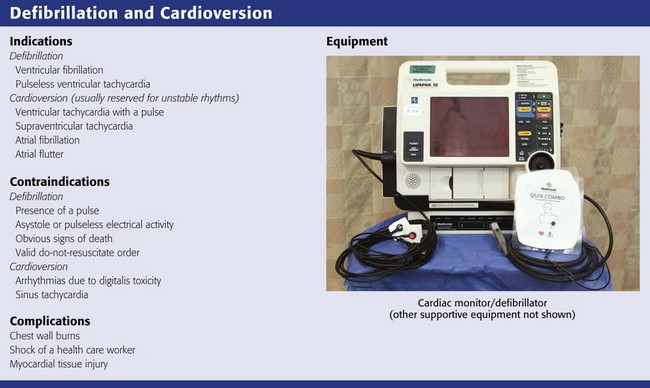
Defibrillation and Cardioversion
Introduction
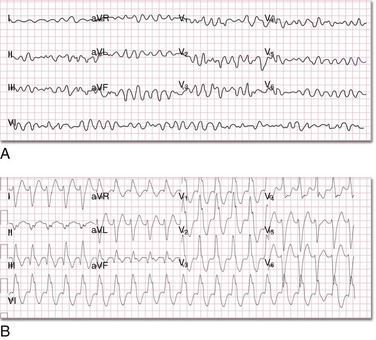
Defibrillation is an emergency procedure performed to terminate ventricular fibrillation (VF) (Fig. 12-1A). VF is a potentially lethal, but survivable “rhythm” commonly found in victims of sudden cardiac arrest (SCA).1,2 VF can be caused by myocardial infarction, myocardial ischemia, undiagnosed coronary artery disease, and electrical injuries. Medications such as tricyclic antidepressants, digitalis, quinidine, and other proarrhythmics can cause QT-segment prolongation and changes in the refractory period of the cardiac cycle that are capable of precipitating VF. Furthermore, chest trauma, hypothermia, cardiomyopathy, electrolyte disturbances, and various toxidromes can induce conditions favoring the development of VF. Hypoxia is another culprit that frequently precipitates VF in adults and the pediatric population. Congenital malformations of the heart and great vessels have also been associated with an increased incidence of VF in young children. The most effective treatment of VF in its early phase is defibrillation.3 It can also be used to terminate pulseless ventricular tachycardia (VT) (Fig 12-1B). Patients with VF or pulseless VT are unresponsive, pulseless, and apneic. These patients sometimes require appropriate integration of cardiopulmonary resuscitation (CPR) with defibrillation to establish the return of spontaneous circulation (ROSC). Other dysrhythmias may also be encountered in patients with SCA, such as pulseless electrical activity (PEA) and even asystole; however, in this chapter discussion is limited to the treatment of VF and pulseless VT.
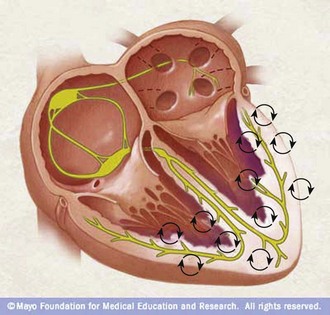
Defibrillation entails passing a therapeutic burst of electrical current across the chest wall through the myocardium for the purpose of terminating the chaotic electromechanical activity that is impeding the ventricles from ejecting blood into the circulation (Fig. 12-2). Failure to recognize and terminate VF promptly makes suppression of VF via defibrillation more difficult.4 For every minute that the heart is in VF without treatment, the potential for the initial defibrillation to be successful and for the victim of SCA to survive decreases by 7% to 10%.5 However, the integration of CPR with defibrillation, when appropriate, increases the chance for successful defibrillation and survival from SCA.6
Cardioversion is performed to suppress dysrhythmias that produce a rapid pulse and cause the patient to become unstable; such dysrhythmias include supraventricular tachycardia (SVT), atrial fibrillation (AF), atrial flutter, and unstable monomorphic VT (Fig. 12-3). These patients do have a pulse, albeit weak, but can rapidly decompensate, become hypotensive, experience chest pain, or have a change in mental status that will require rapid intervention (i.e., cardioversion). CPR is obviously not indicated because these patients have a pulse and their peripheral tissues are being perfused. Cardioversion is very similar to defibrillation; however, the shock is administered during the refractory period of the cardiac cycle. This is accomplished by setting the defibrillator to the synchronized mode.
Principles of Resuscitation
The clinical approach to cardiac resuscitation is an evolving and dynamic endeavor, and guidelines frequently change or are altered. Recommendation from the American Heart Association (AHA) are considered the most reasonable guidelines for the clinician, but many of the principles and caveats are based on minimal data, can be contradictory, and are subject to change; more importantly, any guideline is best applied by considering a specific clinical scenario. Most recently, cardiac resuscitation has been reviewed and new AHA guidelines were released in 2010.7–10 On the basis of the strength of the evidence available, the AHA developed recommendations to support the interventions that showed the most promise. The new algorithms reflect alterations in the sequence of actions to be performed and stress high-quality CPR with compressions of adequate rate and depth that allow complete chest recoil after compressions, minimize interruptions in chest compressions, and avoid excessive overventilation. These modifications stress the interposition of effective CPR (Fig. 12-4) with defibrillation and have been organized in such a way that the time until the first shock is minimized and time to initiation of effective chest compressions is not unnecessarily delayed.
Anatomy, Physiology, and Pathophysiology
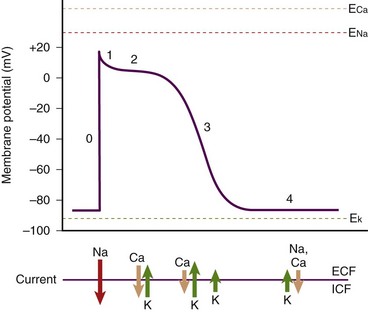
The normal human heart rate (HR) is approximately 80 (±20) beats/min. With each beat the heart ejects a stroke volume (SV) of approximately 70 to 80 mL of blood from each ventricle. Multiplying HR by SV produces a value termed cardiac output (CO) (i.e., HR × SV = CO). The product of CO times total peripheral resistance (TPR) produces the value for mean arterial blood pressure (MABP) (i.e., CO × TPR = MABP). When the HR falls to zero or the heart fails to eject an SV (as in VF), MABP drops precipitously. Subsequently, vital organ perfusion is compromised. Hence, blood flow to the brain, the heart, the lungs, and other peripheral organs ceases. Failure to promptly restore blood flow will lead to significant mortality, morbidity, and SCA. Therefore, any interruption in cardiac contraction must be recognized quickly and corrected promptly. Cardiac contraction occurs as a result of a sequence of electromechanical events occurring in myocytes. The human heart has several unique characteristics that enable it to perform its physiologic role. These myocardial characteristics are automaticity, conductivity, excitability, and contractility. Individual cells have a “variable blend” of these characteristics. Some characteristics are more prominent than others, depending on the anatomic location of the cells in the heart. For example, the “pacemaker cells” have more automaticity, the conduction system has increased conductivity, and ventricular free-wall myocytes have more contractility. The electrical properties of these cells can be assessed by performing regional recordings of the changes in voltage in the tissue with respect to time (i.e., action potentials) (Fig. 12-5). The electrical impulse for myocardial contraction originates spontaneously in the sinoatrial (SA) node and spreads through the atria, which causes it to contract. As the impulse arrives at the atrioventricular (AV) node, it undergoes decremental conduction in which the electrical impulse is slowed down as the atria contract and “preload” the ventricles. Subsequently, the impulse activates the bundle of His and Purkinje fibers, which then causes ventricular contraction via excitation-contraction coupling. The electrical events precede the mechanical events. These events are graphically represented in Figure 12-6, which depicts the change in membrane voltage with respect to time as a result of temporal changes in ion permeability across the myocyte membranes. These sequential changes in ion permeability occur as the membrane potential varies, thereby producing the characteristic cardiac action potential.
