent limp. Excessive femoral anteversion will lead to in-toeing. Hip degenerative changes are caused by loss of cartilage and labral tears secondary to abnormal eccentric joint contact pressure from chronic incongruent acetabular and femoral head articulation; rupture of the ligamentum teres has also been described.8 Degenerative changes caused by hip subluxation typically appear before women reach the age of 40 years and before men reach the age of 55 years; however, symptoms may become apparent during adolescence.2 With completely dislocated hips, many patients may function very well even into late adolescence with a neoacetabulum that forms along the iliac wing.2
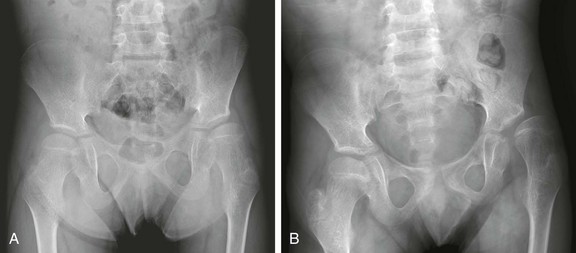
Figure 136-1 Frontal radiographs of the pelvis demonstrating late presentation, and progression of developmental dysplasia of the hip of the left hip.
A, At presentation, in this 11-year-old boy, the left hip is dysplastic, with an upsloping sourcil and mild undercoverage of the femoral head. The femoral head is already noted to be aspherically shaped. B, Three years later, progressive lateral uncovering of the femoral head and progressive secondary dysplastic changes of the femoral head are seen, and the greater trochanter overlies the femoral neck despite proper positioning, indicative of excessive femoral anteversion.
Physical Examination: The physical examination to diagnose DDH consists of maneuvers described by Ortolani, a pediatrician, and by Barlow, an orthopedic surgeon. Ortolani described the hip click in 1937, although hip instability had been reported in 1879 and the clinical test described by Le Damany and Saiget in 1910.5 Barlow described his technique to demonstrate laxity of the dysplastic hip in 1961.
In the Ortolani maneuver, forward pressure is applied to each femoral head with the infant in the supine position and the hips flexed to abduct the hip and to reduce a subluxated or dislocated femoral head. If subluxation or dislocation exists, an audible “clunk” is heard as the femoral head is reduced into the acetabulum. In the Barlow maneuver, backward pressure is applied to each femoral head with the in the infant supine position and the hips flexed to subluxate the hip.9–11 Both these maneuvers may fail to diagnose bilateral irreducible hip dislocations.
Radiography: The infant’s lower extremities should be positioned in neutral extension with longitudinal symmetry. For symmetric imaging, the central ray should be directed above the pubic symphysis in the midline. In the presence of abnormality such as dislocation of the femoral head, a frog-leg view may be useful to evaluate reduction of the femoral head and may provide an opportunity for a second look at acetabular morphology. Positioning the thigh in abduction and internal rotation with a 45-degree angle (the von Rosen view) provides similar information as a frog-leg lateral view.12
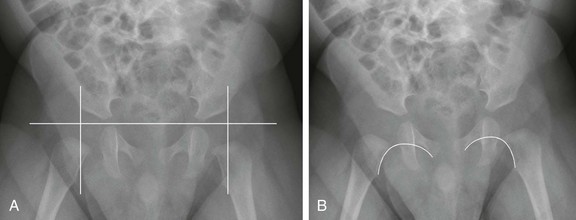
Several lines may be drawn to assist in the assessment of hips, but attention should be made to the overall configuration rather than to the measured angles, which could have varying rates of interobserver and intraobservor variability. The Hilgenreiner line passes horizontally through the superior aspect of the triradiate cartilages. The Perkin line is the vertical line extending from the lateral margin of the acetabulum. The expected location of the femoral head is in the medial, inferior quadrant of the intersection of the Hilgenreiner and Perkin lines (Fig. 136-2, A). The Shenton line curves along the lesser trochanter, femoral neck, and inferior margin of the pubis or obturator foramen and should be smooth (Fig. 136-2, B)
The acetabular index is the angle between the acetabulum (from the superolateral margin of the acetabulum and the superolateral margin of the triradiate cartilage) and the Hilgenreiner line. The acetabular index changes with age: 28 degrees in newborns, 23.5 degrees at age 6 months, 22 degrees at age 1 year, and 20 degrees at age 2 years. The maximal normal measurement for the acetabular index is 30 degrees up to age 4 months and 25 degrees up to age 2 years. The orientation of the pelvis on the radiograph impacts this measurement.13,14
