Chapter 126 Deep Brain Stimulation for Pain
History of DBS for Treatment of Chronic Pain
DBS traces its roots back to the mid-20th century. Heath1 looked to DBS to provide effective treatment for patients afflicted with intransigent psychiatric symptoms. Failure of more established treatments such as electric shock therapy and contemporary pharmacologic regimens prompted Heath to implant electrodes in various subcortical targets. Initial reports of successful diminution of psychotic symptoms in schizophrenics prompted Heath to continue utilizing DBS. During one incident, Heath and Mickle unexpectedly observed analgesic effects following septal stimulation in a young woman.1,2 Over the ensuing years, other studies reported analgesia following intracranial stimulation in humans.3–5 Then in 1969, Reynolds was able to produce analgesia in rats following electric stimulation of the brain.6 By precise stimulation of the lateral margin of the periaqueductal gray (PAG) matter, Reynolds inhibited nociceptive responses in rats undergoing abdominal surgery. This prompted further research into DBS-related analgesia. Shortly after Reynold’s work, several other studies reported consistent results in similar experiments with rats7–10 and in cats.11 Following this research, DBS for pain relief began to be used in clinical trials.
Clinical trials utilizing DBS for the treatment of acute and chronic somatogenic pain of various origins can be categorized into different periods depending on the specific target stimulation. The first period took place between the late 1970s and the late 1990s. Clinical trials utilizing DBS for the treatment of somatogenic pain principally targeted the central gray matter, or periaqueductal gray and periventricular gray (PAG/PVG) matter, and the sensory thalamus (ST).12 The PAG/PVG matter was predominantly stimulated to treat nociceptive pain, while neuropathic pain was primarily treated with stimulation of the ST alone or in combination with PAG/PVG matter stimulation.12 Furthermore, although more infrequent, the internal capsule was stimulated for treatment of somatogenic pain by a number of studies.13–15 Long-term relief of chronic pain symptoms following ST stimulation was first reported by Mazars et al.,16 and such relief following PAG/PVG matter stimulation was reported by Richardson and Akil17 and by Hosobuchi et al.18 During the early 1990s, researchers sought to identify other areas of the brain that could be used as targets for stimulation to relieve chronic pain symptoms.
Another important period for DBS treatment of chronic, intractable pain began in the early 2000s. Cluster headache (CH) is a syndrome that is characterized by severe episodic primary neurovascular headaches.19 Prior to the 21st century, standard treatments for CH were ineffective. Then utilization of positron emission tomography (PET) studies demonstrated to researchers increased cerebral blood flow to the posterior hypothalamus during an acute CH attack.20–22 Taking these findings into account, Leone et al. hypothesized that electric stimulation to the posterior hypothalamus may prevent and/or relieve pain symptoms from a CH attack. Thus, in 2001, Leone et al. applied DBS to the posterior hypothalamus of a 39-year-old man suffering chronic CH.23 The patient experienced reduced pain for 13 months following the procedure. Subsequently, stimulation of the hypothalamus became a highly researched method for the treatment of chronic CH pain. This was not the first time the hypothalamus was targeted for DBS treatment of pain. Heath stimulated the hypothalamus of a pain patient in 1954, reporting one of the first cases of acute pain relief following stimulation. However, the circumstances were much different.1 Soon after Leone et al.’s success, Franzini et al. assembled the first important clinical trial for this procedure.24
In summary, intensive research and clinical trials for DBS in the treatment of chronic pain began primarily with the stimulation of the central gray matter and the ST. More recently, application of DBS to the motor cortex has developed into its own field of stereotactic neurosurgery, remaining highly researched. Lastly, treatment for pain arising from CH via stimulation of the hypothalamus represents the latest and currently most heavily researched applications of DBS.
Types of Pain and Targets
Pain is a common symptom of illness and disease. The International Association for the Study of Pain’s classification of pain elucidates pain as a “negative sensory or emotional experience linked with actual or potential tissue damage or that which is described in terms of such damage.”25 Chronic pain is classified as pain experienced daily for 6 months or longer. Pain is usually categorized as either somatogenic or psychogenic. Somatogenic pain, or “organic pain,” arises from somatogenic lesions resulting from trauma, infection, or other external factors.26 By contrast, the origin of psychogenic is psychological. Often, it is difficult to assign a patient’s pain to a particular category. Although DBS has also been utilized for psychogenic disorders such as tremors, Parkinson’s disease, and epilepsy, this chapter focuses on DBS treatment for somatogenic pain.
