Chapter 7 Developmental Anomalies
Arachnoid Cysts, Dermoids, and Epidermoids
• Treatment for arachnoid cysts is indicated only if the cyst is associated with clinical symptoms.
• When treating arachnoid cysts the choices include
• When resecting dermoids associated with dermal sinus tracts, the entire cyst and sinus tract must be removed in order to prevent recurrence.
• When removing intracranial epidermoids, meticulous care must be undertaken to prevent spillage of the contents into the subarachnoid spaces in order to prevent postoperative chemical meningitis.
Arachnoid Cysts
Arachnoid cysts contain cerebrospinal fluid (CSF) and are most typically enclosed in arachnoid or arachnoid-like membranes and can be located anywhere within the craniospinal axis. With the advent and widespread use of magnetic resonance imaging (MRI), these lesions have been encountered with significant frequency. However, despite being well described throughout the literature, arachnoid cysts are actually rare, accounting for around 1% of intracranial space-occupying lesions. They exhibit a male predominance, at approximately a 3:1 ratio,1,2 and a predilection for the sylvian region. Although these cysts are occasionally bilateral and multiloculated, they are usually unilateral and single.3
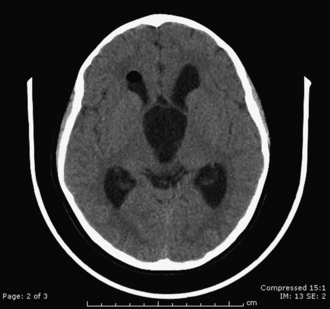
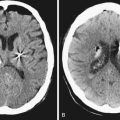
Diagnosis of arachnoid cyst can be made easily with currently available imaging modalities. Computed tomography (CT) and MRI are the gold standard for visualizing and making the diagnosis. Arachnoid cysts will appear markedly hypodense on CT scan (Fig. 7.1) and show low signal on T1 MRI and high signal intensity on T2 MRI (Fig. 7.2), as they are generally isointense with CSF. Diffusion MRI reveals a low signal secondary to high water diffusibility and high apparent diffusion coefficient (ADC). Arachnoid cysts display a smooth, well-demarcated surface and are heterogeneous and nonenhancing, distinguishing them from other cystic lesions such as epidermoids.4 In utero, arachnoid cysts may be visible via ultrasound.5
The embryogenesis of arachnoid cysts remains controversial. Some authors report the arachnoid layer originating strictly from neural crest cells, but others propose the arachnoid originating from two layers: neural crest ectoderm and mesoderm. The primitive mesenchyme, or mesoderm, surrounding the neural tube separates into an endomeninx, which forms the pia-arachnoid membrane, and an ectomeninx.6 The subarachnoid space is then formed by the rupture of the rhombic roof and dissection of the ecto- and endomeninges by CSF pulse pressure from the choroid plexus. Any disruption of this separation is thought to be the initiating event in arachnoid cyst formation.7,8 This disruption can occur anywhere along the neuraxis as evidenced by reports of spinal intradural and extradural arachnoid cysts.9 Congenital spinal arachnoid cysts have been mostly described in patients with neural tube defects.10 Posterior fossa arachnoid cysts have been specifically discussed in the literature along with Chiari malformations and Dandy-Walker syndrome as a possible result of embryonal atresia of the fourth ventricle.4 Two theories predominate in the literature with respect to the pathogenesis of middle fossa arachnoid cysts. Robinson’s theory proposes primary temporal lobe agenesis as the main factor in middle fossa arachnoid cyst formation. Starkman and co-workers propose the arachnoid cyst as the primary abnormality leading to eventual temporal lobe hypoplasia secondary to cyst expansion.11 Both theories are supported equally well throughout the literature.
In 1831, Bright submitted what is considered the first pathological description of arachnoid cysts. He reported the cysts as malformations caused by a splitting of the arachnoid membrane.12 More recently, Rengachary and Watanabe described the structural features of arachnoid cysts after review of several hundred cases: (1) splitting of the arachnoid membrane at the margin of the cyst; (2) thickened collagen layer in the cyst wall; (3) absence of normal arachnoid trabeculations within the cyst; and (4) hyperplastic arachnoid cells in the cyst wall.6,13 On pathological review of optic nerve arachnoid cysts, authors report three common features of the cyst wall: meningothelial cell proliferation, thickened dura, and psammoma bodies.14–16
Galassi and associates introduced a classification scheme for arachnoid cysts based on their communication with the adjacent cisterns. In this classification scheme, type I arachnoid cysts freely communicate with the cisterns. Type II cysts are intermediate and may or may not communicate with the cisterns but it is likely they communicated with the subarachnoid space at one time, before sealing off their communication.17 Type III cysts do not communicate with any region of the subarachnoid space and cause local mass effect.2,18 This classification scheme also hints at a possible treatment guide, with type I cysts rarely needing surgical intervention, owing to the free communication with the subarachnoid space, and type III cysts more frequently requiring surgical intervention secondary to mass effect.
