42 Development of the Cardiovascular System
Embryologic development of the heart requires the coordination of multiple steps, including heart tube formation, cardiac looping, chamber septation, and development of appropriate inflow and outflow tracts. Additionally, the cardiac conduction system and coronary artery system must develop during this time period. After organogenesis is complete, normal fetal circulation is notable for the presence of three levels of communication that normally close after birth, namely the ductus venosus, the foramen ovale, and the ductus arteriosus. Although these communications are vital for fetal circulation, they can be maladaptive in the postnatal period. Functional closure of all three communications generally occurs during the transition to postnatal life. Understanding the complexity of the normal development of the cardiovascular system allows for greater understanding of the development of congenital cardiovascular disease (see Chapters 43 and 44).
Embryologic Development
Heart Tube Formation
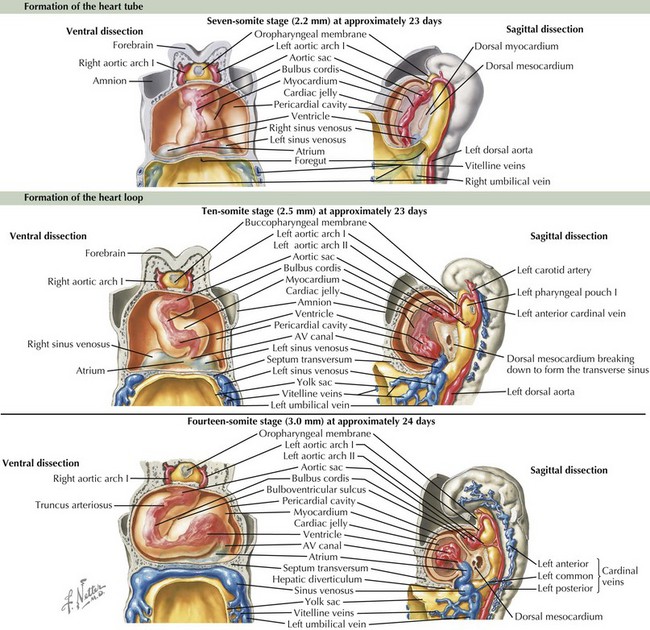
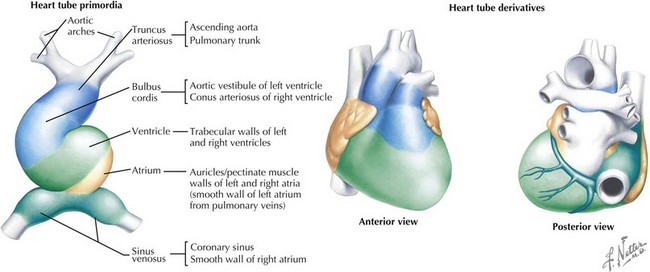
The cardiovascular system is derived primarily from the splanchnic mesoderm, which develops on the 15th day after ovulation. Migrating cells form the cardiogenic crescent, which fuses in the midline as embryonic folding occurs, creating the heart tube by day 20 to 21 of development. The heart begins to beat on day 22 of development. At this time, the heart tube segments begin to differentiate, with constrictions demarcating specific segments of the tube (Figure 42-1). Most cranially, the bulbus cordis leads into the truncus arteriosus, which connects to the aortic sac, the aortic arches, and the dorsal aorta. Caudal to the bulbus cordis is the primitive ventricle followed by the primitive atrium, which is connected in turn to the sinus venosus. Between these segments, transitional zones exist that will become septa, valves, conduction tissue, and fibrous skeleton structures.
Cardiac Looping
The crucial process of cardiac looping evolves over day 23 to 28 of gestation, with the bulbus cordis of the heart tube bending ventrally, caudally, and toward the right of the embryo, forming a D-looped heart (see Figure 42-1). This positions the proximal bulbus cordis to become the future right-sided right ventricle and the primitive ventricle to become the future left-sided left ventricle while also positioning the atria dorsal to the future ventricles (Figure 42-2). The process and direction of looping appear to be under genetic control within the myocardium, with dysregulation of this process potentially resulting in abnormal positioning of the four chambers of the heart, such as occurs in an L-looped heart (i.e., ventricular inversion). Through looping, the transitional zones are brought together along the inner curvature, and the primitive chambers are more predominantly located along the outer curvatures. Shortly after the completion of looping, blood begins circulating within the embryo and cardiac septation begins, as does the formation of the aortic arches.
