Development of the brain
Neural tube formation
The central nervous system is derived from the neural tube, which appears during the fourth week after fertilization. At this early stage the embryo takes the form of a trilaminar germ disc, lying in the floor of the amniotic sac (Fig. 2.1). The germ disc is composed of three layers of tissue from which all the structures of the body originate:
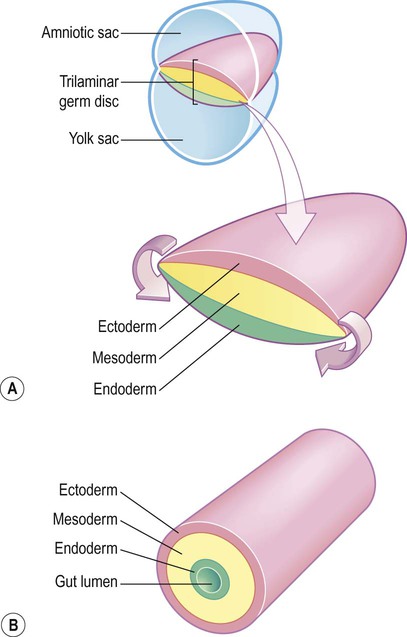
(A) The germ disc is composed of three primitive germ layers from which all body tissues are derived. A complex process of folding (not illustrated) transforms the germ disc so that the ectoderm comes to lie on the outside and the endoderm lines the gut lumen; (B) The body can be represented in cartoon form as a hollow cylinder with the mouth at one end and the anus at the other.
 The ectoderm (Greek: ektos, outside) contributes mainly to the skin, but also gives rise to the central and peripheral nervous systems.
The ectoderm (Greek: ektos, outside) contributes mainly to the skin, but also gives rise to the central and peripheral nervous systems.
 The mesoderm (Greek: misos, middle) is the origin of the cardiovascular, musculoskeletal, urinary and reproductive systems.
The mesoderm (Greek: misos, middle) is the origin of the cardiovascular, musculoskeletal, urinary and reproductive systems.
 The endoderm (Greek: endon, within) contributes chiefly to the respiratory and gastrointestinal tracts, including the liver, gallbladder and pancreas.
The endoderm (Greek: endon, within) contributes chiefly to the respiratory and gastrointestinal tracts, including the liver, gallbladder and pancreas.
The process by which the embryonic ectoderm gives rise to the neural tube is called primary neurulation (Fig. 2.2). It is initiated by the notochord, a rod-like mesodermal structure that helps to define the longitudinal axis of the embryo. The notochord releases soluble mediators including cell adhesion molecules and trophic factors, which influence the overlying ectoderm. This process is termed neural induction.
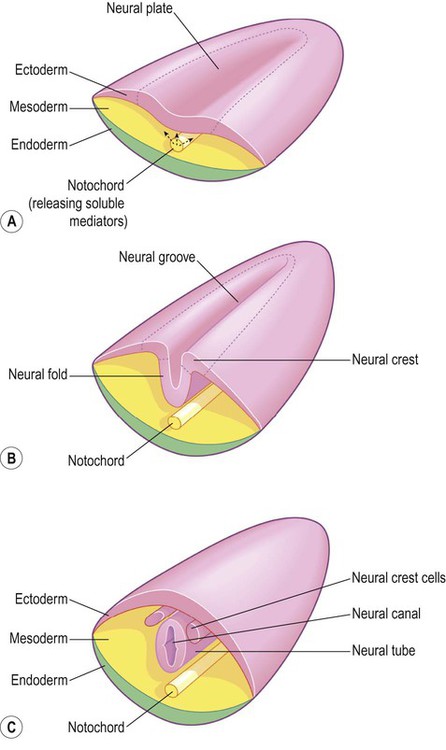
(A) The mesodermal notochord releases soluble mediators that induce neural tube formation in the overlying ectoderm (neural induction); (B) The neural tube forms from the paired neural folds which flank the neural groove; (C) The neural crest cells detach from the dorsolateral margins of the neural tube and will give rise to much of the peripheral nervous system, including the spinal and autonomic ganglia.