The most common location for arachnoid cysts is the middle fossa or sylvian fissure, usually behind the greater wing of the sphenoid bone19 accounting for nearly 50% of arachnoid cysts in one study. Posterior fossa cysts including cerebellopontine angle and the cerebellar vermis comprise 20% to 30% of lesions,20 and supracellar cysts, 9%. Other documented locations include interventricular, optic nerve, cerebral convexity, and clival interpeduncular area arachnoid cysts.12,21 Intraspinal arachnoid cysts are rare and mostly traumatic except in the cases of intramedullary cysts, which have been reported as truly congenital. Most intradural spinal arachnoid cysts occur in the thoracic region (80%) followed by the cervical (15%) and lumbar regions (5%).22
Macrocephaly is one of the most common presenting signs of an arachnoid cyst in infants and can be diagnosed in utero. In older patients, cyst location correlates with presenting symptoms. Headache, secondary to increased intracranial pressure from mass effect, is usually the most common presenting symptom, frequently seen with middle fossa cysts. Middle fossa cysts are also often associated with post-traumatic subdural hemorrhages and may present with signs of mass effect from the hemorrhage.19 Suprasellar, pineal, and posterior fossa region arachnoid cysts present with signs of obstructive hydrocephalus. Suprasellar cysts in particular may present with precocious puberty, hyperinsulinism, and even visual loss.23,24 These cysts are frequently symptomatic and rarely respond to surgical treatment, requiring initiation of long-term hormonal therapy regimens. In cases of optic nerve arachnoid cysts, patients may present with a childhood history of blindness in the affected eye with progressive complaints of proptosis, erythema, and pain.8 There are also several reports of patients presenting with cranial nerve palsies of the occulomotor, trigeminal, abducens, facial, vestibulocochlear, and hypoglossal nerves.25–29 Patients with spinal arachnoid cysts may present with a constellation of symptoms if associated with a congenital syndrome. Others may present with back pain or, rarely, with progressive spastic paraparesis.30
The natural history of arachnoid cysts is not yet clearly delineated given that most are found incidentally and remain static in size over time. Patients are frequently asymptomatic throughout their lives and many are found at autopsy. However, arachnoid cysts are often associated with other congenital disorders and in these cases the natural history may be related to the associated disorder. Glutaric aciduria type I (GAT1) is an inborn metabolic disorder that appears to have a strong association with bitemporal intracranial arachnoid cysts, the most consistent reported finding on all imaging modalities.7 Patients with these findings in combination with macrocephaly, psychomotor development, and dystonic cerebral palsy warrant a detailed metabolic workup. Recently, a case of bitemporal arachnoid cysts was also reported in a patient with tuberous sclerosis. Short-rib polydactyly syndromes and their variants, including Beemer-Langer syndrome, have been associated with arachnoid cysts, along with other genetic syndromes including cri-du-chat, autosomal dominant polycystic kidney disease, Aicardi syndrome, neurofibromatosis, and proteus syndrome. Further supporting a genetic basis of arachnoid cyst formation are reports of familial cysts. Familial arachnoid cysts usually occur in the same location in affected family members and have even been documented as mirror-image cerebellopontine-angle arachnoid cysts in monozygotic twins. An association with behavioral and cognitive disabilities has been recently documented and centers largely around attention-deficit hyperactivity disorder (ADHD), epileptic aphasia (Landau-Kleffner syndrome), and other developmental language disorders. Some authors report positron emission tomography (PET) studies revealing hypometabolism in cortical regions surrounding the cyst. After surgical decompression of the cysts, repeat PET studies demonstrated improvement in cortical metabolism and clinical performance on language testing.19,31
Another area of controversy surrounds arachnoid cysts in middle fossa locations of epileptic patients. Many studies suggest only an incidental association, yet others report improvement in seizures following surgical treatment. There are several reports of epileptic foci over the region of the arachnoid cyst as confirmed by electroencephalography.3 However, other reports describe epileptic patients with temporal lobe arachnoid cysts and seizure onset localization far from the cyst,32–34 varying treatment decisions on among cases. With regard to spinal arachnoid cysts, associations with congenital spinal malformations including caudal regression syndrome and acquired anomalies such as syringomyelia have been documented.35
