Chapter 54 Deficit Therapy
Dehydration, most often due to gastroenteritis, is a common problem in children. Most cases can be managed with oral rehydration (Chapter 332). Even children with mild to moderate hyponatremic or hypernatremic dehydration can be managed with oral rehydration. This chapter focuses on the child who requires intravenous therapy, although many of the same principles are used in oral rehydration.
Clinical Manifestations
The first step in caring for the child with dehydration is to assess the degree of dehydration (Table 54-1), which dictates both the urgency of the situation and the volume of fluid needed for rehydration. The infant with mild dehydration (3-5% of body weight dehydrated) has few clinical signs or symptoms. The infant may be thirsty; the alert parent may notice a decline in urine output. The history is most helpful. The infant with moderate dehydration has clear physical signs and symptoms. Intravascular space depletion is evident from an increased heart rate and reduced urine output. This patient needs fairly prompt intervention. The infant with severe dehydration is gravely ill. The decrease in blood pressure indicates that vital organs may be receiving inadequate perfusion. Immediate and aggressive intervention is necessary. If possible, the child with severe dehydration should initially receive intravenous therapy. For older children and adults, mild, moderate, or severe dehydration represents a lower percentage of body weight lost. This difference occurs because water accounts for a higher percentage of body weight in infants (Chapter 52).
Table 54-1 CLINICAL EVALUATION OF DEHYDRATION
Physical examination findings are usually proportional to the degree of dehydration. Parents may be helpful in assessment of the child for the presence of sunken eyes, because this finding may be subtle. Pinching and gently twisting the skin of the abdominal or thoracic wall detects tenting of the skin (turgor, elasticity). Tented skin remains in a pinched position rather than springing quickly back to normal. It is difficult to properly assess tenting of the skin in premature infants or severely malnourished children. Activation of the sympathetic nervous system causes tachycardia in children with intravascular volume depletion; diaphoresis may also be present. Postural changes in blood pressure are often helpful for evaluating and assessing the response to therapy in children with dehydration. Tachypnea in children with dehydration may be present secondary to a metabolic acidosis from stool losses of bicarbonate or due to lactic acidosis from shock (Chapter 64).
Laboratory Findings
Several laboratory findings are useful for evaluating the child with dehydration. The serum sodium concentration determines the type of dehydration. Metabolic acidosis may be due to stool bicarbonate losses in children with diarrhea, secondary renal insufficiency, or lactic acidosis from shock. The anion gap is useful for differentiating among the various causes of a metabolic acidosis (Chapter 52). Emesis or nasogastric losses usually cause a metabolic alkalosis. The serum potassium concentration may be low as a result of diarrheal losses. In children with dehydration due to emesis, gastric potassium losses, metabolic alkalosis, and urinary potassium losses all contribute to hypokalemia. Metabolic acidosis, which causes a shift of potassium out of cells, and renal insufficiency may lead to hyperkalemia. A combination of mechanisms may be present; thus, it may be difficult to predict the child’s acid-base status or serum potassium level from the history alone.
The blood urea nitrogen (BUN) value and serum creatinine concentration are useful in assessing the child with dehydration. Volume depletion without parenchymal renal injury may cause a disproportionate increase in the BUN with little or no change in the creatinine concentration. This condition is secondary to increased passive resorption of urea in the proximal tubule due to appropriate renal conservation of sodium and water. The increase in the BUN with moderate or severe dehydration may be absent or blunted in the child with poor protein intake, because urea production depends on protein degradation. The BUN may be disproportionately increased in the child with increased urea production, as occurs with a gastrointestinal bleed or with the use of glucocorticoids, which increase catabolism. A significant elevation of the creatinine concentration suggests renal insufficiency, although a small, transient increase can occur with dehydration. Acute tubular necrosis (Chapter 529) due to volume depletion is the most common etiology of renal insufficiency in a child with volume depletion, but occasionally the child may have previously undetected chronic renal insufficiency or an alternative explanation for the acute renal failure. Renal vein thrombosis is a well-described sequela of severe dehydration in infants; possible findings include thrombocytopenia and hematuria (Chapter 513.7).
