Chapter 114 Deep Brain Stimulation in Movement Disorders
Parkinson’s Disease, Essential Tremor, and Dystonia
History
The treatment of movement disorders has intrigued neurosurgeons for more than a century. One of the first case reports, by Horsley1 in 1909, described a resection of the precentral gyrus in a patient with hemiathetosis. The procedure relieved the patient of the athetosis but left him with permanent hemiparesis and dyspraxia. A few decades passed before a renewed interest in this field led Bucy to perform a similar procedure for treatment of tremor. In 1937, Bucy and Case2 resected the motor and premotor regions in a patient with an incapacitating post-traumatic tremor. The procedure abolished the tremor and led Bucy to apply the same technique to parkinsonian tremor. Although effective in treating the tremor, the procedure left patients with impairment of dexterous movements and varying degrees of hemiparesis. Subsequently, locations were sought for interruption of the corticospinal tract that might be associated with less morbidity. Walker3 described sectioning of the lateral two thirds of the cerebral peduncle to treat parkinsonian tremor. Again, this procedure was associated with postoperative hemiparesis, although dexterity seems to have been less affected.
It was not until the 1940s that surgeons first performed surgery on the basal ganglia to treat movement disorders. Before then, most physicians shared Dandy’s view that this region of the brain was the center of consciousness and that damage to the basal ganglia could lead to vegetative states. Meyers4 performed the first direct operation on the basal ganglia in 1939 to treat a patient with postencephalitic tremor. In this procedure, via a transcortical, transventricular approach, he resected two thirds of the caudate nucleus, resulting in good control of the patient’s contralateral tremor. Subsequent surgeries by Meyers for Parkinson’s disease (PD), hemiballism, and choreoathetosis included resections of the caudate, along with portions of the putamen, globus pallidus, and/or ansa lenticularis. By 1949, he had performed 58 such procedures.5 Of his patients, 62% had significant improvement in their tremor and rigidity; however, there was a 12% rate of operative mortality. Meyers determined that the mortality rate was excessive for elective surgery and concluded that open surgery on the basal ganglia had a limited role, although he showed that surgery in this region did not lead to alterations in consciousness or coma.
A few years later, Cooper6 discovered an alternate way of performing Meyers’s procedure. While attempting to perform a pedunculotomy on a patient with PD, he inadvertently transected the anterior choroidal artery and was forced to abandon the operation. However, when the patient awoke from surgery, he had no neurologic deficit and his tremor and rigidity were much improved. Cooper reasoned that sacrificing the artery led to a circumscribed infarct within the basal ganglia and the subsequent improvement of the patient’s symptoms. He went on to perform anterior choroidal artery ligation in a series of 55 patients. Tremor and rigidity were improved in approximately 70% of patients; however, there was an 11% rate of postoperative hemiplegia and a 10% rate of operative mortality.7 The high complication rate was attributed to the great variability in the vascular territory of the artery, and the procedure was soon abandoned.
Perhaps the most significant development in movement disorder surgery came in 1947 when Spiegel and Wycis introduced their stereotactic apparatus.8 Their first cases involved pallidal lesions in patients with Huntington’s chorea and choreoathetosis. This was followed by similar procedures for patients with PD and tremor. In their series of 50 patients, 78% had significant improvement in their tremor, whereas there was only a 4% rate of hemiplegia and 3% rate of operative mortality.9 Spiegel and Wycis proved their stereotactic technique was at least as effective as open resections, with far less morbidity and mortality. They were soon followed by Hassler and Riechert,10 who performed the first stereotactic thalamotomy in 1952. The ventral lateral thalamus soon became the target of choice for the treatment of PD. Thalamotomy proved more effective than pallidotomy for treating tremor and was arguably as effective for rigidity.
The introduction of levodopa in the 1960s led to a sharp decline in surgery for PD. It was not until a few decades later, when the long-term complications of levodopa became apparent, that interest was rekindled in movement disorder surgery. Many technical advances were made in the interim. The use of computed tomography and magnetic resonance imaging (MRI) supplanted ventriculography for target localization. The use of microelectrode recording (MER) became more prevalent and provided an additional method for target confirmation. Even the targets for the lesions themselves had become better defined. The ventral intermediate nucleus (Vim), a portion of the ventral lateral nucleus, became the preferred target for tremor control, and the posteroventral portion of the internal segment of the globus pallidus (GPi) became the preferred target for bradykinesia and rigidity.
The use of subcortical or deep brain stimulation (DBS) is the most recent advance in the treatment of PD and other movement disorders. Since the introduction of stereotactic surgery, electrical stimulation has frequently been performed to improve target localization and predict potential adverse effects before creating a lesion. For example, it was recognized that high-frequency stimulation (more than 100 Hz) in the Vim could suppress tremor. Because of the risk of irreversible neurologic injury and the complications associated with bilateral thalamic lesions, Benabid et al.11 carried out the first large-scale series of thalamic DBS for tremor. The safety and efficacy of this technique fueled interest in applying DBS to other targets such as GPi and the subthalamic nucleus (STN). The remainder of this chapter focuses on the use of DBS at these three sites to treat PD, disabling tremor, and dystonia.
Pathophysiology of PD, Tremor, and Dystonia
Parkinson’s Disease
Although a comprehensive review of the extrapyramidal motor system is beyond the scope of this chapter, a fundamental understanding of the basal ganglia and basal nuclei in regard to their physiologic function and the pathophysiology of movement disorders is important. The basal ganglia integrate and modulate cortical information along multiple parallel channels. Three main parallel systems can be delineated: sensorimotor, associative, and limbic.12 The best understood functions of the basal ganglia are those of the sensorimotor system. The role of the associative and limbic loops in causing the cognitive and behavioral disturbances associated with basal ganglia syndromes is less clear. These systems affect behavior indirectly by feedback to the cerebral cortex and directly by providing information to subcortical centers that influence movements. Their function is primarily through disinhibition of neural activity. Disruption of these channels by disease or injury results in disruption of movement and may be associated with significant deficits in cognition, perception, and mentation.