Somatogenic pain is divided into two main categories, nociceptive and neuropathic pain. Nociceptive pain refers to pain originating via stimulation of peripheral nociceptors, or pain receptors. Nociceptive stimulation then transmits signals to the central nervous system through integral somatosensory pain pathways, causing a person to experience pain.27 DBS treatment for pain of nociceptive origin predominately entails stimulation of the central gray matter, both the PAG matter and the PVG matter. The central gray matter, residing within the tegmentum of the midbrain, surrounds the cerebral aqueduct.28 Neuronal pathways traversing the central gray matter have demonstrated roles in reproduction, defensive behavior, and analgesia.28 Nonetheless, DBS of the central gray matter has proved to elicit analgesic responses.
Neuropathic pain, or deafferentation pain, refers to pain following direct damage to the nervous system. Neuropathic pain can be classified as either peripheral or central.29 Peripheral neuropathic pain originates from damage to the peripheral nervous system. Types of peripheral neuropathic pain include dysesthesia dolorosa, phantom-limb pain, and diabetic neuropathy. Central neuropathic pain originates from damage to the central nervous system, that is, the brain and spinal cord. Specific types of central pain include spinal cord injury, poststroke pain, postherpetic neuralgia, and other forms of neuralgia.29 The ST has been the principal target region for DBS treatment of neuropathic pain. The thalamus, a large, paired mass of gray matter, resides in the diencephalon. It is located in the center of the brain, along the midline, and sits right above the brain stem. The thalamus’s functions include motor control and transmission of sensory stimuli.30 Stimulation of the lateral nuclei, specifically the ventral posterolateral (VPL) nucleus and the ventral posteromedial (VPM) nucleus, has been shown to reduce pain symptoms of neuropathic origin.
CH is a severe primary neurovascular headache disorder causing 10% to 20% of patients with the disorder to experience debilitating headaches.31 Pain arising from this syndrome is indicated by attacks of intense unilateral periorbital pain, often jointly experienced with ipsilateral cranial autonomic disturbance. Through utilization of PET studies, increased blood flow to the posterior hypothalamic region was observed in patients amid an acute CH attack. The hypothalamus, forming the ventral aspect of the diencephalon and located beneath the thalamus, is linked with endocrine and pituitary function.30 DBS of the posterior hypothalamic region was introduced to treat pain arising from acute CH attacks.23
Mechanism of Action (of A DBS Device)
The mechanism by which DBS treats pain symptoms is not fully understood. The most common sites of electric stimulation are the PAG/PVG matter and the ST. More recently, targeted sites have included the posterior hypothalamus and the motor cortex. The original studies of Reynolds in rats showed that stimulation of the lateral margin of the PAG matter inhibited nociceptive responses.6 Further research indicated that this effect was reversible with administration of opioids antagonists.32 Other research studies also reported reverses in pain relief after administration of naloxone.17,18 Two additional studies showed that endogenous opioid levels were elevated in the third ventricle following electric stimulation of the PAG/PVG matter.33,34 Elevated levels of endogenous opioids following PAG/PVG matter stimulation may be critical in producing analgesic effects. However, it is still open to debate whether opioid release is a direct result of PAG/PVG matter stimulation or is a secondary effect.
Although electric stimulation of both the PAG matter and the PVG matter leads to increased levels of endogenous opioids, research has shown that other mechanisms are likely to accompany this stimulation. Specific to PAG matter stimulation, an additional mechanism involves spinal cord stimulation. Although PAG matter neurons do not usually extend directly to the spinal cord, they normally connect with the medullary nucleus raphe magnus (NRM).27 Following stimulation via PAG matter neurons, the NRM projects to the dorsal horn of the spinal cord. Considering that the PAG matter, NRM, and dorsal horn of the spinal cord all contain high levels of opiates, analgesic effects via stimulation are likely to be mediated by these structures. Furthermore, analgesic effects following PAG matter stimulation are terminated if descending pathways to the spinal cord are incised. Because analgesia is produced when opiates permeate the subarachnoid space of the spinal cord and the interventricular space of the brain,35 this action strongly suggests that a signal cascade from the PAG matter to the NRM to the dorsal root of the spinal cord, subsequent to PAG matter stimulation, causes those structures to mediate and possibly sequester opiates to specific areas, inducing pain relief.27
Similar to stimulation of PAG matter, stimulation of PVG matter leads to increased levels of endogenous opiates. However, it is thought that other mechanisms, together with increased opiate levels, result in analgesic effects following PVG matter stimulation.12 One such mechanism involves signaling along ascending pathways from the PVG matter. One study noted that following PVG matter stimulation, there was increased activity in the medial dorsal nucleus of the thalamus. This region is associated with the limbic system and has strong links to the amygdala and cingulated cortex.36 More simply, stimulation of PVG matter may alter a patient’s psychogenic response to pain.12 To summarize, evidence suggests that rising opiate levels, together with an altered psychological response to pain, give rise to analgesic effects.
