Chapter 451 Definitions and Classification of Hemolytic Anemias
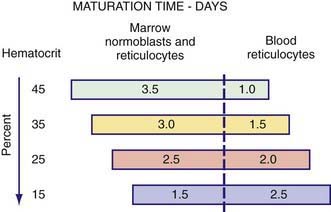
where µ is a maturation factor of 1-3 related to the severity of the anemia (see ![]() Fig. 451-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com). The normal reticulocyte index is 1.0; therefore, the index measures the fold increase in erythropoiesis (e.g., 2-fold, 3-fold).
Fig. 451-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com). The normal reticulocyte index is 1.0; therefore, the index measures the fold increase in erythropoiesis (e.g., 2-fold, 3-fold).
As anemia becomes more severe, the erythropoietin concentration increases and reticulocytes are released from the marrow earlier; they are identifiable as reticulocytes in the blood that last for >1 day. Because the reticulocyte index is essentially a measure of RBC production per day, the maturation factor, µ, provides this correction (see Fig. 451-1). The usual marrow response in acute hemolytic anemia is reflected by a reticulocyte index of 2-3, whereas in long-standing chronic hemolysis, the increase in erythropoiesis is approximately 4–6-fold.
The erythroid hyperplasia resulting from chronic hemolytic anemia in children, especially thalassemia, may be so extensive that the medullary spaces expand at the expense of the cortical bone. These changes may be evident on physical examination or on radiographs of the skull and long bones (see Fig. 456-7). A propensity to fracture long bones can also occur.
Several other plasma, urinary, or fecal chemical alterations reflect the presence of hemolysis. The exaggerated degradation rate of hemoglobin results in increased biliary excretion of heme pigment derivatives and increased urinary and fecal urobilinogen (Fig. 451-2). Gallstones composed of calcium bilirubinate may be formed in children with chronic hemolysis as young as 4 yr of age. Elevations of serum unconjugated bilirubin and lactate dehydrogenase also can accompany hemolysis.
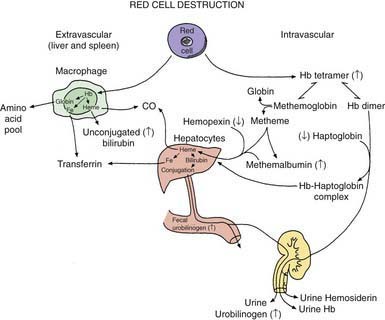
Figure 451-2 Red cell destruction and catabolism of hemoglobin (Hb) based on the description by Hillman and Finch. Fe, iron
(From Hillman RS, Finch CA: Red cell manual, Philadelphia, 1983, FA Davis.)
Three heme-binding proteins in the plasma are altered during hemolysis (see Fig. 451-2). Hemoglobin binds to haptoglobin and hemopexin, both of which are cleared more rapidly as conjugates, resulting in a reduced plasma concentration. Oxidized heme binds to albumin to form methemalbumin, which is increased in the plasma. When the capacity of these binding molecules is exceeded, free hemoglobin appears in the plasma, and the pink color can be seen if the plasma is partitioned after centrifugation in a capillary hematocrit tube. If present, free hemoglobin in the plasma is prima facie evidence of intravascular hemolysis. Free hemoglobin dissociates into dimers and is filtered by the kidneys. When the tubular reabsorptive capacity of the kidneys for hemoglobin is exceeded, free hemoglobin appears in the urine. Even in the absence of hemoglobinuria, iron loss can result from reabsorbed hemoglobin and the shedding of renal epithelial cells in which the iron from hemoglobin is stored as hemosiderin. This iron loss can lead to iron deficiency during chronic intravascular hemolysis. When hemoglobin is degraded, an α-methene bridge is broken in the cyclic tetrapyrrole of the heme moiety, with release of carbon monoxide (CO) (see Fig. 451-2). The amount of CO in the blood or expired air provides a dynamic measure of the hemolytic rate; end-tidal CO is not available in most clinical laboratories to measure hemolysis.
The hematocrit level depends on the severity of hemolysis and on the erythropoietic response. The shortened RBC life span and heightened RBC production result in a marked susceptibility to aplastic or hypoplastic crises, characterized by erythroid marrow failure and reticulocytopenia, accompanied by a rapid reduction in hemoglobin and hematocrit to extremely low levels. The most common cause of aplastic crisis is infection with parvovirus B19, which is erythrocytotropic (Chapters 243, 444, 452, and 456). An aplastic crisis can produce a precipitous and life-threatening decline in hematocrit that usually lasts 10-14 days. Such transient erythroid marrow failure has only a mild effect in persons with a normal RBC life span, but it has a proportionately greater effect if the RBC life span is shortened by hemolysis. A second infection with parvovirus B19 is uncommon, but other infections can compromise erythroid marrow output, resulting in various degrees of hypoplasia or hypoplastic crises.