In the preschool children and older children, the center edge angle may be calculated, and this measures the relative acetabular coverage of the femoral head. To determine the center edge angle, one line is drawn between the center of both femoral heads. Next, a vertical line is drawn 90 degrees at the center of the femoral head, and this serves as one reference line. Next, a line from the femoral head is drawn to the lateral acetabulum, and this serves as the second reference line. Now the center edge angle can be determined. This measurement is used in children over age 5 years. DDH should be suspected when the center edge angle is less than 19 degrees in children aged 6 to 13 years and less than 25 degrees in those over age 13 years.13,15
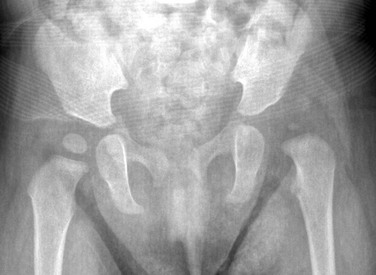
Radiographs are utilized to screen for DDH after age 4 to 6 months, when ultrasonography becomes more challenging and less reliable because of ossification of the femoral heads. Radiographic findings of DDH include an upward slanting sourcil, small capital femoral epiphysis, and superolateral subluxation of the femoral head (Figs. 136-3 and 136-4). Anteroposterior views are sufficient for screening. When abnormal, a frog-leg lateral view or a von Rosen view may be obtained to assess whether the femoral head reduces with hip abduction. When chronic, untreated DDH exists, the ipsilateral capital femoral epiphysis may become disproportionately smaller (Fig. 136-5). Without concentric apposition of two bones of a joint, as may occur with untreated DDH, epiphyseal ossification will be delayed.
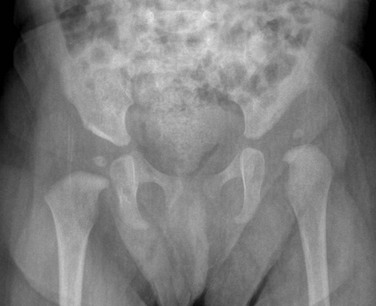
Figure 136-3 An anteroposterior radiograph in an 8-month-old girl demonstrates bilateral developmental dysplasia of the hip, with the left hip worse than the right hip.
Note the upward slanting sourcil bilaterally. The right femoral head is concentrically located within its dysplastic acetabula. The left femoral head is superolaterally dislocated.
Ultrasonography: An orthopedic surgeon, Graf, described the use of ultrasonography in the assessment of the anatomy of the infant hip in 1980.16 Four years later, a group of radiologists including Harcke, described the dynamic technique to assessing infant hips.17 Ultrasonography has the advantages of absence of ionizing radiation, widespread availability, and the feasibility of a dynamic examination.
The examination is typically performed with a linear array transducer using the highest frequency that provides adequate penetration (often 12 megahertz [MHz]). The transducer is placed over the lateral aspect of the hip in either the coronal plane, with the hip in neutral or flexed positions, or in the transverse or axial plane, with the hip in adduction or abduction (e-Fig. 136-6). The infant should be placed in the lateral decubitus or supine position. In the coronal plane, the ilium should appear as a horizontal line, the midportion of the acetabulum visualized to its maximal depth, and the middle of the fibrocartilaginous labrum seen. Without technical accuracy, a normal hip may appear abnormal; the converse is not true. The femoral head should be in close apposition with the acetabulum with at least 50% of the femoral head covered by the acetabulum. Subluxation is present if the femoral head is less than 50% covered but in contact with the acetabulum. In the absence of contact between the femoral head and acetabulum, the hip is dislocated. The hip may reduce with changes in position, typically in abduction and flexion.
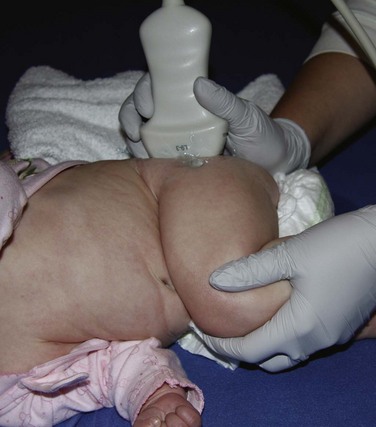
e-Figure 136-6 Positioning of the ultrasound transducer on an infant for examination of the left hip in the coronal plane with flexion.