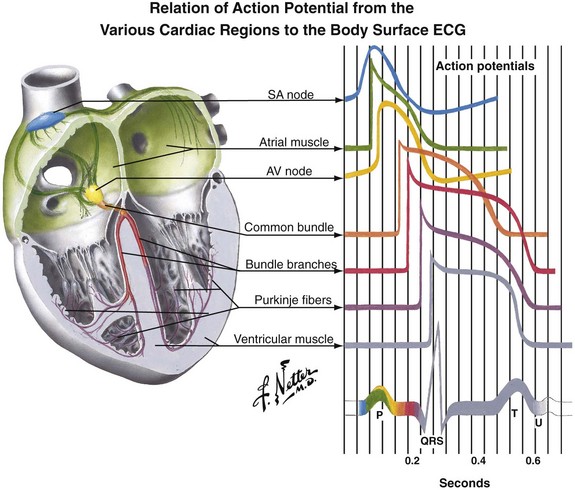
Figure 12-5 Regional action potentials and electrocardiographic correlation. AV, atrioventricular; SA, sinoartrial. (Netter illustration from www.netterimages.com. © Elsevier Inc. All rights reserved.)
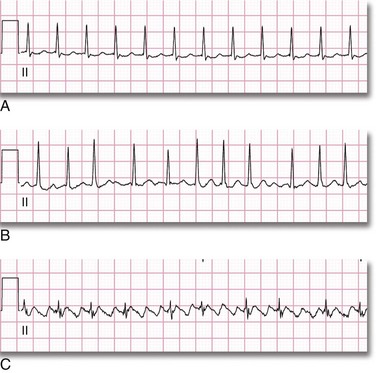
As the original impulse from the SA node travels through the atria and into the ventricles, various action potentials are generated regionally. The summation of all these action potentials produces the characteristic electrocardiographic (ECG) tracing PQRST (see Figs. 12-5 and 12-6). The ECG tracing is a graphic representation of the electrical activity that induces the mechanical activity of systole. Systole occurs as a result of excitation-contraction coupling. Calcium ion levels in the cytoplasm increase and trigger the contractile proteins to interact. As the ion channels reset, the myocytes return to the resting membrane potential (intracellular calcium is resequestered), and diastole occurs. The membrane pumps restore the ion concentrations to normal. This cycle keeps occurring about 80 times per minute. Each “cardiac cycle” lasts approximately 300 msec. During each cardiac cycle there are two periods that need to be addressed: the absolute and relative refractory periods (Fig. 12-7). During the absolute refractory period, the myocytes do not respond to excitatory stimuli because the channels are in full operation. During the relative refractory period, the myocytes can be stimulated with a stimulus that is proportionately larger than usual as more and more ion channels reset. These facts have relevance with regard to cardioversion and will be discussed further later in the chapter.
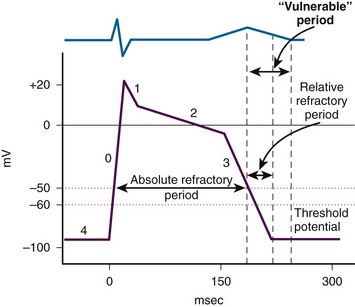
Figure 12-7 Absolute and relative refractory periods.
Mechanisms of Cardiac Dysrhythmias
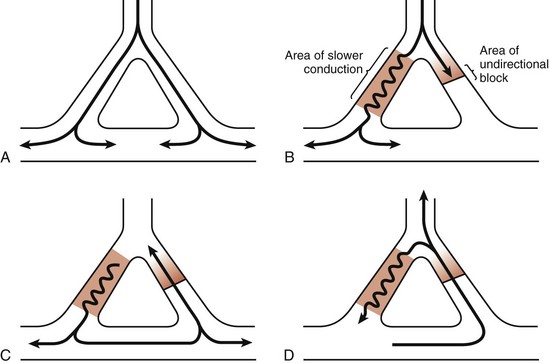
As is evident from the preceding discussion, normal cardiac activity is a compendium of complex, sequential electrochemical, physiologic, and mechanical events. It includes three mechanisms: enhanced automaticity, triggered activity, and reentry. If alterations in the action potential phases or a modification of the refractory periods occurs and another impulse stimulates the myocyte at a time that it is out of synch with the normal depolarization-repolarization process, the coordinated normal excitation-contraction coupling becomes asynchronous. If conditions favor the development of ectopic foci, individual loci in the ventricular free walls and septum become “pacemakers” and the myocardium begins to contract uncontrollably (see Fig. 12-2) and produce an irregular ECG tracing (see Fig. 12-1A). CO falls to zero, with SCA ensuing. Another proposed mechanism that can precipitate the development of a dysrhythmia is a malfunction in propagation secondary to errors in conductivity and excitability and reentry of already propagated impulses (Fig. 12-8).
Cardiopulmonary Resuscitation: Ventricular Fibrillation and Pulseless Ventricular Tachycardia
When SCA occurs and the heart is in VF or pulseless VT, ventricular contraction is absent and circulation of blood comes to a standstill. To initiate CPR, mechanically compress the heart between the sternum and vertebral column. This causes pulsatile ejection of blood into the circulation, including the coronary circulation. For these compressions to be effective, perform them quickly and with sufficient displacement of the sternum (i.e., at least 2 inches) to produce adequate flow. Furthermore, keep interruptions in CPR to a minimum so that adequate perfusion pressure is maintained in the vasculature. Although the flow is not at physiologic levels, enough circulation occurs in the tissues, especially the myocardium, that the by-products of VF are “washed out” and the myocardium becomes less refractory to defibrillation.11 During VF, the myocytes are actually consuming oxygen and adenosine triphosphate at a rate believed to be the same or higher than during normal contraction.12,13 Several other concerns must be reinforced. During chest compressions, make sure that the chest recoils completely to the resting state so that blood can enter from the vena cava and pass into the right atrium. The rate of compressions should exceed 100 compressions/min so that adequate forward flow of blood is produced. Remember that HR × SV = CO.