In incidental cases of arachnoid cysts, and those unrelated to other congenital anomalies, the size and dynamic status of the cyst appear to play a role in the natural history. Several theories have been proposed to explain the mechanism of arachnoid cyst expansion, the most common being the ball-valve theory. In this theory, an anatomical communication exists between the cyst and the subarachnoid space which acts as a unidirectional valve. Multiple reports of MRI studies have demonstrated this effect through cine-mode studies. However, this mechanism has not been observed in all arachnoid cysts and cannot explain the spontaneous resolution of cysts reported in the literature.36–38 Another proposed mechanism is an osmotic gradient between the cyst and the surrounding CSF. This theory is not widely accepted in cases of congenital arachnoid cysts given that the cystic content is quite similar to the composition of CSF. This theory could be plausible in cases of traumatic arachnoid cyst formation, especially in the presence of hemorrhagic or inflammatory foci. A third theory, backed by clinical evidence, is that of fluid production by the cells of the cyst wall. There are many reports of isolated or closed compartment cysts that expand over time and, as discussed previously, the cyst wall is physiologically similar to the subdural and arachnoid granulation neurothelium.13
Another factor affecting the natural history of arachnoid cysts is related to location of the cyst and susceptibility to hemorrhage. Middle fossa arachnoid cysts can be complicated by post-traumatic subdural hemorrhages, regardless of the age of the patient.39 The mechanism of the hemorrhage is likely secondary to the displacement at stretching of the bridging veins by the cyst as they extend from the cortical surface to the dura. As they stretch, and tear, hemorrhage accumulates mostly in the subdural space, and occasionally in the cyst itself.19,40 Some authors cite annual increases in the risk of subdural hemorrhage by 20- to 40-fold in patients with arachnoid cysts.19,41,42
The lack of evidence-based data or studies has led to significant controversies regarding the appropriate treatment protocol for patients presenting with arachnoid cysts. No significant controversy is present when the patient is found to have a small asymptomatic cyst. Even with larger cysts, if the patient has no clinical symptoms, no intervention is necessary. In patients with more dynamic cysts without signs of increased intracranial pressure or focal neurological deficit, nonoperative management may also be considered in conjunction with close follow-up and serial imaging.43
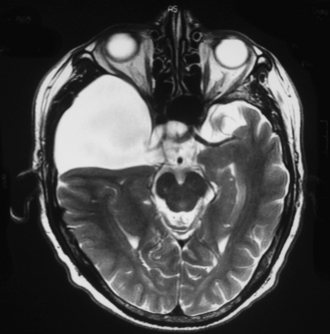
Perhaps the most challenging treatment decision concerns when to treat patients who present with a medium-size arachnoid cyst and have mild symptoms (headaches, dizziness, mild ataxia, etc.) and it is not clear if the symptoms are causally related to the presence of the cyst. Assessment of the patient, looking for signs of elevated intracranial pressure, and clinical judgment play a crucial role in the decision-making process. The easiest clinical scenario is one in which the patient presents with marked symptoms and large cysts producing significant mass effect, midline shift, or obstructive hydrocephalus, as in the case of large suprasellar cysts (Fig. 7.3). Moreover, in these cases controversy exists as to which surgical approach is the best to treat the patient.
Open craniotomy with cyst fenestration has been reported in cases of well-circumscribed cysts without hydrocephalus. This procedure allows for total or subtotal (as is most commonly the case) resection of the cyst wall and pathological diagnosis. Some authors even report arachnoidoplasty via an open craniotomy. The surgeons incised the cyst microsurgically to initially enter the cyst and then again to allow communication with the cisterns followed by subsequent closure of the outermost membrane to prevent CSF leakage.44 Cyst fenestration may also be accomplished via an endoscopic approach with possible marsupialization of the cyst and even varying degrees of resection of the cyst wall. This technique is favored by many, especially in the management of symptomatic suprasellar arachnoid cysts.43,45,46 Shunting of the cyst, most commonly to the peritoneum, allows for appropriate diversion of the CSF and is favored by some for middle fossa cysts and recurrent cysts in the setting of increased intracranial pressure. This technique allows for a gradual decompression of the cyst and concomitant gradual expansion of the brain. Neuronavigation techniques have been combined with most types of surgical treatments for arachnoid cysts yielding a minimally invasive approach and higher precision, particularly with suprasellar and multiloculated cysts.43 With regard to spinal arachnoid cysts, surgical interventions are again usually reserved for symptomatic patients. In the cases of extradural cysts, some authors favor amputation at the stalk near the dural cleft from which the cyst protrudes.47 In other cases, a decompressive laminectomy may be warranted.