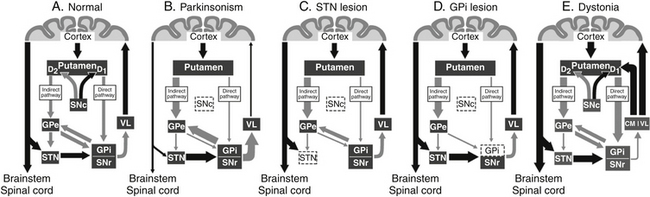
Motor input to the striatum from the neocortex is via excitatory glutamatergic projections that terminate on spiny cells in the putamen; dopaminergic terminals from the SNc coterminate on these cells. Neurons within the putamen send inhibitory γ-aminobutyric acid–ergic (GABAergic) axons to the external segment of the globus pallidus (GPe), GPi, and SNr. Although a matter of some debate, it appears that dopamine increases striatal output to the GPi and SNr (the direct pathway) and decreases transmission to the GPe (the indirect pathway) via subtype-1 and subtype-2 dopamine receptors, respectively13 (Fig. 114-1A).
This represents a simplified description of the basal ganglia motor loop and omits several pathways, such as those from the cortex to the subthalamus and from the subthalamus to the external segment of the globus pallidus. Nevertheless, it provides a basic framework for understanding the basal ganglia’s role in the extrapyramidal system. A delicate balance exists between the direct and the indirect pathways, with the former increasing thalamocortical activity (thalamic disinhibition) and the latter decreasing it (thalamic inhibition). It has been proposed that these two pathways may be involved with scaling or termination of movements.14 Striatal output would first inhibit GPi and SNr neurons via the direct pathway (facilitating movement), followed by disinhibition of the same neurons via the indirect pathway (terminating movement). Alternately, these pathways may be involved in the focusing of movements by simultaneously targeting separate populations of neurons in the GPi and SNr.15 Activity along the direct pathway would lead to inhibition of select GPi and SNr neurons, facilitating an intended movement, whereas activity along the indirect pathway would target surrounding neurons in the GPi and SNr, inhibiting unintended movements.
PD, whose hallmarks include bradykinesia, akinesia, and rigidity, is the most common and best understood disorder of the basal ganglia. The disease correlates with a loss of dopaminergic neurons in the SNc that project to the striatum. According to the DeLong model,16 this loss of dopaminergic input to the putamen leads to increased activity of the indirect pathway while decreasing activity along the direct pathway (Fig. 114-1B). The net effect of this change results in overexcitation of GPi and SNr neurons, which in turn increases their tonic inhibition of thalamocortical cells. This deficit of dopamine in the basal ganglia circuitry leads to the development of the cardinal symptoms of PD. Positron emission tomography studies in parkinsonian patients have demonstrated decreased activity in the supplementary motor area, dorsal prefrontal cortex, and frontal association areas that receive subcortical input from the basal ganglia.17
According to the DeLong model, the increased basal ganglia output causing PD is primarily due to overactivity of the STN and GPi. As such, these nuclei have become the surgical targets for the treatment of PD. Ablation of the GPi18 and more recently the STN19 has been shown to ameliorate many cardinal symptoms associated with the disease. The effects of such ablations in regard to the DeLong model can be seen in Fig. 114-1C and D. Recent surgical treatments have focused on the use of DBS in these nuclei to reduce their overactivity.
Tremor
The Vim has been extensively mapped using MER during thalamic procedures. Two subsets of tremor cells have been identified: kinesthetic cells, which simply fire in response to somatotopographically organized contralateral movements, and autonomous tremor cells, which fire synchronously with the tremor.20 Tremor cells can also be found in other brain structures, such as the GPi and STN, but their relationship to thalamic tremor cells is unclear. The Vim does not receive direct projections from the GPi and appears to receive principally cerebellar input. It has been proposed that Vim thalamotomy or thalamic stimulation may be targeting pallidal outflow fibers en route to other thalamic regions.21 Cells discharging at rates similar to the tremor have been identified in regions of the thalamus that receive input from the basal ganglia.22 The increased inhibition on the thalamus from GPi and SNr in parkinsonian patients may promote bursts in thalamocortical cells,23 and periodic bursting of the reticular nucleus during periods of immobility may enhance this rhythmic oscillation.
Dystonia
Dystonia is a heterogeneous condition characterized by sustained muscle contractions, frequently causing twisting and repetitive movements or abnormal postures. The age of onset reveals a bimodal distribution, with modes at 9 and 45 years and a nadir at 27 years.24 Various schemes have been proposed for its classification, including (1) age of onset (early onset or late onset), (2) distribution of affected body regions (focal, segmental, multifocal, or generalized), and (3) etiology (idiopathic, genetic, or secondary). Although 15 subtypes of dystonia can be distinguished genetically, the most common mutation is the deletion of a GAG triplet from the DYT1 gene.25 It is likely that many dystonias currently classified as idiopathic will also have a genetic basis. The causes of secondary dystonia are numerous and include vascular insults, trauma, Wilson’s disease, Huntington’s disease, tardive dystonia, and demyelination.
Dystonia is a hyperkinetic movement disorder whose pathophysiology is best appreciated in relation to the aforementioned sensorimotor circuit of the basal ganglia. Although the lack of a suitable primate model has limited investigations into the circuit abnormalities, available data indicate that dystonia and PD are roughly similar with regard to their indirect pathway overactivity. However, in contrast to PD, the direct pathway appears to be overactive in dystonia, resulting in a net reduction in GPi activity and subsequent increases in thalamocortical activation (Fig. 114-1E). Reductions in the mean discharge rates in the GPi have been reported in humans with dystonia.26 Based on the previous models of hyperkinetic movement disorders, we could not have predicted that GPi lesions (pallidotomy) would improve dystonia; rather, we would have predicted that GPi lesions would make dystonia worse. Yet ample evidence exists that pallidotomy (and pallidal stimulation) improve symptoms in dystonic patients.27 Because of this, it is unlikely that alterations in firing rates alone explain the benefits of pallidotomy. The patterning and synchrony of the discharge, rather than the frequency alone, are pathophysiologically relevant to dystonia and explain the improvement following pallidotomy or GPi DBS.28
Physiologic Effects of Stimulation
In the region of stimulation, three general classes of neurons can be affected: local cells, afferent inputs, and fibers of passage. Based on animal data, average stimulation currents used in DBS may affect the preceding neural elements for a distance of 2 to 5 mm from the active cathode.29 During stimulation, the firing of the cell body is not necessarily representative of the efferent output via the axon. Work by Holsheimer et al.30 and Mclntyre and Grill31 would suggest that, at the pulse widths and amplitudes used in DBS, stimulation is likely affecting myelinated axons as opposed to cell bodies. Fibers running parallel to the direction of the stimulating current would be preferentially activated as opposed to those running transversely. In addition, given the short refractory period of myelinated axons, frequencies in the 100- to 200-Hz range are unlikely to inhibit conduction. Using a primate model of PD, Hashimoto et al.32 demonstrated that stimulation of the STN produced short-latency excitatory responses that increased the average firing rate and altered the pattern of neuronal activity in both the GPi and the GPe. These results would seem to argue against stimulation-induced blockade in the target nucleus.