Approach to Dehydration
The child with dehydration needs acute intervention to ensure that there is adequate tissue perfusion. This resuscitation phase requires rapid restoration of the circulating intravascular volume and treatment of shock with an isotonic solution, such as normal saline (NS) or Ringer lactate (LR) (Chapter 64). The child is given a fluid bolus, usually 20 mL/kg of the isotonic fluid, over approximately 20 min. The child with severe dehydration may require multiple fluid boluses and may need to receive the boluses as fast as possible. In a child with a known or probable metabolic alkalosis (the child with isolated vomiting), LR should not be used because the lactate would worsen the alkalosis.
Colloids, such as blood, 5% albumin, and plasma, are rarely needed for fluid boluses. A crystalloid solution (NS or LR) is satisfactory, with both less infectious risk and lower cost. Blood is obviously indicated in the child with significant anemia or acute blood loss. Plasma is useful for children with a coagulopathy. The child with hypoalbuminemia may benefit from 5% albumin, although there is evidence that albumin infusions increase mortality in adults. The volume and the infusion rate for colloids are generally modified compared with crystalloids (Chapters 464 and 467).
With adequate intravascular volume, it is appropriate to plan the fluid therapy for the next 24 hr. A general approach is outlined in Table 54-2, with the caveat that there are many different approaches to correcting dehydration. In isonatremic or hyponatremic dehydration, the entire fluid deficit is corrected over 24 hr; a slower approach is used for hypernatremic dehydration (discussed later). To assure that the intravascular volume is restored, the patient receives an additional 20-mL/kg bolus of an isotonic fluid over 2 hr. The child’s total fluid needs are added together (maintenance + deficit). The volume of isotonic fluids that the patient has received is subtracted from this total. The remaining fluid volume is then administered over 24 hr. The potassium concentration may need to be decreased or, less commonly, increased, depending on the clinical situation. Potassium is not usually included in the intravenous fluids until the patient voids. Children with significant ongoing losses need to receive an appropriate replacement solution (Chapter 53).
Monitoring and Adjusting Therapy
The formulation of a plan for correcting a child’s dehydration is only the beginning of management. All calculations in fluid therapy are only approximations. This statement is especially true for the assessment of percentage dehydration. It is equally important to monitor the patient during treatment and to modify therapy on the basis of the clinical situation. The cornerstones of patient monitoring are listed in Table 54-3. The patient’s vital signs are useful indicators of intravascular volume status. The child with decreased blood pressure and an increased heart rate will probably benefit from a fluid bolus. Central venous pressure is an excellent indicator of fluid status in the critically ill child with shock.
The patient’s intake and output are critically important in the dehydrated child. The child who, after 8 hr of therapy, has more output than input because of continuing diarrhea needs to be started on a replacement solution. See the guidelines in Chapter 53 for selecting an appropriate replacement solution. Urine output is useful for evaluating the success of therapy. Good urine output indicates that rehydration has been successful.
Both hypokalemia and hyperkalemia are potentially serious (Chapter 52). Because dehydration can be associated with acute renal failure and hyperkalemia, potassium is withheld from intravenous fluids until the patient has voided. The potassium concentration in the patient’s intravenous fluids is not rigidly prescribed. Rather, the patient’s serum potassium level and underlying renal function are used to modify potassium delivery. The patient with an elevated creatinine value and a potassium level of 5 mEq/L does not receive any potassium until the serum potassium level decreases. Conversely, the patient with a potassium level of 2.5 mEq/L may require additional potassium.
Hyponatremic Dehydration
The initial goal in treating hyponatremia is correction of intravascular volume depletion with isotonic fluid (NS or LR). An overly rapid (>12 mEq/L over the first 24 hr) or overcorrection in the serum sodium concentration (>135 mEq/L) is associated with an increased risk of central pontine myelinolysis (Chapter 52). Most patients with hyponatremic dehydration do well with the same basic strategy that is outlined in Table 54-2. Again, potassium delivery is adjusted according to the initial serum potassium level and the patient’s renal function. Potassium is not given until the patient voids.
