Chapter 99 Deformity Surgery for Ankylosing Spondylitis
Ankylosing spondylitis (AS) is an inflammatory disease that can lead to painful disability and deformity.1 AS is characterized by a strong heretability factor, with most of the risk for susceptibility being connected to the presence of certain genes.2 The pathogenesis is thought to be immune-mediated joint erosion and bone proliferation that primarily affects the axial skeleton, including ligaments and articulations of the pelvis and spinal column. Inflammation of the vertebral joints and intervertebral disc spaces leads to ossification and fusion of the spine characterized by syndesmophyte formation, ankylosis, and the classic hallmark appearance of “bamboo spine.” Concomitant osteoporosis causes the spine to become brittle and susceptible to fracture and progressive spinal deformity. The etiopathogenesis of AS is under intense scrutiny at present, with current efforts under way to determine the exact roles of the mixture of genetic susceptibility, chronic inflammation, and bone-forming pathways.3
Clinical Presentation
AS is a chronic lifelong disease that affects men two to three times more frequently than women, and manifests clinically between the ages of 20 and 30.4 The prevalence of AS is between 0.5% and 1.3% and varies due to definition of the cases (pure AS vs. spondyloarthritis), screening criteria, ethnicity, and presence of the major histocompatability complex class I molecule HLA-B27.5 Although there is a strong correlation between the prevalence of HLA-B27 and AS, it is suspected but not proven that several non HLA-B27 genes are related to the disease progression.
The primary clinical axial spine symptom of AS from chronic inflammatory sacroiliitis is low back pain.6 The pain may be unilateral or bilateral and may include radicular symptoms extending into the buttocks or thigh that rarely extend below the knee. Symptoms are usually worse in the morning and improve with activity, distinguishing AS from mechanical low back pain. It is not uncommon for this back pain to awaken the patient at night, further distinguishing AS from other causes of chronic back pain. In children, of course, AS may present with peripheral arthritis.
Ankylosing spondylitic spinal deformity results from progressive flexion and kyphosis of the lumbar, thoracic, and cervical spine as patients attempt to unload stress from painful spondylitic facet joints.7 Autofusion in kyphosis results in a fixed flexion deformity and global sagittal imbalance with ventral displacement of the patient’s center of gravity. Compensatory flexion contractures of the hips and knees may develop as the patient attempts to maintain an erect posture and adequate field of vision. These strains lead to osseous remodeling, further kyphosis, and progressive deformity.
Inflammation and new bone formation drive vertebral column remodeling in AS.8 Indeed, the first two spinal lesions in AS described by Andersson9 and Romanus and Yden10 are inflammatory in nature. Andersson lesions appear as a spondylodiscitis that destroys the central portion of the intervertebral disc and adjacent vertebral body. Romanus lesions are erosive changes at the ventral and dorsal vertebral end plates that appear on radiographs as “shiny corners.” In late disease, these Romanus lesions lead to destruction and rebuilding of the cortex, resulting in squaring of the vertebral bodies.
Other inflammatory lesions are also characteristic of AS.6 Enthesopathy, or inflammation of the ligamentous insertion points, characterizes AS throughout the axial spine. Indeed, enthesitis is the cause of both Andersson and Romanus lesions. Synovitis occurs at zygapophyseal, costovertebral, and costotransverse joints. Inflammation then promotes ectopic bone formation within affected ligaments, resulting in ossification of spinal ligaments and within intervertebral discs, end plates, and apophyseal structures. Formation of new ectopic bone leads to formation of syndesmophytes (bridging the ossified nucleus pulposus at each disc level) or enthesophytes (osseous outgrowths that do not bridge structures). Therefore, advanced AS is characterized by universal syndesmophytosis and squared vertebral bodies with kyphotic deformity that is aptly termed “bamboo spine.” It is this propensity of AS patients to make new bone that may not be affected by newer biologic agents that provide remarkable symptom relief. This is the challenge to our understanding of the fundamental nature of this disease.3
Osteoporosis in AS is particularly challenging. Early papers hinted of osteoporosis as a late finding, but more recent studies have demonstrated that spinal osteoporosis is found even in early AS without peripheral osteoporosis.11 This axial osteoporosis is linked to early inflammatory remodeling of the spine. Syndesmophyte formation may also correlate with lower bone mineral density of the spine.12 Paradoxically, dual-energy x-ray absorptiometry (DEXA) scans in advanced AS may overestimate bone mineral density due to the increased mineral concentration in syndesmophytes, which provide no real functional support. CT can help correlate osteoporosis and disease duration. The clinical consequences of this osteoporosis are profound. Patients with early AS and mild osteoporosis have a fracture prevalence five times greater than in the normal population.13
The combination of inflammation and osteoporosis promotes AS fractures and is paradoxically related to ossification. Ossification of the disc space occurs centripetally through the anulus fibrosus, and only rarely is the center of the disc involved. This incomplete ossification leads to formation of polysegments in the spine, with resulting long lever arms of force. The combined stress concentration from loss of polysegmental spinal motion and secondary osteopenia predisposes patients to spinal fracture and nonunion. Aseptic spondylodiscitis, presenting as focal pain with coexisting erosive sclerotic changes in adjacent vertebral bodies, is noted at these sites.14 It is uncertain whether aseptic spondylodiscitis is a primary inflammatory process or the result of trauma. Radiographically, the appearances of spondylodiscitis, pseudarthrosis, and discitis are similar.