Indications for and Contraindications to Defibrillation
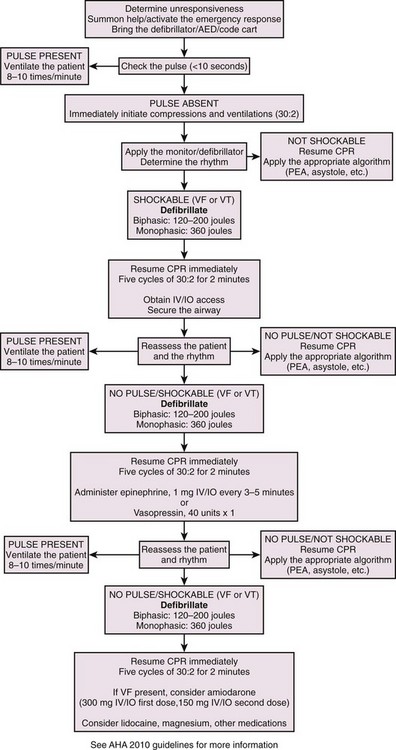
Prompt electrical defibrillation is the most effective treatment of acute SCA and VF.3,4 Prompt initiation of CPR in patients with SCA or VF is also critical for successful resuscitation and ROSC. Starting with the onset of collapse, the survival rate for patients with SCA or VF drops 7% to 10% for every minute of downtime without defibrillation.5 If CPR is initiated, the survival rate declines less rapidly (i.e., 3% to 4% per minute of downtime).4 If an SCA is witnessed and immediate CPR is provided, coupled with immediate defibrillation, survival from such events has been reported to increase up to fourfold.4–6 Therefore, immediate defibrillation is indicated as soon as VF or pulseless VT is diagnosed. Few absolute specific contraindications to early defibrillation exist other than the presence of a pulse, absence of SCA, medical futility for the procedure, or a valid do-not-resuscitate order.
If a patient is found unresponsive, pulseless, and apneic and the “downtime” is unknown, immediately perform good-quality, effective CPR while preparing for defibrillation. Previous recommendations called for immediate defibrillation in lieu of a short period of CPR. The newest development in the 2010 AHA guidelines for CPR is also a change in the basic life support sequence of steps from the “ABCs” (airway, breathing, chest compressions) to “CAB” (chest compressions, airway, breathing) for adults and pediatric patients (children and infants, excluding newborns). As CPR is performed, prepare for rhythm analysis and initiate defibrillation if indicated. After performing CPR for 2 minutes (5 cycles at a rate of 30 compressions to 2 ventilations), perform rhythm analysis. If VF or pulseless VT is diagnosed, promptly perform defibrillation. When the time until the first shock is delayed during prehospital resuscitation (because of prolonged response times), data have demonstrated that the rate of successful defibrillation increases if patients receive bystander CPR before defibrillation.2–6 A scientific evaluation of this information proposed that CPR enhances the defibrillation threshold by restoring substrates to myocytes for the facilitation or resumption of normal excitation-contraction coupling. Furthermore, CPR may wash out myocardial depressants that have built up during prolonged VF. Therefore, administration of CPR before defibrillation in patients with suspected, prolonged VF is recommended in the prehospital setting. Data to substantiate this sequence for in-hospital resuscitation have not been presented. Thus, the issue of unknown downtime, though not a definitive contraindication to immediate defibrillation, may be a factor in the clinician’s decision-making process regarding the resuscitation sequence.
Victims of SCA as a result of traumatic injuries do not usually survive.13 The heart, aorta, and pulmonary arteries may have sustained injury that will prevent resumption of normal cardiovascular function. There is a high probability that the underlying hypovolemia and organ damage may preclude successful resuscitation. However, the cause of the trauma may have been SCA with subsequent loss of consciousness. In such cases, if SCA or VF is present in a trauma patient, attempt treatment with CPR and defibrillation; if unsuccessful, search for and treat the underlying cause of the trauma and pursue the SCA. Therefore, trauma is not a contraindication to defibrillation, although the resuscitative effort may be futile.
If the victim of VF or pulseless VT is a pregnant female, treatment of the mother is critical. Therefore, prompt defibrillation is indicated as per the same guidelines and sequencing as for nonpregnant patients.14 No harm to the fetus has been reported as a result of defibrillation, and thus pregnancy is not a contraindication to defibrillation.
Previous recommendations suggested delivering a “stacked” sequence of up to three shocks without interposed chest compressions if the first shock was unsuccessful in terminating VF. This was done to decrease transthoracic impedance with the monophasic damped sinusoidal (MDS) defibrillators in use and to deliver more current to the myocardium. However, this recommendation has been rescinded because of lack of supporting evidence. Now, with the higher first-shock efficacy (90%) in successfully terminating VF (termination of VF for 5 seconds) through the use of biphasic defibrillators,8 the recommendation to repeat a shock if the first treatment was unsuccessful is harder to justify. Hence, the AHA now recommends a one-shock protocol for VF. Evidence has accumulated that even short interruptions in CPR are harmful. Thus, rescuers should minimize the interval between stopping compressions and delivering shocks and should resume CPR immediately after delivery of a shock.
Defibrillation can be an ignition source for explosion if arcing occurs or if there are any stray or aberrant electrical discharges that occur as a result of paddle or electrode discharge. Therefore, in an environment in which volatile explosive material is present, such as the operating room or other areas of critical care, be careful during defibrillation to avoid electrical arcing and to ensure that electrical conductivity through the patient’s chest is optimal. Avoid using anesthetic agents and oxygen. A potentially explosive environment is a relative contraindication to defibrillation.15
Finally, defibrillation of an “occult” or “false” asystole or a very fine VF not detectable because of paddle or electrode position may be considered but is not recommended.13 Fine VF can occasionally masquerade as ventricular standstill or asystole. This may be a function of perpendicular electrode orientation with respect to the wavefront of depolarization. When evaluating the rhythm of a patient, if there is any doubt or confusion regarding the type of rhythm present, make sure that several leads are checked and rotate the paddles 90 degrees from their original position to ensure that asystole is indeed present before abandoning the possibility of defibrillation. If fine VF is unmasked, consider providing aggressive CPR before defibrillation. Also, place the controls on the ECG monitor on maximal gain to ensure adequate amplification of weak signals.
Conductive Material
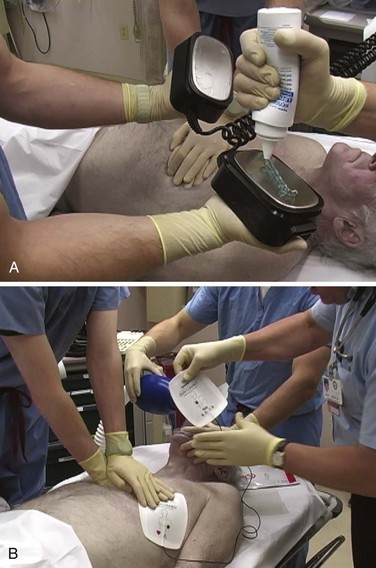
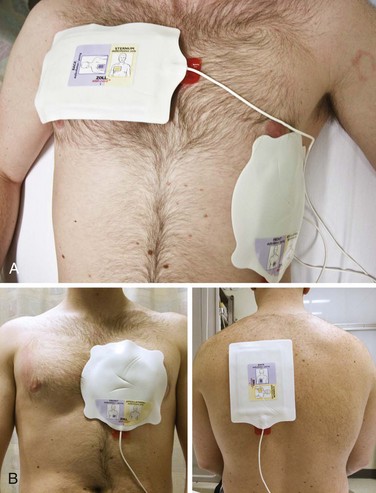
Use of conductive material is important to lower the impedance or resistance to flow of current at the electrode–chest wall interface.16–18 Multiple factors affect the range of impedance (e.g., body weight, chest size, chest hair, moisture on the skin surface of the patient, paddle size [diameter], paddle contact pressure, phase of respiration, and type of conductive material used). High impedance or resistance to flow of current can compromise the amount of current actually delivered to the myocardium and lead to a failed first shock. Inappropriate use of conductive material can result in current bridging or a short circuit and arcing of electrical current secondary to streaking of the material across the chest. This can produce sparks and unnecessary burns on the patient’s skin. In addition, arcing of electricity can become a possible explosion hazard, depending on the circumstances. Conductive material needs to be used with the handheld electrodes. Various electrode gels are available on the market and should be kept in the proximity of the defibrillator, on the prearranged cart ready to use (Fig 12-9A).