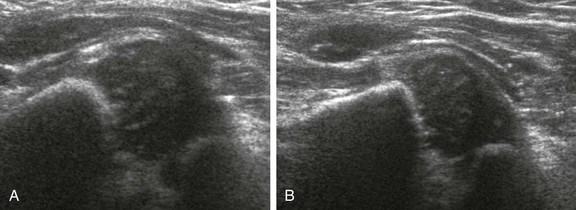
The alpha-angle, which is the slope of the posterior and superior acetabulum relative to the iliac line on the coronal view, should be greater than or equal to 60 degrees (Fig. 136-7). Until age 3 months, 50 to 60 degrees may be physiologic. The majority of these hips develop normally without treatment, but maturation should be confirmed with a follow-up study. The beta-angle, which is the slope of the anterior cartilaginous roof relative to the iliac line on the coronal view, should be less than 55 degrees; the beta-angle is not as significant as the alpha-angle in diagnosis or treatment.
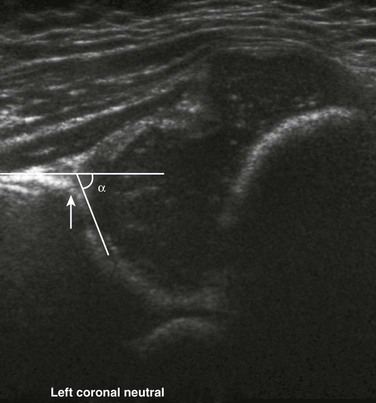
Figure 136-7 Ultrasonographic depiction of a normal hip in the coronal plane with the hip in neutral position and the alpha-angle measured along the iliac bone and the acetabular roof.
The alpha-angle is normal, measuring 64 degrees in this example. The acetabular roof is mature, with a sharply angulated acetabular margin (arrow).
In addition to the abnormally lateral and superior positioning of the femoral head and the abnormal angle of the acetabulum seen with DDH, the labrum may become thick and echogenic. The acetabular rim in normal hips has a sharply defined corner at the intersection of the anterior iliac line and sourcil (see Fig. 136-7). With progressive acetabular dysplasia, this corner becomes rounded and dysplastic (Fig. 136-8, A). The deformed labrum and pulvinar may be interposed between the acetabulum and femoral head (see Fig. 136-8, A).
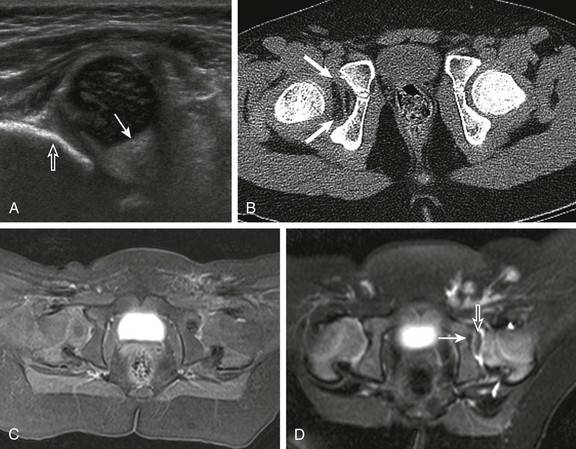
Figure 136-8 Obstacles to hip reduction.
A, A coronal ultrasonographic image of a dysplastic hip with echogenic pulvinar (solid arrow). Note the dysplastic shallow acetabula with blunted obtuse margin (open arrow). B, Low-dose axial computed tomography demonstrating fatty pulvinar (arrows) within the right hip joint. C, An axial T1-weighted 3T magnetic resonance image demonstrates pulvinar, thickened posterior labrum and ligamentum teres within the dysplastic left hip, which was successfully reduced. Symmetric enhancement is seen in this postcontrast image. D, An axial magnetic resonance image with pulvinar between the femoral head and acetabulum (arrow) and thickened ligamentum teres (open arrow).