Ultrasound studies show that in humans the neural tube begins to form at around 21-23 days after fertilization, when the embryo is just 2–3 mm in length. The first change (which occurs at about day 18) is the appearance of the neural plate, a broad area of thickening in the dorsal ectoderm. A shallow longitudinal depression termed the neural groove separates the neural plate into paired neural folds which gradually roll up to form a cylinder. The neural folds ultimately meet in the midline and unite to create the neural tube and neural canal. Fusion begins in the presumptive cervical region and proceeds both rostrally and caudally in a ‘zipper-like’ fashion. The open ends of the neural tube are called the cranial and caudal neuropores, which have normally closed by the beginning of week five. Disorders resulting from faulty neural tube closure are discussed in Clinical Box 2.1.
Origin of neurons and glial cells
The wall of the neural tube can be divided into three concentric zones (Fig. 2.3). The ventricular zone is closest to the fluid-filled neural canal (which will become the cerebral ventricles) and is composed of proliferating neural progenitor cells. These include neuroblasts (neuronal precursors) and glioblasts (glial precursors) that give rise to most of the specialized cells of the central nervous system. Microglia are the resident phagocytes of the brain, but originate from the bone marrow and are of mesodermal rather than ectodermal lineage.
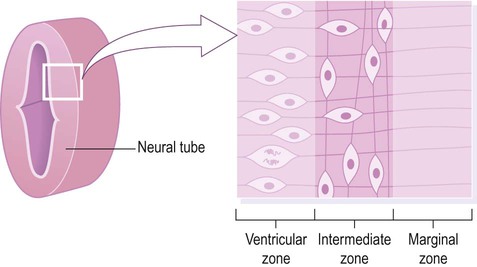
Cell division (mitosis) occurs in the ventricular zone, giving rise to neurons and glia.
Formation of the cerebral cortex
In the cerebral hemispheres the majority of neurons ultimately vacate the intermediate zone or undergo programmed cell death (see Ch. 8) and this region eventually becomes the subcortical white matter. Up to 50% of neurons produced in the developing brain fail to (i) reach their intended targets or (ii) make appropriate functional connections and are consequently deleted by programmed cell death.
Sensory and motor areas
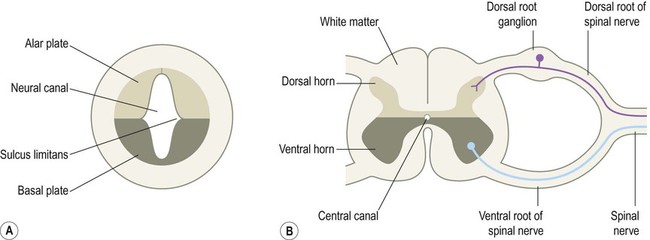
The neural tube has two functional divisions, separated by the sulcus limitans (Fig. 2.4A). The basal plate occupies the ventral portion of the neural tube (anterior to the sulcus limitans) and is predominantly a motor structure; the alar plate is located dorsally and is sensory. This dorsal–ventral division between sensory and motor areas is reflected in the adult spinal cord, with sensory fibres entering via the dorsal roots and motor fibres emerging in the ventral roots (Fig. 2.4B). It is echoed throughout the central nervous system so that motor structures (e.g. cortical areas, tracts, nuclei) tend to be anterior to sensory structures.
The neural crest
The peripheral nervous system is mainly derived from the neural crest. This is a population of cells that detaches from the lateral margins of the neural plate during neurulation (see Fig. 2.2). Neural crest cells that come to lie dorsolateral to the neural tube ultimately become the primary sensory neurons of the dorsal root ganglia. These neurons are initially bipolar but their central and peripheral processes fuse at a common T-shaped extension of the cell body to form a single continuous axon. For this reason they are described as pseudounipolar (Greek: pseudo-, false). The central processes of the dorsal root ganglion cells innervate the alar (sensory) plate of the neural tube, whereas the peripheral processes enter the spinal nerves at each segmental level (see Fig. 2.4B).