Acute traumatic fractures, particularly in the cervical spine, are also widely reported.6 Again, osteoporosis and stress forces due to long, stiff lever arms enhance the susceptibility of the AS patients to acute spinal fracture. The lifetime incidence of acute traumatic fractures is believed to be approximately 14%.15,16 It is reported that 75% of fractures occur in the cervical or cervicothoracic junction, 14% in the thoracic spine, and 5% in the lumbar spine.17,18 Cervical fractures commonly involve both anterior and posterior columns, leading to higher rates of mortality and neurologic complications in AS than in non-AS patients.19,20 Even minor trauma such as a simple slip and fall can cause a major spinal fracture and neurologic injury, with the rate of neurologic deficit ranging from 53% to 83%.21 There should be a high index of suspicion in any AS patient with acute onset of new focal pain or deformity, including any newly observed loss of height. Occult fractures must be suspected any time an abrupt change occurs in the patient’s condition, and CT is often required to fully evaluate the symptomatic areas. Undiagnosed or poorly managed spinal fractures can contribute to worsening kyphosis and deformity, particularly if the fractures heal in flexion.
Spinal deformity leads to disability and subsequent mortality.6 Chin-on-chest deformity seen with fixed cervical flexion significantly hinders forward vision, swallowing, hygiene, and self-esteem. The combination of debilitating disease, deformity, and limited treatment options makes managing these deformities difficult. Although the surgical management of AS deformity is technically challenging and not without risks, the psychological and functional impairment of progressive deformity warrants surgical correction and stabilization when conservative options have been exhausted.
Surgical Management
General Principles
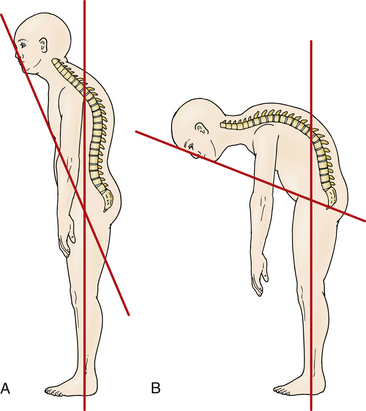
Accurate measurement of the deformity is required for surgical planning. Simmons advocates the chin-brow to vertical (CBV) angle as the most effective and reproducible measurement of deformity.22 The CBV can be evaluated on photographs and is the angle created by (1) the vertical axis of the patient standing with hips and knees extended and (2) the line drawn from the chin to the brow. A greater CBV angle correlates with greater compromise of horizontal gaze and is a critical marker for the degree of deformity (Figs. 99-1A and B).
Normal CBV is 0 degrees but can exceed 90 degrees in severe chin-on-chest deformity. A final corrected angle of approximately 10 degrees of flexion is generally recommended.23 Radiographic evaluation with 36-inch plain radiography is highly recommended.24 Osseous anatomy for instrumentation, existing stenosis requiring decompression, and evaluation of soft tissue or vascular structures like the vertebral arteries can be better delineated on the CT and MRI studies necessary for preoperative planning.25–27 Flexion and extension radiographs can evaluate for instability (particularly atlantoaxial instability) sometimes present in AS.
The technique and location of the osteotomy depends on the region of the spinal deformity that maximally influences sagittal alignment. Overall spinal balance as well as the hips must be evaluated to delineate the primary site of deformity. In some patients, more than one site may contribute to the deformity. The common sites of deformity include the cevicothoracic junction, midthoracic spine, thoracolumbar spine, and hip joints.6 Assuming equal deformity at these levels, lumbar correction surgery should be considered prior to cervical correction surgery because of the lower rate of complications.28
The site of correction depends on the site of deformity. Deformities isolated to the lumbar spine are corrected by a lumbar osteotomy procedure. The osteotomy is preferred below the level of the conus medullaris and is usually performed at L3 to avoid acute angular correction at the cord level.22 Most thoracolumbar kyphotic deformities can be addressed through a single lumbar osteotomy. The correction should be planned so that the plumb line from C7 falls within the body of S1. Even in cases in which the thoracic kyphosis is greater than normal, a compensatory lumbar osteotomy may correct sagittal plane malalignment and allow the patient to have forward gaze with the hips and knees fully extended. In cases of severe thoracic kyphosis, where the lumbar and cervical lordosis have been at least partially maintained, thoracic osteotomy by a combined ventral and dorsal approach may be indicated. It is important to note that due to fixed cervical deformity, overcorrection of the gaze angle can cause significant gait difficulty. When the primary deformity is at the cervicothoracic junction with a chin-on-chest deformity, an osteotomy of the cervical spine is indicated. The C7-T1 junction is the preferred location because it places the osteotomy below the entrance of the vertebral arteries into the transverse processes at C6 and uses the relatively large spinal canal–to–cord area ratio to safely obtain correction.
The influence of severe hip flexion contractures, with or without associated hip joint disease, is critical in the preoperative assessment. Soft tissue release about the hips, or more commonly, total hip joint arthroplasty, may be sufficient in itself to allow the patient to stand reasonably upright and see straight ahead, irrespective of the spinal deformity.29 These procedures should be performed prior to any larger surgical correction of spinal deformity.