Self-adhesive pad electrodes now have a resistance-reducing, conductive material incorporated into the adhesive, thus rendering the use of a gel or other conductive material unnecessary. Firmly applying the self-adhesive electrode pads to the skin will usually be sufficient to minimize impedance, allow adequate ECG acquisition, and if indicated, defibrillate (see Fig. 12-9B).
Procedure
Witnessed Sudden Cardiac Arrest (Figs. 12-10 and 12-11)
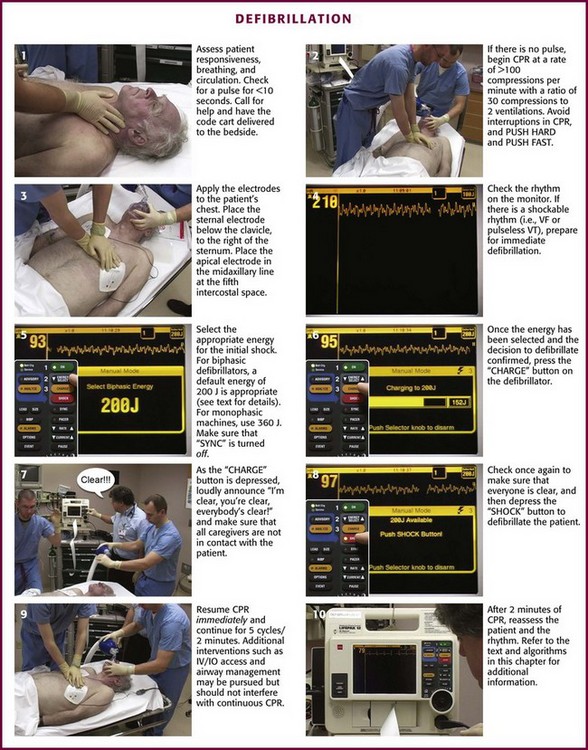
When confronted with a patient who has just become unresponsive, prepare for immediate defibrillation (Fig. 12-12). As soon as the defibrillator is available and the patient is connected to the monitor, assess the rhythm. In the interim, turn on the defibrillation equipment, place the paddles or electrodes on the chest, begin assessment of the patient, and initiate the steps in CPR by applying the CAB principle.7
Cardiopulmonary Resuscitation
Perform a pulse check (<10 seconds; see Fig. 12-11, step 1). If a pulse is definitely present, provide 1 breath for 1 second every 5 to 6 seconds or 8 to 10 breaths/min. The breaths can be delivered with either a bag-valve-mask (BVM) or some type of barrier device. Observe the patient for visible chest wall rise and fall so that the thorax dose not become overinflated. Hyperinflation of the chest can lead to inadvertent pressurization of the esophagus, which can cause lower esophageal sphincter pressure to be exceeded. This can lead to retrograde flow of gastric contents into the esophagus with the potential for subsequent aspiration of acid and debris into the trachea if the airway is not adequately protected. Overventilation of the thorax can also lead to an increase in intrathoracic pressure and impedance of blood flow to and from the heart, which should be avoided. Reassess the patient’s pulse every 2 minutes.
If no pulse is present, begin a sequence of 30 chest compressions followed by 2 ventilations/breaths (see Fig. 12-11, step 2). Keep your hands on the lower half of the sternum and compress it at least 2 inches (5 cm) at a rate of at least 100 compressions/min. The time allotted for compression should be 50%/50% for compression and relaxation of the chest. Watch for full chest recoil to allow adequate ventricular filling (do not lean on the chest) before the next compression. When the defibrillator or AED arrives, continue the 30 : 2 ratio of compressions to ventilations during CPR, attach the patient to the defibrillator via electrodes, pads, and paddles applied to the patient’s chest, and initiate the rhythm check (see Fig. 12-11, step 3). Every attempt should be made to minimize interruption of compressions.
Rhythm Assessment
The correct position for placement of either the handheld quick-look paddle electrodes or the self-adhesive pads is illustrated in Figure 12-13. Frequently, the pads are labeled with a diagram as a guide to placing the electrodes on the chest wall. Using the patient’s right side for orientation, place the sternal electrode just below the clavicle and just to the right of the sternum. Place the apical electrode in the midaxillary line around the fifth or sixth intercostal space. Once the electrodes or pads are in position, set the selector dial or switch on the defibrillator monitor to the appropriate position to acquire the ECG signal from the input source—either the handheld quick-look paddles or the multifunctional electrode pads. Errors sometimes occur when the selector switch is in the position for the patient cable and electrode pads while the operator is attempting to use the handheld paddles. This could lead to misinterpretation of the rhythm, with the operator perceiving that the patient is in asystole, whereas in reality, VF, pulseless VT, or some other rhythm is actually present. Be familiar with the operation of the switches. In addition, adjust the controls for gain of the ECG signal to increase the sensitivity or gain of the ECG amplifier to ensure that fine VF is not interpreted as asystole. As the ECG rhythm appears on the monitor, make a diagnosis of the type of rhythm or lack thereof (see Fig. 12-11, step 4). If a shockable rhythm such as VF or pulseless VT is present, defibrillation is indicated. Proceed to select the appropriate energy level for the anticipated defibrillation.
Energy Selection
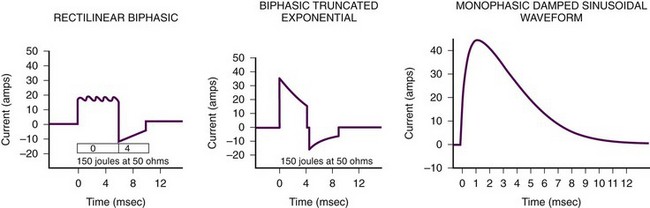
As noted previously, two major types of defibrillators are available: biphasic and monophasic (Fig. 12-14). Currently, the biphasic defibrillator, which is more likely to be found in the clinical setting, produces either a biphasic rectilinear waveform or a biphasic truncated exponential (BTE) waveform. However, there are still monophasic defibrillators present that usually produce an MDS waveform. Current data do not support one waveform over another, but biphasic defibrillators appear to be more efficient in achieving defibrillation with the first shock.
Defibrillate
Continue CPR until the defibrillator is charged and ready to defibrillate. Once the energy level has been selected and the decision made to defibrillate, clear the patient for defibrillation by loudly stating “I’m clear, you’re clear, everybody’s clear,” and then activate the button to charge the defibrillator (see Fig. 12-11, steps 6 and 7). Once the defibrillator has been charged and everyone is clear, apply firm pressure to the defibrillation paddles (25 lb) to increase contact and deflate the lungs to the end-expiration state. This will decrease impedance at the paddle–chest wall interface. Subsequently, depress the defibrillation controls and deliver the shock (see Fig. 12-11, step 8). This will usually be followed by a perceptible whole-body muscle twitch in the patient. If no obvious response or twitch of the patient is seen, check the defibrillator controls to make sure that it is in the unsynchronized mode and that the paddles are activated. If using the multifunctional pads, no pressure is needed.