Divisions of the brain
The three fundamental divisions of the brain can be identified in the neural plate as early as week three, before the neural tube has closed. They are illustrated schematically (at a later stage of development) in Fig. 2.5:
 The prosencephalon (Greek: pro, before) is the precursor of the forebrain and will give rise to the cerebral hemispheres, basal ganglia and thalamic region (diencephalon).
The prosencephalon (Greek: pro, before) is the precursor of the forebrain and will give rise to the cerebral hemispheres, basal ganglia and thalamic region (diencephalon).
 The mesencephalon corresponds to the adult midbrain.
The mesencephalon corresponds to the adult midbrain.
 The rhombencephalon (Greek: rhomboeides, shaped like a rhombus) gives rise to the hindbrain, which includes the pons, medulla and cerebellum.
The rhombencephalon (Greek: rhomboeides, shaped like a rhombus) gives rise to the hindbrain, which includes the pons, medulla and cerebellum.
During weeks four and five the neural tube closes and the cranial portion undergoes an impressive transformation, differentiating into five regions that will become the major anatomical divisions of the adult brain (Fig. 2.6). Each of these components contains a fluid-filled cavity or channel that corresponds to the lumen of the neural tube (Fig. 2.7).
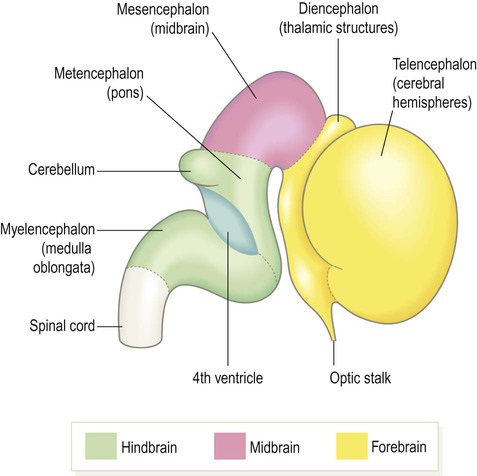
At this stage the embryo is around 10 mm in length.
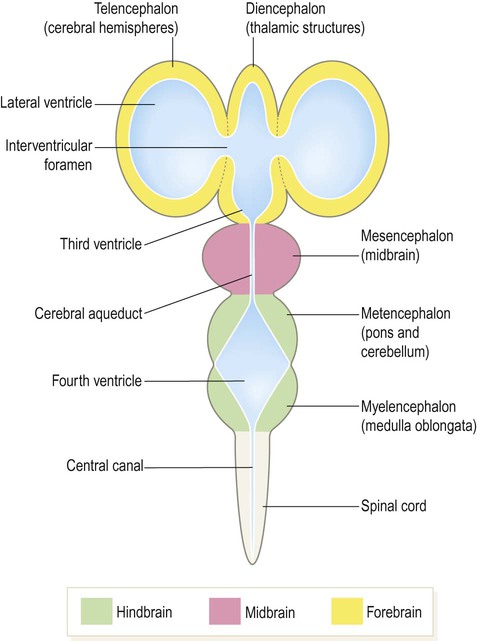
Each part of the brain contains a fluid-filled cavity or channel corresponding to the lumen of the neural tube.
In contrast, the mesencephalon
does not subdivide further. Instead, it retains a somewhat tubular structure as the adult midbrain, traversed by a narrow cerebral aqueduct.
Flexures and the neuraxis
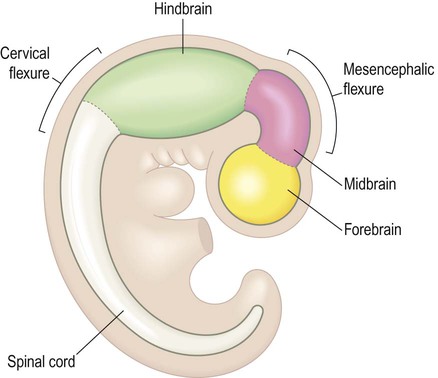
The neural tube develops three flexures (bends). Two forward-flexures (cervical and mesencephalic; Figs 2.5 & 2.6) contribute to the change in the longitudinal axis of the nervous system (the neuraxis). This is vertical in the spinal cord but horizontal in the cerebral hemispheres. The 90-degree bend in the human neuraxis contrasts with that of other animals (partly because of our upright, bipedal stance) and alters the meaning of certain anatomical terms (Fig. 2.8).