Endocardial Cushion Development and Atrial Septation
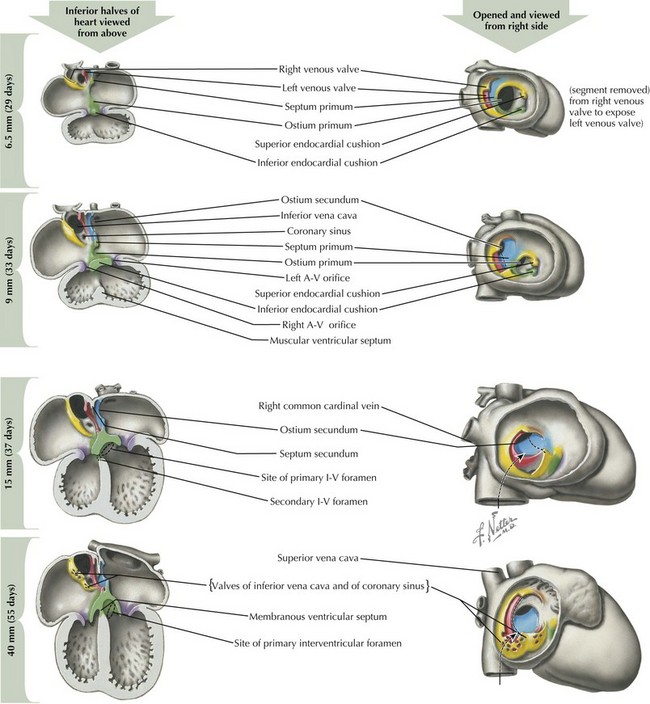
After looping has completed, the division of the four chambers and ventricular outflow tract must begin through division of the atrial and ventricular structures via the endocardial cushions, and division of the right and left sided structures through a complex process of septation (Figure 42-3). The endocardial cushions develop early in this process, appearing at the level of the future atrioventricular (AV) valves. Inferior and superior endocardial cushions grow toward each other, dividing the common AV canal into left and right AV openings. In so doing, they establish the site of the future AV valves, which will develop between the fifth and eighth weeks of development through a process of invagination of the cardiac wall.
Around day 26 to 28, atrial septation begins with formation of a crescent-shaped membrane from the posterior-superior wall of the atria, called the septum primum. On day 35, septum primum begins to extend toward the fusing endocardial cushions, gradually separating the left atrium from the right atrium. The endocardial cushions of the AV canal continue to develop and separate the atria, closing the ostium primum (see Figure 42-3). Failure of the aforementioned processes results in AV canal defects, frequently associated with trisomy 21. Classical embryologic teaching has been that before completing the division between the two atria, perforations develop in the septum primum, creating the foramen secundum, allowing continued flow between the two atria. However, virtually all hearts that have a septum primum display a crescentic superior edge with attachments on both ends of the crescent and no remnant of septum primum above the crescent indicating that there is no hole within septum primum but rather that the crescent shape was there originally.