Dermoid Cysts
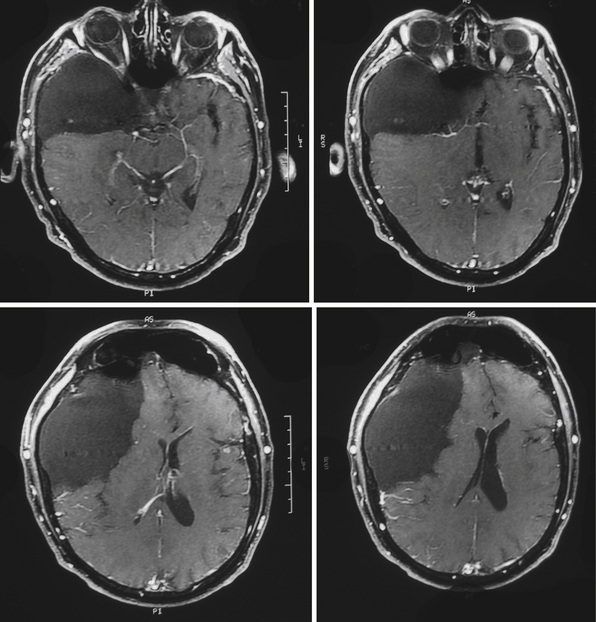
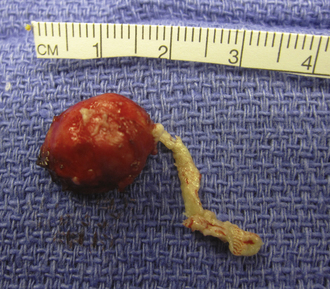
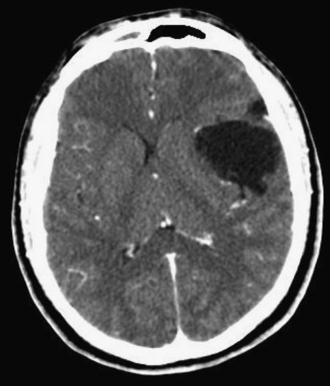
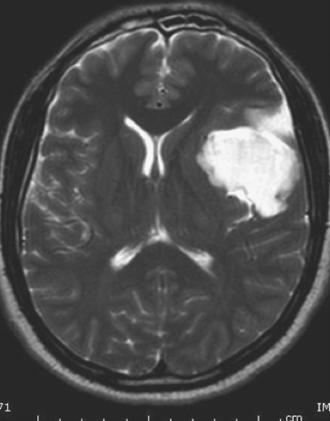
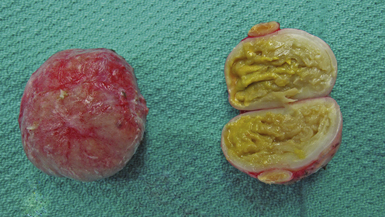
Dermoid cysts are by definition inclusion cysts, which mean that they are made up of implanted epithelial tissue into an area that should not contain it. As such, these cysts can be found in many parts of the body to include the face, nose, scalp, skull, brain, spinal cord, orbits, neck, and oral and nasal cavities. These congenital lesions are thought to arise from misplaced ectodermal elements during the third to fifth week of embryonic life due to failure of the neural tube closure at the midline. They are more commonly associated with dermal sinus tracts (Fig. 7.4) and spinal abnormalities than are epidermoid tumors. Dermoid inclusion cysts account for approximately 0.3% of all brain tumors. The tumors are usually benign slow-growing lesions that rarely undergo malignant transformation, and can occur anywhere along the spinal axis. Histologically, dermoid tumors usually contain desquamated epithelial keratin and some lipid material, which gives its external surface a smooth, lobulated, pearly appearance. These tumors have an outer connective tissue capsule and are lined with stratified squamous epithelium that also contains hair follicles, sebaceous glands, and sweat glands. Because they contain mature tissues, these cysts are almost universally benign (Figs. 7.5, 7.6).
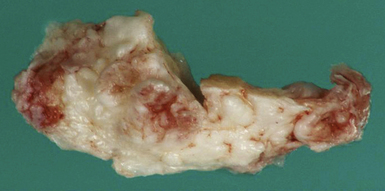
FIGURE 7.5 Gross specimen of dermoid seen in Figure 7.4. Keratinized material is seen along with an outer capsule. Care should be taken to avoid spillage of this material in the subarachnoid spaces.
