Delirium, Sleep, and Mental Health Disturbances in Critical Illness
ACUTE BRAIN DYSFUNCTION OR DELIRIUM
SLEEP DISRUPTION IN THE CRITICALLY ILL PATIENT
Definition and Diagnostic Criteria
Prevalence of ICU-Related Posttraumatic Stress Disorder
Risk Factors for Posttraumatic Stress Disorder
Conceptual Explanations for Posttraumatic Stress Disorder After Critical Illness
Overview
Having acknowledged the preceding realities of the states of survival of critical illness, it is also true that advances in clinical medicine have resulted in dramatic improvements in survival in a host of medical conditions, such as acute respiratory distress syndrome (ARDS) and sepsis. In general, 1- to 3-month mortality rates for both of these common ICU admission diagnoses in most cohorts is now in the 20% to 35% range, whereas in the 1990s these mortality rates were in the 40% to 60% range. As a result of the increased confidence in survival, both the lay public and health care professionals are now increasingly focused on preservation of cognitive abilities, prevention of functional decline, and the quality of life among patients who survive critical illness.1–5 The potential of being left cognitively impaired is a major determinant of patients’ treatment preferences at the end of life, with 9 of every 10 patients preferring death to severe cognitive impairment.6 In support of this reality, in a report from the international “Surviving Intensive Care” 2002 Roundtable Conference held in Brussels,7 the need for future investigations in neurocognitive abnormalities among survivors of intensive care received the strongest recommendation from the international panel of experts. Physicians and health care providers in ICUs are accustomed to recognizing multiple organ dysfunction syndrome (MODS),8–11 with therapy focused on the causes and treatment of respiratory, cardiovascular, renal, and hepatic dysfunction. There are increasing data on the syndrome of brain dysfunction during and following the ICU. This chapter focuses on the cognitive and mental health disturbances of critical illness, with emphasis on delirium and sleep disturbances during critical illness, as well as a brief discussion of PTSD, long-term cognitive impairment (LTCI), and depression after critical illness.
Acute Brain Dysfunction or Delirium
Definition
Delirium is defined by American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 4th revised edition (DSM-IV),12 as a disturbance of consciousness with the cornerstone component of inattention13 being the pivotal feature of the diagnosis. This alarmingly common form of brain dysfunction often develops acutely (hours to days) in critically ill patients and fluctuates over time. Many different terms have been used to describe this spectrum of cognitive impairment in critically ill patients, including ICU psychosis, ICU syndrome, acute confusional state, septic encephalopathy, and acute brain failure.14–16 The current consensus of many authorities is to use the unifying term delirium and subcategorize according to the level of alertness (hyperactive, hypoactive, or mixed).17 We will take this approach in this chapter.
Prevalence and Subtypes
Although the prevalence of delirium in medical ICU cohort studies has been reported to be between 20% and 80%,18–20 more general ranges are practical depending on the severity of illness and the delirium detection instrument used, such as 40% to 60% in nonventilated and 60% to 80% in ventilated ICU patients.21 Unfortunately, because delirium is usually “quietly” manifested by negative symptoms, it remains unrecognized by the clinician in a majority of the patients experiencing this complication,22,23 and it may be incorrectly attributed to dementia, depression, or just an “expected” occurrence in the critically ill, elderly patient.22 Peterson and associates24 reported on delirium subtypes from a cohort of ventilated and nonventilated ICU patients in whom delirium was monitored. They found the rates of these subtypes in the ICU to be 1.6% for hyperactive, 43.5% for hypoactive, and 54.1% for mixed.25
Hyperactive delirium, which is rare in the pure form and associated with a better overall prognosis,26 is characterized by agitation, restlessness, attempting to remove catheters or tubes, hitting, biting, and emotional lability.17,27 This subtype was in the past referred to by the misnomer “ICU psychosis.” Hypoactive delirium, on the other hand, is very common and in many circumstances actually more deleterious for the patient in the long run.26 Unfortunately, it remains unrecognized in 66% to 84% of patients, whether being treated in the ICU, hospital ward, or emergency department.23,28–30 This delirium subtype is characterized by withdrawal, flat affect, apathy, lethargy, and decreased responsiveness.26,31,32 Some authorities continue to refer to the hypoactive delirium as “encephalopathy” and the hyperactive subtype as “delirium or ICU psychosis.” Because of the fluctuating nature of delirium, patients may present with a mixed clinical picture or may sequentially experience both of these subtypes. Many critical care providers would have the incorrect impression that hyperactive delirium is far more common, perhaps because affected patients attract attention because of their immediate threat to self and others. Unless routine monitoring for delirium is implemented as part of an overall ICU bundle such as the ABCDEs (spontaneous Awakening trials, spontaneous Breathing trials, Coordination of care and Choice of sedative, Delirium monitoring and management, and Early mobility),33,34 the majority of delirium episodes, especially the “quiet” (hypoactive) form, will be missed.
Prognostic Significance
In non-ICU populations, the development of delirium in the hospital is associated with an in-hospital mortality rate of 25% to 33%, prolonged hospital stay, and three times greater likelihood of discharge to a nursing home.35–37 In a three-site study of non-ICU medical patients, delirium was found to be an independent predictor of the combined outcome of death or nursing home placement.38 McCusker and colleagues39 found a 2.11 adjusted hazard ratio for dying in association with the development of delirium. This mortality rate increase has now been shown to be independent of dementia status.40 Furthermore, three prospective studies have found that delirium was associated with a higher risk for dementia during the 2 to 3 years after hospitalization in non-ICU patient populations.41–43
Among medical ICU patients, delirium has been shown to be a strong predictor of longer duration of mechanical ventilation, longer length of ICU stay, higher costs, prolonged neuropsychological dysfunction, and even death.44–47 The development of delirium was associated with a threefold increase in risk of death after data were controlled for preexisting comorbid conditions, severity of illness, coma, and the use of sedative and analgesic medications.44 Three separate cohort studies have shown that the duration of delirium (in a dose-dependent sort of relationship) is an independent predictor of increased subsequent death, with the most commonly cited finding being that every day a patient spends in ICU delirium portends a 10% higher risk of death even after adjusting for relevant covariates such as severity of illness, age, psychoactive medication use, and coma.44,48,49 In addition, it is now shown that the duration of delirium is an independent predictor of LTCI in general medical and surgical ICU patients who in the past were not suspected of having long-term dementia-like abnormalities.50
Pathophysiology
Neurotransmitters
From a neuroscience perspective, delirium is thought to be related to imbalances in the synthesis, release, and inactivation of neurotransmitters modulating the control of cognitive function, behavior, and mood.26,32 Three of the neurotransmitter systems involved in the pathophysiology of delirium are dopamine, γ-aminobutyric acid (GABA), and acetylcholine.15,51,52 Whereas dopamine increases excitability of neurons, GABA and acetylcholine decrease neuronal excitability.15 An imbalance in one or more of these neurotransmitters results in neuronal instability and unpredictable neurotransmission. In general, an excess of dopamine and depletion of acetylcholine are two major physiologic problems believed to be central to delirium.53 Other neurotransmitter systems thought to be involved in the development of delirium are serotonin imbalance, endorphin hyperfunction, and increased central noradrenergic activity.26,51
Inflammatory Mediators
Other factors thought to be mechanistically deliriogenic in ICU patients are inflammatory abnormalities induced by endotoxins and cytokines.54–57 The inflammatory mediators produced in sepsis, such as tumor necrosis factor-α (TNF-α), interleukin 1, and other cytokines and chemokines, initiate a cascade that leads to endothelial damage, thrombin formation, and microvascular compromise.58 Animal models show that these inflammatory mediators cross the blood-brain barrier,59 increase vascular permeability in the brain,60 and result in electroencephalography (EEG) changes consistent with those seen in septic patients with delirium.61,62 Release of inflammatory mediators may occur from (1) decreased cerebral blood flow, a result of the formation of microaggregates of fibrin, platelets, neutrophils, and erythrocytes in the cerebral microvasculature, (2) cerebral vasoconstriction occurring in response to (α1-adrenergic receptor activity63; or (3) interference with neurotransmitter synthesis and neurotransmission.64
Impaired Oxidative Metabolism
One hypothesis attempts to explain acute delirium as a behavioral manifestation of a “widespread reduction of cerebral oxidative metabolism resulting in an imbalance of neurotransmission.”65 On the basis of a series of investigations in which they evaluated delirious patients using EEG, Engel and Romano66 postulated that delirium is a state of “cerebral insufficiency,” that is, a global failure of cerebral oxidative metabolism. Their work showed that delirium is associated with diffuse slowing on EEG, a finding believed to represent a reduction in brain metabolism.
Cholinergic Deficiency
Blass and colleagues67 offered a possible link between the state of cerebral insufficiency proposed by Engel and Romano66 and the hypothesis of cholinergic blockade by suggesting that impaired oxidative metabolism in the brain results in a cholinergic deficiency. The finding that hypoxia impairs acetylcholine synthesis supports this hypothesis.68 This reduction in cholinergic function leads to an increase in the level of glutamate, dopamine, and norepinephrine in the brain. Additionally, serotonin and GABA levels are reduced; all of these changes contribute to delirium.
Large Neutral Amino Acid in Delirium
Changes in the plasma levels of various amino acid precursors of cerebral neurotransmitters may affect their function, thus contributing to the development of delirium.69 The amino acid entry into the brain is regulated by a sodium-independent large neutral amino acid transporter type 1 (LAT1).70 The essential amino acid tryptophan, which is the precursor for serotonin, competes with several large neutral amino acids (LNAAs), such as tyrosine, phenylalanine, valine, leucine, and isoleucine, for transport across the blood-brain barrier via the LAT1 transporter.70 This competition determines its uptake into the brain.70 Recently, alterations in plasma tryptophan and tyrosine levels were identified as independent risk factors for the transition to clinical delirium.71 Increased activation of the kynurenine pathway, which is involved in the metabolism of tryptophan and the formation of neurotoxins, has also been linked to fewer days alive and without acute brain dysfunction in critically ill patients.72 Another amino acid that may play an important role in the pathogenesis of delirium is phenylalanine.65 Like tryptophan, phenylalanine competes with the other LNAAs (tyrosine, tryptophan, valine, leucine, and isoleucine) for transport across the blood-brain barrier. An increase in the cerebral uptake of tyrosine and phenylalanine compared with the other LNAAs leads to greater availability of precursors for both dopamine and norepinephrine, two neurotransmitters that have been implicated in the pathogenesis of delirium.65
Inflammation
It is important to realize that although delirium may occur as a result of perturbations in other organ systems, the brain responds to systemic infections and injury with an inflammatory response of its own that also involves cytokine production, cell infiltration, and tissue damage.73,74 Reports indicate that local inflammation in the brain and subsequent activation of these central nervous system immune responses are accompanied by manifestations of systemic inflammation,52,75,76 including production of large amounts of peripherally produced TNF-α, interleukin 10, and interferon-γ.73,77–79 Thus, it is postulated that the brain can become an engine of inflammation, driving the development, resolution, or both, of MODS. Van Gool and associates propose that this self-propelling inflammation, in the context of microglial activation, could help explain the association between delirium and LTCI.80 Surprisingly, van Gool points out that animal models have shown activated microglia can remain primed for months following injury, allowing ample time for ongoing injury long beyond the period of an ICU stay. This factors into hypothesis generation when designing neuroimaging studies in ICU survivors and will serve as fascinating topics for future study.