Ventricular Septation
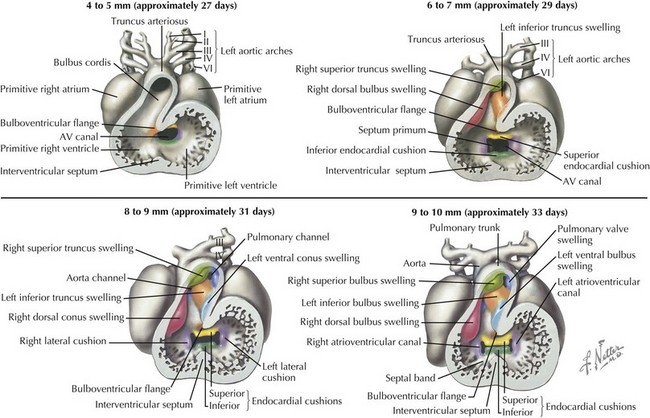
Ventricular septation also involves coordination of multiple converging septal structures (see Figure 42-3). The muscular interventricular septum begins development by day 30 and continues into the seventh week of life. While the muscular ventricular septum is forming, myocardial trabecula develop in both ventricles. The posterior portion of the muscular septum is the inlet ventricular septum, smooth walled and close to the AV canal, and the anterior portion, called the primary ventricular fold or septum, is trabeculated. The muscular septum growth slows in the seventh week of life, but ventricular septation continues between 36 and 49 days of life with the closure of the infundibular septum (separating pulmonary outflow area from aortic) and closure of the membranous ventricular septum (closed by fusion of the left and right bulbar ridges along with the posterior endocardial cushion). Depending on the location, defects in ventricular septation can result in five major types of ventricular septal defects, which are further discussed in Chapter 43.
Development of Major Aortic and Pulmonary Outflow Tracts
Development of the ventricular outflow tract and aorticopulmonary septa require division of the associated left and right-sided structures such that the right ventricle flows into the newly developed pulmonary artery and the left ventricle flows into the aorta. The specifics of this process are controversial, with multiple theories currently being explored, generally involving the formation of bulbar and truncal ridges through a process that appears dependent on neural crest cells (Figure 42-4). During this process, expansion of the bulbus cordis beneath the left-sided pulmonary artery with regression of muscle beneath the right-sided aorta while maintaining the fixed position of the superior aorta relative to the pulmonary artery at the level of the bronchus causes the outflow tract to twist around itself, bringing the pulmonary artery closer to the right-sided morphologic right ventricle and the aorta closer to the morphologic left ventricle. Abnormal migration of neural crest cells in certain conditions, such as DiGeorge syndrome, may account for their associated conotruncal anomalies, such as tetralogy of Fallot, truncus arteriosus communis, and interrupted aortic arch with ventricular septal defect.
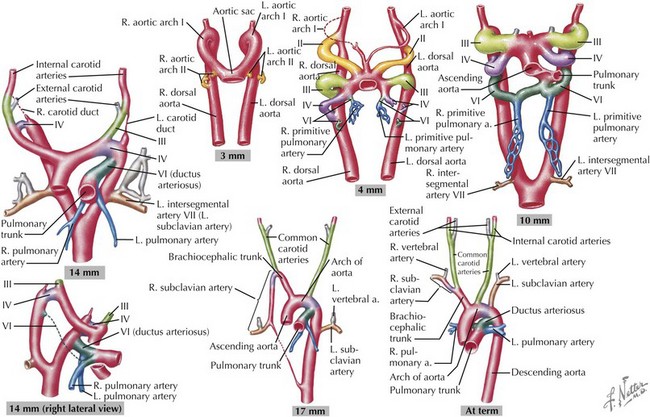
Development of the Great Arteries
The great arteries are formed through the division of the truncus arteriosus, with more distal portions of the aorta and pulmonary arteries developing from the paired embryonic aortic arches. The aortic arches develop sequentially during early gestation, with the final pair appearing by the sixth week of development (Figure 42-5). The first aortic arches develop into the maxillary arteries and portions of the external carotid arteries. The second pair of aortic arches provides circulation to the stapes in the inner ear as well as the hyoid artery. The third pair of aortic arch arteries develops into the common carotids and the proximal portion of the internal carotid arteries. The fourth pair of aortic arch arteries has asymmetric development, with the left side developing into the aortic arch and the right side connecting the right seventh intersegmental artery (precursor of the right subclavian artery) to the right carotid to form an innominate artery. The fifth pair regresses without any known remnant structures in normal humans, although a persistent fifth aortic arch has been noted rarely. The sixth pair of aortic arches develops into the ductus arteriosus on the left with involution on the right. Through sequential development and regression, great artery arrangement is evident by the eighth week of development. Abnormal development during this process can result in coarctation of the aorta, double aortic arch, right-sided aortic arch, and anomalous right subclavian artery.