Resume Cardiopulmonary Resuscitation
Once the shock has been delivered, resume resuscitation with immediate chest compressions (see Fig. 12-11, step 9). Continue chest compressions for approximately 5 cycles of 30 compressions to 2 ventilations, or about 2 minutes of CPR. This facilitates the transition from SCA to ROSC after the heart has been stunned by the defibrillation and may not be functioning at optimal contractility for a few minutes after the shock. If additional monitoring devices are in place such as arterial lines or Swan catheters, modify this step accordingly as dictated by the resuscitation team leader.
Second Defibrillation
If the patient does not have a shockable rhythm, proceed to the appropriate algorithm for VT, PEA, or asystole. Regarding airway management, consider using a supraglottic airway or endotracheal intubation without causing any significant interruption in chest compressions. Also, initiate end-tidal carbon dioxide measurements (capnography) to determine the adequacy of CPR and ROSC when applicable.7
Unwitnessed Arrest
Automated External Defibrillator Application
The availability of AEDs or semi-automated defibrillators in hospitals has increased, especially in non–critical care areas (Fig. 12-15). Although AEDs are designed for lay public use, application of these devices may also occur in the clinical setting. As in the algorithm, assess the patient, summon help, and apply the AED. Operation of the AED is guided by voice and visual prompts. Turn the device on, apply the patient electrodes in the appropriate positions, analyze the rhythm, and deliver a shock if a shockable rhythm is present. The AED will determine the rhythm and choose the energy level. Integrate CPR with the shocks to enhance the potential outcome of SCA resuscitation.

Figure 12-15 Automated external defibrillator.
Medication
As per the 2010 AHA guidelines, there are insufficient data to demonstrate that any drugs or mechanical CPR devices improve long-term outcome after cardiac arrest. There is now some concern that epinephrine, a long-time universally recommended adjunct to CPR, may actually worsen outcomes in patients with SCA. The routine use of medications has been deemphasized, but not abandoned, in the current recommendations for resuscitation of SCA, VF, and pulseless VT (Box 12-1). Whether increased long-term survival from cardiac arrest can be expected with the use of any medications during CPR remains uncertain.
Complications
Complications of defibrillation include soft tissue injury, myocardial injury, and cardiac dysrhythmias. The availability of multifunctional electrode pads and better applicators for electrode gel has decreased the potential for soft tissue injuries such as chest burns.19 In fact, many clinicians now prefer to use the multifunctional electrode pads for ECG acquisition and for defibrillation.
The development of new, energy-efficient biphasic defibrillation waveforms, such as the BTE and the rectilinear biphasic waveform, has increased first-shock success and decreased the incidence of dysrhythmias after defibrillation.8 As a result, fewer shocks are needed to defibrillate the myocardium and less current is applied to the myocardium, which results in less electrical damage to myocytes.
Use of AEDs in public access defibrillation programs has not been reported to have produced any significant mishaps or adverse outcomes.20
Some older recommendations, such as use of the precordial thump, have been retracted. This procedure has been reported to have caused asystole or complete heart block (or both) when applied.21
In addition, the use of procainamide, though not a complication, has fallen out of favor because of long infusion times and mixed results regarding the efficacy of the effects of procainamide during the acute phase of VF and pulseless VT resuscitation.22
Pediatric Defibrillation
Cardiac arrest in infants and children should initially be considered to be secondary to respiratory arrest. SCA, VF, and pulseless VT are much less likely to occur in children than in adults. However, 5% to 15% of pediatric and adolescent SCA events demonstrate VF in the prehospital setting. In in-hospital arrests, a 20% occurrence of VF at some point during the resuscitation is reported. Nonetheless, rapid intervention and defibrillation improve outcomes from SCA. Causes of SCA, VF, and pulseless VT are more diverse in pediatric patients.22 Cardiac arrest does not usually occur as a result of a primary cardiac cause. Therefore, the approach to resuscitation of a pediatric patient in VF or pulseless VT may differ depending on the cause of the arrest.
Ventricular Fibrillation in Children
VF is much less common in children than in adults. The etiology of VF and SCA in children is most likely to be sudden infant death syndrome, respiratory compromise, sepsis, neurologic disease, or injuries from motor vehicle crashes, burns, accidental firearm discharge, and drowning, which are preventable.23,24 The most common terminal rhythms reported in children younger than 17 years are PEA, bradycardia, and asystole.25 The etiology of these pediatric arrhythmias is most often hypoxemia, hypotension, hypoglycemia, and acidemia. In addition, focal electrical ectopy is less likely to initiate VF in a young heart. A significant myocardial mass must be unstable and fibrillating before VF becomes established. In children (from birth to 8 years old) with nontraumatic arrest, only 3% of the dysrhythmias are reported to be VF. In victims aged 8 to 30 years, the number of patients with VF increases by almost sixfold (17%).23 Several subpopulations of pediatric patients at various ages with cardiomyopathy or myocarditis or who have undergone heart surgery are at increased risk for a primary dysrhythmia.
As noted previously, the incidence of VF in cardiac arrest rhythms of pediatric patients is reported to range from 7% to 20%.26 Patients with rhythms who have been defibrillated from VF have been reported to have a higher survival-to-discharge rate than do children who sustained asystole or PEA.27 Therefore, there is a definite indication for early defibrillation in the pediatric population.
Procedure and Technique
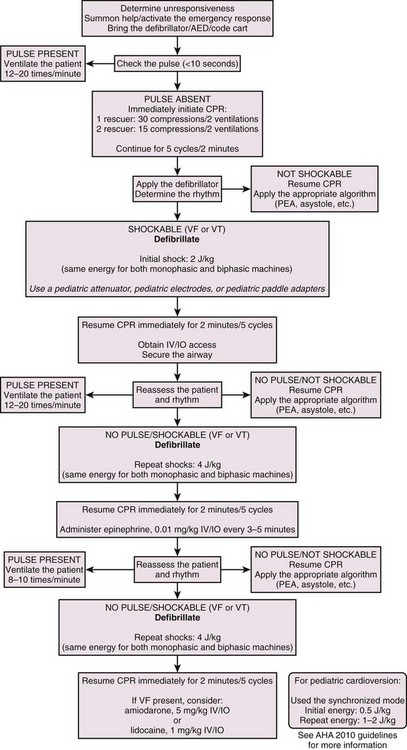
The procedure for pediatric defibrillation is similar to the algorithm for adult defibrillation (Fig. 12-16). However, a few differences must be addressed. These guidelines do not apply to children younger than 1 year.
Pediatric Sudden Cardiac Arrest: When cardiac arrest occurs in a child, it is usually a terminal event associated with respiratory compromise or shock. The probability of SCA resulting from a primary cardiac cause is extremely low.23 Nonetheless, it can and does occur. If resuscitation is prompt, the potential for a positive outcome, including preservation of the patient’s neurologic integrity, is quite high. To enhance the outcome of SCA resuscitation, defibrillation and CPR must be effectively integrated. The pediatric resuscitation guidelines incorporated findings from a comprehensive review of the data.28 Revised steps for the recommended resuscitation sequence are described in the following sections.
