Design and administration of medicines for children and the elderly
Catherine Tuleu and David Wright
Chapter contents
Human development, ageing and drug administration
Paediatric and geriatric populations
Formulation of paediatric and geriatric medicines
Adaptation of existing dosage forms
Future developments in the formulation of paediatric and geriatric medicines
Considerations for the patient-focused approach in formulation development
Key points
Human development, ageing and drug administration
Whilst early development and ageing processes can affect the acceptability and appropriateness of different dosage forms for administration to either paediatric or geriatric populations, each patient should be considered on an individual basis. The fact that someone is young or old does not necessarily mean that they will experience the problems commonly associated with their ‘physical age’. It is however important for the designers of dosage forms to be aware of potential age-related problems and how these affect the suitability of different formulations depending on the ‘behavioural age’ or ‘developmental age’ of the patient. Furthermore, pharmacists and pharmaceutical scientists should be aware of the different options available to them, and be able to provide suitable products and advice on the most appropriate dosage form, formulation or method of administration.
Paediatric and geriatric populations
The paediatric population is generally classified into five age groups (ICH E11):
• Preterm newborn infants – those who are born before 38 weeks of pregnancy
• Term newborn infants – those who are less than one month old
• Infants and toddlers – those who are aged between one month and 2 years of age
• Children – those who are aged 2 to 11 years
• Adolescents – those who are 12 to 16–18 years (dependent on regional definitions).
The geriatric population is arbitrarily defined as comprising patients aged 65 years or older (ICH E7). Nevertheless, patients in the older age range of 75 years and over may need further consideration during the design and administration of medicines.
Swallowing oral dosage forms
Tablets and capsules are the most commonly prescribed solid dosage forms because they are relatively cheap to produce, are portable, can be coated to mask unpleasant flavours or formulated to modify the drug release profile. Furthermore, their dry nature provides a stable environment for the drug, and hence gives the final product a relatively long shelf-life. Whilst such medicines are popular with prescribers they are not always the most suitable for patients with swallowing difficulties that can result from either a psychological aversion to swallowing tablets or a physical impairment to swallowing (dysphagia). Psychological aversion to swallowing tablets, which usually originates in childhood, is prevalent in all age groups, whereas dysphagia is much more common in older people.
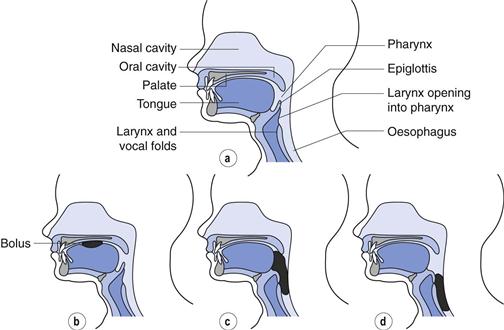
The swallowing process
The swallowing process comprises three phases: the oral, pharyngeal and oesophageal phases (Fig. 43.1). Within the oral phase (Fig. 43.1b) the bolus is manipulated by the tongue in preparation for swallowing, usually breaking larger objects into smaller ones and mixing with saliva to make the bolus both soft and less adhesive. During the pharyngeal phase (Fig. 43.1c) muscle control is sub-conscious and the bolus is moved into the pharynx where the passage to the lungs is automatically closed via movement of the epiglottis. Within the oesophageal phase (Fig. 43.1d) the bolus passes beyond the upper oesophageal sphincter and into the oesophagus. Although movement of the bolus is partially controlled by peristaltic waves within the oesophagus which occur automatically in response to swallowing, the movement of objects within the oesophagus is largely as a result of gravity. Consequently, tablets and capsules are most easily swallowed in an upright position (with the patient sitting or standing up) and this advice is commonly given to patients for tablets or capsules which are known to cause oesophageal irritation, e.g. doxycycline.
Paediatric populations
Swallowing problems are common in young children. Before 4 or 5 months of age, infants possess an extrusion reflex which enables them to swallow only liquids. Moreover, a gag reflex of varying degrees can last up until about seven to nine months of age. Hence eating requires active effort and the child must be able to coordinate sucking, swallowing and breathing. Infants are ready for spoon-feeding of semi-solids by 4 to 6 months of age. Although they are not capable at that age of swallowing a monolithic dosage form (e.g. a tablet), multiparticulates (powders, granules, pellets, minitablets <3 mm) might be given sprinkled into soft food (if compatible). At ages above about 6 years, children are considered able to swallow conventional tablets or capsules. Generally, the smaller the solid dosage form, the easier it is for children to swallow, but inter-individual differences in swallowing ability should be considered.
Geriatric populations
The loss of muscle control associated with the swallowing process is of concern for the administration of tablets and capsules to the elderly. As people get older, saliva production generally reduces and this can make it more difficult to swallow tablets or capsules, which require some lubrication before swallowing. This can however be easily overcome by taking the tablets or capsules with a glass of water; reduction in saliva should not in itself be a reason for patients not to be prescribed such solid dosage forms. Furthermore, tablets or capsules administered without water can be held in the pharynx or oesophagus and this can increase the likelihood of local erosion or irritation and therefore all patients, irrespective of age, should be told take tablets and capsules with water.