Diligent presurgical screening is paramount since AS patients frequently have multiple comorbidities.24 Preoperatively, patients with a fixed thoracic deformity should be screened for cardiac and pulmonary abnormalities that can be associated with extra-articular manifestations of AS. Although pulmonary function abnormalities secondary to decreased thoracic expansion have not carried anesthetic risk for most patients, 10% will have cardiac pathology, generally either aortic stenosis or conduction abnormalities.30 Nonsteroidal anti-inflammatory agents may need to be halted prior to surgery to reduce the risk of pseudarthrosis and nonunion. Nutrition should be optimized, sometimes with tube feeding or parenteral nutrition in extreme cases, especially with postoperative risks of swallowing difficulty.
Surgical Correction
Smith-Petersen Osteotomy
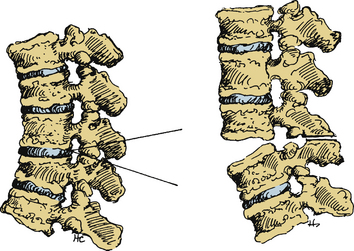
Smith-Petersen and Larson first proposed their osteotomy for the correction of flexion deformity for rheumatoid arthritis in the lumbar spine on six patients in 1945.31 Since that time, the Smith-Petersen osteotomy (SPO), also known as the opening wedge osteotomy and extension osteotomy, has been used extensively and optimized for AS. It has been reported primarily in the lumbar and cervical spine.
Smith-Petersen originally performed a V-shaped wedge resection osteotomy at the L1, L2, and L3 levels (Fig. 99-2).31 In the original operation, the L2 spinous process was removed completely along with the articular processes of L1, L2, and L3. This dorsal osteotomy wedge was then closed and the deformity corrected via forceful manual manipulation through hyperextension. This maneuver used the middle column (e.g., the posterior longitudinal ligament) as a fulcrum and caused disruption of the anterior longitudinal ligament with a monosegmental opening wedge and extension of the anterior column. Local bone grafts were placed across the osteotomy sites, and the patient was immobilized in a postoperative cast for 2 months followed by a back brace for 1 year. Detailed results were not described.
In 1973, McMaster and Coventry reported on 17 patients with an SPO of the lumbar spine using a plaster case with a turnbuckle and hip spica immobilization for postoperative correction (no instrumentation was used).28 They reported an impressive 39-degree correction average, which has been replicated by other authors.32 Twelve of the 17 patients had complications, including 2 deaths and 5 neurologic deficits.
The SPO has also been commonly employed in the cervical spine. In 1953, Mason et al. reported successful correction of flexion deformity of the cervicothoracic spine in a patient with AS.33 They performed the osteotomy distal to C7 to avoid damage to the vertebral arteries. In 1958, Urist reported a successful osteotomy at the cervicothoracic junction in a patient awake under local anesthesia.34 However, it was Simmons that elaborated on the SPO in the first large case series of 42 patients in 1977.22
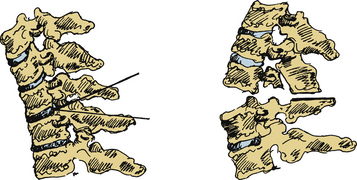
The Simmons SPO modification involved a wide laminectomy from C6 to T1, with osteotomy at the C7-T1 space (Fig. 99-3).22 Simmons resected the entire dorsal arch of C7, the inferior half of C6, and the upper half of T1. The laminae were undercut and foraminotomies performed to prevent impingement of the C8 nerve root. Following bony decompression, Simmons extended the neck and “cracked” the anterior column. Simmons performed the procedure under local anesthesia with halo control and then fixed the halo to a body cast that was worn for 4 months. There were no mortalities, and C8 weakness was the primary morbidity, occurring in 18 patients, with five permanent deficits.
Some authors have performed an initial ventral release prior to a cervical SPO.35,36 Mummaneni et al. have described a staged ventral-dorsal-ventral procedure for cervical osteotomy.37 This consists of a ventral release (C5-6 discectomy and partial wedge resection of C5 and C6 vertebral bodies), followed by a dorsal SPO with controlled correction supplemented by a screw-rod construct, and finally a ventral placement of iliac autograft in the opening wedge defect with a cervical plate and screws.
Several adjustments of the original SPO technique have since become standard. General anesthetic is now frequently used with controlled halo correction, followed by either an intraoperative wake-up test or spinal cord monitoring. Lateral mass screws are used in the cervical spine, with pedicle screws the method of choice elsewhere for internal fixation. Indeed, modern instrumentation is now ubiquitous in deformity surgery. Halo and vest supplementation may or may not be used. Neurologic compression is now minimized by adequate decompression and undercutting of the lamina prior to closure of the osteotomy site and rigid stabilization. Despite these modifications, subluxation caused by rupture of the posterior longitudinal ligament has been associated with nonunion, high neurologic complications, and mortality.38 Although the SPO remains in common use, some surgeons prefer other alternatives.
Polysegmental Wedge Osteotomy
In 1949, Wilson and Turkell reported the first polysegmental wedge osteotomy (PWO) on a patient with thoracolumbar kyphotic deformity attributed to AS.39 Correction was achieved by multiple closing wedges of dorsal lumbar osteotomies including the interlaminar space and by trimming the facet processes. In contrast to the SPO, a PWO leaves the anterior longitudinal ligament (ALL) intact and generates a more gradual multisegment curvature. In the 1980s, Zielke et al. advocated polysegmental lumbar dorsal wedge osteotomies with internal fixation.40,41 He first used Harrington rods and laminar hooks and, more recently, transpedicular screws.