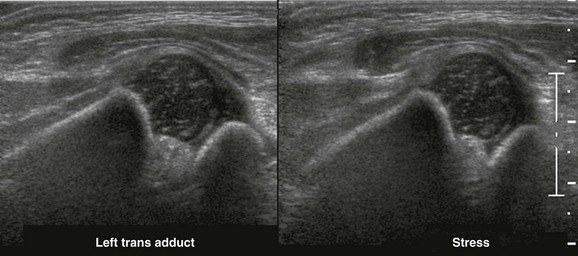
Dynamic examination includes the use of stress maneuvers, which correspond to the physical tests of Ortolani and Barlow. Normally, the femoral head is well seated within the acetabulum at rest and while stress maneuvers are applied. If laxity exists or if the hip is dislocatable, the femoral head moves posterolaterally when stress is applied. The transverse view is excellent for visualization of this movement during the stress maneuver (e-Fig. 136-9). These maneuvers are not performed if the patient is undergoing therapy with a harness.18
Screening: Some advocate for screening of all newborns for DDH and some for screening only those with risk factors or a positive or equivocal clinical examination. Many studies have been published describing the merits of each approach.19–34 General screening may help prevent delayed diagnosis and the need for surgical intervention but may also increase costs and lead to unnecessary treatment. The benefits of screening all newborns are not yet clear, and the current practice in the United Sates is to screen with ultrasonography only patients with an abnormal physical examination result or those who have positive risk factors for DDH.34–36
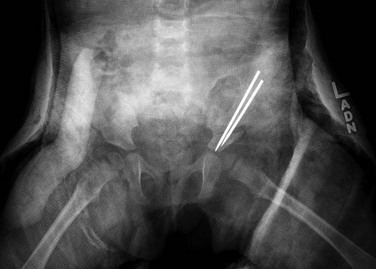
Computed Tomography: Primarily used for patients who have undergone treatment or will undergo open surgical repair with orthopedic hardware for DDH, rather than as a means of obtaining a diagnosis, computed tomography (CT) provides excellent spatial resolution for determining femoral and acetabular version.37 CT may be performed immediately following surgical reduction of DDH and is ideally performed using the low-dose technique and a small scan extent (Fig. 136-10). CT screening after surgical reduction is reserved for patients who have undergone a periacetabular osteotomy or proximal femoral osteotomy or for those who have had orthopedic hardware placed within 2 cm of the hip joint line, thereby precluding the ability to perform magnetic resonance imaging (MRI). Artifacts from metallic hardware are decreased by using higher peak kilovoltage (increasing the penetration of x-rays) and increasing tube current (allowing enough photons to reach the detector); this parameter needs to be manually changed to overcome automatic exposure controls.
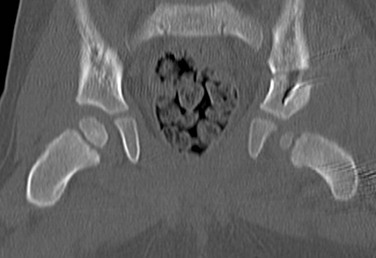
Figure 136-10 A coronal computed tomography image reformatted in a 2-year-old boy following Salter osteotomy for treatment of left-sided developmental dysplasia of the hip.
Note that the bilateral femoral heads are concentrically located. The left femoral head is small, an expected finding in the setting of developmental dysplasia of the hip.
Displacement and subluxation should be described in both the coronal and axial planes. Identification of any orthopedic hardware failures such as pin extrusion into the joint line, migrated interposition bone grafts and identification of any intrinsic or extrinsic causes of failed reduction should be made. The gluteus maximus should have a clear fat plane anteriorly between the muscle and a line tangent to the posterior aspect of the ischia on axial imaging; this fat plane will be interrupted, displaced posteriorly, or both, if the hips are not adequately reduced. Reconstructions of CT data may assist in demonstrating anatomic relationships and are often particularly helpful to the surgeon. Coronal reconstructions are excellent in depicting the acetabular roof.18,37
CT should still be the first line of postoperative imaging in children with DDH who have orthopedic hardware placement or have undergone periacetabular osteotomies to determine bone graft quality and position18,38–40
Magnetic Resonance Imaging: Following reduction and placement of a spica cast, MRI is increasingly being utilized as an evaluation of the anatomy because of lack of ionizing radiation and the ability to perform the study without sedation (Fig. 136-11).41–46 In addition to providing anatomic information regarding the relationship between the femoral head and the acetabulum, obstacles to reduction may be visualized better than with CT (e-Fig. 136-12). Fatty pulvinar, thickened or inverted labrum, transverse acetabular ligaments, and hypertrophied ligamentum teres (see Fig. 136-8) may be present in the hip joint preventing successful reduction. Sometimes, the labrum may be displaced altogether because of trauma from intraoperative femoral head relocation into the acetabular fossa (Fig. 136-13). An abnormal shape of the acetabulum or the capital femoral epiphysis may also be obstacles to reduction. Extrinsic obstacles to reduction include shortened external rotator and adductor muscles, interpositioning of the iliopsoas muscle, and capsular adhesion to the ilium.47 The most useful sequences include T1-weighted and proton density–weighted sequences in the axial and coronal planes.48–50

Figure 136-11 A T2-weighted fat-saturated coronal magnetic resonance image of the hips in a patient who underwent acetabuloplasty of the right hip and is in a spica cast.