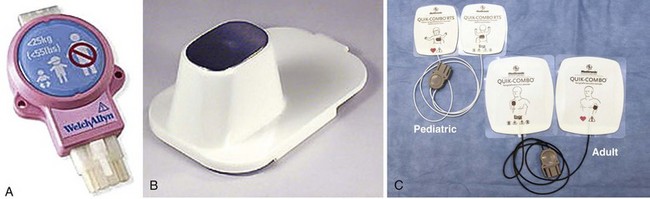
Equipment.: To perform pediatric defibrillation, a defibrillator monitor capable of adjustments in energy appropriate for children is needed. If an AED is to be used, it should have an energy attenuator for adjusting the energy to the appropriate level for a child (Fig. 12-17A). In addition, the quick-look electrode paddles (Fig. 12-17B) should have adapters attached to the adult paddles to ensure appropriate contact with the chest wall in a child without causing the electrodes to overlap. If adhesive pads are used, choose the appropriate size that will not overlap (Fig 12-17C). Use gels as in adults while being careful to prevent bridging across the chest wall from streaks of conductive material that may have been carelessly applied to the chest.
Paddle and Pad Application and Use of Conductive Material.: To acquire the electrical rhythm and subsequently administer an effective defibrillatory shock, place the appropriate-sized pads and paddles correctly on the chest. Use of the appropriate size and placement of paddles or pads will ensure that the appropriate current density is delivered across the myocardium to effectively defibrillate the myocytes. Furthermore, appropriate pad or paddle size—the largest surface area possible without direct electrode-to-electrode contact—will decrease transthoracic impedance and enhance defibrillation.29 To accomplish this, use infant paddles for children weighing less than 10 kg. However, use larger paddles if they do not contact each other. If contact is made between the paddles, an electrical arc or short circuit could occur.30 In children who weigh more than 10 kg (mean age, 1 year), use adult pads or paddles (8 to 10 cm in diameter).29 Use a conductive agent to enhance skin contact and decrease transthoracic impedance. Never use dry paddles because the resistance to flow of current will be very large. However, refrain from using saline-soaked pads in children because they may cause arcing as a result of the proximity of the pads on the chest. Remember that electricity will take the path of least resistance and that the current from defibrillation will travel across the chest if there is a saline bridge between the electrodes. In addition, the use of ultrasound gel and alcohol pads is discouraged because of poor electrical conductivity and potentially high impedance.30
Apply the paddles or pads firmly to the chest, one to the right of the sternum, just below the clavicle, and the other to the left of the left nipple, over the ribs and the apex of the heart (see Fig. 12-13A). An option when using self-adhesive pads is to place one pad just to the left of the sternum and the other over the back so that they approximate the position of the heart (see Fig. 12-13B).
Procedure in an Unresponsive Child: When confronted with an unresponsive child, immediately summon assistance and start the ABCs of CPR. Bring equipment for resuscitation expediently to the patient’s side. If no help is immediately available, first perform about 2 minutes of CPR before leaving the patient’s side. Remember that the arrest may have been the result of respiratory compromise and that performance of CPR may ameliorate the condition.
Rhythm Assessment.: Once the defibrillator or monitor is at the patient’s side, turn it on and place the electrodes on the patient’s chest. The positions of the electrodes on a child correspond to the positions used in an adult (see Fig. 12-13). If quick-look paddles are used, carefully apply the conductive gel to the electrodes’ surface. If self-adhesive multifunctional electrode pads are used, there is no need to use conductive gel. Make sure that the input selector switch is reading from the appropriate source (i.e., paddles or pads). Adjust or increase the gain or sensitivity of the monitor so that fine VF is not missed because of low amplitude. As the ECG rhythm appears on the monitor, assess and diagnose the rhythm. If VF or pulseless VT is present, proceed to select the appropriate energy level for the anticipated defibrillation.
Energy Selection.: As mentioned earlier in the adult section of the chapter, two types of defibrillators are available: biphasic and monophasic. As of this writing, there is no specific, detailed differentiation between energy levels to be used by either type of defibrillator. However, the caveat that biphasic shocks are at least as effective as monophasic shocks and that they are less damaging to the myocardium still applies.
Based on a review of adult and pediatric animal data, when a manual defibrillator is used for the first shock attempt, an energy level of 2 J/kg should be used with either a biphasic or a monophasic defibrillator. If a second or subsequent defibrillation is indicated, 4 J/kg should be used with either type.7,27
Mode Selection.: Before defibrillation, check to make sure that the defibrillator is set to the unsynchronized mode for defibrillation. Most defibrillators default into the unsynchronized mode between shocks. Nonetheless, this control should be checked to make sure that it is in the unsynchronized mode; otherwise, the defibrillator may not discharge when the shock buttons are depressed because it is looking for the QRS complex, which is not present in VF (this is discussed in more detail in the later section “Cardioversion”).
Defibrillate.: Once the energy level has been selected and the decision made to defibrillate, simultaneously clear the patient for defibrillation by loudly stating “I’m clear, you’re clear, everybody’s clear” while the button is activated to charge the capacitor. Continue CPR until ready to shock. Once the defibrillator has been charged and the patient cleared, apply firm pressure to the defibrillation paddles (25 lb) to increase contact and deflate the lungs to the end-expiration state. This will decrease impedance at the paddle–chest wall interface. Subsequently, depress the defibrillation controls and deliver the shock. This will usually be followed by a perceptible whole-body muscle twitch by the patient. If no obvious response or twitch of the patient is seen, check the defibrillator controls to make sure that it is in the unsynchronized mode and that the paddles are activated. No pressure is needed if adhesive multifunctional pads are used.
Resume Cardiopulmonary Resuscitation.: Once the shock has been delivered, resume resuscitation with immediate chest compressions. Continue the compressions for approximately 5 cycles of 30 compressions to 2 ventilations or about 2 minutes of CPR. If two rescuers are available, use a 15 : 2 ratio and switch compressors when the first compressor fatigues. This is done to facilitate the transition from SCA to ROSC after the heart has been stunned by the defibrillation and may not be functioning at optimal contractility for a few minutes after the shock. If additional monitoring devices are in place in the hospital setting, modify this step accordingly as decided by the resuscitation team leader. Continue CPR for approximately 2 minutes.
Reassess the Patient, Manage the Airway, and Gain Intravenous Access.: After 2 minutes of CPR, 5 cycles of 30 : 2, check the patient’s perfusion status or carotid pulses. If no pulse is palpable, resume compressions immediately and prepare to deliver a second defibrillatory shock.
Change in Cardiopulmonary Resuscitation.: Once an advanced airway has been secured, compression and ventilation cycles are no longer delivered. Now, the compressor will continue to deliver compressions at a rate of 100/min continuously without pausing for interposition of ventilations. When delivering the ventilations, provide 8 to 10 breaths/min, but be careful to not overinflate the chest or use too much force during ventilation to avoid overpressurizing the airways and esophagus and potentiating reflux. Lesser force also decreases the possibility of compromising CO as a result of elevated intrathoracic pressure.