Risk Factors for Delirium
Although non-ICU cohort studies have identified numerous risk factors for the development of delirium,37 only a few studies have examined these factors in the ICU population. Baseline risk factors that predispose patients to a greater degree of vulnerability for delirium include Alzheimer’s disease, chronic illness, advanced age, and depression.15,81,82 Dubois and coworkers83 found that preexisting hypertension and smoking (presumably because of relative hypoperfusion and nicotine withdrawal, respectively) were significantly associated with the development of ICU delirium. Another investigation reported that preexisting dementia was a significant risk factor for the development of delirium in the post-ICU period.19 A new area of investigation is genetic predisposition to delirium, as the apolipoprotein E4 polymorphism was found to predict delirium that lasted nearly twice as long as patients without the polymorphism.84 Further studies have continued to suggest this association.85
Precipitating and iatrogenic risk factors represent areas of potential modification and, thus, intervention for delirium prevention and treatment. Precipitating factors are hypoxia, metabolic disturbances, electrolyte imbalances, withdrawal syndromes, acute infection (systemic and intracranial), seizures, dehydration, hyperthermia, sleep deprivation, head trauma, vascular disorders, and intracranial space-occupying lesions.15,81,82
In practical terms, the risk factors for delirium can be divided into the following categories: (1) host factors, (2) the acute illness itself, and (3) iatrogenic or environmental factors (Box 72.1). Although delirium may be a function of patients’ specific underlying illness, it may also be due to medical management issues and thus may have preventable causes. Of these risk factors, sedative and analgesic medications and sleep deprivation appear to be the leading iatrogenic and hence possibly preventable risk factors for delirium. There are conflicting data on the association of anticholinergics, corticosteroids, histamine H2 antagonists, and anticonvulsants with the development of delirium in ICU patients.22,86–88
Sedatives and Analgesic Agents Contributing to Delirium
Sedative and analgesic medications are routinely administered to patients undergoing mechanical ventilation, in accordance with widely recognized clinical practice guidelines by the Society of Critical Care Medicine (SCCM),89 in order to reduce pain and anxiety. Investigations have shown that continuous intravenous sedation is associated with prolonged mechanical ventilation and greater morbidity.90 Similarly, associations between psychoactive medications and worsening cognitive outcomes have been reported in postoperative patients. Marcantonio and colleagues,91 studying postoperative patients in whom delirium developed, found an association between use of benzodiazepines and meperidine and the occurrence of delirium. Dubois and coworkers83 have shown that opiates (morphine and meperidine) administered either intravenously or via an epidural catheter may be associated with the development of delirium in medical/surgical ICU patients. Studies such as these have generated concern about whether such drugs were actually responsible for the development of delirium or were given as a result of delirium.
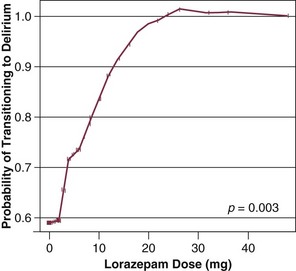
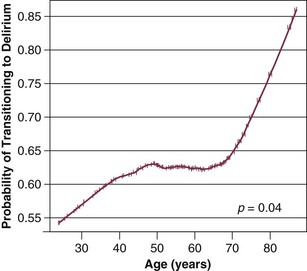
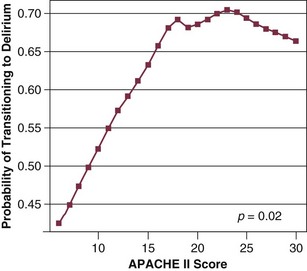
Our group has studied this temporal relationship between the administration of sedatives and analgesics and delirium.92 To do so, one must make repeated cognitive assessments and must be able to assess the risk factors a patient is exposed to in between these assessments in order to study which of these factors is associated with a transition or a change in cognitive status to or from normal, delirium, or coma. In our study, lorazepam was found to be an independent risk factor for daily transition to delirium, and fentanyl, morphine, and propofol were associated with higher but not statistically significant odds ratios for such transition (Fig. 72.1).92 Increasing age and APACHE II (Acute Physiology and Chronic Health Evaluation II) scores were also independent predictors of transitioning to delirium (Figs. 72.2 and 72.3).92 Similar associations between another benzodiazepine, midazolam, and transition to delirium have been found in another study conducted in our trauma and surgical ICU patients.21
At this time it is not clear whether this association between benzodiazepines, and possibly opioids, and delirium is related to the pharmacokinetic properties of the agents or the pharmacodynamics of the drug. Benzodiazepines and propofol have high affinity for the GABA receptor in the central nervous system.93 This GABA-mimetic effect can alter levels of numerous neurotransmitters believed to be deliriogenic.64,94 Novel sedative agents that are GABA receptor-sparing (such as the α2-agonists) may help reduce some of the cognitive dysfunction seen in ICU patients. It is important to note that the data for opioids and delirium are not as consistent as those for the benzodiazepines. Although meperidine has been associated with delirium in most of the published studies, evidence for both fentanyl and morphine has been less convincing.83,91,95 Morrison and colleagues95 conducted a prospective observational trial in patients who had undergone hip surgery and found that patients whose pain was well controlled with morphine were less likely to demonstrate delirium than those who received other opioids. These investigations point to the importance of the judicious use of psychoactive medications, with a focus on adequate analgesia.96 One hopes that ongoing randomized controlled trials will pave the way to the development of sedation and analgesic guidelines for the prevention or reduction of the occurrence of delirium due to the administration of these psychoactive drugs.
Diagnosis
The development of tools such as the Intensive Care Delirium Screening Checklist (ICDSC)18 and the Confusion Assessment Method for the ICU (CAM-ICU)20 have allowed for the rapid diagnosis of delirium in patients by nonpsychiatric physicians and other health care personnel even while such patients are mechanically ventilated. The SCCM has proposed guidelines for more routine and more diligent monitoring of delirium using reliable and validated scales.89
Diagnosis of delirium is a two-step process. Level of arousal is first measured with the use of a standardized sedation scale, like the Richmond Agitation-Sedation Scale (RASS) (Fig. 72.4).97,98 This is a 10-point scale with scores ranging from +4 to –5, score of 0 denoting a calm and alert patient. Positive RASS scores denote positive or aggressive symptomatology ranging from +1 (mild restlessness) to +4 (dangerous agitation). The negative RASS scores differentiate between response to verbal commands (scores –1 to –3) and physical stimulus (scores –4 and –5). If the patient’s RASS score is –4 or –5 or not arousable by verbal commands, no further evaluation for delirium is performed, because the patient is comatose and is unable to be assessed for delirium. For patients who are arousable (RASS scores of –3 and higher), delirium can be assessed with the ICDSC18 or by the CAM-ICU.20 The ICDSC assesses eight features of delirium: altered level of consciousness, inattention, disorientation, hallucinations, psychomotor agitation/retardation, inappropriate mood/speech, sleep/wake cycle disturbance, and symptom fluctuation. The sensitivity and specificity of this tool are 99% and 64%, respectively.18 The CAM-ICU, which can be performed in about 60 to 90 seconds,99 comprises four features that assess the following: acute change or fluctuation of mental status (feature 1), inattention (feature 2), disorganized thinking (feature 3), and an altered level of consciousness (feature 4).
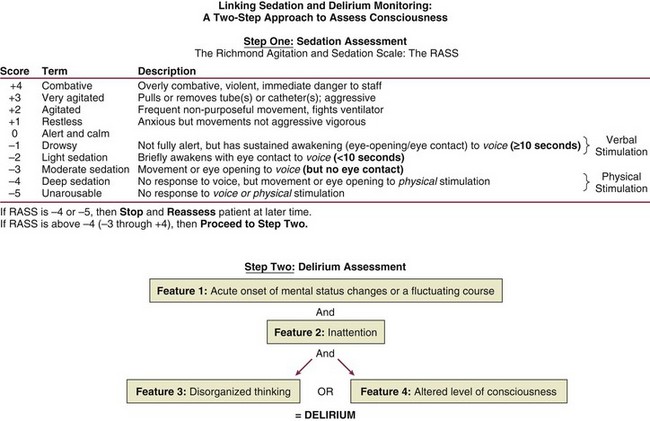
Figure 72.4 Richmond Agitation-Sedation Scale (RASS) and the Confusion Assessment Method for the ICU (CAM-ICU). This sedation scale and delirium instrument can be used together as a two-step approach to assess consciousness and diagnose delirium. Patients are considered to have delirium if they have RASS scores of –3 and higher and are assessed by CAM-ICU as “positive” by having both features 1 and 2 and either feature 3 or feature 4. (Data from references 19, 85, 86, and 148 [available online].)
To be diagnosed as delirious, a patient must have a RASS score of –3 or higher, with an acute change or fluctuation in mental status (feature 1), accompanied by inattention (feature 2) and either disorganized thinking (feature 3) or an altered level of consciousness (feature 4). A complete description of the CAM-ICU as well as training materials, including translations and clinical vignettes, can be found at our website (www.icudelirium.org).
Recently, a meta-analysis evaluated the accuracy of the CAM-ICU and the ICDSC for the diagnosis of ICU delirium.100 This investigation included nine studies evaluating the CAM-ICU (N = 969 total patients) and four studies evaluating the ICDSC (N = 361). The authors calculated the pooled sensitivity of the CAM-ICU to be 80% (95% confidence interval [CI]: 77.1-82.6%) and specificity of 95.9% (95% CI: 94.8-96.8%). The pooled sensitivity and specificity of the ICDSC were 74% (95% CI: 65.3-81.5%) and 81.9% (95% CI: 76.7-86.4%). They concluded that both delirium scales can be used as a screening tool, and that the CAM-ICU was an excellent diagnostic tool and the ICDSC had moderate sensitivity and good specificity.100
Prevention and Management
Primary Prevention and Nonpharmacologic Approaches
In a trial of 852 general medical patients older than 70 years,101 implementation of strategies for primary prevention of delirium resulted in a 40% reduction in the odds for development of delirium (15% in control subjects vs. 9.9% in the intervention patients). The protocol focused on optimization of risk factors via the following methods: repeated reorientation of the patient by trained volunteers and nurses, provision of cognitively stimulating activities for the patient three times per day, a nonpharmacologic sleep protocol to enhance normalization of sleep/wake cycles, early mobilization activities and range-of-motion exercises, timely removal of catheters and physical restraints, institution of the use of eyeglasses and magnifying lenses, use of hearing aids and earwax disimpaction, and early correction of dehydration.101 Unfortunately, this intervention did not show sustained benefit during the 6 months of follow-up.102 Later studies of delirium were unable to reproduce this success in reducing the incidence of delirium.103,104
Milisen and associates105 and Lundstrom and coworkers,106 who studied the implementation of multifactorial and multidisciplinary educational strategies, reported decreases in the duration and severity of delirium in patients cared for by staff who had received delirium-specific education.105,106 Milisen and associates105 measured the impact on delirium of implementing a nurse-led intervention program that involved delirium education for the nursing staff, systematic cognitive screening, availability of consultative services from a delirium resource nurse, and scheduled pain protocol on delirium. These researchers reported that although the program had no effect on the incidence of delirium, the duration and severity of delirium were significantly lower.105 Lundstrom and coworkers106 reported that in patients on a ward where the staff participated in specific educational activities focused on delirium and where the bedside nursing care was reorganized to provide more continuity of patient-centered care, duration of delirium and hospital stay were shorter and mortality rate was lower than in control patients. Cole and colleagues104 found no difference in delirium rates between patients cared for by an intervention nurse and patients who received standard care. All of these nonpharmacologic “protocolization of care” studies focused on non-ICU patient populations. Clearly such investigations must be designed and conducted in the ICU (rather than simply extrapolated from non-ICU studies).