The mechanism by which analgesia is produced following stimulation of the somatosensory thalamus is not completely understood.12 Studies by Benabid and colleagues on induced analgesia following ST stimulation in rats experiencing deafferentation pain provided evidence that the mechanism does not involve rises in endogenous opioid levels.27,37 In experiments with rats, Benabid et al. found that stimulation of the VPL nucleus of thalamus, the ventral caudal thalamus in humans, prevented binding of noxious agonists to their respective receptors in the thalamic intralaminar nucleus, or the nucleus parafascicularis (Pf), through an opioid-independent process.37 Furthermore, Benabid et al.’s findings suggested that dorsal horn neurons were not involved in neural pathways responsible for suppression of Pf pain reception following VPL stimulation. Moreover, the neural pathways involved were not monosynaptic. Some believe that analgesia via ST stimulation occurs due to activation of inhibitory corticofugal fibers following stimulation.38,39 Activation of these fibers would inhibit the sequestration of foreign pain agonists. Gerhart et al. performed experiments with ST stimulation in monkeys to address this hypothesis.40 These experiments focused on the inhibitory effects of lamina I to V spinothalamic tract (STT) neurons following thalamic stimulation. Gerhart et al. sought to observe which neuronal pathways led to activation of inhibitory STT neurons following ST stimulation. They observed that inhibitory effects, subsequent to ST stimulation, of lamina I cells contained within the dorsal horn of the spinal cord were terminated when lesions were made to the dorsolateral funiculi and the ventral funiculus of the ipsilateral funiculus. However, when lesions were applied solely to the dorsolateral funiculus, substantial STT inhibition remained. Gerhart et al. concluded that although neurons projecting through the dorsal lateral funiculus have involvement in STT inhibition following ST stimulation, much of the inhibition is mediated through pathways projecting through the ventral spinal cord. This strongly suggests an inhibitory thalamocortical–corticofugal pathway.12 Also, increases in extracellular serotonin concentrations have been observed in monkeys undergoing VPL stimulation.41 Serotonin is involved in mediation of nociceptive signaling and thus may be involved in pain relief subsequent to ST stimulation. Finally, in some neuropathic pain patients, thalamic bursting is observed, which may be associated with feelings of chronic pain.42–44 ST stimulation may inhibit this bursting, resulting in analgesic effects. Thus, several possible mechanisms could account for the analgesic effects following ST stimulation.
Targeting the posterior hypothalamus for stimulation for treatment of CH is a relatively new practice. Hypothesized mechanisms of action are activation or inhibition of neuronal pathways traversing the posterior hypothalamus. These neuronal pathways include ascending catecholaminergic and descending pathways from the hypothalamus to the brain stem and spinal cord. The posterior hypothalamus also contains cells concentrated with melatonin and opiate peptides.45 Traversing autonomic neuronal pathways through the targeted area led to theories that the induction of analgesia following electric stimulation includes a hormonal mechanism. However, this hypothesis was disproved through close patient assessment following chronic DBS stimulation. Schoenen et al. utilized DBS for hypothalamic stimulation of a small number of patients suffering from chronic CH.46 One week following DBS, two patients showed small decreases in urinary secretion of melatonin but no other hormonal abnormalities. Schoenen et al. concluded that the induced hypoalgesia was independent of a reduction in sensitivity or perception of pain. They also concluded that the mechanism was more complex than any simple pathway leading to hypoalgesia. This idea is in accord with earlier predictions by May and Leone, who suggested that various central pathways are involved.19 Lastly, it was observed that amid a CH attack, there is increased blood flow localized to the hypothalamus.46 Electric stimulation of the hypothalamus could mediate CH attacks by suppressing the surge of blood to the hypothalamus.
Patient Selection and Indications
Criteria for patients receiving DBS vary from one clinical trial to another, although similarities are present. The most common sites stimulated in clinical trials have been the PAG/PVG matter and the somatosensory thalamus. In a thorough review, Bittar et al. stated that patients chosen for DBS stimulation were selected based on three main criteria: the patient’s pain was diagnosed as somatogenic, prior treatment had failed to provide relief, and the patient did not present any psychogenic disorders and/or severe depression.12 Levy et al. stated that between 1972 and 1984, 304 patients who received DBS stimulation had debilitating, chronic, and intractable pain that was untreatable by conventional methods.14 Generally, patients were chosen for PAG/PVG matter stimulation if their pain was nociceptive and for ST stimulation if their pain was neuropathic. Young et al. discussed their selection criteria for 48 patients receiving DBS over a 5-year period, from 1978 to 1983.15 Patients selected for DBS had suffered chronic pain for a mean duration of 4.5 years. Patients were subjected to more established means of treatment prior to receiving DBS. They also received a thorough psychological analysis, and their therapeutic responses to morphine, naloxone, and a placebo were monitored closely. In our study, electrodes were implanted into the PAG/PVG matter, the ST, and the internal capsule. In accordance with Levy et al., the placement of electrodes was based on the pathogenesis of pain (i.e., nociceptive or neuropathic).