Loss of muscle control during the oral phase of swallowing can make it difficult to create a manageable bolus for swallowing, may cause the ‘loss’ of small tablets within the oral cavity or can make it more difficult psychologically to overcome the gag reflex that is necessary to swallow tablets or capsules. Loss of muscle control during the pharyngeal phase, which is controlled subconsciously, can increase the likelihood of choking, or at least the anticipation of choking, if it is not possible to propel the bolus through this phase. During the pharyngeal phase the airway is temporarily closed to allow the safe passage of food or liquids into the stomach, if solid dosage forms lodge in this area, the epiglottis will not open and the patient will asphyxiate.
Loss of control of the epiglottis during the pharyngeal phase can result in it not closing as the bolus passes and consequently the contents may inadvertently be aspirated into the lungs. The main defence mechanism within the lungs is the cough reflex, whereby contents are forcibly removed under pressure. A natural and background production of mucus which transports materials out of the lungs is another mechanism of removing foreign and waste products. However this has limited capacity and is restricted to small particles. Consequently, unintended aspiration of non-sterile foods or medicines can increase the likelihood of pulmonary infections, which are more likely to be fatal in an older person.
In addition to the ageing process, conditions commonly associated with ageing such as dementia, stroke, gastro-oesophageal reflex disease (GORD), Parkinson’s disease and cancer can all cause difficulties when orally administering tablets and capsules. The loss of muscle control associated with the progression of Parkinson’s disease and dementia results in dysphagia in the majority of patients with these diagnoses. It is estimated that almost 70% of people who suffer a stroke will have some form of dysphagia immediately post-stroke. However, in the majority of patients the normal swallow returns and less than 10% of people who experience a stroke are found to have permanent dysphagia. Dementia, stroke and Parkinson’s disease have greatest impact on the oral and pharyngeal phases of swallowing and therefore patients become concerned about choking and aspiration with these conditions. Head, neck and gastrointestinal cancers can all cause dysphagia by blocking access to the stomach, whilst radiotherapy and chemotherapy can affect the oral and gastrointestinal mucosa therefore making swallowing more difficult. Persistent GORD can cause inflammation within the oesophagus and this can result in patients describing foods and medicines ‘sticking in the back of their throats’ with a consequential struggle to swallow.
The nature of the dysphagia is therefore important when deciding the best approach for the patient. For patients who are struggling to manipulate oral doses, increasing the bulk of a medicine or the addition of water to the swallow may make it easier for the patient; whereas those who have dysphagia due to a blockage may require physically very small doses or low viscosity liquids. In those patients who are at risk of aspiration it may be inappropriate to administer tablets with water as the mixed consistencies may increase the likelihood of aspiration. Consequently, a single bolus of a thicker consistency may be more appropriate for such patients.
Assessment of swallowing ability
Speech and language therapists are usually the most appropriate professionals to assess the swallowing process. This may involve a brief bedside assessment via questioning and a test requiring the patient to drink a glass of watert. More invasive procedures include use of a naso-gastric camera (fibre optic endoscopic examination or FEES) or an X-ray of the swallow of a radio opaque liquid material (videofluoroscopy). The assessment should determine whether the oral route is appropriate and if so what the optimal texture of administered foods and medicines should be. This should provide some insight into the best pharmaceutical formulation for that patient.
If dysphagia is not identified in a patient and an inappropriate formulation is prescribed then this may affect the patient’s ability or willingness to self-administer perorally. Evidence suggests that older people frequently deny or ignore swallowing problems as this is indicative of ageing. An older person’s ability to swallow oral medicines should however always be ascertained by the prescriber to ensure that they are given the most appropriate formulation.
Helping patients with swallowing difficulties
Where patients with dysphagia are still able to use the oral route, the prescriber or pharmacist has three main options available to them:
• determine whether the medicine is still required or whether it can be safely discontinued
In reviewing the on-going need for the medicine, the pharmacist, together with the prescriber, should determine if the medicine is effective and if so, whether the benefits of the therapy outweigh any risks during administration. Additionally, if the dysphagia is likely to be acute, as may be seen immediately following a stroke, then medicines may be temporarily stopped until the swallow mechanism reappears.
Formulation of paediatric and geriatric medicines
Liquid peroral dosage forms
Formulating liquid, rather than solid medicines provides some particular problems for the pharmaceutical industry due to the need to keep the drug stable in the usually aqueous environment, to ensure that the final mixture is palatable and to ensure dosing consistency. Selection of a suitable liquid vehicle and environmental conditions requires significant investment, as does the selection of appropriate antimicrobials, stabilizers, suspending agents and flavourings (see Chapter 24). The combination of complex formulation science, relatively short shelf-lives (compared to tablets and capsules) and the relatively small market size, results in a lack of availability of licensed liquid medicines for many drugs. Moreover, liquid formulations often cost significantly more than their solid dosage form counterparts. Where licensed liquid medicines are available the texture may not always be appropriate and therefore this also requires consideration.
Paediatric considerations
In children, liquids are actually easy to administer and offer flexible yet accurate dosing with an appropriate dosing device. The most suitable of these are oral syringes, graduated in mL, which can cater for the heterogeneous paediatric population and for a single child as it grows. However, the taste or smell of the drug can sometimes be difficult to mask in a liquid formulation and this is a strong deterrent to patient compliance.