Although primary prevention of delirium is preferred, some delirium is inevitable in the ICU. In patients exhibiting delirium, the basic tenets of patient management, such as restoration of sleep/wake cycles, timely removal of catheters, early mobilization, minimization of unnecessary noise/stimuli, and frequent reorientation, should be applied liberally. Additionally, it should be emphasized that although sedative and analgesic agents have a very important role in patient comfort, health care professionals must also strive to achieve the right balance of administering these drugs through greater focus on reducing unnecessary or overzealous use. Instituting daily interruption of sedatives and analgesics, protocolizing their delivery, and instituting target-based sedation have all been shown to improve patient outcomes, although the studies reporting these results have not specifically looked at delirium rate or duration.107–109 Studies have also shown a benefit for pain control using morphine in the prevention of delirium, because pain itself can be a risk factor for the development of delirium. Family involvement can also be very helpful in reorienting and soothing some delirious patients. It is important to teach family members about the fluctuating course of delirium as well as how they can detect it. Preventive and management strategies for delirium in the ICU represent an important area for future investigation.
Pharmacologic Therapy
Medications should be used for delirium only after adequate attention has been given to correction of modifiable contributing factors (e.g., sleep disturbance, restraints), as discussed previously. It is important to remember that delirium could be a manifestation of an acute, life-threatening problem that requires immediate attention (such as hypoxia, hypercarbia, hypoglycemia, metabolic derangement, or shock). After such concerns have been addressed, pharmacologic management should be considered. It should be recognized that although agents used to treat delirium are intended to improve cognition, they all have psychoactive effects that may further cloud the sensorium and promote a longer overall duration of cognitive impairment. Therefore, until we have outcomes data that confirm beneficial effects of treatment, these drugs should be used judiciously in the smallest possible dose and for the shortest time necessary, a practice infrequently adhered to in most ICUs. Indeed, some cases prove refractory to all “cocktail” approaches to sedation and delirium therapy, and in these cases, a trial of complete cessation of all psychoactive drugs should be considered. Some reports have described the utility of dexmedetomidine (an α2-agonist) as an adjunct to assist with weaning patients from all psychoactive medications.110 Preliminary results from a prospective, randomized, yet unblinded trial in postoperative patients who had undergone cardiac surgery showed that sedation with dexmedetomidine at sternal closure was associated with an 8% incidence of postoperative delirium compared with 50% for either propofol or benzodiazepines.111
Currently, no drugs are approved by the U.S. Food and Drug Administration (FDA) for the treatment of delirium. The SCCM guidelines89 recommend haloperidol as the drug of choice, with the acknowledgment that this recommendation is based on sparse outcome data from nonrandomized case series and anecdotal reports (i.e., level C data). Nevertheless, haloperidol is a “typical” butyrophenone antipsychotic, which is the most widely used neuroleptic agent for delirium.112 It does not suppress the respiratory drive and works as a dopamine receptor antagonist by blocking the δ2-opioid receptor, resulting in treatment of positive symptoms (hallucinations, unstructured thought patterns, etc.) and producing a variable sedative effect. There are data to suggest that haloperidol may have some anti-inflammatory properties,113,114 though this is theoretical in terms of a helpful pharmacologic effect on disease (such as septic delirium) at this point and requires further study.
In the non-ICU setting, the recommended starting dose of haloperidol is 0.5 to 1.0 mg orally or parenterally, with repeated doses every 20 to 30 minutes until the desired effect is achieved. In the ICU, a recommended starting dose would be 2 to 5 mg every 6 to 12 hours (intravenous, intramuscular, or oral), with maximal effective doses usually in the neighborhood of 20 mg/day. This dose range will usually be adequate to achieve the “theoretically optimal” 60% δ2-receptor blockage115 while avoiding complete δ2-receptor saturation associated with the adverse effects cited later. Because of the urgency of the situation in many ICU patients—due to the potential for inadvertent removal of central lines, endotracheal tubes, or even aortic balloon pumps—much higher doses of haloperidol or a sedative are often used. Unfortunately, there are few data from formal pharmacologic investigations to guide dosage recommendations in the ICU. Once calm, the patient can usually be managed with much lower maintenance doses of haloperidol.
Neither haloperidol nor similar agents (i.e., droperidol and chlorpromazine) have been extensively studied for ICU use.89 Newer “atypical” antipsychotic agents (e.g., risperidone, ziprasidone, quetiapine, and olanzapine) may also prove helpful for delirium.116 The rationale behind use of the atypical antipsychotics rather than haloperidol (especially in hypoactive/mixed subtypes of delirium) is theoretical and centers on the fact that they affect not only dopamine but also other potentially key neurotransmitters such as serotonin, acetylcholine, and norepinephrine.116–119 The use of haloperidol has been reported to have a mortality benefit in a retrospective analysis of critically ill, mechanically ventilated patients.114 Kalisvaart and associates120 showed that low-dose haloperidol prophylaxis reduced the duration and severity of delirium in elderly patients recovering from hip surgery, even though the actual prevalence of delirium was not reduced. A more recent study by Wang and coworkers121 also studied prophylactic haloperidol administration after cardiac surgery and actually did find a lower incidence of postoperative delirium associated with haloperidol, though this study was of a low severity of illness cohort and may not apply to truly critically ill patients with septic shock and ARDS. Skrobik and colleagues116 reported that olanzapine and haloperidol were equally efficacious in treating ICU delirium in both medical and surgical patients but that olanzapine had fewer side effects (again findings from a moderately ill group that must be confirmed and tested against placebo).
Adequately powered prospective, randomized placebo-controlled trials of antipsychotics are not available to date and must be performed to provide clinicians with evidence-based guidelines for preventing and treating delirium. Although there are prospective studies that begin to examine the safety and efficacy of antipsychotics such as amisulpride,122 quetiapine,122–124 and risperidone,125,126 these are generally small and quite heterogeneous in design population studied, dosing regimens, and the use of delirium assessment tools. A National Institutes of Health (NIH)-sponsored large prospective randomized placebo-controlled trial called MIND-USA is currently under way to investigate further the utility, risk, and benefit of both haloperidol and the atypical antipsychotic ziprasidone. The pilot study, called the MIND (Minimizing the Incidence of Delirium) trial,127 established feasibility in a population of mechanically ventilated medical and surgical ICU patients and laid the groundwork for future research.
Kato and coworkers128 reported a case study that suggests that genotyping may affect the choice of antipsychotic drugs. They showed that a patient with the CYP2D6 genotype had persistent delirium and demonstrated severe extrapyramidal symptoms when treated with risperidone. When quetiapine (metabolized by CYP3A4) was used instead, the patient’s delirium cleared within 2 days without side effects. This case report is not proof of a positive effect of antipsychotics; yet it is interesting and suggests that pharmacogenetics may play an important role in medication choices in the near future.
Adverse effects of typical and atypical antipsychotics include hypotension, acute dystonias, extrapyramidal effects, laryngeal spasm, malignant hyperthermia, glucose and lipid dysregulation, and anticholinergic effects such as dry mouth, constipation, and urinary retention. Perhaps the most immediately life-threatening adverse effect of antipsychotics is torsades de pointes, and these agents should not be given to patients with prolonged QT intervals unless absolutely necessary. Patents who receive substantial quantities of typical or atypical antipsychotics or coadministered arrhythmogenic drugs should be monitored closely with electrocardiography. In early 2005, the FDA issued an alert that atypical antipsychotic medications are associated with a mortality risk among elderly patients. This warning was supported by a meta-analysis of a large volume of data from outpatient treatment of patients with dementia who were experiencing psychotic symptoms that resulted in their receiving antipsychotic medications.129 Similar associations with higher stroke risk and fatality have been reported by other investigators.130,131 Subsequently, other studies have suggested that this higher risk of death in non-ICU elderly patients treated with antipsychotics may not be limited to the atypical antipsychotic agents; Schneider and colleagues130 found that the conventional antipsychotic haloperidol had an even higher mortality risk.
Along with haloperidol and the atypical antipsychotics for the treatment of acute brain dysfunction, a growing body of literature suggests a role for sedatives such as the α2-agonist dexmedetomidine in the pharmacologic management of delirium. The MENDS (Maximizing Efficacy of Targeted Sedation and Reducing Neurological Dysfunction) trial132 compared dexmedetomidine to lorazepam for targeted sedation greater than 24 hours in 106 mechanically ventilated patients. Sedation with dexmedetomidine resulted in more days alive without coma or delirium and lower prevalence of coma compared to sedation with lorazepam. Subgroup analysis also demonstrated that sedation with dexmedetomidine reduced the daily risk of delirium, particularly in patients with sepsis.133 The SEDCOM (Safety and Efficacy of Dexmedetomidine Compared with Midazolam) trial134 compared dexmedetomidine to midazolam, another commonly used benzodiazepine in the ICU. This large prospective trial showed significantly lower prevalence of delirium in patients receiving dexmedetomidine compared to midazolam, an effect observed within 1 day of initiating sedation and sustained throughout the week of study drug administration.134 In 2012, the MIDEX and PRODEX trials compared midazolam to dexmedetomidine (MIDEX) and propofol (preferred usual care) to dexmedetomidine (PRODEX) in mechanically ventilated patients requiring long-term sedation. These large trials, which together enrolled nearly 1000 patients, showed that dexmedetomidine was not inferior to midazolam or propofol for targeted light to moderate sedation and was found to be associated with improved communication between the patients and others involved in their care. Dexmedetomidine also reduced time to extubation and was associated with fewer neurocognitive disorders (including delirium) than propofol.135 In each trial, the reproducible significant adverse effect associated with dexmedetomidine was bradycardia, which typically resolved without event and infrequently required adjunctive treatment beyond discontinuation of the drug.135
Other agents such as the cholinesterase inhibitors have proved unsuccessful in the management of ICU delirium. Given the hypothesis that delirium was associated with excess dopamine and a central cholinergic deficiency, it was logical to test the cholinesterase inhibitors such as rivastigmine, used in treating dementia, for the management of ICU delirium. However, in 2010, a large placebo-controlled trial evaluating rivastigmine as an adjunct to usual care with haloperidol was halted early due to increased mortality risk and longer duration of delirium in patients receiving rivastigmine.136 Other agents such as ondansetron137 and melatonin138 are being considered for the treatment of ICU delirium but thus far have little support in the literature.