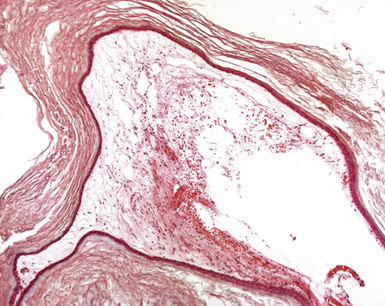
FIGURE 7.6 Histological slide of the same intracranial dermoid shown in Figures 7.4 and 7.5, showing multiple layers of attenuated epithelium that shed the anucleated squames that make up most of the mass.
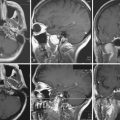
On CT scans, dermoids are usually rounded, well-circumscribed, extremely hypodense lesions with a Hounsfield unit of 220 to 2140, in keeping with their lipid content (Fig. 7.7). Peripheral capsular calcification is frequent. Enhancement after contrast agent administration is rare but has been reported. On MR images, dermoids are typically hyperintense on T1-weighted images but vary from hypo- to hyperintense on T2-weighted studies (Figs. 7.8, 7.9). There is usually no associated vasogenic edema or contrast enhancement. Serpiginous hypointense elements may be seen if the lesion contains hair. Mural calcification can sometimes be identified. Orakcioglu and colleagues noted that diffusion-weighted imaging (DWI) hyperintensity in dermoid cysts is related to a decrease of water proton diffusion and should be used for both the diagnosis and follow-up of these lesions.48 Imaging findings vary, depending on whether the cyst has ruptured. On both CT and MR images, fat-density droplets may be seen throughout the subarachnoid space and in the ventricular system if rupture of the cyst has occurred. Extensive pial enhancement can be seen from chemical meningitis caused by ruptured cysts.
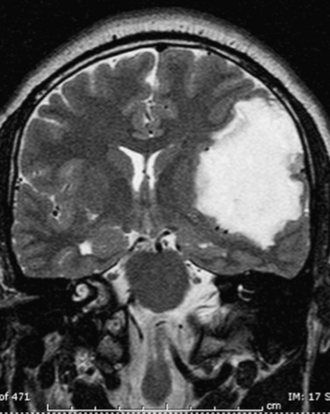
FIGURE 7.9 Coronal T2-weighted magnetic resonance image of same patient in Figure 7.8 demonstrates a high signal intensity lesion displacing the surrounding frontal and temporal lobes. The lesion was found to be a dermoid cyst at surgery.
Morbidity depends on the location of the tumor and on the involvement of adjacent structures. Dermoid tumors can rupture, releasing lipid contents into the ventricular or subarachnoid spaces (Fig 7.5). This causes a chemical meningitis that can lead to recurrent symptoms, most commonly headache. The subsequent meningeal inflammation may result in arterial vasospasm, possible stroke, and death. Orakcioglu and colleagues reviewed the charts of five men and two women with intracranial dermoid cysts and found that clinical presentations included focal neurological deficits, epileptic seizures, persistent headache, mental changes, and psycho-organic syndromes. One patient underwent delayed ventriculoperitoneal shunting after ruptured fatty particles caused obstructive hydrocephalus. In three patients, despite dermoid rupture into the subarachnoid space, hydrocephalus did not develop. In one patient, diffuse vascular supratentorial lesions occurred as a result of aseptic meningitis. In addition, they noted that although rupture does not necessarily cause hydrocephalus, radical removal of the tumor and close monitoring of ventricular size are necessary.
Epidermoid Cysts
Epidermoids are well-circumscribed, smooth, lobulated, encapsulated lesions. Histologically, their internal layer is composed of stratified squamous epithelium with a fibrous capsule. They tend to slowly enlarge as epithelial cells desquamate, with the formation of keratin and cholesterol crystals in the center of the lesion. Handu and co-workers analyzed the aspirates of epidermal inclusion cysts (EICs) to identify cytological features.49 The aspirates showed a clear background with high cellularity, along with nucleate and anucleate squames. In some cases, keratinous material was present but less than the cellular elements. In 31 cases, a diagnosis of infected EIC was made on the basis of dense inflammatory infiltrate in addition to the squames. Of 56 cases for which histopathological data were available, 45 cases of EIC were diagnosed, 5 cases of dermoid cyst, 2 cases of branchial cyst, 2 cases of pilomatricoma, 1 case of sebaceous cyst, and 1 case of thyroglossal cyst.