Several authors have demonstrated good results with the PWO. Van Royen et al. reported a mean correction of 36.3 degrees overall (9.5 degrees per level) in 21 patients treated with PWOs in the thoracic and lumbar spine.42 At last follow-up, however, there was a mean loss of 10.7 degrees, with a significant rate of pedicle fractures, deep wound infections, and pseudarthrosis. Hehne and Zielke described 177 patients with AS and 44-degree overall correction (9.5 degrees per segment) without resulting pseudarthrosis and no loss of correction over the long term.41 Chen reported an average correction of 25.8 degrees overall (5 degrees per level) with a 25% pseudarthrosis rate.43 These results suggest that PWOs are reasonable when gradual correction is necessary over multiple levels. There may be concern for insufficient correction, however, especially if the intervertebral discs are calcified.44
Smith-Petersen pointed out in 1945 that single-stage dorsal thoracic osteotomy correction is compromised by stiffness of the costovertebral joints.31 An alternative involves a two-stage procedure that consists of a first-stage transthoracic approach creating osteotomies through the ossified thoracic disc spaces. Ventral interbody fusion is performed with an autogenous cancellous bone graft. This is followed at the same sitting or 1 week later by PWOs with segmental instrumentation. Dural adhesions to the lamina that formed during the inflammatory phase of the disease can be encountered during dorsal osteotomy and likewise may make passage of sublaminar wires used in the Luque technique more difficult. Hook-rod or screw-rod compression instrumentations are alternatives commonly used today. The approach is similar to that used for severe juvenile kyphosis.45,46
Pedicle Subtraction Osteotomy
The pedicle subtraction osteotomy (PSO), also known as the decancellization procedure, eggshell osteotomy, or closing wedge osteotomy, has been well described in the literature.47–49 Today, the PSO is primarily performed at the upper lumbar and more recently in the cervicothoracic junction. The PSO is a mainstay in correcting deformity due to iatrogenic kyphosis, traumatic kyphosis, rheumatoid arthritis, and AS.
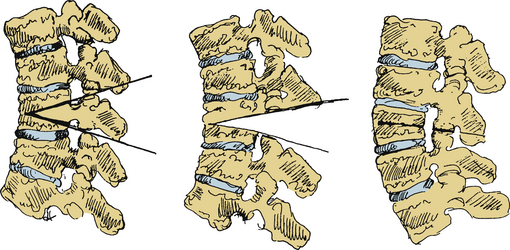
A PSO involves first removing a wedge of the dorsal elements and bilateral pedicles, followed by resection of the dorsal vertebral cortex as well as the cancellous bone of the vertebral body (Fig. 99-4). The ALL and ventral cortex of the vertebral body are left intact. In contrast to the SPO, the ALL is the fulcrum for closure and results in three-column bone-on-bone closure. Closure effectively shortens the spinal canal and achieves angular correction at a single level. Moreover, removal of the pedicle creates a “superforamen,” which transmits the nerve roots from the adjacent segments and decreases the chance for root compression. Generous undercutting and decompression of the supra- and subadjacent laminar edges are performed to ensure adequate space for the redundant dura that may be produced during closure of the osteotomy. Segmental spinal fixation using screw-rod or hook-rod constructs is used to allow for immediate patient mobilization. The surgical table is carefully extended, closing the osteotomy. If necessary, closure can be augmented by pressure on the patient’s shoulders or legs and by compression between the pedicle screws once the rods are placed. A wake-up test or neuromonitoring is routinely performed to assess neurologic function. Finally, a local bone graft is applied and augmented with iliac crest autograft or banked bone, as needed.
The PSO is generally well tolerated in the lumbar spine. Thomasen reported 12 to 50 degrees of correction in 11 patients, with 5 of the 11 having a correction of less than 35 degrees.48 Other reports show corrections of 30 to 40 degrees in the lumbar spine.50 The ability to correct all three columns through a single dorsal approach, correction of more than 30 degrees at a single level, and correction through a prior fusion mass make the PSO a favored procedure in the lumbar spine.
PSOs of the cervicothoracic junction deserve special focus. Recently, Samudrala et al. reported on eight patients who underwent PSO for correction of CT junction kyphosis, achieving a mean correction of 35 degrees, restoration of forward gaze, and significant reduction of pain.51 As with Simmons’ cervical SPO for AS, the site for a cervical PSO is recommended at C7-T1 since the spinal canal is relatively wide, the C8 roots are mobile, and the vertebral artery is rostral to the C7 foramen transversarium. The modern cervical PSO offers a dorsal fusion system in the cervicothoracic junction that avoids stretch injury to critical ventral structures such as the trachea and esophagus while promoting fusion through all three columns.
Ventral Release and Osteotomy
Some authors advocate using an initial ventral release prior to SPOs.24 Ventral release and osteotomy have the advantages of allowing a controlled correction with neck extension without the abrupt fracture from an SPO. The osteotomy is controlled to a specific site, whereas correction of SPO can lead to a fracture at a random, undesired level. Finally, ventral exposure allows for placement of a structural graft.