Patients receiving posterior hypothalamic stimulation suffered from chronic CH. Patients diagnosed with CH generally displayed increased vascular perfusion to the posterior hypothalamic region during a spontaneous or nitroglycerin-induced CH attack from PET scanning utilizing a H21 marker.21,22 Franzini et al. composed one of the first teams of neurosurgeons to observe the therapeutic effects of hypothalamic stimulation on CH patients. They arranged a multidisciplinary team of neurologists to select patients for clinical trials. Neurologists chose patients presenting symptoms of chronic CH in accordance with the International Headache Society. Patients had to have suffered CH for at least 1 year with no response to prior medical treatment, including corticosteroids, lithium, methysergide, ergotamine, calcium channel blockers, beta-blocking agents, tricyclic antidepressants, melatonin, and nonsteroidal anti-inflammatory drugs.24 Potential subjects also underwent psychological screening and were informed of alternative neurosurgical treatments. A more recent clinical trial undertaken by Starr et al. at the University of California at San Francisco chose four patients for hypothalamic stimulation under similar guidelines. Trial patients were diagnosed with chronic CH via the criteria presented by the International Headache Society, had chronic CH episodes for at least 6 months in the previous 2 years, had at least seven unbearable headaches (6 on visual analogue scale of 1-10), and did not respond to several prophylactic and abortive therapies.31
Classifying Pain (Patient Screening)
Patients who took part in clinical trials utilizing DBS for the treatment of chronic pain received thorough physical and psychological evaluations, as described previously. If initial evaluations deemed patients were qualified to participate in the clinical trials, they were screened into two groups based on the etiology of their pain (i.e., nociceptive vs. neuropathic). Nociceptive pain patients principally received DBS in the PAG/PVG matter, and neuropathic pain patients received DBS in the ST. Research by Hosobuchi et al. provided the key method for localizing patients into the aforementioned groups. In one of their early clinical trials, Hosobuchi et al. noted that analgesia invoked via electric stimulation of the central gray matter was reversed following administration of naloxone, an opioid antagonist.34 Combining this knowledge with the finding that levels of endogenous opioids were increased in the third ventricle subsequent to PAG matter stimulation,33,34 it was concluded that PAG/PVG matter stimulation produced analgesia through an opioid-dependent mechanism. However, other research found that stimulation of the ST worked through an opioid-independent mechanism.37 Although contradictory, reports published by Young and Chambi demonstrated conflicting results concerning stimulation of the PAG/PVG matter. Within their findings, they suggested that nonopioid-dependent mechanisms accounted for the majority of analgesia produced via PAG/PVG matter stimulation.47 However, most clinical studies followed research demonstrating that nociceptive pain responded more strongly to PAG/PVG matter stimulation. Thus, a principal screening test performed to discriminate patients based of etiology of pain was the morphine saturation test.12 The pentaorbital test was utilized as well in some studies.12
Hosobuchi introduced the morphine saturation test. Patients underwent a double-blind evaluation.32 Intravenous morphine was administered through a catheter, with saline as a placebo, in 5-mg increments until a 30-mg dose was reached. Administration lasted 30 to 45 minutes. Following administration, subjective evaluation by patients was measured on a visual analogue scale of 0 to 10 (the greater the number, the greater the level of pain). Naloxone was then administered. Patients who experienced significant pain relief following administration of 0 to 10 mg of morphine were excluded from DBS therapy. Patients who demonstrated pain relief following 10 to 30 mg of morphine were selected for PAG/PVG matter stimulation. Patients who expressed no pain relief following morphine administration and demonstrated symptoms of neuropathic pain were chosen for ST stimulation. Neuropathic pain was not difficult to diagnose. Clinical diagnoses such brachial plexus injury and thalamic syndrome provided strong evidence that pain was of neuropathic origin.48 Some patients demonstrating signs of both neuropathic pain and nociceptive pain had electrodes implanted in both the thalamus and the central gray matter.14 Nevertheless, several chief studies screened patients in this manner.13,14,32
Clinical trials conducting hypothalamic stimulation for CH pain utilized PET to properly implicate stimulation of the hypothalamus. Initial PET studies demonstrated increased vascular perfusion to the posterior hypothalamus during an acute CH attack.21,22 This finding, in addition to selection criteria discussed earlier, constituted screening patients before they received hypothalamic stimulation for treatment of their chronic CH pain.