Selection of appropriate excipients
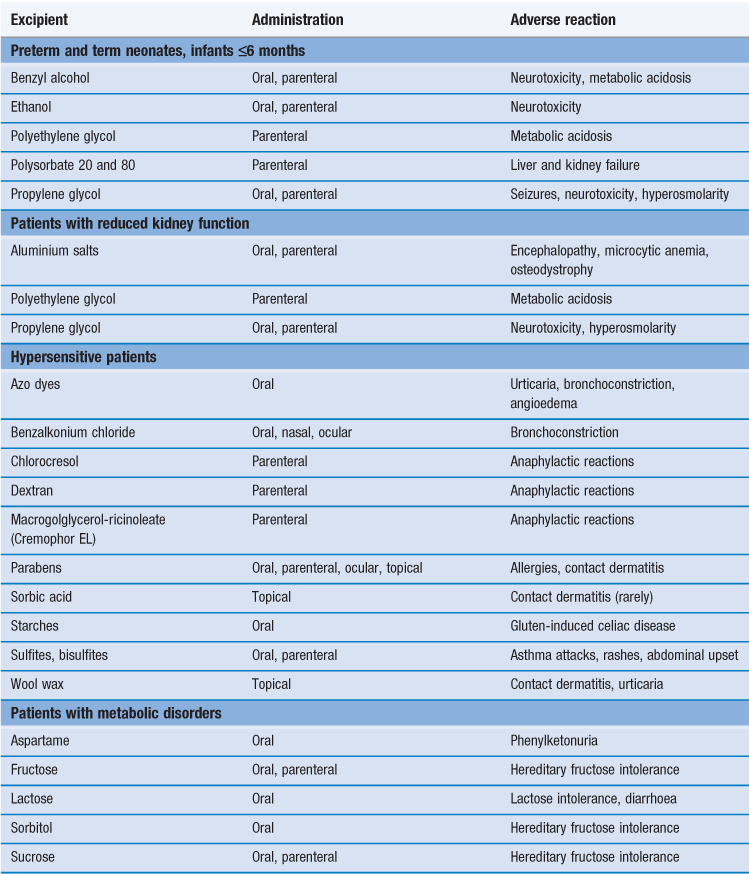
Formulation of liquids usually requires many excipients. These excipients can present a significant toxicological risk to a patient, depending on numerous factors including: the age of the child and their clinical condition, the route of administration, the exposure (dose and dosing frequency) and the safety profile of the excipient (allergies and sensitization, acute and cumulative toxicity) as illustrated in Table 43.1. The formulator of a liquid medicine needs to make rational choices of added excipients; deciding on their purpose and, if their use is inappropriate, an alternative must be sought. A striking example is the case of elixirs; these liquid formulations generally contain an appreciable percentage of ethanol and have historically been used (and some still are) in neonates and infants. The inclusion of ethanol in paediatric medicines should be avoided, especially for babies and vulnerable patients who are not able to metabolize it as efficiently as adults.
Sweeteners and flavouring agents.
These are used to mask the taste of liquids and tablets, such as chewable and dispersible tablets. A sweet flavour can be achieved by the use of sweeteners that may be categorized as follows:
1. nutritive
• sugars (e.g. sucrose, dextrose, fructose, lactose)
• natural intense sweeteners (e.g. glycyrrhizin, thaumatin, rebaudioside A).
Not all sweeteners have received regulatory authority approval in all countries and this, alongside consideration of the safety profile, is an important factor when selecting which to include in a formulation. By blending different sweeteners in combination with other ingredients, such as flavours and texture enhancers, the sensory characteristics of a drug product can be optimized. For example, a sugar-free product can be prepared that is comparable in properties and taste to a sugar-containing version. However, this is not a simple task and requires very specific sensory expertise.
Sugar-free sweeteners.
Due to the cariogenic (causing dental caries) nature of the sugars traditionally used as sweeteners, ‘sugar-free’ alternatives are now increasingly used in liquid medicines. Products that do not contain fructose, glucose or sucrose are considered to be ‘sugar-free’. In addition, preparations containing hydrogenated glucose syrup (lycasin), maltitol, sorbitol or xylitol may also be considered as ‘sugar-free’, as there is evidence that they do not cause dental caries (i.e. they are non-cariogenic). However, preparations containing hydrogenated glucose syrup or maltitol, although they are non-cariogenic, are not strictly ‘sugar-free’ as they are both metabolized to glucose.
Polyols can cause problems of digestive intolerance. These effects are dose-dependent, and particular care must be taken when these are used in medicines for children having a low body weight.
If it is necessary to give a sugar-containing product to a child at bedtime, it is recommended to subsequently rinse the child’s mouth.
Colouring agents.
The use of colouring agents in medicines, particularly those intended for use by children, is widely debated. Colouring agents (colourants) are largely incorporated into pharmaceutical products to improve their appearance and/or sometimes to match the colour of a medicine with its taste. Examples being the addition of a red colour with red-fruit flavours, yellow with lemon, purple with blackcurrant, etc. By default, paediatric medicines should normally not be coloured, except in some very specific and justifiable cases. Colouring agents permitted for use in foodstuffs are also allowed in medicines (providing they are approved by the relevant local regulatory bodies). However, azo-dyes are not considered acceptable.
Geriatric considerations
Liquid medicines are the logical alternative to tablets and capsules in patients with swallowing difficulties and are recommended where a swallow mechanism via the oral route is still available, and when a suitable texture which minimizes the likelihood of aspiration can be obtained.
Clearly many of the formulation issues pertaining to liquid paediatric medicines, described above, equally apply to medicines for use by elderly patients. Particular attention should be paid to the suitability of any excipients used in liquid or dispersible medicines.