Automatic External Defibrillators in Children
As mentioned previously, the incidence of VF and pulseless VT in children is low. Nonetheless, the presence of VF or pulseless VT is an indication for using an AED or defibrillator. The age range for use of an AED or defibrillator is 1 to 8 years. No recommendations for the use of a defibrillator or AED in children younger than 1 year have been provided as of this writing.31 A pediatric energy dose attenuator (see Fig. 12-17A) should be used to prevent the delivery of too much current to the myocardium. If a pediatric dose attenuator is not immediately available, a standard defibrillator should be used at the lowest appropriate setting.
Cardioversion
Indications and Contraindications
A reentrant tachydysrhythmia should be suspected when a sudden change in HR occurs within a few beats. Unless the dysrhythmia is noted while the patient is being monitored, it can be inferred only from the patient’s history of a sudden onset of symptoms. In the unusual case of sinus node reentrant tachycardia, rapid onset and offset may be the only clues.32 Other clues to the presence of a reentrant dysrhythmia are a history of Wolff-Parkinson-White (WPW) syndrome or another known accessory pathway syndrome. Ventricular rates in excess of those predicted for age strongly suggest an accessory pathway.
In digoxin toxicity, not only is cardioversion ineffective, but it is also associated with a higher incidence of post-shock VT and VF.33 However, in a patient with a therapeutic digoxin level, the risk associated with cardioversion is now thought to be no different from that of other patients. Digoxin is still generally withheld for 24 hours before cardioversion as a precaution against inadvertently elevated levels. Pregnancy at any stage is not a contraindication to cardioversion.15
Treatment
Therapy is dictated by the specific wide-complex tachycardia and the patient’s clinical findings (Fig. 12-18). The initial approach must always be led—and modified if necessary—by the patient’s signs and symptoms and subsequent changes. Synchronized monophasic or biphasic cardioversion is the appropriate first choice of treatment for unstable patients.11 In patients deemed to be stable, the therapeutic options are more diverse. Stable, wide-complex tachycardia can always be considered VT and treated according to current VT algorithms.34 A reasonable treatment protocol for stable patients may be the use of adenosine, procainamide, lidocaine, and finally, cardioversion. Amiodarone is effective for most SVTs, and its use for stable unknown wide-complex SVT is both appropriate and safe.7
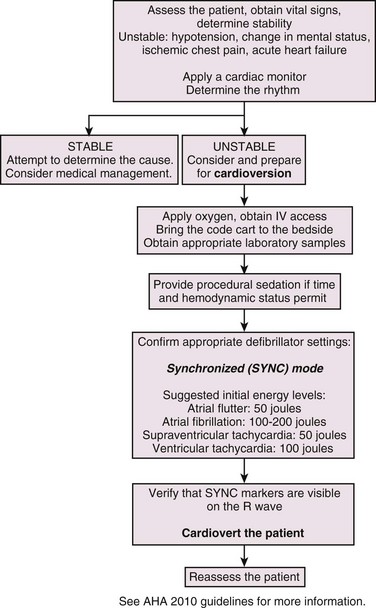
Figure 12-18 Cardioversion algorithm. IV, intravenous.
Equipment and Setup
The critical components of preparation for cardioversion are IV access, airway management equipment, drugs for sedation, monitoring, and DC delivery equipment (cardioverter) (Fig. 12-19, step 1).

Figure 12-19 Cardioversion.
Technique
Sedation
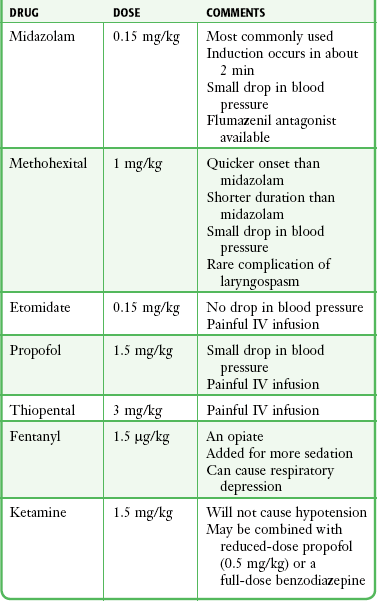
Cardioversion may be extremely painful or terrifying, and patients must be adequately sedated before its use (see Fig. 12-19, step 2). Patients who are not adequately sedated may experience extreme anxiety and fear.35 Several IV medications are available for sedation of patients before cardioversion, including etomidate (0.15 mg/kg), midazolam (0.15 mg/kg), methohexital (1 mg/kg), propofol (0.5-1.0 mg/kg), and thiopental (3 mg/kg). In addition, IV ketamine (1.5 mg/kg), with or without a benzodiazepine or slightly reduced-dose propofol, and IV fentanyl (1.5 µg/kg), a synthetic opioid analgesic, may be administered 3 minutes before induction (Table 12-1).
In elderly patients, the pharmacodynamics and kinetics are altered by coexisting illness and polypharmacy rather than by any intrinsic effect of old age.36 Drug doses should be reduced in these patients.
Cardioverter Use
Selection of the synchronized or nonsynchronized mode is the next critical step (see Fig. 12-19, step 3). In the synchronized mode, the cardioverter searches for a large positive or negative deflection, which it interprets as the R or S wave. It then automatically discharges an electric current that lasts less than 4 msec, thereby avoiding the vulnerable period during repolarization when VF can easily be induced. Once the cardioverter is set to synchronize, a brief delay will occur after the buttons are pushed for discharge as the machine searches for an R wave. This delay may be disconcerting to an unaware operator.
Energy Requirements
The amount of energy required for cardioversion varies with the type of dysrhythmia, the degree of metabolic derangement, and the configuration and thickness of the chest wall (see Fig. 12-19, step 4). Obese patients may require a higher energy level for cardioversion, and the anteroposterior paddle position is sometimes more effective in these patients. If patients are shocked while in the expiratory phase of their respiratory cycle, energy requirements may also be lower.
VT in a hemodynamically stable patient should be treated with amiodarone, 150 mg intravenously, and this can be repeated as needed up to a dose of 2.2 g/24 hr. If unsuccessful, cardioversion is then performed. Cardioversion with 10 to 20 J is successful in converting VT in more than 80% of cases. Cardioversion will be accomplished with 50 J in 90% of cases, and conversion should initially be attempted at this energy level.7 Cardioversion should be synchronized unless the T wave is large and could be misread as the R wave by the cardioverter. If the initial attempts at electrical cardioversion are unsuccessful, the energy level should be doubled—and doubled again if necessary—until a perfusing rhythm is restored. Immediately after conversion of VT, antidysrhythmic medications should be given to prevent recurrence.
Patients with pulseless VT should be initially shocked with 200 J, followed by 300 J if the first shock is not successful. Reentrant SVTs generally respond to low energy levels. Atrial flutter, for example, usually requires less than 50 J for conversion.7 Cardioversion of atrial flutter in the emergency department (ED) is indicated when the ventricular rate is not slowing in response to pharmacologically enhanced AV node blockade or if the patient is unable to tolerate the aberrant rhythm.