Ventral exposure in AS may be inappropriate in some cases. Ventral exposure may be unnecessary since an ankylosed, osteoporotic spine often will fracture without excessive force. Chin-on-chest deformity also limits the exposure and operative corridor with a ventral approach. Finally, syndesmophyte formation limits a surgeon’s ability to distinguish normal from abnormal anatomic landmarks of the disc spaces. The added risk of patient repositioning and extended anesthesia must also be considered. Finally, the use of standard dorsal screw-rod instrumentation provides greater mechanical stability than ventral constructs.52,53
Anesthesia, Neuromonitoring, and Intraoperative Care
Although local anesthesia has been reported in the treatment of these spinal deformities, general anesthesia is preferred.54,55 With endotracheal intubation, the airway access is secured, allowing for the procedure to be performed in a prone rather than sitting position, which facilitates placement of instrumentation, reduces the risk of air embolism, and ensures patient comfort. Intubation is facilitated by the use of fiberoptic guidance where cervicothoracic kyphosis complicates easy passage of the endotracheal tube. Awake intubation allows for constant neurologic monitoring and limits the risk of neurologic injury.
The ability to monitor neurologic function is critical in deformity correction since neurologic injury can occur due to translation of the spine or by compression on closing of the osteotomy. McMaster has described using the Stagnara wake-up test as the gold standard for intraoperative neurologic evaluation.23 However, anesthetic limitations often hinder timely and safe wake-up during a prolonged procedure, especially during a period when critical neural compression may go unrecognized.
Neuromonitoring is a useful adjunct for any deformity correction. Somatosensory-evoked potentials (SSEPs) and motor-evoked potentials (MEPs) are routine in complex spine procedures. SSEPs provide monitoring of the dorsal column but do not provide information regarding motor pathways, and several reports have demonstrated postoperative neurologic deficits despite normal intraoperative SSEPs.56 MEP monitoring, in contrast, evaluates the corticospinal tracts. Langeloo et al. evaluated MEP monitoring in 16 patients undergoing SPO using a 20% decrease in MEP measurements as a threshold.57 Langeloo addressed any MEP decreases by first evaluating for technical problems and optimizing hemodynamics. Persistent decreases were addressed by reversing the surgical maneuver that preceded the MEP change. In his 16 patients, he found 9 events in 7 patients. One patient had spontaneous recovery of MEP amplitude without sequelae. In six patients, the cervical extension maneuver preceded the MEP change. Upon change, the extension was reversed, leading to recovery in five of the six patients. One patient did not recover MEPs and had persistent C6 cord injury despite reversing the maneuver and performing a secondary ventral decompression the same day. There were no cases of stable intraoperative MEPs with postoperative neurologic sequelae.
Internal Fixation
Early reports of correction often used external immobilization with either a halo cast or halo vest without internal fixation. However, the lack of supplemental internal fixation poses the risk of delayed subluxation with potential neurologic injury or pseudarthrosis, particularly in the situation of an SPO. McMaster compared 12 SPO patients with halo immobilization alone with 3 patients with a halo body cast with internal Luque rod and wiring fixation.23 He found four cases of postoperative C7-T1 subluxation and two cases of nonunion in the patients treated with halo immobilization alone, compared with no cases of subluxation or pseudarthrosis in patients treated with internal fixation. Similarly, Belanger et al. reported on 26 SPO patients, 7 with halo vest alone and 19 with internal fixation.58 There were five cases of subluxation reported, all of which occurred in the patients treated with halo vest alone.
Modern screw-rod constructs are the mainstay of deformity correction. The use of dorsal wiring has been well described, although the use of sublaminar wires in the thoracic spine represents a high risk of cord injury with limited immobilization. Multilevel fixation is crucial given the long lever arms and osteoporosis prominent in AS.59
Complications
It has been stated in review of several series that mortality has varied from 8% to 10%, and neurologic complications have occurred in up to 30% of patients. However, these quotes may be misleading. We performed an analysis of the 14 largest series consisting of five or more cases reported. A total of 427 cases were found with a 4% incidence of neurologic complications and a 5% mortality rate.30,31,33,48,55,60–68 In the single largest study, 177 patients reported by Hehne and Zielke, there was a 2.3% mortality rate and a 2.3% rate of irreversible root lesions.41 Based on the authors’ review of the published data and their own experience, it appears that neurologic complications and mortality can be greatly lessened if not prevented altogether by careful attention to four critical factors: (1) avoiding compression of neurologic tissue, (2) monitoring neurologic function during the osteotomy, (3) using internal fixation, and (4) avoiding translational displacement at the osteotomy site.