No sedation was used for this study. The femoral head is concentrically located. There is globular increased signal within the superior labrum at the chondro-osseous junction (arrow) related to labral injury incurred during reduction.
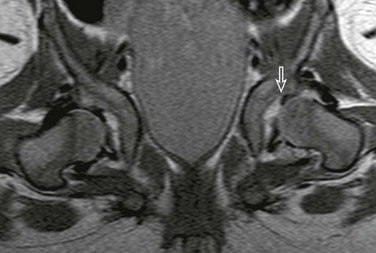
e-Figure 136-12 Magnetic resonance spica proton density imaging sequence in an 8-month-old girl demonstrates an inverted labrum on the left (arrow).
Susceptibility artifact noted in right hip joint space related to iodinated contrast from intraoperative arthrography performed prior to magnetic resonance imaging.
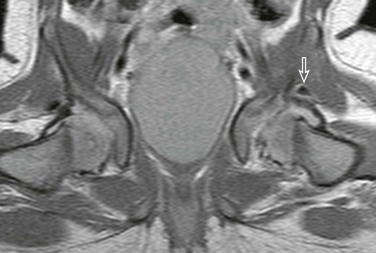
Figure 136-13 Magnetic resonance spica proton density imaging sequence in a 6-month-old girl demonstrates a floating superior labrum after reduction (arrow).
Note the hyaline cartilage lateral sourcil margin, as the superior labrum has been displaced. Normal right superior labrum for comparison.
With the administration of contrast, MRI can detect ischemia within the femoral head and possibly identify patients at risk for epiphyseal osteonecrosis, which is the most common serious complication of hip reduction and may occur to some degree in more than 70% of cases. The risk of epiphyseal osteonecrosis is increased with greater hip abduction, which has led to the concept of the “safe zone,” which is the amount of abduction that is large enough to prevent redislocation and small enough to prevent avascular necrosis, usually at 55 degrees maximal hip abduction.46 Abduction may compromise the blood supply to the femoral head, as the blood supply arises primarily from the deep medial femoral circumflex vessels. This ischemia may be detected by low-signal on postcontrast T1-weighted images (Fig. 136-14). Because of the lack of intraepiphyseal vascular anastomoses, regions of poor enhancement are often well delineated by immediately adjacent well-perfused areas. Performance of this examination immediately after reduction is currently the recommended practice.45
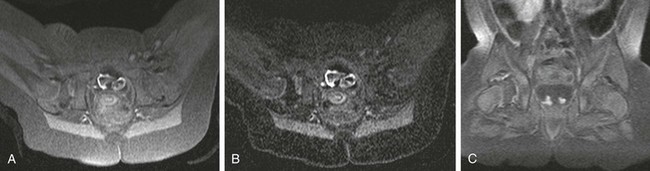
Figure 136-14 Magnetic resonance imaging of the hips obtained in a 1.5 Tesla magnet after placement of a spica cast without sedation.
A, An axial T1-weighted, fat-suppressed image demonstrates lower signal in the right femoral head. B, A subtraction image more clearly demonstrates the asymmetric perfusion of the femoral heads. C, A coronal T1-weighted, fat-suppressed image demonstrates decreased perfusion on the right.
Treatment: The objective of DDH treatment is concentric reduction of the femoral head into the acetabulum. The method of achieving this outcome depends on the age of the patient as well as the severity of the presentation. Up to age 6 months, orthoses, often a Pavlik harness, are used to place the hip in flexion and abduction. This treatment aids in developing the acetabulum along the lateral edge. The risk of epiphyseal osteonecrosis increases with greater hip abduction, as discussed above. The harness is often worn for 3 to 6 weeks following successful reduction; both clinical examination and ultrasonography are performed to evaluate the effectiveness of therapy. In the patient in a harness, stress maneuvers are not performed during ultrasonography.