Likewise, the theory of synaptic depression due to neurotransmitter depletion has been called into question. in vivo experiments have shown increases in neurotransmitter release and changes in firing of efferent nuclei consistent with activation of neurons around the electrode and subsequent synaptic action on their targets during stimulation.32,33 Given the similar effects of ablation and DBS, it was not unreasonable to assume that stimulation had an inhibitory effect on the target nucleus. However, there appears to be mounting evidence that the effects of DBS are not due to depolarizing blockade, synaptic inhibition, or synaptic depression.
Thus, although it appears that DBS preferentially stimulates axons, the ultimate effect that it produces is difficult to predict. The variable volume of the neuropil that is affected by stimulation further confounds this. However, work done by Deuschl et al.34 and Vitek and Giroux35 suggests that the effects are generated by altered firing patterns, increased neuronal synchronization, and low-frequency rhythmic oscillation of neurons within the basal ganglia and thalamus. While ablation and DBS result in similar therapeutic benefits, it is likely that they achieve their results through very different mechanisms.
Patient Selection
Proper patient selection is one of the most important factors in ensuring successful outcomes after DBS. Although the ultimate decision to perform surgery is the responsibility of the neurosurgeon, input from the neurologist and the neuropsychologist is invaluable. Presurgical evaluation by a neurologist with experience in movement disorders ensures that the patient has undergone appropriate medical management before progressing to surgical intervention. In addition, a neurologist can screen for those patients with atypical parkinsonian syndromes such as progressive supranuclear palsy or multisystem atrophy. These “Parkinson plus” syndromes do not typically respond well to DBS. Neuropsychological evaluation can identify those patients with cognitive dysfunction, as this may worsen postoperatively and compromise the likelihood of success.
1. Patients should have significant disability despite maximal medical therapy.
2. Patients should be in generally good health without significant cardiac, pulmonary, or renal risk factors. In addition, many centers use an age of 70 years as a cutoff, although this is becoming less stringent.
3. Patients should not be demented or have significant cognitive impairment, nor should they have uncontrolled psychiatric illness such as anxiety or mood disorders.
4. Patients should be able to comprehend the risks of the proposed procedure. In addition, they should have reasonable expectations concerning the outcome from surgery.
5. Patients should not have severe atrophy or extensive white matter disease on preoperative MRI, as these findings may increase the risk of intracerebral hemorrhage or postoperative cognitive impairment.
VIM Stimulation
Ablation of the Vim of the thalamus (thalamotomy) has been used as a treatment for both ET and parkinsonian tremor for more than 50 years. The procedure is highly effective, significantly reducing contralateral tremor in approximately 85% of patients.36 However, thalamotomy is associated with a high rate of transient and, to a lesser degree, permanent neurologic deficits. Series have shown as many as 60% of patients experience transient deficits, lasting as long as 3 months, and permanent deficits in up to 23%.37 These deficits include weakness, dysarthria, ataxia, and sensory deficits. The risks are even greater for bilateral thalamotomy, with more than 50% of patients experiencing bulbar and cognitive effects. Because of these complications, Andy38 suggested that chronic stimulation of the thalamus might be preferable to lesioning. The use of intraoperative stimulation to identify lesioning targets has been well established.39 Stimulation of the Vim at low frequencies would exacerbate patients’ tremor, whereas high-frequency stimulation (more than 100 Hz) in the same location would suppress the tremor. The efficacy of stimulation in suppressing tremor and the side effects associated with bilateral thalamotomy led Benabid et al.11 to carry out the first large-scale trial of thalamic stimulation for parkinsonian tremor and ET.
Targeting and Lead Placement
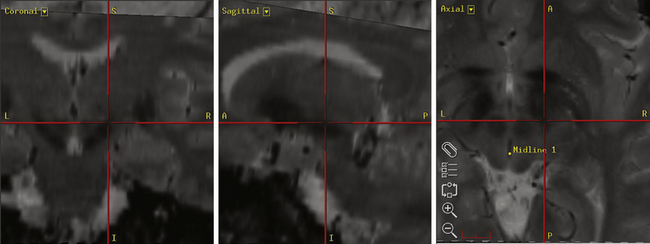
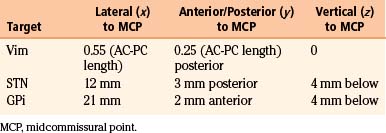
Our preferred target for Vim stimulation is at the level of the anterior commissure–posterior commissure (AC-PC) line, 25% the length of the AC-PC line anterior to the PC, and 55% of the AC-PC length laterally (Fig. 114-2). Corrections are made based on imaging if the target is too close to the internal capsule or the patient has a particularly wide third ventricle. The lead is inserted under local anesthetic (see the Surgical Technique section for further details) and macrostimulation is carried out to assess efficacy and side effects. Stimulation at frequencies of less than 5 Hz is used to look for motor effects that may indicate that the lead is too lateral or within the internal capsule. Next, stimulation is carried out at 50 Hz to assess the sensory threshold. Paraesthesias at less than 1 V indicate that the lead may be too posterior. Finally, stimulation is performed at 180 Hz to assess tremor suppression. Because of the consistency of anatomic targeting and simplicity of performing macrostimulation, we do not routinely perform MERs for Vim lead implants. The lead is finally positioned so that the middle two contacts bracket the final target.