Complications related to surgical correction are related to patient, site, and procedure attempted. Van Royen and De Gast identified 856 patients surgically treated for thoracolumbar kyphotic deformity in 41 articles from 1945 to 1998 using SPO, PWO, and PSOs in the lumbar spine for AS.44 They classified three main categories of complications (1) loss of correction and implant failure, (2) vascular complications, and (3) neurologic complications. Loss of correction and implant failure are related to osteoporosis and present as implant loosening and pullout. This failure places additional stress on the fusion construct and remaining instrumentation, increasing the likelihood of nonunion and loss of correction. Vascular complications, primarily rupture of the aorta and its branches, were found in 0.9% (4 of 450 patients) of lumbar SPO at L1-2 and L2-3, but not below L3.66,69,70 Finally, neurologic deficit due to displacement of the vertebral body was reported in six patients with SPO (2.7%) and in one patient treated by PSO (2.0%).48,71,72
Correction of cervical deformity in AS has its own set of complications. In 2008, Hoh et al. reviewed the literature for case series of at least 10 patients with AS who underwent cervical SPO.24 The review found 5 of 183 patients had significant spinal cord injury (1 paraparesis, 1 hemiparesis, and 3 tetraparesis), several cases of transient postoperative weakness with spontaneous recovery, and 35 cases of C8 sensory disturbances with most resolving over several months.23,26,57,58 Six deaths were noted within 3 months of the operation. Other complications included postoperative dysphagia and pseudarthroses as reported by Simmons et al. and McMaster only in cases treated without internal fixation.23,26
Summary
Deformity in AS is progressive and ultimately debilitating. Inflammation is a major factor leading to structural remodeling of the spine, although only a few of the pathways involved in the new bone formation that is widespread in this disease are known. In order to halt the spiral to deformity, neurologic decline, and loss of function, surgical treatment must be considered when conservative measures have failed. A grasp of the surgical indications, options, and techniques is essential to optimize AS therapy.
Hehne H.J., Zielke K. Polysegmental lumbar osteotomies and transpedicled fixation for correction of long-curved kyphotic deformities in ankylosing spondylitis: report on 177 cases. Clin Orthop Relat Res. 1990;258:49-55.
Samudrala S., Vaynman S., Thiayananthan T., et al. Cervicothoracic junction kyphosis: surgical reconstruction with pedicle subtraction osteotomy and Smith-Petersen osteotomy. Presented at the 2009 Joint Spine Section Meeting. Clinical article. J Neurosurg Spine. 2010;13:695-706.
Simmons E.D., DiStefano R.J., Zheng Y., et al. Thirty-six years experience of cervical extension osteotomy in ankylosing spondylitis: techniques and outcomes. Spine (Phila Pa 1976). 2006;31:3006-3012.
Tokala D.P., Lam K.S., Freeman B.J., et al. C7 decancellisation closing wedge osteotomy for the correction of fixed cervico-thoracic kyphosis. Eur Spine J. 2007;16:1471-1478.
van Royen B.J., de Kleuver M., Slot G.H. Polysegmental lumbar posterior wedge osteotomies for correction of kyphosis in ankylosing spondylitis. Eur Spine J. 1998;7:104-110.
1. Stafford L., Youssef P.P. Spondyloarthropathies: an overview. Intern Med J. 2002;32:40-46.
2. Evans D.M., Reveille J.D., Brown M.A., et al. The genetic basis of spondyloarthritis: SPARTAN/IGAS 2009. J Rheumatol. 2010;37:2626-2631.
3. Schett G., Coates L.C., Ash Z.R., et al. Structural damage in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: traditional views, novel insights gained from TNF blockade, and concepts for the future. Arthritis Res Ther. 2011;13(Suppl 1):S4.
4. Feldtkeller E., Khan M.A., van der Heijde D., et al. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23:61-66.
5. Helmick C.G., Felson D.T., Lawrence R.C., et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15-25.
6. Mundwiler M.L., Siddique K., Dym J.M., et al. Complications of the spine in ankylosing spondylitis with a focus on deformity correction. Neurosurg Focus. 2008;24:E6.
7. Simkin P.A., Downey D.J., Kilcoyne R.F. Apophyseal arthritis limits lumbar motion in patients with ankylosing spondylitis. Arthritis Rheum. 1988;31:798-802.
8. Jacobs W.B., Fehlings M.G. Ankylosing spondylitis and spinal cord injury: origin, incidence, management, and avoidance. Neurosurg Focus. 2008;24:E12.
9. Andersson O. Röntgenbilden vid spondylarthritis ankylopoetica. Nord Med Tidskr. 1937.
10. Romanus R., Yden S. Destructive and ossifying spondylitic changes in rheumatoid ankylosing spondylitis (pelvo-spondylitis ossificans). Acta Orthop Scand. 1952;22:88-99.
11. Will R., Palmer R., Bhalla A.K., et al. Osteoporosis in early ankylosing spondylitis: a primary pathological event? Lancet. 1989;2:1483-1485.
12. Karberg K., Zochling J., Sieper J., et al. Bone loss is detected more frequently in patients with ankylosing spondylitis with syndesmophytes. J Rheumatol. 2005;32:1290-1298.
13. Donnelly S., Doyle D.V., Denton A., et al. Bone mineral density and vertebral compression fracture rates in ankylosing spondylitis. Ann Rheum Dis. 1994;53:117-121.
14. Toussirot E., Chataigner H., Pepin L., et al. Spinal cord compression complicating aseptic spondylodiscitis in ankylosing spondylitis. Clin Exp Rheumatol. 2009;27:654-657.
15. Hitchon P.W., From A.M., Brenton M.D., et al. Fractures of the thoracolumbar spine complicating ankylosing spondylitis. J Neurosurg. 2002;97:218-222.
16. Mitra D., Elvins D.M., Speden D.J., Collins A.J. The prevalence of vertebral fractures in mild ankylosing spondylitis and their relationship to bone mineral density. Rheumatology (Oxford). 2000;39:85-89.