DBS Target Selection
Targets chosen for electric stimulation were correlated with the classification of pain of diagnosed patients. The PAG/PVG matter is principally targeted for chronic nociceptive pain; the ST and the motor cortex are targeted for chronic neuropathic pain. More recently, the hypothalamus has been targeted for patients suffering chronic CH. The precise spots in the areas mentioned were often determined specifically by the research groups performing the clinical trials. In 1969, Reynolds was the first to induce analgesia in rats by targeting the lateral line of the PAG matter.6
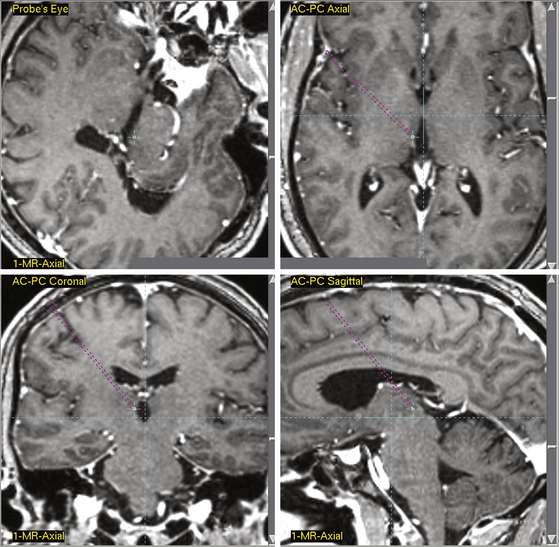
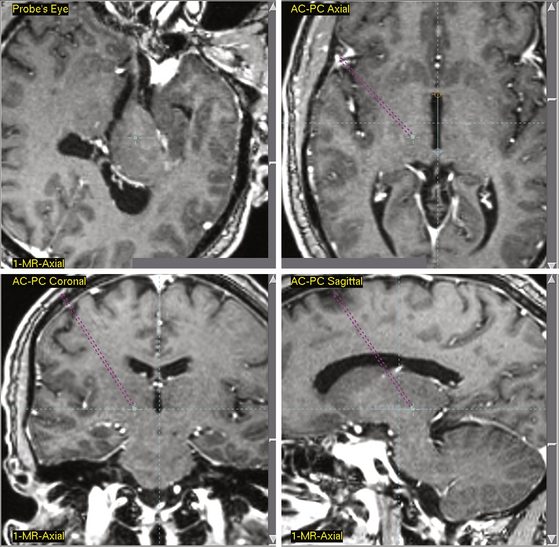
Central gray matter stimulation points have generally been determined in respect to the anterior commissure–posterior commissure (AC-PC) point, a radiologic landmark located in the third ventricle. Levy et al. determined targets in their studies by advancing a ventricular cannula into the lateral ventricle to obtain a ventriculography.14 Coordinates for stimulation in relation to the ventriculography were determined from the Schaltenbrand and Bailey stereotactic atlas.49 Four platinum-pole electrodes were directly implanted, with the most distal electrode localized 1 mm inferior and 1 mm posterior to the PC line and 2 to 3 mm lateral to the lateral wall of the third ventricle. To determine correct electrode placement, patients received 5 to 6 mA of stimulation. Subsequent feelings of pain relief and bodily warmth indicated correct electrode placement. Hosobuchi et al. stimulated the PAG matter, proximal to the canal of the sylvian aqueduct, 3 mm from the midline.18 They determined this target by comparing the contrast ventriculograms of patient’s heads with parameters set forth by the Schaltenbrand and Bailey atlas.18,49 Improper placement of electrodes was often indicated by patients experiencing fear and anxiety. Richardson and Akil also identified target points by comparison of ventriculograms of patient’s heads and parameters in the Schaltenbrand and Bailey atlas.17,49,50 They targeted points ventral to the PVG matter. During initial stimulation tests, 5 × 1.1 stainless steel monopolar electrodes were placed 20 mm from the target point and were moved 5 mm at a time until the target point had been reached. X-ray images reinforced proper placement of electrodes. In a recent publication delineating current standards for stereotactic neurosurgery procedures, Starr et al., using 3387 and/or 3389 quadrapolar leads, outlined specific coordinates for contemporary DBS application.51 In this publication, coordinates were selected to correspond to radiologic landmarks as defined in the Schaltenbrand and Bailey stereotactic atlas.49,51 The published coordinates for PAG/PVG matter stimulation are 0 to 3 mm anterior–posterior from the PC line, 3 mm lateral to the midline of the third ventricle, and −2 mm interior to 3 mm superior in reference to the AC-PC plane.51 Although Starr et al. indicated that specific targeting often required slight adjustments from these coordinates, they stated that deviation from these coordinates should never extend beyond 1 to 2 mm. This guideline is consistent for all other coordinates discussed in this chapter as defined by Starr et al. Figure 126-1 displays a stereotactic target point in a patient for PVG matter stimulation. Figure 126-2 displays a stereotactic target point in the same patient for PAG matter stimulation. Precise initial coordinates should take into account AC-PC scaling for each patient, as well as patient-specific radiographic anatomy.