Outcomes
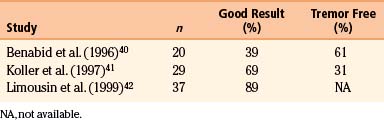
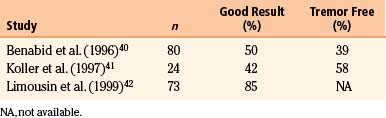
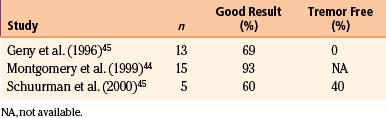
The original application of Vim stimulation as reported by Benabid et al.11 was for parkinsonian tremor in patients who had previously undergone contralateral thalamotomy. The technique, which proved to be as effective as thalamotomy, was subsequently used bilaterally in the treatment of both parkinsonian tremor and ET. Results for ET show good to excellent control of tremor in 89% to 100% of patients40–42 (Table 114-1). One series reported 61% of patients to be tremor free at 6-month follow-up,40 although there is some evidence that patients develop tolerance to stimulation over time. Equally impressive are the results for PD, which have good to excellent control in 85% to 100% of patients40–42 (Table 114-2), with as many as 58% of patients being tremor free.41 Tremor suppression appears to remain stable during 2-year follow-up. In general, control of resting tremor is better than that of postural or action tremor, distal is better than proximal or axial tremor, and upper is better than lower extremity tremor. As expected, thalamic stimulation does little to improve rigidity or bradykinesia in patients with PD.40 Vim DBS has also been used to treat secondary tremor from a variety of disorders, including multiple sclerosis, cerebrovascular accidents, and traumatic brain injury. Secondary tremors are often severe, postural, and kinetic and have a major proximal component. Multiple sclerosis represents the majority of secondary tremor cases treated using DBS. Good results have been obtained in 60% to 93% of patients43–45 (Table 114-3). However, the tremor is less consistently or effectively suppressed by thalamic DBS than ET or parkinsonian tremor.40 Patients with multiple sclerosis also tend to develop tolerance to the effects of stimulation and require frequent reprogramming.44 There are only sporadic reports of Vim DBS in the treatment of other secondary tremors. The consensus appears that it is less effective than stimulation for PD or ET. Nonetheless, given the severe disability of these patients and the lack of alternatives, DBS for secondary tremor may be gratifying.
STN Stimulation
According to the DeLong model,16 the motor symptoms seen in PD can be attributed to overactivity of the indirect pathway due to a deficiency of dopamine. This phenomenon occurs because the STN is released from its tonic inhibition and provides increased excitatory input to GPi and SNr. Appreciation of the STN’s role in the pathophysiology of PD has led to interest in the STN as a target for the treatment of the disease. Bergman et al.46 demonstrated that lesions of the STN in monkeys with MPTP-induced parkinsonism could improve their motor symptoms. Further animal studies demonstrated that high-frequency stimulation of the STN in MPTP monkeys reversed rigidity and akinesia.47 Concerns of creating hemiballismus by lesioning the STN and the safety and efficacy of Vim stimulation for tremor prompted Limousin et al. to attempt STN stimulation in parkinsonian patients.48 Since this first publication, much interest has been generated in DBS of the STN, and it has become the procedure of choice for treating PD.
Targeting and Lead Placement
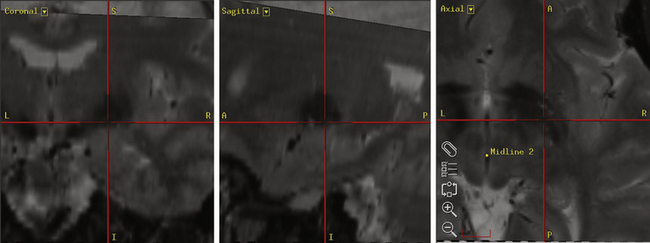
Our preferred target for STN stimulation is 4 mm below the level of the AC-PC line, 3 mm posterior to the midcommissural point, and 12 mm lateral to the AC-PC line (Fig. 114-3). Corrections are made to the final target if it appears too anterior within the cerebral peduncle or too medial with respect to the red nucleus. Physiologic mapping and lead placement are performed under local anesthetic (see the Surgical Technique section for further details). MER is performed to identify the reticular nucleus of the thalamus, the zona incerta, the STN, and the SNr. The reticular nucleus typically contains cells that exhibit brief bursts of spontaneous of activity. The subthalamic white matter is relatively electrically quiet except for the zona incerta, which shows some increased cellular activity. The STN is identified by high-frequency (30-70 Hz), irregularly firing cells that may respond to contralateral passive limb movements. Cells with an even high frequency and regular discharge pattern mark entrance into the SNr. Once the target nucleus has been identified, the permanent electrode is positioned so that the second contact lies within the MER-defined center of the STN.
Outcomes
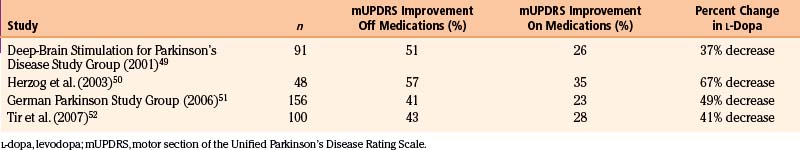
STN stimulation has been reported to improve all motor symptoms of PD, including rigidity, bradykinesia, tremor, and fenestrating gait. Improvements in the scores in the motor section of the Unified Parkinson’s Disease Rating Scale in the off-medication state range from 41% to 57% in most series49–52 (Table 114-4). However, improvements in the on-medication state after STN DBS show less dramatic change (23%-35%).49,50,52 Given that STN stimulation does less to improve on-medication motor function, it is important to counsel patients that the effects of stimulation will likely be equal to their best on-medication state. Much of the benefit derived from STN stimulation comes from reductions in off time and on/off fluctuations.49 Time in the off-medication state was reduced by more than 60% in one study.49 In addition, STN DBS significantly increases on-medication time without dyskinesias.49,53 Overall, dyskinesia scores tend to decrease by 50% after stimulation. Much of the improvement in regards to dyskinesia may be related to significant reductions in levodopa requirements by patients after STN stimulation. Most series report a reduction in daily levodopa doses by approximately 40% to 70%.49–52 Fewer studies have specifically measured the effects of STN stimulation on tremor; those that have typically show a 70% to 80% reduction of tremor in the off-medication state.49,53
GPI Stimulation
Pallidotomy has been used to treat the akinesia and rigidity associated with PD for more than 6 decades. However, with the introduction of levodopa in the 1960s, the procedure was largely abandoned. Despite advances in medical therapy, patients often developed severe medication-induced dyskinesias and motor fluctuations. In 1992, Laitinen et al.54 reintroduced the procedure and found it to be effective in treating both the motor symptoms and the dyskinesias of PD. However, most patients with advanced PD have bilateral disease with prominent gait and balance disturbances. These symptoms are not adequately treated by unilateral surgery. Bilateral pallidotomy is only rarely performed due to the increased risk of cognitive impairment and speech disorders.55 This prompted Siegfried and Lippitz56 to perform high-frequency bilateral GPi stimulation for the treatment of PD. Previous work by Benabid et al.11 had shown that thalamic stimulation had effects similar to those of thalamotomy and could safely be performed bilaterally. Pallidal DBS offered the ability to provide the therapeutic benefits of bilateral pallidotomy without the associated morbidity. Based on the successful use of pallidal stimulation to treat PD and the previous experience with pallidotomy for dystonia,27 DBS for dystonia was started in the late 1990s with promising results.57
Targeting and Lead Placement
Our preferred target for GPi stimulation is 4 mm below the level of the AC-PC line, 2 mm anterior to the midcommissural point, and 21 mm lateral to the AC-PC line (Fig. 114-4). Corrections are made as necessary to ensure that final target does not abut the optic tract. Physiologic mapping and lead placement are performed under local anesthetic (see the Surgical Technique section for further details). MER is performed to identify the external and the internal segments of the globus pallidus, as well as the subpallidal white matter. The GPe typically displays large-voltage spontaneous activity at low frequency interspersed with rapid bursts. The transition zone between the external and the internal segments of the globus pallidus contains border cells demonstrating slow discharge rates. The GPi is marked by highly active, high-frequency (80-90 Hz) neurons. Once the target nucleus has been identified, the permanent electrode is implanted so that its distalmost contact lies at the base of the GPi.