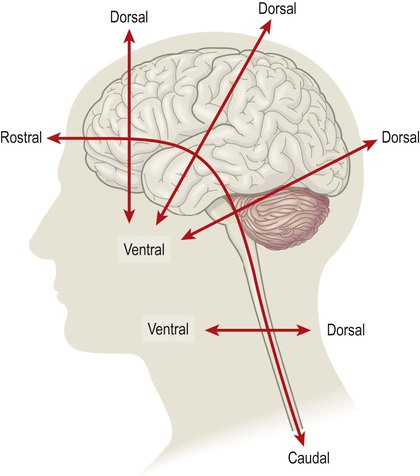
This is partly due to our upright, bipedal posture and alters the meaning of certain directional terms in the dorsoventral and rostrocaudal axes (i.e. in the spinal cord, dorsal = posterior and ventral = anterior; whereas in the brain, dorsal = superior and ventral = inferior).
The pontine flexure bends in the opposite direction to the other two and marks the boundary between the pons and medulla. It also causes the neural tube to split along its relatively weak line of fusion and ‘gape open’ posteriorly (Fig. 2.9; see also Fig. 2.6). The pontine flexure thus contributes to the rhomboid shape of the fourth ventricle, which is the origin of the term rhombencephalon.
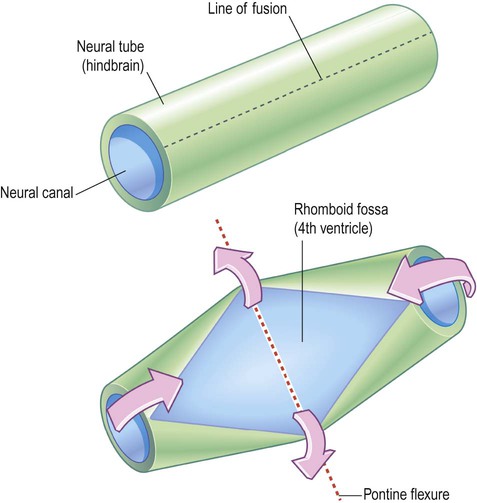
The neural tube is seen from the posterior aspect. This view would normally be obscured by the cerebellum which covers the rhomboid fossa and contributes to the roof of the fourth ventricle. [This can be reproduced by bending a piece of rolled-up A4 paper.]
Expansion of the telencephalon
The cerebral hemispheres and lateral ventricles become C-shaped as a result of the non-uniform expansion of the telencephalon (Fig. 2.10). As the frontal, parietal, temporal and occipital lobes grow the telencephalon expands like an inflating balloon. A small island of tissue overlying the basal ganglia expands comparatively little and is progressively ‘swallowed up’ by the surrounding frontal, parietal and temporal lobes. This region corresponds to the insula, which is hidden within the depths of the lateral sulcus in the mature brain (Latin: insula, island).
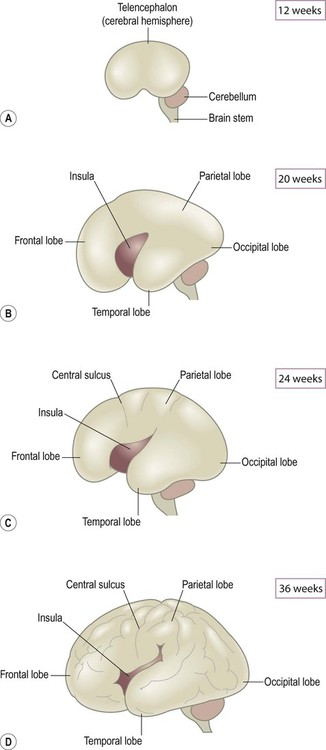
(A) At 12 weeks the surface of the brain is still smooth; (B) By 20 weeks the four lobes are just discernible and the insula can be seen within the lateral sulcus; (C) The central sulcus is evident at 24 weeks, marking the boundary between the frontal and parietal lobes; (D) By 36 weeks the surface convolutions are well-developed and the insula is mostly hidden within the lateral sulcus.