Somatosensory thalamus stimulation targets were chiefly located in the contralateral ST, or ventral caudalis (Vc).12 Hosobuchi et al. described the target area for ST stimulation to be the region where the medial lemniscus connects with the ST.18 This connection is found in the VPM of facial deafferentation pain patients and in the VPL for extremity deafferentation pain patients. Levy et al. identified points through correlation of ventriculographies with the Schaltenbrand and Bailey stereotactic atlas.14,49 Neuropathic pain patients received stimulation at points with respect to parameters previously described by Hosobuchi et al.18 Facial deafferentation pain patients had electrodes implanted in the VPM nucleus. Electrodes were localized 8 mm posterior, 8 mm lateral, and 1 to 3 mm superior to the mid–AC-PC line. Extremity deafferentation pain patients had electrodes implanted in the VPL nucleus. The target point was located 9 mm posterior, 10 to 12 mm lateral, and 2 to 5 mm superior to the mid–AC-PC line. Upon initial stimulation, if paresthesia was not induced in the region of pain, electrode placement was carefully adjusted until such effects were felt. Current established coordinates for stimulation of the Vc, as defined by Starr et al., are 4 to 6 mm anterior to the PC line, 12 to 14 mm lateral to the midline at the third ventricle, and 0 mm superior–inferior to the AC-PC plane51 (Fig. 126-3).
The first series of clinical trials testing DBS for treatment of chronic pain also targeted the centromedian nucleus and the internal capsule, although more infrequently in respect to the ST and PAG/PVG matter. However, the current established coordinates in respect to these areas are important. As defined by Starr et al., stimulation of the centromedian nucleus is measured −1 anterior to 10 mm posterior to the PC line, 8 to 10 mm lateral to the midline at the third ventricle, and 0 mm in respect to the AC-PC plane. Stimulation of the internal capsule is measured 4 to 6 mm anterior to the PC line, 18 to 20 mm lateral to the midline at the third ventricle, and 0 mm in respect to the AC-PC plane.51
Leone et al. performed the first series of hypothalamic stimulations in 2001. They published coordinates that corresponded to 6 mm inferior to the midcommissural point, 2 mm lateral to the midline, and 8 mm inferior to the commissural plane.23 In 2003, Franzini et al. identified a new target for stimulation of the posterior hypothalamus via H21 PET scanning. PET scans indicated increased blood perfusion amid a CH attack. Magnetic resonance imaging (MRI) scans were fused with 2-mm-thick slices obtained from PET, and coordinates for stimulation were indicated.24 The target coordinates correlated to 3 mm posterior and 5 mm inferior to the midcommissural point and 2 mm lateral to the midline. Starr et al. utilized these coordinates in 2007 for their clinical trial. Furthermore, these coordinates define the current standard target in the posterior hypothalamus for treatment of CH pain.51 In their 2007 publication, Starr et al. also discussed the debate concerning the defined region wherein the coordinated target is located. They indicated that the target point in their trial and Franzini et al.’s trial24 was anteromedial to the dorsal part of the red nucleus and 4 mm posterior to the mammillothalamic tract (MTT). The anatomic location of the MTT is not universally defined. Some sources state that the MTT is localized on the posterior border of the hypothalamus,45,46 whereas others believe the MTT extends several millimeters above the posterior hypothalamus. Starr et al. indicated that due to this discrepancy, the target point introduced by Franzini et al. in 200324 could be defined as being either at the posterior hypothalamus or within the anterior periventricular gray matter.31
Surgical Technique
To date, there has been no universally accepted technique of performing DBS. However, there are principal components to all DBS procedures. In a meta-analysis of the first series of clinical trials, Bittar et al. described the chief components of all DBS surgery. The fundamental aspects include defining of target, placement of electrodes, and stimulation tests following placement.12 As target selection has previously been discussed, excessive details are not given here. One important point to clarify is that targeting procedures were greatly improved in the late 1970s. Hosobuchi explained that it was not until after 1976 that computed tomography (CT) and MRI scans were utilized on a broad scale to target sites for stimulation.32 The same holds true today. Patients who underwent DBS prior to 1976 principally utilized air/contrast ventriculographies and/or ventriculograms to specify targets.14,32
To provide a sufficient general outline of the DBS procedure, details from a number of individual clinical trials (PAG/PVG matter and ST stimulation) are incorporated herein. Patients were first put under local anesthesia. By doing this, subjective evaluation following trial stimulation could be obtained. Then, a stereotactic head frame (e.g., Leskell13,14,32 or Todd-Wells13) was placed and then attached to the patient’s head. An entrance incision was then made, followed by the drilling of bur holes through which electrodes were placed. Depending on the etiology of pain, electrodes were placed either into the central gray matter, often bilaterally, or into the contralateral ST.12 Electrodes were then bonded, but not permanently, into the bur holes. Hosobuchi bonded intracranial electrodes through utilization of methyl methacrylate.32 Leads were then drawn out of the skull, usually through a second entrance incision.