Outcomes
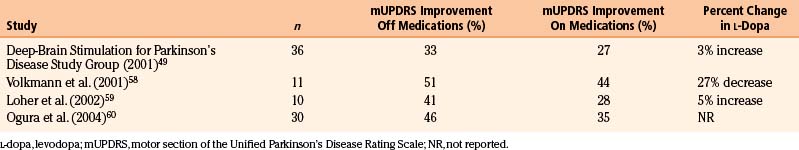
Much like STN stimulation, GPi DBS has been shown to be efficacious in treating the contralateral motor symptoms of PD. Improvements in the off-medication state show a 33% to 51% reduction in the motor section of the Unified Parkinson’s Disease Rating Scale scores49,58–60 (Table 114-5). These reductions were significant for tremor, rigidity, bradykinesia, gait, and postural stability. Improvements in the on-medication state show a more modest reduction of 27% to 44%.49,58–60 Unlike the case with STN stimulation, there is some evidence of a synergistic effect between pallidal stimulation and levodopa in the on-medication state.53 GPi stimulation also increases the length of the on-medication period without dyskinesias.49 In addition, patients’ total on time increases with a concomitant decrease in on/off fluctuations.49 To date, there have been only two prospective, randomized studies comparing GPi with STN DBS.53,61 The initial study with 10 patients and a follow-up study with 20 patients showed no significant difference in benefit between the two therapies. The only outcome measure that was significantly different between the GPi and the STN groups was medication use. The mean levodopa intake at 12 months was reduced by 50% in the STN group but remained unchanged in the GPi group. Similar effects on medication dosing have been noted by others49,59,60 and suggest that the mechanism of interaction between levodopa and DBS may be very different at the two sites.
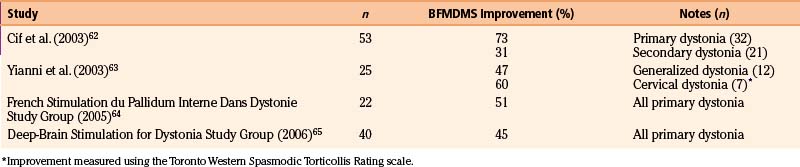
GPi stimulation is also being used to treat dystonia. Given the heterogeneity of the disorder, the results differ significantly depending on whether the etiology is idiopathic, genetic, or secondary. For primary dystonia (both idiopathic and genetic), improvements in the Burke-Fahn-Marsden Dystonia Movement Scale (BFMDMS) following DBS tend to range from about 45% to 75%62–65 (Table 114-6). Similar improvements were seen regardless of whether the dystonia was generalized or segmental. The results for secondary forms of dystonia are far less promising, with changes in BFMDMS scores of about 10% to 30%.62,66 In contrast to the immediate benefit seen with DBS for PD and tremor, the effects of GPi stimulation on dystonia take time to manifest. Often, clinical improvement is not seen for up to a month following the initiation of stimulation, with fixed dystonic postures slowly improving over the course of several months.
Surgical Technique
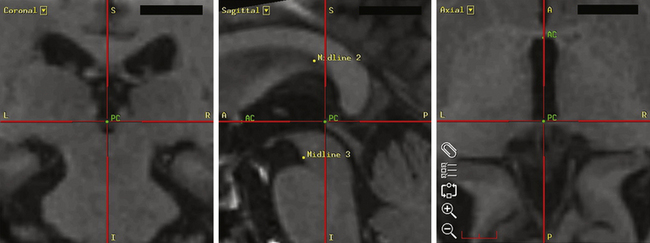
The Leksell Model G (Elekta, Stockholm, Sweden) stereotactic base ring and localizer are affixed to the patient’s skull under local anesthesia. MRI is then performed using a spoiled gradient recalled acquisition in the steady state sequence. It is our preference to use MRI for targeting, but the use of positive-contrast ventriculography and computed tomography are well described. The images are then transferred to the Stealth Station (Medtronic, Minneapolis, MN) and merged with a high-resolution, T2-weighted, fast spin echo sequence performed preoperatively on a 3-tesla MRI. This preoperative imaging sequence allows for direct visualization of the STN or GPi. Targeting is then carried out with the aid of the system’s Frame Link software. The AC and PC are identified on axial images (Fig. 114-5), as are several midline structures. The target coordinates are then calculated based on fixed relationships to these structures (Table 114-7). The software superimposes the target onto corresponding axial, sagittal, and coronal MRI slices. Modifications are then made if the target appears to be too close to critical structures (e.g., internal capsule or optic tract).
For STN and GPi implants, MER is performed using the MicroGuide (Alpha Omega, Nazareth, Israel) system. Recordings are begun using a single-channel electrode system, approximately 25 mm above the presumed target. Initial recordings are taken in 0.5-mm increments until the target nucleus is encountered and then are taken every 0.1 to 0.2 mm. Recordings continue until either the reticular portion of the SN (for the STN) or the subpallidal white matter (for the GPi) is identified. We do not routinely conduct MER for placement of electrodes within the Vim of the thalamus. The consistency of targeting based on anatomic structures and the ease of performing intraoperative macrostimulation to assess efficacy have, in our experience, made MER unnecessary in these cases. However, many physicians still advocate the use of MER for the placement of Vim electrodes.