Typical imaging findings on CT include a round/lobulated mass with a density resembling CSF; the mass may have a crenated margin,which, when present, could be characteristic. Calcification is reported to be present in approximately 10% of all intracranial epidermoids,50 which may be due to saponification of the desquamated debris. In their review of reported intracerebral epidermoids, Kaido and associates found high density in 2 out of 13 patients with a CT description51 and Watanabe and colleagues52 described a right frontal lobe epidermoid with nodular peripheral calcification. On MRI epidermoid cysts appear hypointense on T1-weighted images and hyperintense on T2-weighted images. There is usually some internal heterogeneity, which is best seen in the proton-density and fluid attenuated inversion recovery (FLAIR) images, and this finding could help distinguish these cysts from arachnoid cysts. In a review of the MRI appearance of epidermoids, signal heterogeneity was observed on T1 and proton-density weighted images in 65% of cases. MRI may also show insinuating margins of the cyst, extending into the adjacent cisterns or fissures, features that are not usually associated with arachnoid cysts. Lesions typically do not enhance. When present, contrast enhancement is minimal and peripheral; it has been seen in up to 35% of cases.53 DWI is the most helpful imaging sequence in diagnosing an epidermoid cyst. Because of a combination of T2 and diffusion effects, epidermoid tumors appear markedly hyperintense compared with CSF and brain tissue on diffusion-weighted images. Epidermoid tumors demonstrate an ADC that is similar to that of gray matter and lower than that of CSF. In contrast, arachnoid cysts or other cystic intracranial lesions do not show restricted diffusion and follow the CSF signal on DWI and ADC maps.
Epidermoid cysts are benign, slowly but ineluctably growing tumors that require surgical treatment. Similar to dermoid cysts, morbidity of epidermoids depends on the location of the tumor and on the involvement of adjacent structures. Lopes and co-workers reviewed the postoperative morbidity and mortality rates in 44 patients (22 men and 22 women) between 1980 and 2000. Their postoperative morbidity rate was 13.6% and the mortality rate was 8.9%, with a median follow-up period of 8 years and a recurrence rate of 4.5%. Morbidity and mortality rates for epidermoid cysts seem to be unrelated to classical aseptic meningitis (22.7% in their series) or hydrocephalus (10%). They concluded prolonged cerebral retraction could be one of the responsible factors for increased morbidity and mortality rates.54
Other common locations for epidermoids include the scalp and skull. These slow-growing lesions commonly present in infancy or childhood and grow unabated until adulthood. They may be located anywhere in the scalp and may be multicentric (Fig. 7.10). As opposed to dermoids which may also contain hair, teeth, and skin glands, epidermoids typically only contain epidermal tissue and keratin debris (Fig. 7.11). Gross total resection is curative and provides excellent results.
Akor C.A., Wojno T.H., Newman N.J., Grossniklaus H.E. Arachnoid cysts of the optic nerve. Ophthal Plast Reconstr Surg. 2003;19:466-469.
Cincu R., Agrawal A., Eiras J. Intracranial arachnoid cysts: current concepts and treatment alternatives. Clin Neurol Neurosurg. 2007;109:837-843.
Peraud A., Ryan G., Drake J.M. Rapid formation of a multi-compartment neonatal arachnoid cyst. Pediatr Neurosurg. 2003;39:139-143.
Piatt J.H.Jr. Unexpected findings on brain and spine imaging in children. Pediatr Clin North Am. 2004;51:507-527.
Tatli M., Guzel A. Bitemporal arachnoid cysts associated with tuberous sclerosis complex. J Child Neurol. 2007;22:775-779.
Please go to expertconsult.com to view complete list of references.
1. Redla S., Husami Y., Colquhoun I. Apparent paradoxical vault changes with middle cranial fossa arachnoid cysts—implication for aetiology. Clin Radiol. 2001;56:851-855.
2. Peraud A., Ryan G., Drake J.M. Rapid formation of a multi-compartment neonatal arachnoid cyst. Pediatr Neurosurg. 2003;39:139-143.
3. Tatli M., Guzel A. Bitemporal arachnoid cysts associated with tuberous sclerosis complex. J Child Neurol. 2007;22:775-779.
4. Dutt S.N., Mirza S., Chavda S.V., Irving R. Radiologic differentiation of intracranial epidermoids from arachnoid cysts. Oto Neurol. 2002;23:84-92.
5. Bretelle F., Senat M.V., Bernard J.P., et al. First-trimester diagnosis of fetal arachnoid cyst: prenatal implication. Ultrasound Obstet Gynecol. 2002;20:400-402.
6. Rengachary S.S., Watanabe I. Ultrastructure and pathogenesis of intracranial arachnoid cysts. J Neuropathol Exp Neurol. 1981;40:61-83.
7. Martinez-Lage J.F., Casas C., Fernandez M.A., et al. Macrocephaly, dystonia, and bilateral temporal arachnoid cysts: glutaric aciduria type 1. Childs Nerv Syst. 1994;10:198-203.