For instance, sorbitol is commonly used as a sweetener in liquid formulations, and a dose exceeding 15 g/day in adults can result in bloating, flatulence and diarrhoea. This is the case for many polyols. In adults, their intake can lead to usually mild and temporary gastrointestinal symptoms. The cumulative amount administered should be checked when the patient is polymedicated. Medicines taken concomitantly, especially if they are liquids, should be checked for their polyol content. Gastrointestinal transit may be accelerated by these excipients and there is the potential for drug absorption to be decreased.
When using dispersible tablet formulations, the sodium content may be clinically important. For example, 4 g daily of paracetamol, administered as eight dispersible 500 mg tablets, provides up to 160 mmol of sodium.
Diabetic considerations
Diabetes UK advises that when medicines are taken in small quantities, for limited periods, the sugar content is unlikely to cause problems for patients with diabetes. This is because the sugar content of medicines is low in relation to the total carbohydrate content of a normal diet. Sugar-free alternatives should be recommended if the medicine is for long-term use. Care should be taken with preparations containing hydrogenated glucose syrup or maltitol because, as mentioned above, they are both metabolized to glucose. Additionally, they offer no real advantage to patients with diabetes as they contain significant amounts of calories and carbohydrates. Therefore, patients with diabetes should treat these products as if they contain sugar. Artificial sweeteners are virtually free of calories, and do not raise blood glucose levels.
Non-peroral dosage forms
Parenteral routes
Parenteral routes of administration are discussed in Chapter 36. As the oral route is not usable in seriously ill patients, including neonates, intravenous administration via peripheral, umbilical or ‘long’ peripheral lines is frequently used. The addition of medications needs to be taken into account (i.e. their volume and ion contribution) in the context of the patient’s complex fluid, electrolyte and nutrition management. Intramuscular administration is not recommended when muscle mass and perfusion is not optimal, for instance in neonates, as this may lead to erratic bioavailability and importantly to pain during injection and risk of tissue damage. Moreover, children tend to be needle phobic. The majority of the reconstitution of injectable drugs is carried out immediately before administration to the patient, and the risks and errors related to the preparation and administration of injectable drugs are numerous in paediatric settings. The formulation characteristics (pH, viscosity, osmolarity), use of inappropriate concentrations requiring complex calculation and serial dilutions or measurement of small volumes, the site of injection, and, if relevant, the needle thickness and needle length and the infusion rate all have to be scrutinized closely. The importance of being aware of the excipients to which the patient will be exposed cannot be over-emphasized (Table 43.1).
Older people tend to have more fragile veins, largely due to muscle wasting and loss of supportive tissues. Consequently, siting and maintaining injection lines can be harder than in a younger person. Furthermore, the risk of vein rupture and extravasation is higher in the elderly. Similarly, subcutaneous injections are more difficult to administer to this patient group, due to loss of cutaneous tissue and collagen. The subcutaneous space is harder to find when the skin is pinched due to the overall skin structure lacking thickness and to the fragility of the epidermis resulting from collagen loss.
Administering medicines via enteral feed tubes
In extreme cases, where it is deemed no longer safe to administer medicines orally to elderly patients, enteral tubes which bypass the oral route may be inserted. Enteral tubes, which are primarily designed for the administration of foods and liquids, can be sited either through the nasal cavity to the exit site (see below) or directly into the stomach or jejunum through the abdominal wall. They provide a significant resistance during the administration of medicines, due to both their length and internal diameter. Administration of inappropriate formulations of medicines via these tubes can cause tube blockage, which at the very least prevents the patient from accessing food and water until the tube is unblocked, and can in extreme cases cause rehospitalization of patients to have a new tube sited.
Advice for administration of medicines via this route is specialized and can be found in Smythe (2010) and White & Bradnam (2010), or by contacting an appropriate medicines information centre.
Enteral feeding tubes are commonly used to maintain or supplement nutritional and fluid intake in patients with significant dysphagia. Within acute care, a nasogastric tube is most frequently used, the internal diameter usually being 1.5 to 2.5 mm. Enteral feeding tube external diameters (D) are typically measured in ‘French units’ or ‘French gauge’. 1 French unit (Fr) = 0.33 mm, thus D (mm) = Fr/3 or Fr = D (mm) × 3. In general, the range of small-bore tube sizes for paediatric use is 6 Fr to 10 Fr. A size of 6 Fr is used for standard feeds and 7 Fr is used for higher density and fibre feeds. The tubes have a range of lengths, usually 550 mm, 750 mm or 850 mm. In intensive care neonatal and paediatric units, the tubes employed are likely to be smaller in length and diameter.
Within the community, permanent gastrostomy devices are most commonly used as these have minimal risk of displacement. Additionally these tubes are usually of slightly wider bore. Increasingly, jejunal tubes are being used in patients who also have delayed gastric emptying, gastroparesis or pancreatic disease.
Several factors need to be considered when choosing a medicine and formulation for administration via an enteral feeding tube; the dimensions of the tube, the exit site, the excipients in the formulation and any possible interaction of the formulation with components of the enteral feed.
For enteral tube administration, a solution is preferred. This will be administered via the tube, without resistance or blockage. Liquids with a high viscosity can be diluted immediately prior to administration to facilitate administration.