As the telencephalon continues to expand it eventually envelops and fuses with the diencephalon. Once this has happened, axons can pass directly between the cerebral hemispheres and brain stem, traversing the basal ganglia and partially dividing them (see Ch. 3). Many internal hemispheric structures are distorted by the expansion of the cerebral hemispheres and take on the same C-shaped profile as the lateral ventricles. These include the hippocampus and its outflow pathway, the fornix (Fig. 2.11) which are involved in memory formation (see Ch. 3).
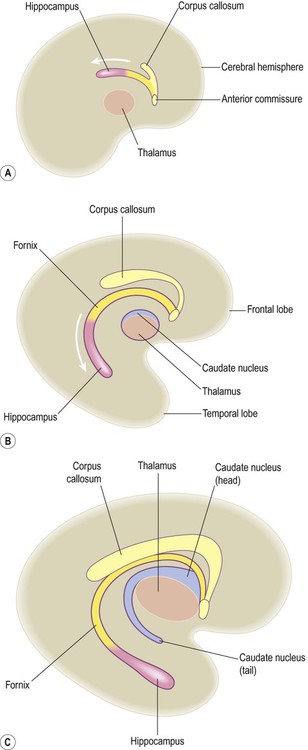
(A) The hippocampus originates as a dorsal midline structure, superior to the thalamus and in other species remains in this position; (B) As the human telencephalon expands, the hippocampus is carried downwards and forwards, coming to lie in the floor of the temporal lobe; (C) The hippocampus leaves behind an arching trail of white matter, the fornix, which echoes the C-shaped curvature of the cerebral hemisphere and lateral ventricle. Modified from Fitzgerald: Clinical Neuroanatomy and Neuroscience 5e (2006) with permission.
Development of the cerebellum
The cerebellum originates from a dorsal outgrowth of the metencephalon (in the hindbrain). It thus overlies the pons and fourth ventricle (Fig. 2.12). The alar (sensory) plates of the metencephalon (including the rhombic lips, which overhang the upper part of the fourth ventricle) fuse to form the cerebellar plate. Expansion of the cerebellar plate gives rise to the cerebellar hemispheres and vermis which eventually cover the rhomboid fossa, forming the roof of the fourth ventricle. In keeping with its origin as an alar (sensory) plate derivative, the cerebellum has many more afferent than efferent connections and has no direct role in movement initiation.
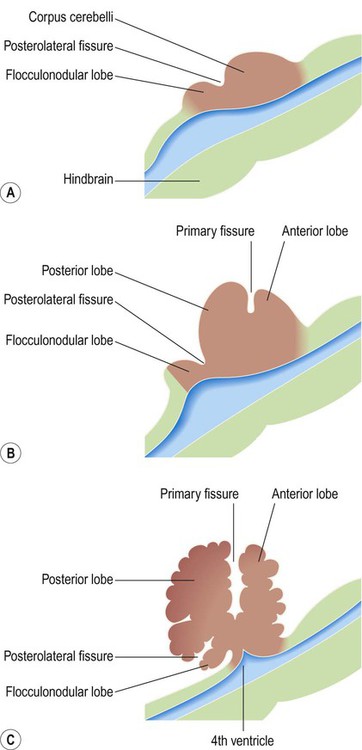
(A) The cerebellum arises from the alar plate of the metencephalon and is initially divided into the corpus cerebelli and flocculonodular lobe by the posterolateral fissure; (B, C) The primary fissure further subdivides the corpus cerebelli into anterior and posterior lobes. In the mature human brain, the flocculonodular lobe is very small.