17. Hunter T., Dubo H. Spinal fractures complicating ankylosing spondylitis. Ann Intern Med. 1978;88:546-549.
18. Hyman S.A., Rogers W.D. Bullington JCr. Cervical osteotomy and manipulation in ankylosing spondylitis: successful general anesthesia after failed local anesthesia with sedation. J Spinal Disord. 1990;3:423-426.
19. Bohlman H.H. Acute fractures and dislocations of the cervical spine. An analysis of three hundred hospitalized patients and review of the literature. J Bone Joint Surg [Am]. 1979;61:1119-1142.
20. Osgood C., Martin L.G., Ackerman E. Fracture-dislocation of the cervical spine with ankylosing spondylitis. Report of two cases. J Neurosurg. 1973;39:764-769.
21. Weinstein P.R., Karpman R.R., Gall E.P., Pitt M. Spinal cord injury, spinal fracture, and spinal stenosis in ankylosing spondylitis. J Neurosurg. 1982;57:609-616.
22. Simmons E.H. Kyphotic deformity of the spine in ankylosing spondylitis. Clin Orthop Relat Res. 1977;128:65-77.
23. McMaster M.J. Osteotomy of the cervical spine in ankylosing spondylitis. J Bone Joint Surg [Br]. 1997;79:197-203.
24. Hoh D.J., Khoueir P., Wang M.Y. Management of cervical deformity in ankylosing spondylitis. Neurosurg Focus. 2008;24:E9.
25. El Saghir H., Boehm H. Surgical options in the treatment of the spinal disorders in ankylosing spondylitis. Clin Exp Rheumatol. 2002;20:S101-S105.
26. Simmons E.D., DiStefano R.J., Zheng Y., Simmons E.H. Thirty-six years experience of cervical extension osteotomy in ankylosing spondylitis: techniques and outcomes. Spine (Phila Pa 1976). 2006;31:3006-3012.
27. Tokala D.P., Lam K.S., Freeman B.J., Webb J.K. C7 decancellisation closing wedge osteotomy for the correction of fixed cervico-thoracic kyphosis. Eur Spine J. 2007;16:1471-1478.
28. McMaster M.J., Coventry M.B. Spinal osteotomy in akylosing spondylitis. Technique, complications, and long-term results. Mayo Clin Proc. 1973;48:476-486.
29. Bisla R.S., Ranawat C.S., Inglis A.E. Total hip replacement in patients with ankylosing spondylitis with involvement of the hip. J Bone Joint Surg [Am]. 1976;58:233-238.
30. Feltelius N., Hedenstrom H., Hillerdal G., Hallgren R. Pulmonary involvement in ankylosing spondylitis. Ann Rheum Dis. 1986;45:736-740.
31. Smith-Petersen M.N., Larson C.B. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. J Bone Joint Surg. 1945;27:1-11.
32. Herbert J.J. Vertebral osteotomy; technique, indications and results. J Bone Joint Surg [Am]. 1948;30:680-690.
33. Mason C., Cozen L., Adelstein L. Surgical correction of flexion deformity of the cervical spine. Calif Med. 1953;79:244-246.
34. Urist M.R. Osteotomy of the cervical spine: report of a case of ankylosing rheumatoid spondylitis. J Bone Joint Surg [Am]. 1958;40:833-843.
35. Bhojraj S.Y., Dasgupta D., Dewoolkar L.V. One-stage “front” and “back” correction for rigid cervical kyphosis. A safer technique of correction for a rare case of adult-onset Still’s disease. Spine (Phila Pa 1976). 1993;18:1904-1908.
36. Bouchard J.A., Feibel R.J. Gradual multiplanar cervical osteotomy to correct kyphotic ankylosing spondylitic deformities. Can J Surg. 2002;45:215-218.
37. Mummaneni P.V., Mummaneni V.P., Haid R.W.J., et al. Cervical osteotomy for the correction of chin-on-chest deformity in ankylosing spondylitis. Technical note. Neurosurg Focus. 2003;14:e9.
38. Herbert J.J. Vertebral osteotomy for kyphosis, especially in Marie-Strumpell arthritis: a report on fifty cases. J Bone Joint Surg [Am]. 1959;41:291-302.
39. Wilson M.J., Turkell J.H. Multiple spinal wedge osteotomy: its use in a case of Marie-Strumpell spondylitis. Am J Surg. 1949;77:777-782.
40. Halm H., Metz-Stavenhagen P., Zielke K. Results of surgical correction of kyphotic deformities of the spine in ankylosing spondylitis on the basis of the modified arthritis impact measurement scales. Spine (Phila Pa 1976). 1995;20:1612-1619.
41. Hehne H.J., Zielke K. Polysegmental lumbar osteotomies and transpedicled fixation for correction of long-curved kyphotic deformities in ankylosing spondylitis: report on 177 cases. Clin Orthop Relat Res. 1990;258:49-55.
42. van Royen B.J., de Kleuver M., Slot G.H. Polysegmental lumbar posterior wedge osteotomies for correction of kyphosis in ankylosing spondylitis. Eur Spine J. 1998;7:104-110.
43. Chen P.Q. Correction of kyphotic deformity in ankylosing spondylitis using multiple spinal osteotomy and Zielke’s VDS instruments. Taiwan Yi Xue Hui Za Zhi. 1988;87:692-699.