Children over 6 months but less than 2 years of age are often treated with a spica cast following closed or open reduction. Intraoperative arthrography is often performed to assess for structures that my impede reduction and to assess the anatomy (e-Fig. 136-15). A total contrast volume of 3 to 4 milliliters is typically all that is required; too much contrast may give the impression of capsular laxity.18 Positioning of the femoral heads following placement of the cast may be ascertained with CT or MRI, with MRI having the advantages of no radiation and the possibility of assessing perfusion of the femoral head. Radiography may also detect late features of avascular necrosis. Recurrent dislocation may occur in up to 8% of patients and is more common in those with bilateral or right-sided DDH, decreased abduction in the spica cast, and large pelvic width.51
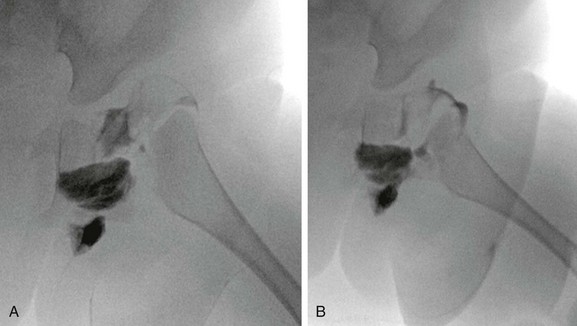
e-Figure 136-15 Intraoperative arthrogram obtained before (A) and after (B) reduction of the left hip in this child with Larsen syndrome.
DDH that is diagnosed late or that persists despite treatment may require surgical osteotomy. For patients with persistent subluxation and mild to moderate acetabular dysplasia, an innominate or Salter osteotomy may be indicated. This technique includes a horizontal osteotomy of the ilium superior to the acetabulum to create a wedge, which is filled with bone from the iliac crest (e-Fig. 136-16). Frequently utilized in children with cerebral palsy caused by posterolateral deficiency, the Dega osteotomy is made in the lateral ilium and cannot be distinguished from the Pemberton osteotomy radiographically (e-Fig. 136-17). Patients over 8 years of age may require a Chiari osteotomy, which increases the size of the acetabulum with a supraacetabular osteotomy. For adolescents and adults, the Ganz or periacetabular osteotomy may be necessary to improve anteversion and redirect the acetabulum.
Dezateux, C, Rosendahl, K. Developmental dysplasia of the hip. Lancet. 2007;369:1541–1552.
Grissom, L, Harcke, HT, Thacker, M. Imaging in the surgical management of developmental dislocation of the hip. Clin Orthop Relat Res. 2008;466:791–801.
Karmazyn, BK, Gunderman, RB, Coley, BD, et al. ACR Appropriateness Criteria on developmental dysplasia of the hip—child. J Am Coll Radiol. 2009;6:551–557.
Shipman, SA, Helfand, M, Moyer, VA, et al. Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics. 2006:117–e557.
Tiderius, C, Jaramillo, D, Connolly, S, et al. Post-closed reduction perfusion magnetic resonance imaging as a predictor of avascular necrosis in developmental hip dysplasia: a preliminary report. J Pediatr Orthop. 2009;29:14–20.
References
1. Record, RG, Edwards, JH. Environmental influences related to the aetiology of congenital dislocation of the hip. Brit J Prevent Soc Med. 1958;12:8.
2. Murray, KA, Crim, JR. Radiographic imaging for treatment and follow-up of developmental dysplasia of the hip. Semin Ultrasound CT MR. 2001;22:306–340.
3. Perry, DC, Tawfiq, SM, Roche, A, et al. The association between clubfoot and developmental dysplasia of the hip. J Bone Joint Surg Br. 2010;92:1586–1588.
4. Minihane, KP, Grayhack, JJ, Simmons, TD, et al. Developmental dysplasia of the hip in infants with congenital muscular torticollis. Am J Orthop (Belle Mead NJ). 2008;37:E155. [E158].
5. Dezateux, C, Rosendahl, K. Developmental dysplasia of the hip. Lancet. 2007;369:1541–1552.
6. Sankar, WN, Neubuerger, CO, Moseley, CF. Femoral head sphericity in untreated developmental dislocation of the hip. J Pediatr Orthop. 2010;30:558–561.