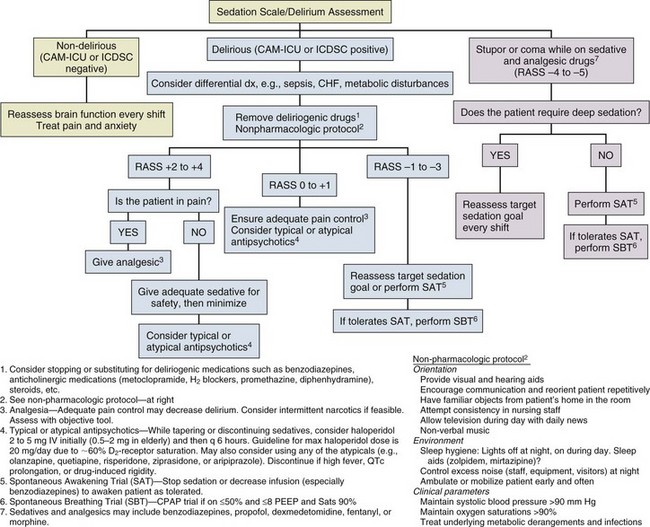
Protocols and evidence-based strategies for prevention and treatment of delirium will no doubt emerge as more evidence becomes available from ongoing randomized clinical trials of both nonpharmacologic and pharmacologic strategies. To assist readers in developing a delirium management algorithm in their respective clinical arenas, we have provided an empiric protocol largely based on the current SCCM clinical practice guidelines (Fig. 72.5). At this time, we have few data as to which antipsychotic medications are most suitable for delirium. The nonpharmacologic interventions recommended in this protocol have shown beneficial results in non-ICU patients, but the extrapolation to ICU populations is speculative. Nevertheless, such data emphasize the need for more research in this area and underscore the importance of exercising caution in the treatment of delirium. We wish to emphasize that protocols such as the ABCDEs of critical care33,34 must be updated regularly with new data and also individualized at each medical center to form an integrated approach to delirium monitoring, sedation targeting, and delirium management in critically ill ICU patients.
By way of summary regarding delirium management, the main thrust of our approach as health care professionals must begin with treatment of the underlying disease processes that are leading to brain dysfunction, then drug removal, and then modification of the environmental factors (hearing and vision aids, removal of restraints and early mobility, and sleep hygiene), which can be summarized via the mnemonic Dr. DRE (Diseases, Drug Removal, Environment). These are nicely summarized and operationalized in the evidence-based ABCDEs of ICU care, which stands for spontaneous Awakening trials, spontaneous Breathing trials, Coordination of care and Choice of sedative, Delirium monitoring and management, and Early mobility.33,34 Only after these issues are addressed is it appropriate to add another medication such as an antipsychotic into the mix of the individual pharmacopeia for any patient.
Sleep Disruption in the Critically Ill
The sleep cycle is divided into rapid eye movement (REM) sleep and non-REM (NREM) sleep.139 NREM sleep is further divided into four stages according to an increasing depth of sleep.111 A normal sleep cycle lasts approximately 90 minutes, cycling continuously between REM and NREM sleep. Stages 3 and 4 of the NREM sleep represent slow-wave or more restful sleep.139
Critically ill patients have severe sleep deprivation with disruption of sleep architecture. The average amount of sleep in the ICU has been measured to be about 2 hours out of 24 hours, with less than 6% of it spent in REM sleep. In a study by Cooper and associates,140 the majority of the patients had abnormal sleep patterns. The causes of sleep deprivation in the ICU have been extensively reported. They consist of excessive noise and lighting, patient care activities such as procedures and baths, metabolic consequences of critical illness, mechanical ventilation, and the sedative and analgesic medications that are administered to these patients.141 This disturbance in duration and quality of sleep has detrimental effects on protein synthesis, cellular and humoral immunity, and energy expenditure, resulting in respiratory and hemodynamic effects as well as cognitive function.141,142 Studies that have looked at sleep disturbances due to noise, patient activities, and light have found that only about 30% of the sleep arousals were a result of these environmental factors, suggesting that other patient factors or management issues play an important role.143 Of these, it is interesting that the psychoactive medications are common risk factors for both delirium and sleep disturbances, whereas sleep deprivation can itself lead to delirium.
Neurotransmission in Sleep
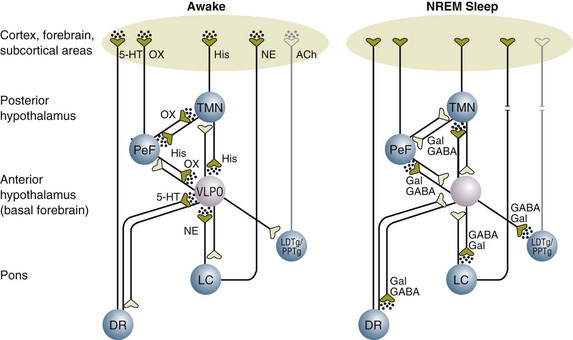
The ventrolateral preoptic (VLPO) nucleus in the anterior hypothalamus is the major area of the brain that controls sleep induction and maintenance.144 Its major neurotransmitter is GABA, and during the awake state, the GABA release from the VLPO nucleus is inhibited by norepinephrine (NE) from the locus coeruleus.144 With the inhibition of GABA, neurotransmitters such as orexin, serotonin, histamine, and acetylcholine are released, resulting in a state of wakefulness (Fig. 72.6). During NREM sleep, norepinephrine release diminishes, thus removing the inhibitory effect on release of GABA from the VLPO nucleus. The firing of GABA neurons inhibits the neurotransmitters of wakefulness (orexin, serotonin, histamine, and acetylcholine), resulting in NREM sleep (see Fig. 72.6). REM sleep, on the other hand, is facilitated by neurons in the pons that release acetylcholine. Studies show that serotonin and norepinephrine inhibit these neurons, suppressing REM sleep.
Sedative and analgesic medications are routinely administered to critically ill patients to promote sleep. However, although patients appear sedated, their sleep architecture is often adversely affected.139 Benzodiazepines and propofol prolong stage 2 NREM sleep while decreasing slow-wave sleep and REM sleep. Opioids, on the other hand, increase stage 1 NREM sleep while decreasing slow-wave and REM sleep. It is hypothesized that one of the reasons that dexmedetomidine allows for less time in acute brain dysfunction (delirium) is that its effect in the locus coeruleus (rather than the VLPO nucleus, which is the site of action of benzodiazepines and propofol) leads to a more normal pattern of sleep. This effect has not been convincingly shown beyond animal models to date. Numerous other medications routinely administered to critically ill patients affect sleep architecture. These include antiarrhythmic agents, inotropes and vasopressors, antibiotics, antidepressants, steroids, anticonvulsants, and bronchodilators.139 The effects of some of these compounds on sleep patterns are summarized in Table 72.1.
Table 72.1
Drugs Commonly Used in the Intensive Care Unit and Their Effects on Sleep Patterns
| Drug Class/Individual Drug | Sleep Disorder Induced/Reported | Possible Mechanism |
| Benzodiazepines | ↓ REM, ↓ SWS | γ-Aminobutyric acid type A (GABA[A]) receptor stimulation |
| Opioids | ↓ REM, ↓ SWS | µ-Opioid receptor stimulation |
| Clonidine | ↓ REM | α2-Adrenergic receptor stimulation |
| Nonsteroidal anti-inflammatory drugs | ↓ TST, ↓ SE | Prostaglandin synthesis inhibition |
| Norepinephrine/epinephrine | Insomnia, ↓ REM, ↓ SWS | α1-Adrenergic receptor stimulation |
| Dopamine | Insomnia, ↓ REM, ↓ SWS | δ2-Opioid receptor stimulation/α1-adrenergic receptor stimulation |
| β-Blockers | Insomnia, ↓ REM, nightmares | Central nervous system β-blockade by lipophilic agents |
| Amiodarone | Nightmares | Unknown mechanism |
| Corticosteroids | Insomnia, ↓ REM, ↓ SWS | Reduced melatonin secretion |
| Aminophylline | Insomnia, ↓ REM, ↓ SWS, ↓ TST, ↓ SE | Adenosine receptor antagonism |
| Quinolones | Insomnia | GABA(A) receptor inhibition |
| Tricyclic antidepressants | ↓ REM | Antimuscarinic activity and α1-adrenergic receptor stimulation |
| Selective serotonin reuptake inhibitors | ↓ REM, ↓ TST, ↓ SE | Increased serotonergic activity |
| Phenytoin | ↑ Sleep fragmentation | Inhibition of neuronal calcium influx |
| Phenobarbital | ↓ REM | Increased GABA(A) activity |
| Carbamazepine | ↓ REM | Adenosine receptor stimulation and/or serotonergic activity |
REM, rapid eye movement (sleep); SE, sleep efficiency; SWS, slow-wave sleep; TST, total sleep time.
Reproduced with permission from Bourne RS, Mills GH: Sleep disruption in critically ill patients—Pharmacological considerations. Anaesthesia 2004;59:374-384.
Although they lack anxiolytic properties, ω1-receptor agonists such as zolpidem may preserve REM as well as slow-wave sleep.145 Similarly, mirtazapine, a noradrenergic and specific serotonergic antidepressant, has been studied in healthy volunteers and has been shown to improve sleep efficiency, reducing the number and duration of awakenings.146 The slow-wave sleep time was also increased, whereas stage 1 sleep time was reduced significantly. This agent had no significant effect on REM sleep variables.147 An investigation of the use of dexmedetomidine (an α2-agonist) in rats shows that it mimics and increases NREM sleep144 but decreases REM sleep. By acting on the locus coeruleus, dexmedetomidine inhibits the release of norepinephrine, thus causing GABA output from the VLPO nucleus and inhibition of the neurotransmitters of wakefulness to produce a NREM sleep pattern. In contrast, benzodiazepines and propofol exert their sedative action on the VLPO nucleus to increase GABA and decrease the neurotransmitters such as orexin, histamine, and serotonin but without affecting norepinephrine release from the locus coeruleus. Further clinical trials are required to ascertain the role of these medications in improving the quality and quantity of sleep in critically ill patients and to see whether such improvement may better the cognitive outcomes in ICU patients.