Hardware
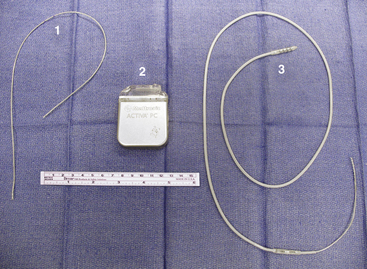
The lead currently used for implantation at our institution is the Medtronic model 3387S quadripolar DBS electrode with four platinum–iridium contacts, each 1.5 mm long and separated by 1.5 mm (Fig. 114-6). Also available is the Medtronic model 3389S electrode with contacts spaced 0.5 mm apart. Both leads come with a central stylet to provide increased rigidity during insertion. Also included is the implantable nylon, 14-mm Stimloc bur hole cover to secure the lead. The lead is connected to the generator by extension cables of varying lengths (Fig. 114-6). Activa PC (Medtronic) is a fully implantable pulse generator powered by a hybrid combined silver vanadium oxide battery (Fig. 114-6). It is housed in a titanium casing with an epoxy heading where the extension cable inserts. The device allows for a range of stimulus parameters through one or two leads and is externally programmable in either voltage or current modes. Frequencies can be set from 2 to 250 Hz in voltage mode and 30 to 250 Hz in current mode. Pulse widths range from 60 to 450 μsec, and amplitudes range from 0 to 10.5 V. Typical stimulation parameters are a frequency of 130 to 185 Hz, pulse width of 60 to 90 msec, and amplitude of 1 to 3 V. Given these settings, battery life averages approximately 3 to 5 years. Also available is the Activa RC, which is powered by a battery capable of being recharged externally and has an anticipated battery life of 9 years. Both devices come with an external programmer to allow the patient to turn the device on or off as desired.
Programming
For their initial programming session, patients with PD have their medication withheld for at least 12 hours. Baseline motor assessments are then performed, evaluating the patient for rigidity, bradykinesia, gait, and postural stability. The electrode contacts are then sequentially evaluated in a monopolar configuration. Frequency and pulse width are typically kept at constant settings of 180 Hz and 90 μsec, respectively. The amplitude is then steadily increased to the tolerance level of the patient or until side effects occur. Repeat motor evaluation is then performed to assess efficacy of stimulation. This process is carried out for each of the four electrode contacts. Between trials of separate contacts, 10 to 15 minutes are permitted to pass to allow for at least partial washout of any previous stimulation effects. If a satisfactory result cannot be achieved with monopolar stimulation, more complex arrays consisting of bipoles, tripoles, or multiple cathodes are tried. Once an effective program has been established, patients are given an appropriate dose of levodopa and are observed for dyskinesias. Further programming adjustments are then made as required to treat any perceived drug-induced dyskinesias.
Complications
The complications from DBS can be broken down into three main categories: (1) procedure related, (2) hardware related, and (3) stimulation related. The overall incidence of complications from DBS is approximately 30%,49,67 but the majority are relatively minor or amenable to changes in stimulation parameters. Mortality rates are less than 1%, with intracerebral hemorrhage being the most common cause of death. The complication rates are essentially the same regardless of the target with the exception of some stimulation-related adverse events.
The most common surgical complications are intracerebral hemorrhage and infection. The incidence of intracerebral hemorrhage ranges between 1% and 5%.49,67 Fortunately, most of these hemorrhages are small and cause minimal to no symptoms. As with any procedure involving the implantation of hardware, infection is a major concern. Reported infection rates range from 3% to 13%.49,67 Superficial wound infections may be treated with intravenous antibiotics, but infections that involve the device generally require wound debridement and explantation of the stimulator. Care must also be taken to prevent a secondary infection of the stimulator; patients with DBS undergoing routine dental work and minor surgical procedures should be given prophylactic antibiotics.
Complications related to the hardware include lead fractures, lead migration, skin erosion, and hardware malfunctions. Reports of equipment failure range from approximately 5% to 13% in most series.49,67 Fracture of the electrode is the most common cause of device failure. This is becoming less of a problem, because newer electrodes are made entirely of platinum. In our early experience, lead fracture occurred most commonly just proximal to the connection with the extension cable. We found that ensuring this connection was placed over the skull, as opposed to the soft tissues of the neck, eliminated this problem. Another problem related to the hardware can be erosion of the skin overlying the device. Most often this occurs at the site of the electrode–extension cable connection but may also occur over the lead anchoring caps in individuals with thin scalps. The newer low-profile extension cable will likely reduce the incidence of erosion at the connection site.
Stimulation-related complications are typically related to activation of adjacent nuclei or fiber tracts. Such adverse reactions generally respond to adjustments in stimulation parameters, although sometimes at the expense of therapeutic benefit. For thalamic stimulation, the most common reactions are paresthesia involving the face or limb, dysarthria, dysmetria, and ataxia.32 Reported complications after STN stimulation include dysarthria, diplopia, apraxia of eyelid opening, and depression.49,58 Stimulation of the globus pallidus has been reported to cause dyskinesia, dystonia, and ataxia.49,58
Anderson M.E., Postupna N., Rufio M. Effects of high frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol. 2003;89:11501160.
Anderson V.C., Burchiel K.J., Hogarth, et al. Pallidal vs. subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol. 2005;62:554-560.
Benabid A.L., Pollack P., Gao D., et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84:203-214.
Cif L., El Fertit H., Vayssiere N., et al. Treatment of dystonic syndromes by chronic electrical stimulation of the internal globus pallidus. J Neurosurg Sci. 2003;47:52-55.
Deep Brain for Dystonia Study Group. Pallidal deep brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978-1990.
Deep-Brain Stimulation for Parkinson’s Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345:956-963.
French Stimulation du Pallidum Interne dans la Dystonie Study Group. Bilateral deep brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459-467.
Geny C., Nguyen J.P., Pollin B., et al. Improvement of severe postural cerebellar tremor in multiple sclerosis by chronic thalamic stimulation. Mov Disord. 1996;11:489-494.
German Parkinson Study Group. A randomized trial of deep brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896-908.
Hashimoto T., Elder C.M., Okun M.S., et al. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916-1923.
Herzog J., Volkmann J., Krack P., et al. Two-year follow-up of subthalamic nucleus deep brain stimulation in Parkinson’s disease. Mov Disord. 2003;18:1332-1337.
Holsheimer J., Demeulemeester H., Nuttin B., de Sutter P. Identification of the target neuronal elements in electrical deep brain stimulation. Eur J Neurosci. 2000;12:4573-4577.