7. Fujii, M, Nakashima, Y, Sato, T, et al. Pelvic deformity influences acetabular version and coverage in hip dysplasia. Clin Orthop Relat Res. 2011;469:1735–1742.
8. Fujii, M, Nakashima, Y, Jingushi, S, et al. Intraarticular findings in symptomatic developmental dysplasia of the hip. J Pediatr Orthop. 2009;29:9–13.
9. Barlow, TG. Neonatal hip dysplasia—treatment, results and complications. Proc R Soc Med. 1975;68:475.
10. Barlow, TG. Congenital dislocation of the hip in the newborn. Proc R Soc Med. 1966;59:1103–1106.
11. Barlow, TG. Early diagnosis and treatment of congenital dislocation of the hip. Proc R Soc Med. 1963;56:804–806.
12. Greenspan, A. Orthopedic radiology: a practical approach. Philadelphia, PA: Lippincott Williams & Wilkins; 2000.
13. Noordin, S, Umer, M, Hafeez, K, et al. Developmental dysplasia of the hip. Orthop Rev (Pavia). 2010;2:e19.
14. van der Bom, MJ, Groote, ME, Vincken, KL, et al. Pelvic rotation and tilt can cause misinterpretation of the acetabular index measured on radiographs. Clin Orthop Relat Res. 2011;469:1743–1749.
15. Pedersen, DR, Lamb, CA, Dolan, LA, et al. Radiographic measurements in developmental dysplasia of the hip: reliability and validity of a digitizing program. J Pediatr Orthop. 2004;24:156–160.
16. Graf, R. The diagnosis of congenital hip-joint dislocation by the ultrasonic compound treatment. Arch Orthop Trauma Surg. 1980;97:117–133.
17. Harcke, HT, Clarke, NM, Lee, MS, et al. Examination of the infant hip with real-time ultrasonography. J Ultrasound Med. 1984;3:131–137.
18. Grissom, L, Harcke, HT, Thacker, M. Imaging in the surgical management of developmental dislocation of the hip. Clin Orthop Relat Res. 2008;466:791–801.
19. Clinical practice guideline: early detection of developmental dysplasia of the hip. Committee on Quality Improvement, Subcommittee on Developmental Dysplasia of the Hip. American Academy of Pediatrics. Pediatrics. 2000;105:896–905.
20. Goldberg, MJ. Early detection of developmental hip dysplasia: synopsis of the AAP Clinical Practice Guideline. Pediatr Rev. 2001;22:131–134.
21. Pashapour, N, Golmahammadlou, S. Study on the diagnosis time of developmental dysplasia of the hip. East Mediterr Health J. 2007;13:465–469.
22. Karmazyn, BK, Gunderman, RB, Coley, BD, et al. ACR Appropriateness Criteria on developmental dysplasia of the hip—child. J Am Coll Radiol. 2009;6:551–557.
23. Shipman, S, Helfand, M, Nygren, P, et al, Screening for developmental dysplasia of the hip. Agency for Healthcare Research and Quality, 2006. [PMID 20722139].
24. Shipman, SA, Helfand, M, Moyer, VA, et al. Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics. 2006;117:e557–e576.
25. Maxwell, SL, Ruiz, AL, Lappin, KJ, et al. Clinical screening for developmental dysplasia of the hip in Northern Ireland. BMJ. 2002;324:1031–1033.
26. Sewell, MD, Eastwood, DM. Screening and treatment in developmental dysplasia of the hip-where do we go from here? Int Orthop. 2011;35:1359–1367.
27. Shorter, D, Hong, T, Osborn, DA. Screening programmes for developmental dysplasia of the hip in newborn infants. Cochrane Database Syst Rev. (9):2011. [CD004595].
28. Godward, S, Dezateux, C. Surgery for congenital dislocation of the hip in the UK as a measure of outcome of screening. MRC Working Party on Congenital Dislocation of the Hip. Medical Research Council. Lancet. 1998;351:1149–1152.
29. Holen, KJ, Tegnander, A, Bredland, T, et al. Universal or selective screening of the neonatal hip using ultrasound? A prospective, randomised trial of 15,529 newborn infants. J Bone Joint Surg Br. 2002;84:886–890.
30. Eastwood, DM. Neonatal hip screening. Lancet. 2003;361:595–597.
31. Gray, A, Elbourne, D, Dezateux, C, et al. Economic evaluation of ultrasonography in the diagnosis and management of developmental hip dysplasia in the United Kingdom and Ireland. J Bone Joint Surg Am. 2005;87:2472–2479.