Posttraumatic Stress Disorder
Definition and Diagnostic Criteria
The DSM-IV12 defines PTSD as a potentially debilitating psychiatric condition that develops as the result of exposure to a traumatic occurrence, in which the person experienced, witnessed, or was confronted with an event or events that involved actual or threatened death or serious injury, or a threat to the physical integrity of self or others and that generates intense feelings of fear, helplessness, or horror in the person exposed to the trauma. This condition is characterized by a constellation of symptoms in the following three domains:
• Symptoms of reexperiencing (e.g., intrusive thoughts and upsetting recollections of the trauma, recurrent dreams or nightmares, and flashbacks)
• Symptoms of avoidance and emotional numbing (e.g., efforts to avoid conversations, places, and thoughts associated with the trauma; detachment from others; and a restricted range of affect)
• Symptoms of increased arousal (e.g., sleep disruption, hypervigilance, and exaggerated startle response)
These symptoms must meet two criteria to be diagnosed as PTSD, as follows:
Prevalence of ICU-Related Posttraumatic Stress Disorder
The prevalence rates reported in studies on PTSD after critical illness vary widely (5% to >50%) and differ according to whether the specific outcome in question is a diagnosis of PTSD or merely the presence of PTSD symptoms. In the eight investigations that focused on the identification of subjects with PTSD (as opposed to those with PTSD symptoms), prevalence rates ranged from 9.7% to approximately 40%.147 The prevalence of PTSD differed depending on the time of assessment, with higher rates identified in closer proximity to the time of hospital discharge. In investigations that included serial evaluations, patients generally demonstrated a decrease in symptoms over time. For example, Kapfhammer and colleagues148 reported that 43.5% of study subjects had PTSD at discharge (although according to the DSM-IV, PTSD cannot be diagnosed until 4 weeks after exposure to the “traumatic stressor”), whereas 23.9% suffered from PTSD an average of 8 years later. Prevalence rates tend to be higher among some specific ICU populations, such as patients with sepsis or ARDS (25% to 40% at follow-up), in comparison with general medical ICU cohorts.148
Risk Factors for Posttraumatic Stress Disorder
A number of subject characteristics appear to raise the risk of PTSD symptoms or an actual diagnosis of PTSD, although consensus among the studies reviewed is limited. Reported risk factors include delusional memories of the ICU, a greater number of traumatic memories of the ICU, longer ICU length of stay or duration of mechanical ventilation or both, younger age, prior mental health history, female gender, and higher levels of sedation and neuromuscular blockade.149 No studies have investigated the association between delirium and PTSD.
Conceptual Explanations for Posttraumatic Stress Disorder After Critical Illness
Although the stresses associated with ICU hospitalization may differ from events typically described as “traumatic experiences,” there are a variety of conceptual explanations for the development of PTSD following critical illness. They include (1) profound feelings of helplessness associated with the experience of critical illness, (2) high rates of preexisting psychiatric morbidity that may characterize ICU patients,150 (3) cognitive impairment, including decreased working memory, which may limit individual abilities to suppress intrusive thoughts,150 and (4) multiple traumatic episodes associated with anxiety, panic, or pain that occur during ICU hospitalization, such as intubations, extubations, nightmares, and respiratory distress.151,152
The frequency of anxiety-provoking episodes during ICU hospitalization might partly explain the association between higher levels of sedation and increased PTSD symptoms, in that anxious patients may receive more sedatives. Brewin and Holmes153 suggest that immobilization with incomplete sedation promotes a dissociative experience that may be a PTSD risk factor.
A significant percentage of patients treated in the ICU have chronic medical illnesses,154,155 which may increase anxiety or susceptibility to anxiety reactions. The presence of such stressors may have a cumulative effect on an individual’s coping resources, leading to a greater vulnerability to PTSD. This concept is bolstered by evidence from numerous studies as well as the theoretical work of Turner and Lloyd,156 which supports the notion that healthy coping responses are impeded by the “accumulated burden of adversity” associated with illness or disease.157–159 Although the contribution of cumulative illness to the development of PTSD has not been assessed in ICU populations, investigations support an association between accumulated medical burden and higher risk of PTSD. For example, accident victims (without chronic medical illnesses) who are admitted to the trauma ICU have a lower prevalence of PTSD than their medical ICU counterparts (with multiple chronic diseases).160 Alternatively, patients with chronic cardiovascular disease who have undergone cardiac surgery have relatively high rates of PTSD, which correlate positively with preoperative disease severity.151
The relationship between cognitive function and PTSD is a subject of ongoing debate, particularly as it relates to the role of memory in mediating the development of PTSD.161 The importance of specific, explicit memories (memories pertaining to facts and events, which are accessible to consciousness)162,163 in the generation and maintenance of PTSD is difficult to estimate because they are the basis for nightmares, flashbacks, and intrusive thoughts and contribute to symptoms of avoidance and reexperiencing. Although a detailed treatment of these issues is beyond the scope of this chapter, we briefly discuss several key findings from the literature as they relate to ICU populations.
The preponderance of evidence suggests that the absence of episodic memory for a traumatic event is protective against the development of PTSD, because a majority of studies have shown that the risk of PTSD is markedly lower in individuals unable to recall a traumatic event than in those with explicit memory of it.164–168 The literature is not unanimous and is narrow in scope, with virtually all relevant studies having been conducted on victims of motor vehicle accidents or other traumas with concomitant traumatic brain injury.169 Theories of information processing suggest that traumatic memories can be encoded implicitly during periods of impaired consciousness and may provide the basis for the generation of PTSD symptoms even if patients are not consciously aware of the memories.170–173 Additionally, during periods of impaired consciousness, the encoding of emotional experiences such as panic and severe pain appears to be sufficient for the generation of PTSD symptoms.174
Many ICU patients report little if any conscious awareness of their critical illness, although as Jones and associates175 have reported, delusional memories, often with violent and paranoid themes, are pervasive among such patients. Distorted and fragmentary memories may be vestiges of the nightmares, hallucinations, or delusions induced by critical illness, a variety of medications, or other causes and may be particularly likely to occur during delirium, which is ubiquitous in critically ill, mechanically ventilated patients.16,176 Sedative medications may constitute one factor that mediates the development of delusional memories.177 Delusional memories may exist in the absence of factual memories, which provide markers of reality and may serve to orient the patient. For example, daily sedative interruption has been found to be associated with fewer symptoms of PTSD,178 suggesting that even limited factual memories from brief awakening may reduce PTSD. In addition, delusional memories tend to be stable over time and are significantly more persistent than factual memories.179 Delusional memories may be more refractory to the normal cognitive processes of habituation and reappraisal because they are not well integrated into the long-term memory. Although research is limited, the presence of delusional memories of the ICU is associated with higher levels of anxiety and PTSD.180,181
Long-Term Cognitive Impairment after Critical Illness
Medical and surgical management of critical illnesses can, and frequently does, result in de novo neurocognitive impairments. Approximately one third or more of ICU survivors have LTCI.182 It is difficult to make comparisons among studies owing to differences in definitions of neurocognitive sequelae, administered neuropsychological tests, time to follow-up, patient population, study design (prospective vs. retrospective), and inclusion of a control group. Nevertheless, current data suggest that neurocognitive impairments are extremely common in survivors of critical illness.
Currently, 10 cohort studies totaling approximately 455 patients have assessed LTCI after critical illness.182–192 The populations of the patient cohorts include patients with ARDS, acute lung injury, and respiratory failure in the ICU182,183,187,189–192 as well as two studies in general ICU patients.184,188 The time to neurocognitive assessments was variable, with the majority occurring during the first year after hospital discharge. Three studies assessed patients more than 1 year later. A prospective longitudinal study monitored patients at hospital discharge and then at 1 and 2 years after hospital discharge,193 and two retrospective studies assessed the patients at approximately 6 years after hospital discharge.187,191
The evidence from the 10 cohorts suggests that 25% to 78% of ICU survivors experience neurocognitive impairments.182–192 Among specific populations, such as patients with ARDS, the prevalence of LTCI is even greater; it may be as high as 78% at hospital discharge, 46% at 1 year,183 and 25% at 6 years.191 Hopkins and colleagues183 assessed ARDS patients’ premorbid estimated intelligence quotient (IQ) and found it was significantly higher than the patients’ measured IQ at hospital discharge. However, the patients’ measured IQ improved to their premorbid level by 1-year follow-up, with no additional improvement at 2 years. The finding that patients recovered over time with regard to intelligence does not necessarily suggest a comparable recovery in all cognitive domains, because data from traumatic and anoxic brain injury literature suggest that some neurocognitive abilities are more likely to improve than others.194
A 2004 study found that neurocognitive impairments occur in 70% of ARDS patients at hospital discharge, 45% at 1 year, and 47% at 2 years.193 The neurocognitive test scores of the ARDS survivors with neurocognitive sequelae (≈50% of survivors) fell below the sixth percentile of the normal distribution of cognitive function. These ARDS survivors had marked difficulty with tasks that require executive function, memory, attention, or quick mental processing. The neurocognitive impairments in critically patients are similar to those reported in medical ICU survivors182 after carbon monoxide poisoning195 and several years after elective coronary artery bypass graft surgery.196
In addition to ARDS survivors, neurocognitive impairments have been reported in the general population of critically ill patients. Jackson and colleagues,182 evaluating 34 medical ICU survivors at 6 months, found that 33% had LTCI (using a very conservative definition of impairment as two test scores 2 standard deviations below the mean or three test scores 1.5 standard deviations below the mean). The neurocognitive impairments were similar to those reported in ARDS survivors; they included mental processing speed, memory, language, and visuospatial abilities. Additional support for cognitive impairment in the general critically ill population comes from a prospective cohort of 32 critically ill patients who underwent long-term mechanical ventilation (>5 days). The patients were evaluated at hospital discharge and 6 months later.185 Of the patients who received long-term mechanical ventilation, 91% at hospital discharge and 41% at 6 months had impairments in attention, memory, mental processing speed, and executive function.185
Girard and colleagues showed that the duration of delirium was an independent predictor of LTCI at 1-year follow-up even after adjusting for relevant covariates.50 It is hoped that other ongoing investigations will provide information about the risk factors for the development of LTCI and help guide interventions for prevention of the rather debilitating sequelae of critical illness that affect a person’s quality of life.
Depression
Many ICU survivors experience significant affective symptoms, such as depression and anxiety.197 The prevalence and severity of affective disorders, including symptoms of depression and anxiety in ICU survivors, range from less than 10% to 58%.147,182,183,190,198 Depression has been reported to occur in up to 30% of ICU survivors,182 and it is estimated that 47% have clinically significant anxiety.197 Indeed, the high rates of depression among ICU survivors may be related to the cognitive impairment they experience, although this issue has not been evaluated in ICU cohorts. Affective disorders such as depression, PTSD, and anxiety may adversely affect test performance, especially if severe.199,200 Moderate to severe depression may result in decreased effort and low motivation, which may in turn lower neuropsychological test scores in cognitive domains such as psychomotor speed and attention.201,202 Moderate to severe anxiety, however, may lead to increased distractibility and blocked thoughts or words.203,204 In some cases, severe depression may mimic symptoms of cognitive impairment, although there are important differences between these conditions. In general, individuals with depression retain the ability to learn and do not forget as rapidly, do not display significant decrements in language, are inconsistent with regard to orientation to time and date, and are typically more self-aware than their cognitively impaired counterparts.205–207
A variety of instruments are available for use in the assessment of affective function. Tools such as the Geriatric Depression Scale-Short Form (GDS-SF),208 the Beck Depression Inventory (BDI),209 the Center for Epidemiologic Studies Depression Scale (CES-D),210 and the Hospital Anxiety-Depression Scale (HADS)211 assess depression. Anxiety can be assessed with the HADS211 or the Beck Anxiety Inventory (BAI).212 Discussion of these tools is beyond the scope of this chapter.
References
1. Comarow, A. You’re never too old: Surgery on patients of 80, 90, and up? It’s gaining acceptance. US News World Rep. 2004; 137:83–88.
2. National Research Council. The Aging Mind: Opportunities in Cognitive Research. Washington, DC: National Academy Press; 2000.