Koller W., Pahwa R., Busenbark K., et al. High-frequency unilateral thalamic stimulation in the treatment of essential and parkinsonian tremor. Ann Neurol. 1997;42:292-299.
Limousin P., Speelman J.D., Gielen F., et al. Multicentre European study of thalamic stimulation in parkinsonian and essential tremor. J Neurol Neurosurg Psychiatry. 1999;66:289-296.
Loher T.J., Burgunder J.M., Pohle T., et al. Long-term pallidal deep brain stimulation in patients with advanced Parkinson’s disease: 1-year follow-up study. J Neurosurg. 2002;96:844-853.
McIntyre C.C., Grill W.M. Selective microstimulation of central nervous system neurons. Ann Biomed Eng. 2000;28:219-233.
Merello M., Cerquetti D., Cammarota A., et al. Neuronal globus pallidus activity in patients with generalized dystonia. Mov Disord. 2004;19:548-554.
Montgomery E.B., Baker K.B., Kinkel R.P., Barnett G. Chronic thalamic stimulation for the tremor of multiple sclerosis. Neurology. 1999;53:625-628.
Ogura M., Nakao N., Nakai E., et al. The mechanism and effect of chronic electrical stimulation of the globus pallidus for treatment of Parkinson’s disease. J Neurosurg. 2004;100:997-1001.
Schuurman P.R., Bosch D.A., Bossuyt M.M., et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342:461-468.
Starr P.A., Rau G.M., Davis V., et al. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. J Neurophysiol. 2005;93:3165-3176.
Tir M., Devos D., Blond S., et al. Exhaustive, one-year follow-up of subthalamic nucleus deep brain stimulation in a large, single-center cohort of Parkinson’s patients. Neurosurg. 2007;61:297-304.
Vitek J.L., Giroux M. Physiology of hypokinetic and hyperkinetic movement disorders: model for dyskinesia. Ann Neurol. 2000;47:S131-S140.
Volkman J., Allert N., Voges J., et al. Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology. 2001;56:548-551.
Yianni J., Bain P., Giladi N., et al. Globus pallidus interna deep brain stimulation for dystonic conditions: a prospective audit. Mov Disord. 2003;18:436-442.
1. Horsley V. The function of the so-called motor area of the brain. BMJ. 1909;2:125-132.
2. Bucy P.C., Case T.J. Tremor, physiologic mechanism and abolition by surgical means. Arch Neurol Psychiatry. 1939;41:721-746.
3. Walker A.E. Cerebral pedunculotomy for the relief of involuntary movements: parkinsonian tremor. J Nerv Ment Dis. 1952;116:766-775.
4. Meyers R. A surgical procedure for the alleviation of postencephalitic tremor, with notes on the physiology of the premotor fibers. Arch Neurol Psychiatry. 1940;44:455-459.
5. Meyers R. Historical background and personal experiences in the surgical relief of hyperkinesias and hypertonus. In: Fields W.S., editor. Pathogenesis and Treatment of Parkinsonism. Springfield, IL: Charles C Thomas; 1958:229-270.
6. Cooper I.S. Ligation of the anterior choroidal artery for involuntary movements of parkinsonism. Psychiatr Q. 1953;27:317-319.
7. Cooper I.S. The Neurosurgical Alleviation of Parkinsonism. Springfield, IL: Charles C Thomas; 1956.
8. Spiegel E.A., Wycis H.T., Marks M., Lee A.J. Stereotactic apparatus for operations in the human brain. Science. 1947;106:349-350.
9. Wycis H.T., Spiegel E.A. Long range results of pallidoansotomy in paralysis agitans and parkinsonism. In: Fields W.S., editor. Pathogenesis and Treatment of Parkinsonism. Springfield, IL: Charles C Thomas; 1958:294-298.
10. Hassler R., Riechert T. Indikationen an lokalosations—methode der gezielten Hirnoperationen. Nervearzt. 1954;25:441-447.
11. Benabid A.L., Pollak P., Loveau A., et al. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344-346.
12. Alexander G.E., Crutcher M.D. Functional architecture of basal ganglia circuits: neural substrates for parallel processing. Trends Neurosci. 1990;13:266-271.
13. Gerfen C.R. Dopamine receptor function in the basal ganglia. Clin Neuropharmacol. 1995;18:S162-S177.
14. Mink J.W. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381-425.
15. Wenger K.K., Musch K.L., Mink J.W. Impaired reaching and grasping after focal inactivation of the globus pallidus pars interna in the monkey. J Neurophysiol. 1999;82:2049-2060.
16. DeLong M.R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281-285.
17. Brooks D.J. Functional imaging of Parkinson’s disease: is it possible to detect brain areas for specific symptoms? J Neural Transm. 1999;56:139-153.
18. Laitinen L.V. Pallidotomy for Parkinson’s disease. Neurosurg Clin N Am. 1995;6:105-112.
19. Su P.C., Tseng H.M., Liu H.M., et al. Treatment of advanced Parkinson’s disease by subthalamotomy: one-year results. Mov Disord. 2003;18:531-538.
20. Tasker R.R., Davis K.W., Hutchison W.D., et al. Subcortical and thalamic mapping in functional neurosurgery. In: Gildenberg P.L., Tasker R.R. Textbook of Stereotactic and Functional Neurosurgery. New York: McGraw-Hill; 1998:883-969.
21. DeLong M.R., Wichmann T., Vitek J.L. Pathophysiological basis of neurosurgical treatment of Parkinson’s disease. In: Gildenberg P.L., Tasker R.R. Textbook of Stereotactic and Functional Neurosurgery. New York: McGraw-Hill; 1998:1139-1146.
22. Pare D., Curro’Dossi R., Steriade M. Neuronal basis of the parkinsonian resting tremor: a hypothesis and its implications for treatment. Neuroscience. 1990;35:217-226.
23. Buzsaki G., Smith A., Berger S., et al. Petit mal epilepsy and parkinsonian tremor: hypothesis of a common pacemaker. Neuroscience. 1990;36:1-14.
24. Bressman S.B., de Leon D., Brin M.F. Idiopathic torsion dystonia among Ashkenazi Jews: evidence for autosomal dominant inheritance. Ann Neurol. 1989;26:612-620.
25. Tarsy D., Simon D.K. Dystonia. N Engl J Med. 2006;355:818-829.
26. Starr P.A., Rau G.M., Davis V., et al. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. J Neurophysiol. 2005;93:3165-3176.