44. Van Royen B.J., De Gast A. Lumbar osteotomy for correction of thoracolumbar kyphotic deformity in ankylosing spondylitis. A structured review of three methods of treatment. Ann Rheum Dis. 1999;58:399-406.
45. Bohm H., Harms J., Donk R., Zielke K. Correction and stabilization of angular kyphosis. Clin Orthop Relat Res. 1990;258:56-61.
46. Bradford D.S. Kyphosis: current orthopaedic management. W.J. Kane. New York: Churchill-Livingstone, 1981.
47. Scudese V.A., Calabro J.J. Vertebral wedge osteotomy. Correction of rheumatoid (ankylosing) spondylitis. JAMA. 1963;186:627-631.
48. Thomasen E. Vertebral osteotomy for correction of kyphosis in ankylosing spondylitis. Clin Orthop Relat Res. 1985;194:142-152.
49. Ziwjan J.L. [The treatment of flexion deformities of the spine in Bechterew disease]. Beitr Orthop Traumatol. 1982;29:195-199.
50. Gill J.B., Levin A., Burd T., Longley M. Corrective osteotomies in spine surgery. J Bone Joint Surg [Am]. 2008;90:2509-2520.
51. Samudrala S., Vaynman S., Thiayananthan T., et al. Cervicothoracic junction kyphosis: surgical reconstruction with pedicle subtraction osteotomy and Smith-Petersen osteotomy. Presented at the 2009 Joint Spine Section Meeting. Clinical article. J Neurosurg Spine. 2010;13:695-706.
52. Kotani Y., Cunningham B.W., Abumi K., McAfee P.C. Biomechanical analysis of cervical stabilization systems. An assessment of transpedicular screw fixation in the cervical spine. Spine (Phila Pa 1976). 1994;19:2529-2539.
53. Vaccaro A.R., Falatyn S.P., Scuderi G.J., et al. Early failure of long segment anterior cervical plate fixation. J Spinal Disord. 1998;11:410-415.
54. Jackson R.P., Simmons E.H. Dural compression as a cause of paraplegia during operative correction of cervical kyphosis in ankylosing spondylitis. Spine (Phila Pa 1976). 1991;16:846-848.
55. Thompson W.A., Ingersoll R.E. Osteotomy for correction of deformity in Marie-Struempell arthritis. Surg Gynecol Obstet. 1950;90:552-556.
56. Lesser R.P., Raudzens P., Luders H., et al. Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann Neurol. 1986;19:22-25.
57. Langeloo D.D., Journee H.L., Pavlov P.W., de Kleuver M. Cervical osteotomy in ankylosing spondylitis: evaluation of new developments. Eur Spine J. 2006;15:493-500.
58. Belanger T.A., Milam R.A.4th, Roh J.S., Bohlman H.H. Cervicothoracic extension osteotomy for chin-on-chest deformity in ankylosing spondylitis. J Bone Joint Surg [Am]. 2005;87:1732-1738.
59. Cornefjord M., Alemany M., Olerud C. Posterior fixation of subaxial cervical spine fractures in patients with ankylosing spondylitis. Eur Spine J. 2005;14:401-408.
60. Emnéus H. Wedge osteotomy of spine in ankylosing spondylitis. Acta Orthop Scand. 1968;39:321-326.
61. Fang D., Leong J.C., Ho E.K., et al. Spinal pseudarthrosis in ankylosing spondylitis. Clinicopathological correlation and the results of anterior spinal fusion. J Bone Joint Surg [Br]. 1988;70:443-447.
62. Fast A., Parikh S., Marin E.L. Spine fractures in ankylosing spondylitis. Arch Phys Med Rehabil. 1986;67:595-597.
63. Goel M.K. Vertebral osteotomy for correction of fixed flexion deformity of the spine. J Bone Joint Surg [Am]. 1968;50:287-294.
64. Law W.A. Osteotomy of the spine. Clin Orthop Relat Res. 1969;66:70-76.
65. Lawrence J.S. The prevalence of arthritis. Br J Clin Pract. 1963;17:699-705.
66. Lichtblau P.O., Wilson P.D. Possible mechanism of aortic rupture in orthopaedic correction of rheumatoid spondylitis. J Bone Joint Surg [Am]. 1956;38:123-127.
67. Murray G.C., Persellin R.H. Cervical fracture complicating ankylosing spondylitis: a report of eight cases and review of the literature. Am J Med. 1981;70:1033-1041.
68. Simmons E.H. The surgical correction of flexion deformity of the cervical spine in ankylosing spondylitis. Clin Orthop Relat Res. 1972;86:132-143.
69. Klems H., Friedebold G. [Rupture of the abdominal aorta following a corrective spinal operation for ankylopoeitic spondylitis]. Z Orthop Ihre Grenzgeb. 1971;108:554-563.
70. Weatherley C., Jaffray D., Terry A. Vascular complications associated with osteotomy in ankylosing spondylitis: a report of two cases. Spine (Phila Pa 1976). 1988;13:43-46.
71. Chapchal G. Columnotomy in severe Bechterew kyphosis. Acta Orthop Belg. 1972;58:55-58.
72. Lazennec J.Y., Saillant G., Saidi K., et al. Surgery of the deformities in ankylosing spondylitis: our experience of lumbar osteotomies in 31 patients. Eur Spine J. 1997;6:222-232.