3. Mehta, KM, Yaffe, K, Covinsky, KE. Cognitive impairment, depressive symptoms, and functional decline in older people. J Am Geriatr Soc. 2002; 50:1045–1050.
4. Inouye, SK, Peduzzi, PN, Robison, JT, et al. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998; 279:1187–1193.
5. Inouye, SK, Bogardus, ST, Baker, DI, et al. The Hospital Elder Life Program: A model of care to prevent cognitive and functional decline in older hospitalized patients. J Am Geriatr Soc. 2000; 1697–1706.
6. Fried, TR, Bradley, EH, Towle, VR, Allore, H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002; 346:1061–1066.
7. Angus, DC, Carlet, J. Surviving intensive care: A report from the 2002 Brussels Roundtable. Intensive Care Med. 2003; 29:368–377.
8. Bone, RC, Grodzin, CJ, Balk, RA. Sepsis: A new hypothesis for pathogenesis of the disease process. Chest. 1997; 112:235–243.
9. Bone, RC. Sepsis, the sepsis syndrome, multiorgan failure: A plea for comparable definitions. Ann Intern Med. 1991; 114:332–333.
10. Bone, RC. Multiple system organ failure and the sepsis syndrome. Hosp Pract. 1991; 26:101–109.
11. Russell, JA, Singer, J, Bernard, G, et al. Changing pattern of organ dysfunction in early human sepsis is related to mortality. Crit Care Med. 2000; 28:3405–3411.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th rev. ed. Washington, DC: American Psychiatric Association; 2000.
13. Meagher, DJ, Leonard, M, Donnelly, S, et al. A longitudinal study of motor subtypes in delirium: Relationship with other phenomenology, etiology, medication exposure and prognosis. J Psychosom Res. 2011; 71(6):395–403.
14. Granberg, A, Engberg, B, Lundberg, D. Intensive care syndrome: A literature review. Intensive Crit Care Nurse. 1996; 12:173–182.
15. Webb, JM, Carlton, EF, Geeham, DM. Delirium in the intensive care unit: Are we helping the patient? Crit Care Nurs Q. 2000; 22:47–60.
16. Ely, EW, Siegel, MD, Inouye, SK. Delirium in the intensive care unit: An underrecognized syndrome of organ dysfunction. Semin Respir Crit Care Med. 2001; 22:115–126.
17. Milisen, K, Foreman, MD, Godderis, J, et al. Delirium in the hospitalized elderly: Nursing assessment and management. Nurs Clin North Am. 1998; 33:417–436.
18. Bergeron, N, Dubois, MJ, Dumont, M, et al. Intensive care delirium screening checklist: Evaluation of a new screening tool. Intensive Care Med. 2001; 27:859–864.
19. McNicoll, L, Pisani, MA, Zhang, Y, et al. Delirium in the intensive care unit: Occurrence and clinical course in older patients. J Am Geriatr Soc. 2003; 51:591–598.
20. Ely, EW, Inouye, SK, Bernard, GR, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001; 286:2703–2710.
21. Pandharipande, P, Cotton, B, Costabile, S, Ely, EW. Prevalence of delirium in surgical ICU patients. Crit Care. 2005; 33:167–S.
22. Francis, J, Martin, D, Kapoor, WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990; 263:1097–1101.
23. Inouye, SK. The dilemma of delirium: Clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. 1994; 97:278–288.
24. Peterson, JF, Truman, BL, Shintani, A, et al. The prevalence of hypoactive, hyperactive, and mixed type delirium in medical ICU patients. J Am Geriatr Soc. 2003; 51:S174.
25. Peterson, JF, Pun, BT, Dittus, RS, et al. Delirium and its motoric subtypes: A study of 614 critically ill patients. J Am Geriatr Soc. 2006; 54:479–484.
26. Meagher, DJ, Trzepacz, PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000; 5:75–85.
27. O’Keeffe, ST, Lavan, JN. Clinical significance of delirium subtypes in older people. Age Ageing. 1999; 28:115–119.
28. Marcantonio, ER, Goldman, L, Mangione, CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994; 271:134–139.
29. Sanders, AB. Missed delirium in older emergency department patients: A quality-of-care problem. Ann Emerg Med. 2002; 39:338–341.
30. Hustey, FM, Meldon, SW. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med. 2002; 39:248–253.
31. Meagher, DJ, Hanlon, DO, Mahony, EO, et al. Relationship between symptoms and motoric subtype of delirium. J Neuropsychiatry Clin Neurosci. 2000; 12:51–56.
32. Justic, M. Does “ICU psychosis” really exist? Crit Care Nurse. 2000; 20:28–37.
33. Vasilevskis, EE, Ely, EW, Speroff, T, et al. Reducing iatrogenic risks: ICU-acquired delirium and weakness—Crossing the quality chasm. Chest. 2010; 138(5):1224–1233.
34. Vasilevskis, EE, Pandharipande, PP, Girard, TD, et al. A screening, prevention, and restoration model for saving the injured brain in intensive care unit survivors. Crit Care Med. 2010; 38(10 Suppl):S683–S691.
35. Inouye, SK, Schlesinger, MJ, Lydon, TJ. Delirium: A symptom of how hospital care is failing older persons and a window to improve quality of hospital care. Am J Med. 1999; 106:565–573.
36. Levkoff, SE, Evans, DA, Liptzin, B, et al. Delirium: The occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med. 1992; 152:334–340.
37. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999; 156:1–20.
38. Inouye, SK, Rushing, JT, Foreman, MD, et al. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998; 13:234–242.
39. McCusker, J, Cole, M, Abrahamowicz, M, et al. Delirium predicts 12 month mortality. Arch Intern Med. 2002; 162:457–463.
40. Fick, DM, Agostini, JV, Inouye, SK. Delirium superimposed on dementia: A systematic review. J Am Geriatr Soc. 2002; 50:1723–1732.
41. Rockwood, K, Cosway, S, Carver, D. The risk of dementia and death after delirium. Age Ageing. 1999; 28:551–556.
42. Rahkonen, T, Luukkainen-Markkula, R, Paanilla, S, Sulkava, R. Delirium episode as a sign of undetected dementia among community dwelling subjects: A 2 year follow up study. J Neurol Neurosurg Psychiatry. 2000; 69:519–521.
43. McCusker, J, Cole, M, Dendukuri, N, et al. Delirium in older medical inpatients and subsequent cognitive and functional status: A prospective study. Can Med Assoc J. 2001; 165:575–583.
44. Ely, EW, Shintani, A, Truman, B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004; 291:1753–1762.
45. Lin, SM, Liu, CY, Wang, CH, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004; 32:2254–2259.
46. Milbrandt, EB, Deppen, S, Harrison, PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004; 32:955–962.
47. Ely, EW, Gautam, S, Margolin, R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001; 27:1892–1900.
48. Pisani, MA, Kong, SY, Kasl, SV, et al. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009; 180:1092–1097.
49. Shehabi, Y, Riker, RR, Bokesch, PM, et al. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care unit patients. Crit Care Med. 2010; 38:2311–2318.
50. Girard, TD, Jackson, JC, Pandharipande, PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010; 38:1513–1520.
51. Crippen, D. Treatment of agitation and its comorbidities in the intensive care unit. In: Hill NS, Levy MM, eds. Ventilator Management Strategies for Critical Care. New York: Marcel Dekker; 2001:243–284.
52. Pavlov, VA, Wang, H, Czura, CJ, et al. The cholinergic anti-inflammatory pathway: A missing link in neuroimmunomodulation. Mol Med. 2003; 9:125–134.
53. Hshieh, TT, Fong, TG, Marcantonio, ER, Inouye, SK. Cholinergic deficiency hypothesis in delirium: A synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008; 63:764–772.
54. Fulkerson, W, MacIntyre, N, Stamler, J, Crapo, J. Pathogenesis and treatment of the adult respiratory distress syndrome. Arch Intern Med. 1996; 156:29–38.
55. Bellingan, G. The pulmonary physician in critical care—6: The pathogenesis of ALI/ARDS. Thorax. 2002; 57:540–546.
56. Arvin, B, Neville, LF, Barone, FC, Feurstein, GZ. Brain injury and inflammation: A putative role for TNF-alpha. Ann N Y Acad Sci. 1995; 116:62–71.
57. Fink, MP, Evans, TW. Mechanisms of organ dysfunction in critical illness: Report from a round table conference held in Brussels. Intensive Care Med. 2002; 28:369–375.
58. Wheeler, AP, Bernard, GR. Treating patients with severe sepsis. N Engl J Med. 1999; 340:207–214.
59. Papadopoulos, MC, Lamb, FJ, Moss, RF, et al. Faecal peritonitis causes oedema and neuronal injury in pig cerebral cortex. Clin Sci (Lond). 1999; 96:461–466.
60. Huynh, HK, Dorovini-Zis, K. Effects of interferon-gamma on primary cultures of human brain microvessel endothelial cells. Am J Pathol. 1993; 142:1265–1278.
61. Krueger, JM, Walter, J, Dinarello, CA, et al. Sleep-promoting effects of endogenous pyrogen (interleukin-1). Am J Physiol. 1984; 246:R994–R999.
62. Papadopoulos, MC, Davies, DC, Moss, RF, et al. Pathophysiology of septic encephalopathy: A review. Crit Care Med. 2000; 28:3019–3024.
63. Breslow, MJ, Miller, CF, Parker, SD, et al. Effect of vasopressors on organ blood flow during endotoxin shock in pigs. Am J Physiol. 1987; 252:H291–H300.
64. Van Der Mast, RC. Pathophysiology of delirium. J Geriatr Psychiatry Neurol. 1998; 11:138–145.
65. Lipowski, ZJ. Delirium: Acute Confusional States, rev ed. New York: Oxford University Press; 1990.
66. Engel, GL, Romano, J. Delirium, a syndrome of cerebral insufficiency. J Chronic Dis. 1959; 9:260–277.
67. Blass, JP, Gibson, GE, Duffy, TE, Plum, F. Cholinergic dysfunction: A common denominator in metabolic encephalopathies. In: Pepeu G, Ledinsky H, eds. Cholinergic Mechanisms: Phylogenetic Aspects, Central and Peripheral Synapses, and Clinical Significance. New York: Plenum Press; 1981:921–928.
68. Gibson, GE, Peterson, C, Sansone, J. Decreases in amino acids and acetylcholine metabolism during hypoxia. J Neurochem. 1981; 37:192–201.
69. Van Der Mast, RC, Fekkes, D, Moleman, P, Pepplinkhuizen, L. Is postoperative delirium related to reduced plasma tryptophan? Lancet. 2003; 338:851–852.
70. Wurtman, RJ, Hefti, F, Melamed, E. Precursor control of neurotransmitter synthesis. Pharmacol Rev. 1980; 32:315–335.
71. Pandharipande, PP, Morandi, A, Adams, JR, et al. Plasma tryptophan and tyrosine levels are independent risk factors for delirium in critically ill patients. Intensive Care Med. 2009; 35:1886–1892.
72. Adams Wilson, JR, Morandi, A, Girard, TD, et al. The association of the kynurenine pathway of tryptophan metabolism with acute brain dysfunction during critical illness. Crit Care Med. 2012; 40(3):835–841.