8. Wang P.-J., Lin H.-C., Liu H.-M., et al. Intracranial arachnoid cysts in children: related signs and associated anomalies. Pediatr Neurol. 1998;19:100-104.
9. Lmejjati M., Aniba K., Haddi M., et al. Spinal intramedullary arachnoid cyst in children. Pediatr Neurosurg. 2008;44:243-246.
10. Kumar R., Singh V. Benign intradural extramedullary masses in children in northern India. Pediatr Neurosurg. 2005;43:22-28.
11. Starkman S.P., Brown T.C., Linell E.A. Cerebral Arachnoid Cyst. Neuropathol Exp Neurol. 1958;17:484-500.
12. Mason T.B.A., Chiriboga C.A., Feldstein N.A., et al. Massive intracranial arachnoid cyst in a developmentally normal infant: case report and literature review. Pediatr Neurol. 1997;16:59-62.
13. Gosalakkal J. Intracranial arachnoid cysts in children: a review of pathogenesis, clinical features, and management. Pediatr Neurol. 2002;26:93-98.
14. Wolter J.R., McKenny M.J. Collateral hyperplasia and cyst formation of orbital leptomeninx and cyst formation of orbital leptomeninx. Am J Ophthalmol. 1964;57:1037-1042.
15. Miller N.R., Green W.R. Arachnoid cysts involving a portion of the intraorbital optic nerve. Arch Ophthalmol. 1975;93:1117-1121.
16. Akor C.A., Wojno T.H., Newman N.J., Grossniklaus H.E. Arachnoid cysts of the optic nerve. Ophthal Plast Reconstr Surg. 2003;19:466-469.
17. Oberbauer R.W., Haase J., Pucher R. Arachnoid cysts in children. A European co-operative study. Childs Nerv Syst. 1992;8:281-286.
18. Galassi E., Tognetti F., Gaist G., et al. CT scan and metrizamide CT cisternography in arachnoid cysts of the middle cranial fossa: classification and pathophysiologic aspects. Surg Neurol. 1982;17:363-369.
19. Piatt J.H.Jr. Unexpected findings on brain and spine imaging in children. Pediatr Clin North Am. 2004;51:507-527.
20. Lancon J.A., Ellis A.L. Giant posterior fossa arachnoid cyst. Pediatr Neurosurg. 2004;40:151-152.
21. Maiuri F., Iaconette G., Gangemi M. Arachnoid cyst of the lateral ventricle. Surg Neurol. 1997;48:401-404.
22. Aithala G.R., Sztriha L., Amirlak I., et al. Spinal arachnoid cyst with weakness in the limbs and abdominal pain. Pediatr Neurol. 1999;20:155-156.
23. Adan L., Bussieres L., Dinand V., et al. Growth, puberty and hypothalamic-pituitary function in children with suprasellar arachnoid cyst. Eur J Pediatr. 2000;159:348-355.
24. Weil R. Rapidly progressive visual loss caused by a sellar arachnoid cyst: reversal with transsphenoidal microsurgery. Southern Med J. 2001;94:1118-1121.
25. Ashker L., Weinstein J.M., Dias M., et al. Arachnoid cyst causing third cranial nerve palsy manifesting as isolated internal ophthalmoplegia and iris cholinergic supersensitivity. J Neuro-Ophthalmol. 2008;28:192-197.
26. Jacob M., Gujar S., Trobe J., Gandhi D. Spontaneous resolution of a Meckel’s cave arachnoid cyst causing sixth cranial nerve palsy. J Neuro-Ophthalmol. 2008;28:186-191.
27. Boudewynsa A.N., Declaua F., De Ridderb D., et al. Case report: “auditory neuropathy” in a newborn caused by a cerebellopontine angle arachnoid cyst. Int J Pediatr Oto. 2008;72:905-909.
28. Cartwright M.J., Eisenberg M.B., Page L.K. Posterior fossa arachnoid cyst presenting with an isolated twelfth nerve paresis. Clin Neurol Neurosurg. 1991;93:69-72.
29. Genc E., Dogan E.A., Kocaogullar Y., Emlik D. A case with prepontine (clival) arachnoid cyst manifested as trigeminal neuralgia. Headache. 2008;48:1525-1539.
30. Prevo R.L., Hageman G., Bruyn R.P.M., et al. Extended extradural spinal arachnoid cyst: an unusual cause of progressive spastic paraparesis. Clin Neurol Neurosurg. 1999;101:260-263.