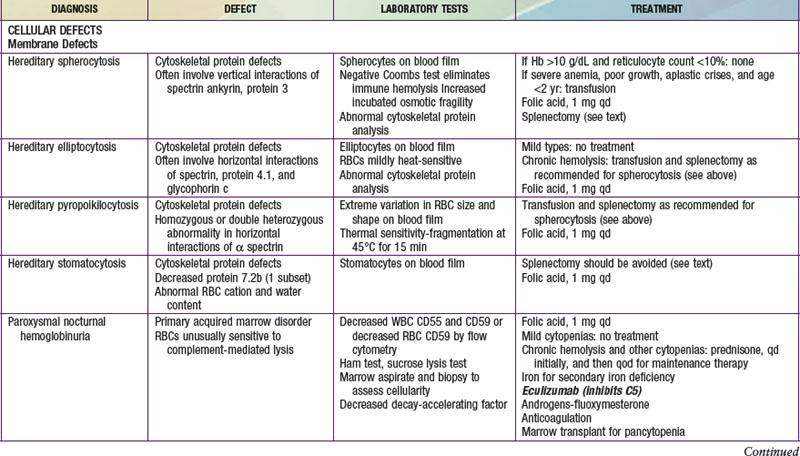
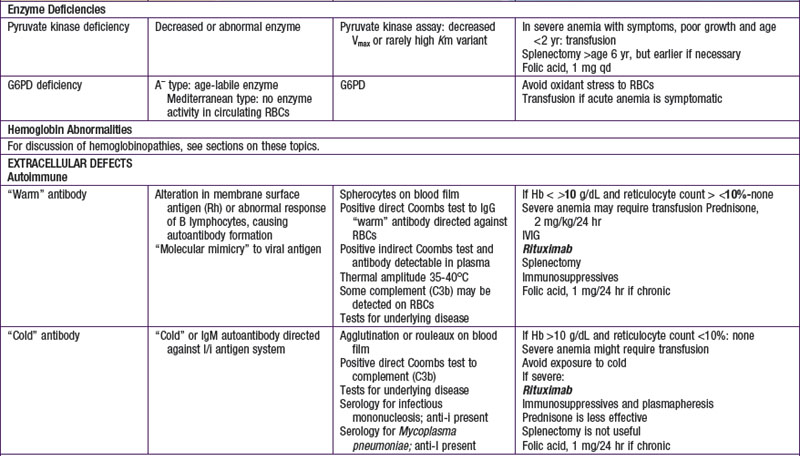
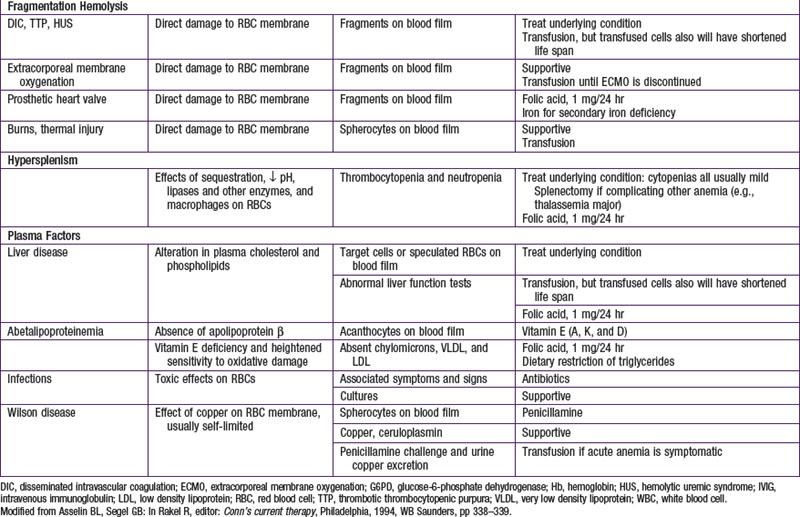
Hemolytic anemias may be classified as either cellular, resulting from intrinsic abnormalities of the membrane, enzymes, or hemoglobin; or extracellular, resulting from antibodies, mechanical factors, or plasma factors. Most cellular defects are inherited (paroxysmal nocturnal hemoglobinuria is acquired), and most extracellular defects are acquired (abetalipoproteinemia with acanthocytosis is inherited). Table 451-1 shows the most common hemolytic anemias, their underlying defects, the diagnostic laboratory tests, and the current recommendations for treatment.