73. Perry, VH, Andersson, B, Gordon, S. Macrophages and inflammation in the central nervous system. Trends Neurosci. 1993; 16:268–273.
74. Rothwell, NJ, Luheshi, G, Toulmond, S. Cytokines and their receptors in the central nervous system: Physiology, pharmacology and pathology. Pharmacol Ther. 1996; 69:85–95.
75. Czura, CJ, Friedman, SG, Tracey, KJ. Neural inhibition of inflammation: The cholinergic anti-inflammatory pathway. J Endotoxin Res. 2003; 9:409–413.
76. Munford, RS, Tracey, KJ. Is severe sepsis a neuroendocrine disease? Mol Med. 2002; 8:437–442.
77. Woiciechowsky, C, Asudullah, K, Nestler, D, et al. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced brain injury. Nat Med. 1998; 4:808–813.
78. Woiciechowsky, C, Schoening, B, Daberkow, N, et al. Brain IL-1 beta induces local inflammation but systemic anti-inflammatory response through stimulation of both hypothalamic-pituitary-adrenal axis and sympathetic nervous system. Brain Res. 1999; 816:563–571.
79. Nicholson, TE, Renton, KW. The role of cytokines in the depression of CYP1A activity using cultured astrocytes as an in vitro model of inflammation in the CNS. Drug Metab Dispos. 2002; 30:42–46.
80. van Gool, WA, van de Beek, D, Eikelenboom, P. Systemic infection and delirium: When cytokines and acetylcholine collide. Lancet. 2010; 375(9716):773–775.
81. Francis, J. Drug-induced delirium: Diagnosis and treatment. CNS Drugs. 1996; 5:103–114.
82. Inouye, SK, Charpentier, PA. Precipitating factors for delirium in hospitalized elderly persons: Predictive model and interrelationship with baseline vulnerability. JAMA. 1996; 275:852–857.
83. Dubois, MJ, Bergeron, N, Dumont, M, et al. Delirium in an intensive care unit: A study of risk factors. Intensive Care Med. 2001; 27:1297–1304.
84. Ely, EW, Girard, TD, Shintani, AK, et al. Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Crit Care Med. 2007; 35:112–117.
85. van Munster, BC, Korevaar, JC, Zwinderman, AH, et al. The association between delirium and the apolipoprotein E epsilon 4 allele: New study results and a meta-analysis. Am J Geriatr Psychiatry. 2009; 17:856–862.
86. Lipowski, ZJ. Delirium in the elderly patient. N Engl J Med. 1989; 320:578–582.
87. Inouye, SK, Viscoli, CM, Horwitz, RI, et al. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. 1993; 119:474–481.
88. Francis, J, Kapoor, WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990; 5:65–79.
89. Jacobi, J, Fraser, GL, Coursin, DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002; 30:119–141.
90. Kollef, MH, Levy, NT, Ahrens, TS, et al. The use of continuous i. v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998; 114:541–548.
91. Marcantonio, ER, Juarez, G, Goldman, L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994; 272:1518–1522.
92. Pandharipande, P, Shintani, A, Truman, P, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006; 104:21–26.
93. Mihic, S, Harris, R. GABA and the GABAA receptor. Alcohol Health Res World. 1997; 21:127–131.
94. Van Der Mast, RC. Delirium: The underlying pathophysiological mechanisms and the need for clinical research. J Psychosom Res. 1996; 41:109–113.
95. Morrison, RS, Magaziner, J, Gilbert, M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003; 58:76–81.
96. Pandharipande, P, Ely, EW. Narcotic-based sedation regimens for critically ill mechanically ventilated patients. Crit Care. 2005; 9:247–248.
97. Sessler, CN, Gosnell, M, Grap, MJ, et al. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care patients. Am J Respir Crit Care Med. 2002; 166:1338–1344.
98. Ely, EW, Truman, B, Shintani, A, et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003; 289:2983–2991.
99. Pun, BT, Gordon, SM, Peterson, JF, et al. Large-scale implementation of sedation and delirium monitoring in the intensive care unit: A report from two medical centers. Crit Care Med. 2005; 33:1199–1205.
100. Gusmao-Flores, D, Salluh, JI, Chalhub, RA, Quarantini, LC. The Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) and Intensive Care Delirium Screening Checklist (ICDSC) for the diagnosis of delirium: A systematic review and meta-analysis of clinical studies. Crit Care. 2012; 16(4):R115.
101. Inouye, SK, Bogardus, ST, Jr., Charpentier, PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999; 340:669–676.
102. Bogardus, ST, Desai, MM, Williams, CS, et al. The effects of a targeted multicomponent delirium intervention of postdischarge outcomes for hospitalized older adults. Am J Med. 2003; 114:383–390.
103. Marcantonio, ER, Flacker, JM, Wright, RJ, Resnick, NM. Reducing delirium after hip fracture: A randomized trial. J Am Geriatr Soc. 2001; 49:516–522.
104. Cole, MG, McCusker, J, Bellavance, F, et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: A randomized trial. Can Med Assoc J. 2002; 167:753–759.
105. Milisen, K, Foreman, MD, Abraham, IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001; 49:523–532.
106. Lundstrom, M, Edlund, A, Karlsson, S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005; 53:622–628.
107. Kollef, MH, Levy, NT, Ahrens, T, et al. The use of continuous IV sedation is associated with prolongation of mechanical ventilation. Chest. 1999; 114:541–548.
108. Brook, AD, Ahrens, TS, Schaiff, R, et al. Effect of a nursing implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999; 27:2609–2615.
109. Kress, JP, Pohlman, AS, O’Connor, MF, Hall, JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000; 342:1471–1477.
110. Siobal, MS, Kallet, RH, Kivett, VA, Tang, JF. Use of dexmedetomidine to facilitate extubation in surgical intensive-care-unit patients who failed previous weaning attempts following prolonged mechanical ventilation: A pilot study. Respir Care. 2006; 51:492–496.
111. Maldonado, JR, van der Starre, PJ, Wysong, A. Postoperative sedation and the incidence of ICU delirium in cardiac surgery patients. Anesthesiology. 2003; 99:A465.
112. Ely, EW, Stephens, RK, Jackson, JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: A survey of 912 healthcare professionals. Crit Care Med. 2004; 32:106–112.
113. Song, C, Lin, A, Kenis, G, et al. Immunosuppressive effects of clozapine and haloperidol: Enhanced production of the interleukin-1 receptor antagonist. Schizophr Res. 2000; 42:157–164.
114. Milbrandt, EB, Kersten, A, Kong, L, et al. Haloperidol use is associated with lower hospital mortality in mechanically ventilated patients. Crit Care Med. 2005; 33:226–229.
115. Kapur, S, Remington, G, Jones, C, et al. High levels of dopamine d2 receptor occupancy with low-dose haloperidol treatment: A pet study. Am J Psychiatry. 1996; 153:948–950.
116. Skrobik, Y, Bergeron, N, Dumont, M, Gottfried, SB. Olanzapine vs haloperidol: Treating delirium in a critical care setting. Intensive Care Med. 2004; 30:444–449.
117. Tune, L. The role of antipsychotics in treating delirium. Curr Psychiatric Rep. 2002; 4:209–212.
118. Foreman, M, Milisen, K, Marcantonia, EM. Prevention and treatment strategies for delirium. Primary Psychiatry. 2004; 11:52–58.
119. Alao, AO, Soderberg, M, Pohl, EL, Koss, M. Aripiprazole in the treatment of delirium. Int J Psychiatry Med. 2005; 35:429–433.
120. Kalisvaart, KJ, de Jonghe, JF, Bogaards, MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: A randomized placebo-controlled study. J Am Geriatr Soc. 2005; 53:1658–1666.
121. Wang, W, Li, HL, Wang, DX, et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: A randomized controlled trial. Crit Care Med. 2012; 40(3):731–739.
122. Lee, KU, Won, WY, Lee, HK, et al. Amisulpride versus quetiapine for the treatment of delirium: A randomized, open prospective study. Int Clin Psychopharmacol. 2005; 20(6):311–314.
123. Pae, CU, Lee, SJ, Lee, CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol. 2004; 19(2):125–127.
124. Devlin, JW, Roberts, RJ, Fong, JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: A prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010; 38(2):419–427.
125. Nasrallah, HA, White, T, Nasrallah, AT. Lower mortality in geriatric patients receiving risperidone and olanzapine versus haloperidol: Preliminary analysis of retrospective data. Am J Geriatr Psychiatry. 2004; 12(4):437–439.
126. Han, CS, Kim, YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004; 45(4):297–301.
127. Girard, TD, Pandharipande, PP, Carson, SS, et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: The MIND randomized, placebo-controlled trial. Crit Care Med. 2010; 38(2):428–437.
128. Kato, D, Kawanishi, C, Kishida, I, et al. Delirium resolving upon switching from risperidone to quetiapine: Implication of the CYP2D6 genotype. Psychosomatics. 2005; 46:374–375.
129. Wang, PS, Schneeweiss, S, Avorn, J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005; 353:2335–2341.
130. Schneider, LS, Dagerman, KS, Insel, P. Risk of death with atypical antipsychotic drug treatment for dementia: Meta-analysis of randomized placebo-controlled trials. JAMA. 2005; 294:1934–1943.
131. Sink, KM, Holden, KF, Yaffe, K. Pharmacological treatment of neuropsychiatric symptoms of dementia: A review of the evidence. JAMA. 2005; 293:596–608.
132. Pandharipande, PP, Pun, BT, Herr, DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA. 2007; 298(22):2644–2653.
133. Pandharipande, PP, Sanders, RD, Girard, TD, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: An a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010; 14:R38.
134. Riker, RR, Shehabi, Y, Bokesch, PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: A randomized trial. JAMA. 2009; 301(5):489–499.
135. Jakob, SM, Ruokonen, E, Grounds, RM, et al. Dexmedetomidine vs. midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA. 2012; 307(11):1151–1160.
136. Van Eijk, MM, Roes, KC, Honing, ML, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: A multicentre, double-blind, placebo-controlled randomised trial. Lancet. 2010; 376:1829–1837.
137. Tagarakis, GI, Voucharas, C, Tsolaki, F, et al. Ondansetron versus haloperidol for the treatment of postcardiotomy delirium: A prospective, randomized, double-blinded study. J Cardiothorac Surg. 2012; 7:25.
138. Bellapart, J, Boots, R. Potential use of melatonin in sleep and delirium in the critically ill. Br J Anaesthesia. 2012; 108(4):572–580.
139. Bourne, RS, Mills, GH. Sleep disruption in critically ill patients—Pharmacological considerations. Anaesth. 2004; 59:374–384.
140. Cooper, AB, Thornley, KS, Young, GB, et al. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000; 117:809–818.
141. Gabor, JY, Cooper, AB, Crombach, SA, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003; 167:708–715.
142. Helton, MC, Gordon, SH, Nunnery, SL. The correlation between sleep deprivation and the intensive care unit syndrome. Heart Lung. 1980; 9:464–468.
143. Freedman, NS, Gazendam, J, Levan, L, et al. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001; 163:451–457.
144. Nelson, LE, Lu, J, Guo, T, et al. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003; 98:428–436.
145. Langtry, HD, Benfield, P. Zolpidem: A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential. Drugs. 1990; 40:291–313.