31. Millichap J.G. Temporal lobe arachnoid cyst attention deficit disorder syndrome: role of the electroencephalogram in diagnosis. Neurology. 1997;48:1435-1439.
32. Sztriha L., Gururaj A. Hippocampal dysgenesis associated with temporal lobe hypoplasia and arachnoid cyst of the middle cranial fossa. J Child Neurol. 2005;20:926-930.
33. Yalcin A.D., Oncel C., Kaymaz A., et al. Evidence against association between arachnoid cysts and epilepsy. Epilepsy Res. 2002;49:255-260.
34. Arroyo S., Santamaria J. What is the relationship between arachnoid cysts and seizure foci? Epilepsia. 1997;38:1098-1102.
35. Tsugu H., Fukushima T., Oshiro S., et al. A case report of caudal regression syndrome associated with an intraspinal arachnoid cyst. Pediatr Neurosurg. 1999;31:207-212.
36. Pandey P., Tripathi M., Chandra P.S., et al. Spontaneous decompression of a posterior fossa arachnoid cyst: a case report. Pediatr Neurosurg. 2001;35:162-163.
37. Arunkumar M.J., Haran R.P., Chandy M.J. Spontaneous fluctuation in the size of a midline posterior fossa arachnoid cyst. Br J Neurosurg. 1999;13:326-328.
38. Russo N., Domeniucci M., Beccaglia M.R., Santoro A. Spontaneous reduction of intracranial arachnoid cysts: a complete review. Br J Neurosurg. 2008;22:626-629.
39. Bilginer B., Onal M.B., Oguz K.K., Akalan N. Arachnoid cyst associated with subdural hematoma: report of three cases and review of the literature. Childs Nerv Syst. 2009;25:119-124.
40. Ziaka D., Kouyialis A.T., Boviatsis E.J., Sakas D.E. Asymptomatic massive subdural hematoma in a patient with bitemporal agenesis and bilateral arachnoid cysts. Southern Med J. 2008;101:324-326.
41. Parsch C.S., Krauss J., Hoffman E., et al. Arachnoid cysts associated with subdural hematomas and hygromas: analysis of 16 cases, long-term follow-up, and review of the literature. Neurosurgery. 1997;40:483-490.
42. Wester K., Helland C.A. How often do chronic extra-cerebral haematomas occur in patients with arachnoid cysts? J Neurol Neurosurg Psychiatry. 2008;79:72-75.
43. Cincu R., Agrawal A., Eiras J. Intracranial arachnoid cysts: Current concepts and treatment alternatives. Clin Neurol Neurosurg. 2007;109:837-843.
44. Shigemori M., Okura A., Takahasi Y., Tokutomi T. New surgical treatment of middle fossa arachnoid cyst. Surg Neurol. 1996;45:189-192.
45. Spacca B., Kandasamy J., Mallucci C.L., Genitori L. Endoscopic treatment of middle fossa arachnoid cysts: a series of 40 patients treated endoscopically in two centres. Childs Nerv Syst. 2010;26:163-172.
46. Shim K.-W., Lee Y.-H., Park E.-K., et al. Treatment option for arachnoid cysts. Childs Nerv Syst. 2009;25:1459-1466.
47. Chang I- C. Surgical experience in symptomatic congenital intraspinal cysts. Pediatr Neurosurg. 2004;40:165-170.
48. Orakcioglu B., Halatsch M.E., Fortunati M., et al. Intracranial dermoid cysts: variations of radiological and clinical features. Acta Neurochir. 2008;150(12):1227-1234. discussion 1234. Epub 2008 Nov 20
49. Handa U., Chabra S., Mohan H. Epidermal inclusion cyst: cytomorphological features and differential diagnosis. Diagn Cytopathol. 2008;36(12):861-863.
50. Osburn A.G., Preece M.T. Intracranial cysts: radiologic-pathologic correlation and imaging approach. Radiology. 2006;239:650-664.
51. Kaido T., Okazaki A., Kurokawa S., Tsukamoto M. Pathogenesis of intraparenchymal epidermoid cyst in the brain: a case report and review of the literature. Surg Neurol. 2003;59:211-216.
52. Watanabe K., Wakai S., Nagai M., Muramatsu H. Epidermoid tumor with unusual CT and MR findings—case report. Neurol Med Chir (Tokyo). 1990;30:977-979.
53. Schaefer P.W., Grant P.E., Gonzalez R.G. Diffusion-weighted MR imaging of the brain. Radiology. 2000;217:331-345.
54. Lopes M., Capelle L., Duffau H., et al. Surgery of intracranial epidermoid cysts. Report of 44 patients and review of the literature. Neurochirurgie. 2002;48(1):5-13.