The exit site of the enteral feeding tube can potentially influence the bioavailability and tolerability of the medication. Medication delivered directly to the jejunum may be incompletely absorbed or result in a rapid peak in plasma concentration depending on the site of drug absorption. In addition, liquid formulations with a high osmolarity; for instance syrup-based formulations, can have an osmotic laxative effect if delivered undiluted into the jejunum.
There are many clinically important interactions between enteral feeds and medication. These are usually the result of the medication binding to the protein or electrolytes in the feed, or competition with amino acids for absorption. Significant interactions occur with phenytoin, theophylline, warfarin, L-dopa, quinolones, tetracyclines and rifampicin. Specific guidance should be given when supplying these medications to minimize the effect of the interaction. This is usually achieved through administering the medication during a break in the feeding regimen.
Respiratory routes
There are many aerosol generating medical devices. Apart from the formulation itself, the ability of the patient to use the device must be taken in account.
Pulmonary delivery.
Inhalation (Chapter 37) is the preferred route of administration when treating asthma – the most common long-term medical condition, affecting almost 10% of children in UK. It is important that the choice of the appropriate inhaler device and interface for patients is informed by the age of the patient, as presented in Table 43.2.
Table 43.2
Choosing an inhaler device and interface for patients of different ages
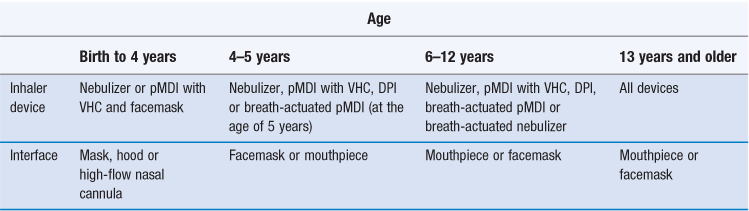
DPI: Dry powder inhaler; pMDI: Pressurized metered-dose inhaler; VHC: Valved holding chamber.
(Data from Ari and Fink, 2011.)
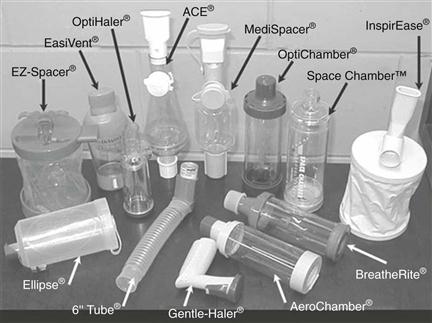
For children less than five years old, the preferred inhalation device would be a pressurized metered-dose inhaler (pMDI) with a mouth piece, spacer (see below) or with a facemask for younger patients. The same applies for nebulizers, with modern designs tending to be smaller, more portable and more appealing to children than in the past. Dry powder inhalers (DPIs) are only suitable if sufficient inspiratory flow is achievable by patients, which are typically school-age children. Spacers are large plastic containers, usually in two halves that click together with a mouthpiece or facemask at one end and a hole for inserting the mouthpiece of an inhalation device at the other end (Fig. 43. 2 and Fig. 37.4). They are used without the need to coordinate breathing and actuating a pMDI to ease administration, to decrease oropharyngeal impaction and improve deposition of the drug in the lung (see also Chapter 37).
The ageing process can result in reduced visual acuity, reduced ability to manipulate small objects and cognitive impairment. All three can affect how a person uses any medicine and/or delivery device and should therefore always be considered when selecting the most appropriate formulation for an older person. Muscle wasting in the older person results in a lack of ability to use accessory muscles (intercostals) effectively when using breath-actuated or non-passive inhalation devices. Similarly, an older person with poor manual dexterity may have difficulties in coordinating inhalation technique due to visual/auditory/cognitive impairment. Finally, standard measures of lung function naturally decline over time (FEV1, FVC, etc.) suggesting loss of elastic recoil, loss of pulmonary volume and loss of muscular control which would affect the ability to utilize an inhalation device or medication effectively. Devices exist (e.g. Haleraid®) which are placed over a conventional pMDI, to allow patients to actuate the device, when strength in the hands is impaired, for instance in arthritis.
Nasal delivery.
Nasal administration (Chapter 38) can also be difficult, but this needle-free approach may be employed as an alternative to parenteral administration in acute situations prior to intravenous catheter insertion, or in situations where intravenous access is not likely to be required. Recent applications are in analgesia, anxiolysis, and anticonvulsants. For example, diamorphine and fentanyl nasal formulations have been administered to treat acute pain in children as an alternative to parenteral administration. The taste of nasal formulations can be an issue as nasally administered preparations are partially swallowed. An appropriate device and formulation can improve nasal retention (see Chapter 38). The limited nasal capacity restricts the volumes that can be instilled, especially in children. Formulators need to ensure the biocompatibility and safety of any excipients, such as preservatives, used in these products.
Delivery to and through the skin
Delivery to and through the skin is discussed in Chapter 39. If babies are born at term their stratum corneum is fully functional, but during the first years after birth, the skin is more perfused and hydrated than in adults. Great care should be taken in preterm neonates whose skin barrier is not efficient, and which could permit the absorption of undesirable materials. Another consideration is the reduced body surface to body mass ratio (cm2/kg) and smaller volume of distribution for very young children, which explains why absorption can be especially enhanced in neonates and infants, even more so if there is unintentional occlusion, for instance by the use of nappies, etc.
The transdermal route of administration is potentially useful, as it is passive. However, drugs suitable for delivery by this route need particular characteristics (Chapter 39) and few products are currently available. Transdermal patches are easy to use, but adapting the dosage requirements for children over a range of body weight, is a possible limitation of their use. The nature of patch design (Chapter 39) means that patches intended for use in adults cannot simply be cut to modify the dose to administration to a child.