146. Aslan, S, Isik, E, Cosar, B. The effects of mirtazapine on sleep: A placebo controlled, double-blind study in young healthy volunteers. Sleep. 2002; 25:677–679.
147. Schelling, G, Stoll, C, Haller, M, et al. Health-related quality of life and posttraumatic stress disorder in survivors of the acute respiratory distress syndrome. Crit Care Med. 1998; 26:651–659.
148. Kapfhammer, HP, Rothenhausler, HB, Krauseneck, T, et al. Posttraumatic stress disorder and health-related quality of life in long-term survivors of acute respiratory distress syndrome. Am J Psychiatry. 2004; 161:45–52.
149. Ozer, EJ, Best, SR, Lipsey, TL, Weiss, DS. Predictors of posttraumatic stress disorder and symptoms in adults: A meta-analysis. Psychol Bull. 2003; 129:52–73.
150. Brewin, CR, Smart, L. Working memory and suppression of intrusive thoughts. J Behav Ther Exp Psychiatry. 2005; 36:61–68.
151. Stoll, C, Kapfhammer, HP, Rothenhausler, HB, et al. Sensitivity and specificity of a screening test to document traumatic experiences and to diagnose post-traumatic stress disorder in ARDS patients after intensive care treatment. Intensive Care Med. 1999; 25:697–704.
152. Murray, MJ, Cowen, J, Deblock, H, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2002; 30:142–156.
153. Brewin, CR, Holmes, EA. Psychological theories of posttraumatic stress disorder. Clin Psychol Rev. 2005; 23:339–376.
154. Zilberberg, MD, Epstein, SK. Acute lung injury in the medical ICU: Comorbid conditions, age, etiology, and hospital outcome. Am J Respir Crit Care Med. 1998; 157:1159–1164.
155. Rosenberg, AL, Hofer, TP, Hayward, RA, et al. Who bounces back? Physiologic and other predictors of intensive care readmission. Crit Care Med. 2001; 29:511–518.
156. Turner, RJ, Lloyd, DA. Lifetime traumas and mental health: The significance of cumulative adversity. J Health Soc Behav. 2005; 36:360–376.
157. Alonzo, AA. The experience of chronic illness and post-traumatic stress disorder: The consequences of cumulative adversity. Soc Sci Med. 2000; 50:1475–1484.
158. Lloyd, DA, Turner, RJ. Cumulative adversity and posttraumatic stress disorder: Evidence from a diverse community of young adults. Am J Orthopsychiatry. 2003; 73:381–391.
159. Miranda, J, Green, BL. The need for mental health services research focusing on poor young women. J Ment Health Policy Econ. 1999; 2:73–80.
160. Schnyder, U, Moergeli, H, Trentz, O, et al. Prediction of psychiatric morbidity in severely injured accident victims at one-year follow-up. Am J Respir Crit Care Med. 2001; 164:653–656.
161. Foreman, M, Milisen, K. Improving recognition of delirium in the elderly. Primary Psychiatry. 2004; 11:46–50.
162. Squire, L. Declarative and non-declarative memory: Multiple brain systems supporting learning and memory. J Cogn Neurosci. 1992; 4:232–243.
163. Parkin, A. Human memory. Curr Biol. 1999; 9:582–585.
164. Sbordone, R, Liter, J. Mild traumatic brain injury does not produce post traumatic stress disorder. Brain Inj. 1995; 9:405–412.
165. Sbordone, R, Seyraniniana, GD, Ruff, RM. Are the subjective complaints of traumatically brain injured patients reliable? Brain Inj. 1998; 12:505–512.
166. Malt, U. The long term psychiatric consequences of accidental injury. Br J Psychiatry. 1988; 153:810–818.
167. Ursano, R, Fullerton, C, Epstein, R. Acute and chronic posttraumatic stress disorder in motor vehicle victims. Am J Psychiatry. 1999; 156:589–595.
168. Bontke, C, Rattok, J, Boake, C. Do patients with mild brain injury have post-traumatic stress disorder too? J Head Trauma Rehabil. 1996; 11:95–102.
169. Klein, E, Caspi, Y, Gil, S. The relation between memory of the traumatic brain injury and PTSD: Evidence from studies of traumatic brain injury. Can J Psychiatry. 2003; 48:28–33.
170. Bryant, RA. Posttraumatic stress disorder and traumatic brain injury: Can they co-exist? Clin Psychol Rev. 2001; 21:931–948.
171. Brewin, CR. A cognitive neuroscience account of posttraumatic stress disorder and its treatment. Behav Res Ther. 2001; 39:373–393.
172. Brewin, CR, Dalgleish, T, Josepth, S. A dual representation theory of posttraumatic stress disorder. Psychol Rev. 1996; 103:670–686.
173. Schacter, DL, Chiu, CYP, Ochsner, KN. Implicit memory: A selective review. Neuroscience. 1993; 16:159–182.
174. Sessler, C. Top ten list in sepsis. Chest. 2001; 120:1390–1393.
175. Jones, C, Griffiths, RD, Humprhis, G. Disturbed memory and amnesia related to intensive care. Memory. 2000; 8:79–94.
176. Ely, EW, Margolin, R, Francis, J, et al. Evaluation of delirium in critically ill patients: Validation of the confusion assessment method for the intensive care unit (CAM-ICU). Crit Care Med. 2001; 29:1370–1379.
177. Capuzzo, M, Valpondi, V, Cingolani, E, et al. Post-traumatic stress disorder-related symptoms after intensive care. Minerva Anestesiol. 2005; 71:167–179.
178. Kress, JP, Gehlbach, B, Lacy, M, et al. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003; 168:1457–1461.
179. Capuzzo, M, Valpondi, V, Cingolani, E, et al. Application of the Italian version of the intensive care unit memory tool in the clinical setting. Crit Care. 2004; 8:R48–R55.
180. Jones, C, Griffiths, RD, Humphris, G, Skirrow, PM. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med. 2001; 29:573–577.
181. Jones, C, Skirrow, PM, Griffiths, RD, et al. Rehabilitation after critical illness: A randomized, controlled trial. Crit Care Med. 2003; 31:2456–2461.
182. Jackson, JC, Hart, RP, Gordon, SM, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003; 31:1226–1234.
183. Hopkins, RO, Weaver, LK, Pope, D, et al. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999; 160:50–56.
184. Jones, C, Griffiths, RD, Slater, T, et al. Significant cognitive dysfunction in non-delirious patients identified during and persisting following critical illness. Intensive Care Med. 2006; 32:923–926.
185. Hopkins, RO, Jackson, JC, Wallace, C. Neurocognitive impairments in ICU patients with prolonged mechanical ventilation. J Int Neuropsychol Soc. 2005; 11(Suppl 1):60.
186. Hopkins, RO. Relationship of cognitive dysfunction to quality of life. J Int Neuropsychol Soc. 2003; 10:1005–1017.
187. Suchyta, MR, Hopkins, RO, White, J, et al. The incidence of cognitive dysfunction after ARDS. Am J Respir Crit Care Med. 2004; 169:A18.
188. Sukantarat, KT, Burgess, PW, Williamson, RC, Brett, SJ. Prolonged cognitive dysfunction in survivors of critical illness. Anaesthesia. 2005; 60:847–853.
189. Marquis, KA, Curtis, JR, Caldwell, E, et al. Neuropsychological sequelae in survivors of ARDS compared with critically ill control patients. Am J Respir Crit Care Med. 2000; 161:A383.
190. Al-Saidi, F, McAndrews, MP, Cheung, AM, et al. Neuropsychological sequelae in ARDS survivors. Am J Respir Crit Care Med. 2003; 167:A737.
191. Rothenhausler, HB, Ehrentraut, S, Stoll, C, et al. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: Results of an exploratory study. Gen Hosp Psychiatry. 2001; 23:90–96.
192. Christie, JD, Biester, RC, Taichman, DB, et al. Formation and validation of a telephone battery to assess cognitive function in acute respiratory distress syndrome survivors. J Crit Care. 2006; 21:125–132.
193. Hopkins, RO, Weaver, LK, Chan, KJ, Orme, JF, Jr. Quality of life, emotional, and cognitive function following acute respiratory distress syndrome. J Int Neuropsychol Soc. 2004; 10:1005–1017.
194. Whitley, E, Ball, J. Statistics review 1: Presenting and summarizing data. Crit Care Forum. 2002; 6:66–71.
195. Weaver, LK, Hopkins, RO, Chan, KJ, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med. 2002; 347:1057–1067.
196. Newman, MF, Kirchner, JL, Phillips-Bute, B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001; 344:395–402.
197. Scragg, P, Jones, A, Fauvel, N. Psychological problems following ICU treatment. Anaesthesia. 2001; 56:9–14.
198. Skodol, AE. Anxiety in the medically ill: Nosology and principles of differential diagnosis. Semin Clin Neuropsychiatry. 1999; 4:64.
199. Brandes, D, Ben-Schachar, G, Gilboa, A, et al. PTSD symptoms and cognitive performance in recent trauma survivors. Psychiatry Res. 2002; 110:231–238.
200. Ravnkilde, B, Videbech, P, Clemmensen, K, et al. Cognitive deficits in major depression. Scand J Psychol. 2002; 43:239–251.
201. Massman, PJ, Delis, DC, Butters, N, et al. The subcortical dysfunction model of memory deficits in depression: Neuropsychological validation in a subgroup of patients. J Clin Exp Neuropsychol. 1992; 14:687–706.
202. Richards, PM, Ruff, RM. Motivational effects on neuropsychological functioning: Comparison of depressed versus nondepressed individuals. J Consult Clin Psychol. 1989; 57:396–402.
203. Buckelew, SP, Hannay, HJ. Relationships among anxiety, defensiveness, sex, task difficulty, and performance on various neuropsychological tasks. Percept Mot Skills. 1986; 63:711–718.
204. Eysenck, MW. Anxiety and cognitive functioning: A multifaceted approach. In: Lister RG, Weingartner HJ, eds. Perspectives of Cognitive Neuroscience. New York: Oxford University Press, 2003.
205. Hart, RP, Kwentus, JA, Taylor, JR, Harkins, SW. Rate of forgetting in dementia and depression. J Consult Clin Psychol. 1997; 55:101–105.
206. McGlynn, SM, Schacter, DL. Unawareness of deficits in neuropsychological syndromes. J Clin Exp Neuropsychol. 1989; 11:143–205.
207. Jones, RD, Tranel, D, Benton, A, Paulsen, J. Differentiating dementia from pseudo-dementia early in the clinical course: Utility of neuropsychological tests. Neuropsychology. 1992; 6:13–21.
208. Sheikh, JL, Yesavage, JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol. 1986; 5:165–173.
209. Beck, AT. BDI-II Depression Inventory Manual, 2nd ed. New York: Harcourt Brace; 1996.
210. Radloff, LS. The CES-D Scale: A self report depression scale for research in the general population. Appl Psychol Meas. 1977; 1:385–401.
211. Zigmond, AS, Snaith, RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983; 67:361–370.
212. Beck, AT, Brown, G, Steer, RA. An inventory for assessing clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988; 56:893–897.