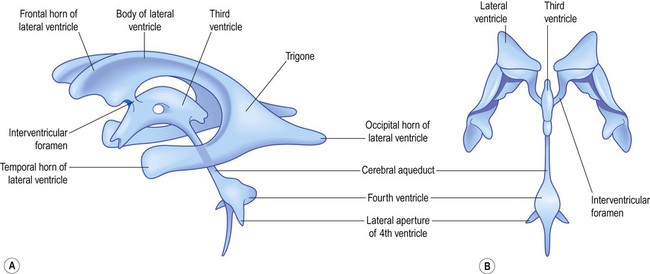
Ventricular system
The fluid-filled cavity of the neural tube is represented in the adult brain by the ventricular system. This consists of four interconnected cavities or ventricles which contain around 30 mL of colourless cerebrospinal fluid (CSF) (Fig. 2.13).
Circulation of CSF
CSF escapes from the fourth ventricle (to the subarachnoid space) via three openings: the single median aperture and the two lateral apertures. It is ultimately reabsorbed into the venous system via the arachnoid granulations which run along the superior aspect of the cerebral hemispheres (Fig. 2.14). These correspond to the arachnoid villi, finger-like projections into a large venous channel called the superior sagittal sinus. These structures allow the reabsorption of CSF into the venous circulation.
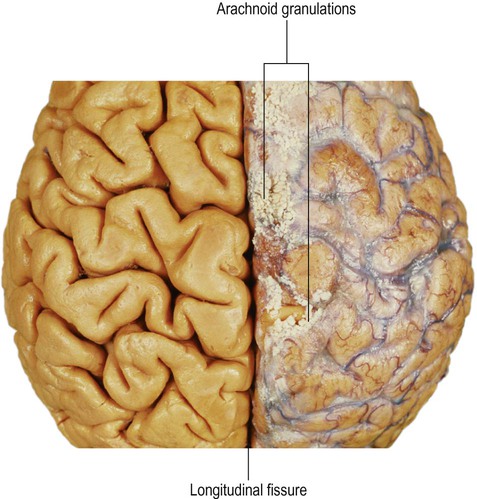
The tough, leathery dura is not seen in this image (it is tightly adherent to the periosteum and is usually left inside the skull when the brain is removed) but the pia and arachnoid membranes are present on the right-hand side, including the arachnoid granulations. On the left side, the membranes have been stripped to reveal the underlying gyri and sulci. From Crossman: Neuroanatomy ICT 4e (Churchill Livingstone 2010) with permission.
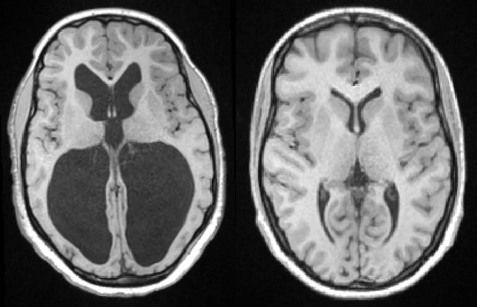
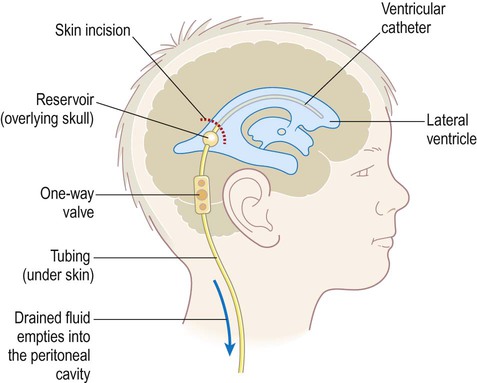
The total volume of intracranial CSF is around 140 mL, most of which is in the subarachnoid space; the spinal CSF volume is more variable and difficult to estimate. Due to a constant cycle of production and reabsorption, the CSF is replaced up to four times each day. Interference with CSF production or drainage can lead to ventricular dilation and raised intracranial pressure (Clinical Box 2.2).