Self-administration of patches by older people can create problems due to difficulties in removing them from their packaging and removal of their backing strips, as well as difficulties in applying them to appropriate areas in rotation. Remembering to remove one patch before applying the next may be a problem in the cognitively impaired, and patients may have difficulties physically reaching patches which have been applied by a carer.
Suppositories
The acceptability of preparations such as suppositories to older persons must be taken into account. In fact, rectal administration (Chapter 42) has many inherent limitations but can be an option for paediatric patients, especially to avoid the oral route or when this route is not usable due to, for example, vomiting or unconsciousness. The rectal route has been used for treatment of epilepsy, constipation, analgesia, inflammatory bowel disease, malaria, fever and nausea. In younger children, lack of self-control of retention of the dosage form can be a drawback.
Oral preparations
Alternative presentations can be administered directly in or to the mouth. These include buccal formulations, (oro)dispersible, soluble or chewable tablets, sprinkle capsules or ‘stickpacks’. However, due to the relatively small size of the market, the range of such options is still limited.
Other routes
The ocular (Chapter 41) and otic/aural routes of administration are complicated by the difficulties in the handling of administration devices and often self-administration is not possible. Although usually not very well accepted, these routes are frequently unavoidable as products administered via these routes are for a local therapeutic effect. A balance between the manual dexterity of the person administering the dose and the cooperation of the patient needs to be struck and this is often problematic for the young and the old.
Alternative dosage forms and routes of administration which avoid the need to swallow are increasingly being made available.
Adaptation of existing dosage forms
If a specific paediatric or geriatric product is not available then the manipulation of existing products, usually tablets or capsules, prior to administration has to be considered. The process of tablet crushing or capsule opening and mixing with food or water is a form of unlicensed manufacture (i.e. the procedure has not received approval by the appropriate regulatory authority) and therefore can only be authorized by a prescriber, though in reality this does not always occur. While in some situations it may be safe to undertake this practice, there are many tablet and capsules formulations where it would generally be considered unwise to tamper with the product prior to administration.
Firstly, it is unsafe to recommend that cytotoxic or hormonal products (e.g. finasteride, tamoxifen, methotrexate) are tampered with prior to administration. Hormonal and cytotoxic products can be aerosolized and inhaled, or absorbed through the skin and therefore the administrator can be exposed to a dose of the drug, however small, which may be unsafe. Similarly, products which can cause contact skin sensitization, such as chlorpromazine, should not be crushed prior to administration. Consequently, when considering either recommending or authorizing the manipulation of an existing dosage form, the potential danger to the individual tampering with the product, and the individual administering it should always be considered.
Dosage form issues
Immediate-release film coated tablets
Immediate-release film coats are placed on medicines for various reasons (see Chapter 32). These include:
In some cases (when the coat is non-functional and is there, for instance, only for colour recognition) damaging the film coat immediately prior to administration is unlikely to adversely affect patient care. Removing a taste masking coating may decrease palatability, whilst film coats which protect against contact sensitization should not be disrupted.
Gastro-resistant (enteric) coated tablets
Gastro-resistant coats (Chapters 31 and 32) are placed on tablets either:
• to protect the stomach from the active ingredient within the medicine
• to protect the active ingredient from the hostile environment of the stomach
Furthermore, products such as sulfasalazine which are prescribed for Crohn’s disease are designed to be absorbed at the site of action, and therefore it is never appropriate to recommend tampering with gastro-resistant coated formulations.
Modified-release products
Modified-release products are carefully designed to release the drug over an extended period of time, in order to reduce the peak drug concentrations in the blood observed with immediate-release products, and reduce the required frequency of dosing (discussed further in Chapter 31). Reduction in peak plasma concentrations minimizes side effects, e.g. for nifedipine and theophylline, whereas reducing dosing frequency improves patient compliance.
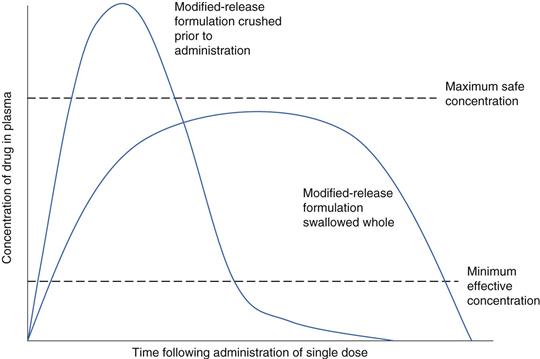
Where a particular formulation is intended to extend the duration of drug release, a larger dose of the drug is incorporated compared with an immediate-release product. Consequently, anything that damages the designed release mechanism (such as crushing tablets) could increase the likelihood of adverse events in the patient resulting from a larger than usual dose dump (Fig. 43.3). Where dose dumping of this type occurs, elimination of the drug is quicker and thus the patient will experience a time period between doses when drug levels are sub-therapeutic (Chapters 22 and 31). Consequently, tampering with any modified-release product is not recommended, unless the manufacturer clearly indicates that this is allowable. Modified-release morphine capsules (MXL®), for example, contain modified-release pellets which can be safely released from the outer shell of the capsule, providing they are not themselves tampered with or chewed, as this will affect their release properties.