32. Elbourne, D, Dezateux, C, Arthur, R, et al. Ultrasonography in the diagnosis and management of developmental hip dysplasia (UK Hip Trial): clinical and economic results of a multicentre randomised controlled trial. Lancet. 2002;360:2009–2017.
33. Rosendahl, K, Toma, P. Ultrasound in the diagnosis of developmental dysplasia of the hip in newborns. The European approach. A review of methods, accuracy and clinical validity. Eur Radiol. 2007;17:1960–1967.
34. Woolacott, NF, Puhan, MA, Steurer, J, et al. Ultrasonography in screening for developmental dysplasia of the hip in newborns: systematic review. BMJ. 2005;330:1413.
35. Treiber, M, Tomazic, T, Tekauc-Golob, A, et al. Ultrasound screening for developmental dysplasia of the hip in the newborn: a population-based study in the Maribor region, 1997-2005. Wien Klin Wochenschr. 2008;120:31–36.
36. von Kries, R, Ihme, N, Altenhofen, L, et al. General ultrasound screening reduces the rate of first operative procedures for developmental dysplasia of the hip: a case-control study. J Pediatr. 2012;160(2):271–275.
37. Jia, J, Li, L, Zhang, L, et al. Three dimensional-CT evaluation of femoral neck anteversion, acetabular anteversion and combined anteversion in unilateral DDH in an early walking age group. Int Orthop. 2012;36(1):119–124.
38. Eggli, KD, King, SH, Boal, DK, et al. Low-dose CT of developmental dysplasia of the hip after reduction: diagnostic accuracy and dosimetry. AJR Am J Roentgenol. 1994;163:1441–1443.
39. Koenig, JK, Pring, ME, Dwek, JR. MR evaluation of femoral neck version and tibial torsion. Pediatr Radiol. 2012;41(1):113–115.
40. van Bosse, HJ, Lee, D, Henderson, ER, et al. Pelvic positioning creates error in CT acetabular measurements. Clin Orthop Relat Res. 2011;469:1683–1691.
41. Laor, T, Roy, DR, Mehlman, CT. Limited magnetic resonance imaging examination after surgical reduction of developmental dysplasia of the hip. J Pediatr Orthop. 2000;20:572–574.
42. McNally, EG, Tasker, A, Benson, MK. MRI after operative reduction for developmental dysplasia of the hip. J Bone Joint Surg Br. 1997;79:724–726.
43. Wakabayashi, K, Wada, I, Horiuchi, O, et al. MRI findings in residual hip dysplasia. J Pediatr Orthop. 2011;31:381–387.
44. Mitchell, PD, Chew, NS, Goutos, I, et al. The value of MRI undertaken immediately after reduction of the hip as a predictor of long-term acetabular dysplasia. J Bone Joint Surg Br. 2007;89:948–952.
45. Westhoff, B, Wild, A, Seller, K, et al. Magnetic resonance imaging after reduction for congenital dislocation of the hip. Arch Orthop Trauma Surg. 2003;123:289–292.
46. Tiderius, C, Jaramillo, D, Connolly, S, et al. Post-closed reduction perfusion magnetic resonance imaging as a predictor of avascular necrosis in developmental hip dysplasia: a preliminary report. J Pediatr Orthop. 2009;29:14–20.
47. Desai, AA, Martus, JE, Schoenecker, J, et al. Spica MRI after closed reduction for developmental dysplasia of the hip. Pediatr Radiol. 2011;41:525–529.
48. Lincoln, TL, Vandevenne, JE, Rinsky, LA, et al. Dynamic magnetic resonance guided treatment of developmental dysplasia of the hip. J Pediatr Orthop B. 2002;11:279–283.
49. Chin, MS, Shoemaker, A, Reinhart, DM, et al. Use of 1.5 Tesla and 3 Tesla MRI to evaluate femoral head reduction in hip dysplasia. J Pediatr Orthop. 2011;31:633–637.
50. Kim, HT, Kim, IB, Lee, JS. MR-based parameters as a supplement to radiographs in managing developmental hip dysplasia. Clin Orthop Surg. 2011;3:202–210.
51. Sankar, WN, Young, CR, Lin, AG, et al. Risk factors for failure after open reduction for DDH: a matched cohort analysis. J Pediatr Orthop. 2011;31:232–239.