27. Lozano A.M., Kumar R., Gross R.E., et al. Globus pallidus internus pallidotomy for generalized dystonia. Mov Disord. 1997;12:865-870.
28. Merello M., Cerquetti D., Cammarota A., et al. Neuronal globus pallidus activity in patients with generalized dystonia. Mov Disord. 2004;19:548-554.
29. Ranck J.B. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975;98:417-440.
30. Holsheimer J., Demeulemeester H., Nuttin B., de Sutter P. Identification of the target neuronal elements in electrical deep brain stimulation. Eur J Neurosci. 2000;12:4573-4577.
31. McIntyre C.C., Grill W.M. Selective microstimulation of central nervous system neurons. Ann Biomed Eng. 2000;28:219-233.
32. Hashimoto T., Elder C.M., Okun M.S., et al. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916-1923.
33. Anderson M.E., Postupna N., Rufio M. Effects of high frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol. 2003;89:11501160.
34. Deuschl G., Raethjen J., Lindemann M., et al. The pathophysiology of tremor. Muscle Nerve. 2001;24:716-735.
35. Vitek J.L., Giroux M. Physiology of hypokinetic and hyperkinetic movement disorders: model for dyskinesia. Ann Neurol. 2000;47:S131-S140.
36. Jankovic J., Cardoso F., Grossman R.G., Hamilton W.J. Outcome after stereotactic thalamotomy for parkinsonian, essential and other types of tremor. Neurosurgery. 1995;37:680-687.
37. Hallet M., Litvan I. A task force on surgery for Parkinson’s disease. Evaluation of surgery for Parkinson’s disease. Neurology. 1999;53:1910-1921.
38. Andy O.J. Thalamic stimulation for control of movement disorders. Appl Neurophysiol. 1983;46:107-111.
39. Cooper I.S., Upton A.R., Amin I. Reversibility of chronic neurologic deficits. Some effects of electrical stimulation of the thalamus and internal capsule in man. Appl Neurophysiol. 1980;43:244-258.
40. Benabid A.L., Pollack P., Gao D., et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84:203-214.
41. Koller W., Pahwa R., Busenbark K., et al. High-frequency unilateral thalamic stimulation in the treatment of essential and parkinsonian tremor. Ann Neurol. 1997;42:292-299.
42. Limousin P., Speelman J.D., Gielen F., et al. Multicentre European study of thalamic stimulation in parkinsonian and essential tremor. J Neurol Neurosurg Psychiatry. 1999;66:289-296.
43. Geny C., Nguyen J.P., Pollin B., et al. Improvement of severe postural cerebellar tremor in multiple sclerosis by chronic thalamic stimulation. Mov Disord. 1996;11:489-494.
44. Montgomery E.B., Baker K.B., Kinkel R.P., Barnett G. Chronic thalamic stimulation for the tremor of multiple sclerosis. Neurology. 1999;53:625-628.
45. Schuurman P.R., Bosch D.A., Bossuyt M.M., et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342:461-468.
46. Bergman H., Wichman T., DeLong M.R. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436-1438.
47. Benazzouz A., Gross C., Feger J., et al. Reversal of rigidity and improvement of motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci. 1993;5:382-389.
48. Limousin P., Pollak P., Benazzouz A., et al. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91-95.
49. Deep-Brain Stimulation for Parkinson’s Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345:956-963.
50. Herzog J., Volkmann J., Krack P., et al. Two-year follow-up of subthalamic nucleus deep brain stimulation in Parkinson’s disease. Mov Disord. 2003;18:1332-1337.
51. German Parkinson Study Group. A randomized trial of deep brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896-908.
52. Tir M., Devos D., Blond S., et al. Exhaustive, one-year follow-up of subthalamic nucleus deep brain stimulation in a large, single-center cohort of Parkinson’s patients. Neurosurg. 2007;61:297-304.
53. Burchiel K.J., Anderson V.C., Favre J., et al. Comparison of pallidal and subthalamic nucleus deep brain stimulation for advanced Parkinson’s disease: results of a randomized, blinded pilot study. Neurosurgery. 1999;45:1375-1382.
54. Laitinen L.V., Bergenheim A.T., Hariz M.I. Leksell’s posteroventral pallidotomy in the treatment of Parkinson’s disease. J Neurosurg. 1992;76:53-61.
55. Favre J., Burchiel K.J., Taha J.M., et al. Outcome of unilateral and bilateral pallidotomy for the treatment of Parkinson’s disease: patient assessment. Neurosurgery. 2000;46:344-353.
56. Siegfried J., Lippitz B. Bilateral chronic electrostimulation of ventro-posterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery. 1994;35:1126-1129.
57. Kumar R., Dagher A., Hutchinson W.D., et al. Globus pallidus deep brain stimulation for generalized dystonia: clinical and PET investigation. Neurology. 1999;53:871-874.
58. Volkman J., Allert N., Voges J., et al. Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology. 2001;56:548-551.
59. Loher T.J., Burgunder J.M., Pohle T., et al. Long-term pallidal deep brain stimulation in patients with advanced Parkinson’s disease: 1-year follow-up study. J Neurosurg. 2002;96:844-853.
60. Ogura M., Nakao N., Nakai E., et al. The mechanism and effect of chronic electrical stimulation of the globus pallidus for treatment of Parkinson’s disease. J Neurosurg. 2004;100:997-1001.
61. Anderson V.C., Burchiel K.J., Hogarth, et al. Pallidal vs. subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol. 2005;62:554-560.
62. Cif L., El Fertit H., Vayssiere N., et al. Treatment of dystonic syndromes by chronic electrical stimulation of the internal globus pallidus. J Neurosurg Sci. 2003;47:52-55.
63. Yianni J., Bain P., Giladi N., et al. Globus pallidus interna deep brain stimulation for dystonic conditions: a prospective audit. Mov Disord. 2003;18:436-442.
64. French Stimulation du Pallidum Interne dans la Dystonie Study Group. Bilateral deep brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459-467.
65. Deep-Brain Stimulation for Dystonia Study Group. Pallidal deep brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978-1990.
66. Eltahawy H., Saint-Cyr J., Giladi N., et al. Primary dystonia is more responsive than secondary dystonia to pallidal interventions: outcome after pallidotomy or pallidal deep brain stimulation. Neurosurg. 2004;54:613-621.
67. Levy R., Lamb S., Adams J. Treatment of chronic pain by deep brain stimulation: long term follow-up and review of the literature. Neurosurg. 1987;21:885-893.