Drugs that have a small therapeutic window can switch from being effective to causing adverse events just by small changes to their bioavailability. Crushing or dispersing digoxin tablets which have a bioavailability of just less than 70% could in theory increase this to 100% and effectively increase the dose received in the patient by almost 50%. Where formulations of drugs having a small therapeutic window are manipulated, the clinical effects should be monitored to ensure that toxicity does not ensue.
Cutting uncoated tablets
To facilitate administration, sometimes tablets are cut into smaller segments, for instance to achieve a smaller dose for a child. The limitations described above equally apply. Additionally, it should be checked that if a score line is present, it is appropriate and safe (i.e. clearly stated in the Summary of Product Characteristics for a particular product) for a part-tablet to be administered. If this is not the case, the manufacturer should be contacted to confirm content uniformity and stability of tablet segments.
Mixing medicines with food and beverages
Mixing of solid dosage forms with food and beverages can be undertaken either to facilitate administration to provide a pleasant vehicle, or to try to reduce the poor taste of a medication. Dispersing crushed tablets or capsule contents in water, beverages or soft food is common in clinical paediatric practice, even if there is often very limited information to support this. There is a need to be pragmatic, but the effect of food on bioavailability should be checked, if data are available. Immediate incompatibilities, such as mixing the medicine with acidic food or drinks (e.g. orange juice), dairy products or with warm/cold foodstuffs need to be assessed. Decisions are often made with little evidence-based data, but by applying common sense, and where possible with the help of the pharmaceutical manufacturer.
Unlicensed products
Where suitable licensed alternative formulations are not available, it may be necessary to prepare, by extemporaneous compounding or by outsourcing (‘specials’), unlicensed formulations to ensure that a patient obtains access to a prescribed drug. However, the quality of unlicensed products, patient acceptability, shelf-life and cost can vary considerably. It is therefore necessary for pharmacists to have some understanding of the quality of the available products, as frequently there may be a choice available to them.
Pharmacy compounding includes the preparation, mixing, assembling, packaging or labelling of a drug in response to a prescription written by an authorized prescriber. It remains one of the highest-risk activities carried out in pharmacy, as the risks of unlicensed medicines are combined with the inherent risks associated with the compounding of a formulation. The quality, safety and efficacy of compounded preparations are jeopardized by the lack of standardization of compounding practices, harmonization of formulations or information on stability.
‘Specials’ have a similar status but are usually made in larger volumes by licensed manufacturers and suitably licensed hospital units (the license is issued by the appropriate regulatory authority). However, these products are not always subjected to full quality assurance test procedures, especially if not produced as a batch.
The preparation of the same drug as an unlicensed liquid medicine could range from crushing a tablet in a suspending agent with a two-week shelf-life to a sourced pure drug placed in a carefully formulated base with an extended shelf-life. In all cases, however, bioequivalence of the liquid medicine compared to a tablet or capsule will not have been demonstrated, and therefore once a patient has been given and stabilized on a certain unlicensed product it is preferable to always source the same product for them.
The main measure of quality of an unlicensed product is via the certificate with which it is supplied. A certificate of analysis is supplied when the special has been produced in a batch and the supplier has quality assured the final product by analysing the ingredients to confirm (quantitatively) the content of the active ingredient and excipients in the final product. A certificate of conformity basically confirms that in making a one-off product, the production formula and process was followed. Any problems with sourced ingredients will not be identified within such a process, nor will mistakes in production. Consequently, a certificate of analysis is always preferable to a certificate of conformity.
There might be an appropriate product with a licence available in another country, which can be imported, yet remains unlicensed in the country of import. However, this option is not without logistic issues (product information provided in the local language, recall systems in place, time to obtain it, cost, etc.).
When sourcing and supplying unlicensed medicines, pharmacists need to aware of their increased legal liability. Where licensed medicines are used within the terms of their licence, liability for any subsequent patient harms reside with the manufacturer. If however a patient is harmed following receipt of an unlicensed medicine, the liability is shared between the prescriber and supplier, with the actions of both being carefully considered in any subsequent legal action.
Administering crushed tablets via enteral feed tubes
The administration of medicines via enteral tubes is usually an unlicensed use, as most medicines have not been tested for administration via this route. Consequently, the practice of crushing or dispersing tablets, or opening capsules prior to administration, does not significantly increase the legal liability associated with this activity. Due to the small internal diameter of these tubes, the risk of tube blockage from the administration of an inappropriate formulation is high. This is not just inappropriately crushed tablets, but also granular liquids, such as Ciproxin®, or dispersible tablets with a large particle size, e.g. Pentasa®. Interestingly, even if the taste of dispersed or crushed mixtures is theoretically of less concern, patients with enteral tubes do report tasting medicines administered via this route.
Such procedures should be considered as a last resort and all reasonable steps should be taken to obtain an appropriate formulation, or change to an alternative medication that is available in an appropriate formulation.
Future developments in the formulation of paediatric and geriatric medicines
Considerations for the patient-focused approach in formulation development
The current provision for specifically designed and tested paediatric and geriatric medicines is poor. However, moves are taking place to improve the present situation. The changes in the regulatory environment for paediatric medicines in the USA and in Europe will ultimately increase the availability of medicines authorized for children as well as increasing the information available for the use of medicinal products in the paediatric population.
Children, especially those in the younger age groups, require age-appropriate formulations that pharmaceutical manufacturers should develop. The gold standard is that these formulations and presentations should allow both safe and accurate dose administration to children and adults of all groups.