Following stereotactic placement, trial stimulation was carried out postoperatively over a period of 7 to 14 days to set stimulation parameters that would induce optimal pain relief.12 Hosobuchi reported standard stimulation parameters for the patients who underwent his clinical trials. For PAG matter stimulation, he reported parameters of 3 V, 30 Hz, and a 0.2-msec pulse width duration. For ST stimulation, he reported broader parameters of 2 to 5 V, 50 to 75 Hz, and 0.2-msec pulse width.32 Levy et al. reported somewhat similar parameters. Their PAG/PVG matter stimulation parameters were 1 to 5 V and 5 to 15 Hz, and their ST stimulation parameters were 3 to 8 V and 20 to 100 Hz.14 Proper electrode placement, effects, and stimulation parameters were delineated by feelings of relaxation and warmth in those receiving DBS of the PAG/PVG matter and by expression of paresthesia at the location of pain in those receiving ST stimulation.12 Chronic PAG/PVG matter stimulation was commonly done two to three times a day at low-frequency, short (20-30 minutes) durations.13 Chronic ST stimulation was commonly done three or more times a day at high-frequency, long (1-2 hours) durations.13 Following trial stimulation, patients who demonstrated sufficient pain relief with minimal adverse effects were selected for chronic stimulation. Electrodes were first fixed into their positions. Next, leads were connected to an external stimulator, which was then internalized in a subcutaneous pocket, often the anterior chest wall.14,32 Earlier procedures utilized a radiofrequency generator connected to external transmitter stimulation switch, while later trials utilized the Itrel pulse generator.12 Contemporary DBS surgery is usually performed in a similar manner. Starr et al. provided excellent descriptions of contemporary DBS surgery.51 CT and MRI imaging, in addition to PET studies, are utilized to define a specific stimulation site. One notable difference compared to the information previously discussed was that external stimulator placement is done intraclavicularly. However, Starr et al. emphasized that the specifics of the surgery are largely the preference of the neurosurgeon.
DBS of the hypothalamus does not deviate significantly from the technique previously discussed. Patient screening and target selection are as discussed previously. The most significant aspect is the optimal stimulation parameters to induce analgesia via CH pain. Starr et al., basing their parameters on previously published research,24,46 defined their stimulation parameters (monopolar) as 1 to 3 V, 185-Hz frequency, and 0.6-msec pulse width.51
Outcomes
Clinical trials utilizing DBS of the somatosensory thalamus (or Vc) and the central gray matter (PAG/PVG matter) provided the majority of successful cases of pain relief through electric intracranial stimulation during the first several decades of clinical trials. Patients suffering nociceptive pain benefitted the most from PAG/PVG matter stimulation. To cite a few individual studies, Hosobuchi implanted electrodes into the PAG matter of 65 patients.32 All implantations were done bilaterally. (Of these patients, 36 also had electrodes simultaneously implanted in the ST. The following outcomes discussed are relative to pain of peripheral origin.) All 65 patients expressed pain relief from initial stimulation. Of these patients, 50 (77%) experienced long-term pain relief (2 years), and 39 had long-term relief from chronic lower back and leg pain. Lumbar radicular pain, nonmalignant abdominal pain, and axial back pain due to osteoporosis of the spine are examples of other types of pain that were successfully treated. Of the 12 patients who were unsuccessful in obtaining long-term relief, 9 developed tolerance to stimulation 6 to 12 months following implantation; the other 3 had their electrodes explanted subsequent to initial stimulation for other reasons. Levy et al. implanted electrodes in the PAG/PVG matter of 57 patients over the course of their clinical trials.14 Of these 57 patients, 51 suffered from lower back and skeletal pain, while the other 6 suffered from cancer-related pain; 29 lower back/skeletal pain patients and 3 cancer-related pain patients expressed initial pain relief, with 16 (32%) and 2 (33%), respectively, expressing long-term pain relief at a mean follow-up of 6.8 years. Young et al. implanted electrodes into the PAG/PVG matter of 45 patients and followed their treatment for a year.15 Of the patients, 38 expressed initial pain relief, with 22 (49%) expressing significant long-term pain relief. Successful treatment of chronic pain via PAG/PVG matter stimulation has been reported in a number of other individual studies.13,17,50 On a broader scale, a meta-analysis published in 1998 by Bendock and Levy analyzed 1114 patients within 13 clinical trials utilizing DBS for treatment of chronic pain. Of the 419 patients treated for pain of peripheral/nociceptive origin, 148 received successful long-term pain relief following PAG/PVG matter stimulation.52