Fetal Circulation
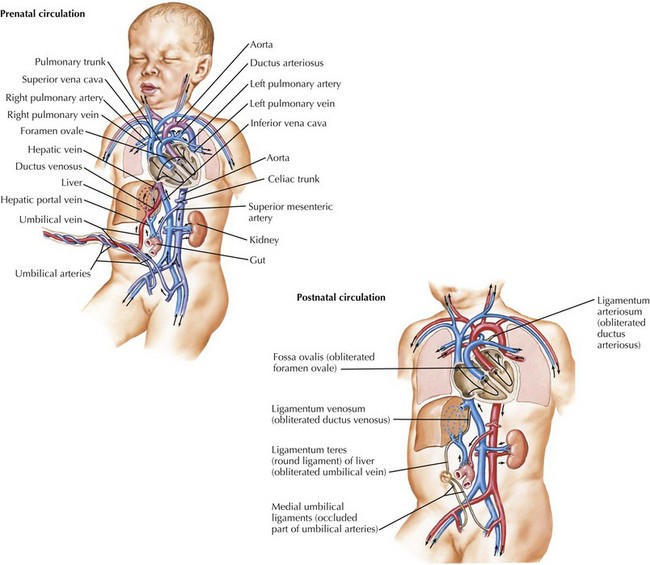
Because the source of oxygenated blood in fetal life is the placenta, rather than the lung, fetal circulation uses specific mechanisms to preferentially direct more oxygenated blood from the placenta toward specific structures such as the heart and the brain. Specifically, three communications mentioned previously, the ductus venosus, the foramen ovale, and the ductus arteriosus, are required for normal fetal circulation (Figure 42-6). The right ventricle receives returning deoxygenated systemic blood flow from the brain via the superior vena cava and right atrium. This blood exits via the pulmonary arteries into the ductus arteriosus for return to the placenta. The pulmonary artery also supplies a minimal amount of flow to the lungs. Oxygenated blood from the placenta returns to the right atrium via the ductus venosus and proximal inferior vena cava. This oxygenated blood is directed preferentially to the left atrium through the foramen ovale and is then pumped by the left ventricle into the aorta, where it is delivered primarily into the coronary circulation and upper body, including the developing brain. In fetal circulation, the right ventricular output is slightly larger than that of the left, resulting in relative right ventricular hypertrophy.
Postnatal Transitions And Neonatal Circulation
After delivery, several transitions must occur because structures required for fetal circulation become maladaptive for extrauterine life. The three previously beneficial communications must close to establish the final configuration of the human cardiovascular system (see Figure 42-6). The foramen ovale, being a flap-valve between the septum primum and septum secundum, functionally closes shortly after delivery when left atrial pressure increases as a result of increased pulmonary venous return after birth. This causes septum primum to become pressed against septum secundum. Anatomic closure of the foramen ovale is a more prolonged process, and it is estimated that 25% to 30% of adults still have a patent foramen ovale. Although this is largely hemodynamically insignificant, it can be a portal for embolic stroke later in life with transient elevation of right atrial pressure.
Abdulla R, Blew GA, Holterman MJ. Cardiovascular embryology. Pediatr Cardiol. 2004;25:191-200.
Gittenberger-de Groot AC. Basics of cardiac development for the understanding of congenital heart malformations. Pediatr Res. 2005;57(2):169-176.
Kiserud T. Physiology of the fetal circulation. Semin Fetal Neonat Med. 2005;10:493-503.
Manner J. The anatomy of cardiac looping: a step towards the understanding of the morphogenesis of several forms of congenital cardiac malformations. Clin Anat. 2009;22:21-35.
McFadden DG, Olson EN. Heart development: learning from mistakes. Curr Opin Genet. 2002;12:328-335.
Sander TL, Klinkner DB, Tomita-Mitchell A, Mitchell ME. Molecular and cellular basis of congenital heart disease. Pediatr Clin North Am. 2006;53(5):989-1009.