The European Medicines Agency (2005 and 2011) (reinforced by the World Health Organization, 2011) in support of the ‘Make Medicines Child Size’ initiative has reviewed the various considerations for the pharmaceutical development of paediatric medicines. They advise that special attention should be paid to answer the following questions:
These questions lead to the following considerations:
• the choice of excipients (qualitative and quantitative composition, Table 43.1) will depend on the availability of safety data for excipients relevant to the target age group(s). It is frequently the case that the available safety data are not as comprehensive as for adults.
On this basis, the most sensitive development aspects are likely to arise in paediatric medicines for long-term use in the most vulnerable patient groups (neonates, infants and young children). There is also an increased interest in elderly patients, particularly how their specific physiological and medical conditions are taken into account in the development and evaluation of new medicines, in order to fill in for the lack of clinical trials in the (very) old, and the potential health impact of the extrapolation of research findings to this population. However, the same level of scrutiny as is now occurring for the young has yet to be applied to rationalize formulation development for the elderly population.
Summary
Tablets and capsules, which are the most commonly prescribed dosage forms, may not be the most appropriate for individuals who have difficulties in swallowing, either due to their young age, the ageing process or due to conditions associated with ageing. The extent of swallowing function should always be ascertained, if necessary by a speech and language therapist, as this will enable the most appropriate formulation to be identified.
A licensed formulation should be identified and recommended if suitable and available. Unlicensed medicines are used, however their quality can vary considerably and a certificate of analysis should always be obtained in preference to a certificate of conformity.
Sometimes, although quite prevalent for children, manipulation of an existing pharmaceutical product may be the only option available to meet the needs of a particular patient. However, the appropriateness of this approach should always be carefully considered, as certain formulations such as gastro-resistant coated or extended-release products should generally not be modified prior to administration. The purpose of a film coat for a particular product should be ascertained. Patients receiving drugs with a narrow therapeutic window from formulations which have been manipulated should be monitored for toxicity. Generally, modified-released products should never be crushed or tampered with prior to administration.
Enteral tubes which have been traditionally used to bypass the swallow mechanism post-surgery and for patients with significant trauma are increasingly presenting in the community setting. Primarily designed for the administration of liquids and food, they create an additional barrier to the administration of medicines. Incorrectly administered medicines can cause tube blockage and rehospitalization, and consequently specialist reference sources should be used to provide advice in these circumstances.
Excipient exposure needs to be ascertained for the most vulnerable.
It is expected that with the latest international regulatory changes incentivizing the pharmaceutical industry to develop better medicines for children (Regulation (EC) No 1901/2006) and the growing focus on our increasingly ageing population, more appropriate dosage forms will be authorized in the near future, to help healthcare professionals, parents and carers support medicines use in children and the elderly.
References
1. Ari A, Fink JB. Guidelines for aerosol devices in infants, children and adults: which to choose, why and how to achieve effective aerosol therapy. Expert Review of Respiratory Medicine. 2011;5:561–572.
2. Asmus MJ, Liang J, Coowanitwong I, Hochhaus G. In vitro performance characteristics of valved holding chamber and spacer devices with a fluticasone metered-dose inhaler. Pharmacotherapy. 2004;24(2):159–166.
3. Breitkreutz J, Tuleu C. Pediatric and geriatric pharmaceutics and formulation. In: Florence AT, Siepmann J, eds. Modern Pharmaceutics. 5th edn New York: Informa Healthcare; 2009;221–257.
4. European Medicines Agency (2005) Reflection Paper on Formulations of Choice for the Paediatric Population (EMEA/196218/05).
5. European Medicines Agency (2011) Draft Guideline on Pharmaceutical Development of Medicines for Paediatric Use (EMA/CHMP/QWP/180157/2011.
6. ICH topic E7: Note for Guidance on Studies in Support of Special Populations: Geriatrics (CPMP/ICH/379/95).
7. ICH topic E11 Clinical Investigation of Medicinal Products in the Paediatric Population (CPMP/ICH/2711/99).
8. Smyth JA. The NEWT Guidelines for Administration of Medication to Patients with Enteral Feeding Tubes or Swallowing Difficulties. North East Wales NHS Trust 2010.
9. Handbook of Drug Administration via Enteral Feeding Tubes. In: White R, Bradnam V, eds. 2nd edn London: The Pharmaceutical Press; 2010.
10. World Health Organization (WHO) Development of Paediatric Medicines: Points to Consider in Pharmaceutical Development. Working document QAS/08.257/Rev.3 (August 2011).
Bibliography
1. European Medicines Agency (2007) Guideline on the Role of Pharmacokinetics in the Paediatric Population (CHMP/EWP/147013/04).
2. Tomlin S, Cockerill H, Costello I, et al. Making Medicines Safer for Children – Guidance for the use of Unlicensed Medicines in Paediatric Patients. Medendium Group Publishing Ltd, Berkhamsted 2009.
3. Tuleu C. Paediatric formulations in practice. ULLA Postgraduate Pharmacy series In: Florence AT, Moffat AC, eds. Paediatric Drug Handling. London: Pharmaceutical Press; 2007;43–74.
4. Tuleu C, Solomonidou D, Breitkreutz J. Paediatric formulations. In: Rose K, Van den Anker JN, eds. Guide to Paediatric Clinical Research. Basel: S. Karger AG; 2009;65–77.