The patient’s sodium concentration is monitored closely to ensure appropriate correction, and the sodium concentration of the fluid is adjusted accordingly. Patients with ongoing losses require an appropriate replacement solution (Chapter 53). Patients with neurologic symptoms (seizures) as a result of hyponatremia need to receive an acute infusion of hypertonic (3%) saline to increase the serum sodium concentration rapidly (Chapter 52).
Hypernatremic Dehydration
Hypernatremic dehydration is the most dangerous form of dehydration due to complications of hypernatremia and of therapy. Hypernatremia can cause serious neurologic damage, including central nervous system hemorrhages and thrombosis. This damage appears to be secondary to the movement of water from the brain cells into the hypertonic extracellular fluid, causing brain cell shrinkage and tearing blood vessels within the brain (Chapter 52).
To minimize the risk of cerebral edema during the correction of hypernatremic dehydration, the serum sodium concentration should not decrease by >12 mEq/L every 24 hr. The deficits in severe hypernatremic dehydration may need to be corrected over 2-4 days (Table 54-4).
To avoid cerebral edema during correction of hypernatremic dehydration, the fluid deficit is corrected slowly. The rate of correction depends on the initial sodium concentration (see Table 54-4). There is no general agreement on the choice or the rate of fluid for correcting hypernatremic dehydration. The choice and the rate of fluid administration are not nearly as important as vigilant monitoring of the serum sodium concentration and adjustment of the therapy according to the result (see Table 54-4). The rate of decrease of the serum sodium concentration is roughly related to the “free water” delivery, although there is considerable variation between patients. Free water is water without sodium. NS contains no free water, half-normal saline (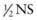 ) is 50% free water, and water is 100% free water. Smaller patients, to achieve the same decrease in the sodium concentration, tend to need higher amounts of free water delivery per kilogram because of higher insensible fluid losses. Five percent dextrose (D5) with
) is 50% free water, and water is 100% free water. Smaller patients, to achieve the same decrease in the sodium concentration, tend to need higher amounts of free water delivery per kilogram because of higher insensible fluid losses. Five percent dextrose (D5) with 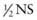 is usually an appropriate starting solution for a patient with hypernatremic dehydration. Some patients, especially infants with ongoing high insensible water losses, may need to receive D5 0.2NS. Others require a higher sodium concentration than is present in D5
is usually an appropriate starting solution for a patient with hypernatremic dehydration. Some patients, especially infants with ongoing high insensible water losses, may need to receive D5 0.2NS. Others require a higher sodium concentration than is present in D5 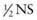 . A child with dehydration due to pure free water loss, as usually occurs with diabetes insipidus, usually needs a more hypotonic fluid than a child with depletion of both sodium and water due to diarrhea.
. A child with dehydration due to pure free water loss, as usually occurs with diabetes insipidus, usually needs a more hypotonic fluid than a child with depletion of both sodium and water due to diarrhea.
Adjustment in the sodium concentration of the intravenous fluid is the most common approach to modifying the rate of decrease in the serum concentration (see Table 54-4). For difficult patients with severe hypernatremia, having 2 intravenous solutions (D5 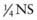 and D5 NS, both with the same concentration of potassium) at the bedside can facilitate this approach by allowing for rapid adjustments of the rates of the 2 fluids. If the serum sodium concentration decreases too rapidly, the rate of D5 NS can be increased and the rate of D5
and D5 NS, both with the same concentration of potassium) at the bedside can facilitate this approach by allowing for rapid adjustments of the rates of the 2 fluids. If the serum sodium concentration decreases too rapidly, the rate of D5 NS can be increased and the rate of D5 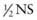 can be decreased by the same amount. Adjustment in the total rate of fluid delivery is another approach to modifying free water delivery. For example, if the serum sodium concentration is decreasing too slowly, the rate of the intravenous fluid can be increased, thereby increasing the delivery of free water. There is limited flexibility in modifying the rate of the intravenous fluid because patients generally should receive 1.25-1.5 times the normal maintenance fluid rate. Nevertheless, in some situations, it can be a helpful adjustment.