In patients with AF, the response to cardioversion is dependent on the duration of the AF and its underlying cause. Most patients with AF do not require cardioversion in the ED unless their ventricular response is high because of a bypass tract, as in WPW syndrome. They may also require cardioversion when sequelae of rapid ventricular contraction are present or anticipated and the ventricular rate is not responding to drug therapy aimed at slowing AV node conduction. Conversion of AF generally requires more energy than reentrant SVTs do (≈100 J in most cases).7
Complications
Chest wall burns resulting from electrical arcing are generally superficial partial-thickness burns, although deep partial-thickness burns have occurred.37 They are preventable by adequate application of conductive gel and firm pressure on the paddles. Paddles should not be placed over medication patches or ointments, especially those containing nitroglycerin, because electrical discharge may cause ignition and result in chest burns.38
References
1. Lloyd-Jones, D, Adams, RJ, Brown, TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2010;121:e46–e215.
2. Valenzuela, TD, Roe, DJ, Cretin, S, et al. Estimating effectiveness of cardiac arrest interventions: a logistic regression survival model. Circulation. 1997;96:3308–3313.
3. Swor, RA, Jackson, RE, Cynar, M, et al. Bystander CPR, ventricular fibrillation, and survival in witnessed, unmonitored out-of-hospital cardiac arrest. Ann Emerg Med. 1995;25:780–784.
4. Holmberg, M, Holmberg, S, Herlitz, J. Incidence, duration and survival of ventricular fibrillation in out-of-hospital cardiac arrest patients in Sweden. Resuscitation. 2000;44:7–17.
5. Larsen, MP, Eisenberg, MS, Cummins, RO, et al. Predicting survival from out-of-hospital cardiac arrest: a graphic model. Ann Emerg Med. 1993;22:1652–1658.
6. Stiell, IG, Wells, GA, Field, B, et al. Advanced cardiac life support in out-of-hospital cardiac arrest. N Engl J Med. 2004;351:647–656.
7. 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(suppl 3):S639–S946.
8. Kudenchuk, PJ, Cobb, LA, Copass, MK, et al. Transthoracic incremental monophasic versus biphasic defibrillation by emergency responders (TIMBER): a randomized comparison of monophasic with biphasic waveform ascending energy defibrillation for the resuscitation of out-of-hospital cardiac arrest due to ventricular fibrillation. Circulation. 2006;114:2010–2018.
9. Chan, PS, Jain, R, Nallmothu, BK, et al. Rapid response teams: a systematic review and meta-analysis. Arch Intern Med. 2010;170:18–26.
10. Hazinski, MF, Idris, AH, Kerber, RE, et al. Lay rescuer automated external defibrillator (“public access defibrillation”) programs: lessons learned from an international multicenter trial: advisory statement from the American Heart Association; Emergency Cardiovascular Committee; the Council on Cardiopulmonary, Perioperative, and Critical Care; and the Council on Clinical Cardiology. Circulation. 2005;111:3336–3340.
11. Dittrich, HC, Erickson, JS, Scheidermann, T, et al. Echocardiographic and clinical predictors for outcome of electrical cardioversion of atrial fibrillation. Am J Cardiol. 1989;63:193.
12. Chan, PS, Krumholz, HM, Nichol, G, et al. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358:9–17.
13. International Liaison Committee on Resuscitation. 2005 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2005;112:IV146.
14. Nanson, J, Elcock, D, Williams, M, et al. Do physiologic changes in pregnancy change defibrillation energy requirements? Br J Anaesth. 2001;87:237.
15. Ward, ME. Risk of fires when using defibrillators in an oxygen rich atmosphere. Resuscitation. 1996;31:173.
16. Kerber, RE, Kouba, C, Martins, J, et al. Advanced prediction of transthoracic impedance in human defibrillation and cardioversion: importance of impedance in determining the success of low-energy shocks. Circulation. 1984;70:303.
17. Kerber, RE, Grayzel, J, Hoyt, R, et al. Transthoracic resistance in human defibrillation: influence of body weight, chest size, serial shocks, paddle size, and paddle contact pressure. Circulation. 1981;63:676.
18. Sirna, SJ, Ferguson, DW, Charbonnier, F, et al. Factors affecting transthoracic impedance during electrical cardioversion. Am J Cardiol. 1988;62:1048.
19. Lown, B, Perlroth, MG, Kaidbey, S, et al. Cardioversion of atrial fibrillation. N Engl J Med. 1963;269:325.
20. American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112(Suppl):IV1–IV203.
21. Pennington, JE, Taylor, J, Lown, B. Chest thump for reverting ventricular tachycardia. N Engl J Med. 1970;283:1192.
22. Wu, D, Amat-y-leon, F, Denes, P, et al. Demonstration of sustained sinus and atrial re-entry as a mechanism of paroxysmal supraventricular tachycardia. Circulation. 1975;51:234.
23. Kuisma, M, Suominen, P, Korpela, R. Paediatric out-of-hospital cardiac arrests: epidemiology and outcome. Resuscitation. 1995;30:141.
24. Pressley, JC, Barlow, B. Preventing injury and injury-related disability in children and adolescents. Semin Pediatr Surg. 2004;13:133.
25. Sirbaugh, PE, Pepe, PE, Shook, JE, et al. A prospective, population-based study of the demographics, epidemiology, management, and outcome of out-of-hospital pediatric cardiopulmonary arrest [published erratum appears in Ann Emerg Med.33:358]. Ann Emerg Med. 1999;33:174.
26. Mogayzel, C, Quan, L, Graves, JR, et al. Out-of-hospital ventricular fibrillation in children and adolescents: causes and outcomes. Ann Emerg Med. 1995;25:484.
27. Young, KD, Seidel, JS. Pediatric cardiopulmonary resuscitation: a collective review. Ann Emerg Med. 1999;33:195.
28. Appleton, GO, Cummins, RO, Larson, MP, et al. CPR and the single rescuer: at what age should you “call first” rather than “call fast.”. Ann Emerg Med. 1995;25:492.
29. Atkins, DL, Sirna, S, Kieso, R, et al. Pediatric defibrillation: importance of paddle size in determining transthoracic impedance. Pediatrics. 1988;82:914.
30. Caterine, MR, Yoerger, DM, Spencer, KT, et al. Effect of electrode position and gel-application technique on predicted trans-cardiac current during transthoracic defibrillation. Ann Emerg Med. 1997;29:588.
31. Harken, AH, Honigman, B, Van Way, CW, 3rd. Cardiac dysrhythmias in the acute setting: recognition and treatment or anyone can treat cardiac dysrhythmias. J Emerg Med. 1987;5:129.
32. Chauhan, VS, Krahn, AD, Klein, GJ, et al. Supraventricular tachycardia. Med Clin North Am. 2001;85:193.
33. Waldecker, B, Brugada, P, Zehender, M, et al. Dysrhythmias after direct-current cardioversion. Am J Cardiol. 1986;57:120.
34. Kastor, JA. Multifocal atrial tachycardia. N Engl J Med. 1990;322:1713.
35. Kowey, PR. The calamity of cardioversion of conscious patients [editorial]. Am J Cardiol. 1988;13:1106.
36. Gurwitz, JH, Avorn, J. The ambiguous relation between aging and adverse drug reactions. Ann Intern Med. 1991;114:956.
37. Reisin, L, Baruchin, AM. Iatrogenic defibrillator burns. Burns. 1990;116:128.
38. Wrenn, K. The hazards of defibrillation through defibrillation patches. Ann Emerg Med. 1990;19:1327.