can be decreased by the same amount. Adjustment in the total rate of fluid delivery is another approach to modifying free water delivery. For example, if the serum sodium concentration is decreasing too slowly, the rate of the intravenous fluid can be increased, thereby increasing the delivery of free water. There is limited flexibility in modifying the rate of the intravenous fluid because patients generally should receive 1.25-1.5 times the normal maintenance fluid rate. Nevertheless, in some situations, it can be a helpful adjustment.
Because increasing the rate of the intravenous fluid increases the rate of decline of the sodium concentration, signs of volume depletion are treated with additional isotonic fluid boluses. The serum potassium concentration and the level of renal function dictate the potassium concentration of the intravenous fluid; potassium is withheld until the patient voids. Patients with hypernatremic dehydration need an appropriate replacement solution if they have ongoing, excessive losses (Chapter 53).
Seizures are the most common manifestation of cerebral edema from an overly rapid decrease of the serum sodium concentration during correction of hypernatremic dehydration. Acutely, increasing the serum concentration via an infusion of 3% sodium chloride can reverse the cerebral edema. Each 1-mL/kg of 3% sodium chloride increases the serum sodium concentration by approximately 1 mEq/L. An infusion of 4 mL/kg often results in resolution of the symptoms. This strategy is similar to that used for treating symptomatic hyponatremia (Chapter 52).
In patients with severe hypernatremia, oral fluids must be used cautiously. Infant formula, because of its low sodium concentration, has a high free water content, and especially if added to intravenous therapy, it may contribute to a rapid decrease in the serum sodium concentration. Less hypotonic fluid, such as an oral rehydration solution, may be more appropriate initially (Chapter 332). If oral intake is allowed, its contribution to free water delivery must be taken into account, and adjustment in the intravenous fluid is usually appropriate. Judicious monitoring of the serum sodium concentration is critical.
Alam NH, Islam S, Sattar S, et al. Safety of rapid intravenous rehydration and comparative efficacy of 3 oral rehydration solutions in the treatment of severely malnourished children with dehydrating cholera. J Pediatr Gastroenterol Nutr. 2009;48:318-327.
Chen L, Kim Y, Santucci KA. Use of ultrasound measurement of the inferior vena cava diameter as an objective tool in the assessment of children with clinical dehydration. Acad Emerg Med. 2007;14:841-845.
Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
Hartling L, Bellemare S, Wiebe N, et al: Oral versus intravenous rehydration for treating dehydration due to gastroenteritis in children, Cochrane Database Syst Rev (3):CD004390, 2006.
Madati PJ, Bachur R. Development of an emergency department triage tool to predict acidosis among children with gastroenteritis. Pediatr Emerg Care. 2008;24:822-830.
Modi N. Avoiding hypernatraemic dehydration in healthy term infants. Arch Dis Child. 2007;92:474-475.
Reid SR, Losek JD. Rehydration: role for early use of intravenous dextrose. Pediatr Emerg Care. 2009;25:49-52.
Shavit I, Brant R, Nijssen-Jordan C, et al. A novel imaging technique to measure capillary-refill time: improving diagnostic accuracy for dehydration in young children with gastroenteritis. Pediatrics. 2006;118:2402-2408.
Steiner MJ, Nager AL, Wang VJ. Urine specific gravity and other urinary indices: inaccurate tests for dehydration. Pediatr Emerg Care. 2007;23:298-303.
van Dommelen P, van Wouwe JP, Breuning-Boers JM, et al. Reference chart for relative weight change to detect hypernatraemic dehydration. Arch Dis Child. 2007;92:490-494.