During the first several decades, the somatosensory thalamus was primarily stimulated to treat pain of neuropathic origins, although it was often utilized in combination with PAG/PVG matter stimulation. Citing specific studies, Hosobuchi targeted the ST to treat neuropathic deafferentation pain of 76 patients; 52 (68%) expressed pain relief following initial stimulation, with 44 (58%) reporting long-term pain relief.32 Furthermore, 17 patients suffering from central deafferentation who had electrodes implanted in the ST had electrodes simultaneously implanted in the PAG/PVG matter. Although all 17 patients reported pain relief following ST stimulation, none reported such effects following PAG/PVG matter stimulation. Levy et al. treated neuropathic pain via DBS by either ST stimulation alone or with PAG/PVG matter stimulation.14 Turnbull et al. treated 19 patients with neuropathic pain of different origins via ST stimulation.53 Of these patients, 14 (78%) reported initial pain relief. Following internalization of electrodes, 12 (67%) patients reported long-term pain relief. Of the 84 patients treated for neuropathic pain, 25 suffered from thalamic pain syndrome and received ST stimulation exclusively (3 of these patients had simultaneously implanted PAG/PVG matter electrodes, but they were removed soon after due to ineffectiveness). Of these 25 patients, 15 (63%) expressed initial pain relief, with 6 (24%) expressing continuous long-term pain relief. The other 69 patients suffering from a range of deafferentation pain syndromes were generally treated with stimulation of the ST and the PAG/PVG matter simultaneously. Of these 69 patients, 37 (54%) patients reported initial pain relief following electric stimulation, with 18 (26%) reporting long-term pain relief. On a larger scale, Bendock and Levy’s meta-analysis stated that 644 of the 1114 patients were treated for neuropathic pain.52 Of these 644 patients, 195 expressed long-term relief of pain symptoms following ST stimulation. In addition, 80 patients expressed long-term relief of neuropathic pain following PAG/PVG matter stimulation.
In 2001, after reviewing a number of recent publications on the etiology of CHs, Leone et al. successfully provided long-term pain relief to a 39-year-old man suffering from the neurovascular headache disorder.23 Following this initial success, Franzini et al. utilized the procedure with slight adjustments in a clinical trial consisting of five patients suffering from chronic, intractable CH.24 Franzini et al. reported very positive results. All five patients reported initial complete pain relief from CH attacks following high-frequency, hypothalamic stimulation. Furthermore in follow-up visits ranging from 2 to 22 months, all five patients continued to report excellent pain relief via chronic hypothalamic stimulation. Soon after, Schoenen et al. conducted a clinical trial giving DBS in the posterior hypothalamus in six patients suffering chronic CH.46 With one patient experiencing significant adverse effects following initial electrode placement, five patients had electrodes implanted. After 6 months of chronic stimulation, Schoenen et al. reported that two patients were pain free, one patient had significant but not total pain relief, and one patient suffered occasional remissions of CH pain. The fifth patient passed away prior to follow-up visits. Lastly, Starr et al. published results from a clinical trial conducting DBS stimulation for chronic CH in four patients.31 Following 1 year of chronic stimulation, two patients experienced significant long-term pain relief, one experienced moderate long-term pain relief, and one did not respond to long-term DBS therapy. To date, a high percentage of CH patients who have participated in clinical trials conducting DBS stimulation of the posterior hypothalamus have experienced significant pain relief from their otherwise untreatable pain symptoms. However, it is imperative that more studies be done and more results be published regarding hypothalamic stimulation to make a concrete determination of the efficacy of the procedure.
Complications
DBS is an invasive surgical procedure, and various complications and adverse effects have been observed throughout the course of clinical trials. Clinical trials that stimulated the PAG/PVG matter and the ST reported the most serious complication to be intracranial hemorrhage.12 Citing individual studies, in his analysis of the 122 patients who underwent DBS, Hosobuchi reported five cases of intracranial hemorrhage following electrode implantation.32 Three cases constituted ventricular hemorrhage, with one proving fatal, and two cases constituted intracranial hemorrhage, with one proving fatal. Levy et al., in a broad analysis of the 141 patients treated with DBS, reported 5 cases of intracranial hemorrhage.14 In 3 of the cases, hemorrhaging was intracranial. For the other 2 cases, 1 constituted epidural hemorrhaging and the other periventricular hemorrhaging. Of the 5 cases of intracranial hemorrhaging, 1 proved fatal; 2 resulted in severe, permanent neurologic damage; and 2 recovered completely. Kumar et al., in their analysis of the 68 patients treated with DBS for chronic pain, reported only 1 case of intracranial hemorrhaging.13 Treatment of hematomas subsequent to intracranial hemorrhaging was generally done by evacuation via craniotomy or by placement of a shunt. A number of other side effects were reported in these studies, all of which were considered milder than hemorrhaging. Hosobuchi and Levy et al. reported the highest frequency of adverse effects to be infection.14,32 Hosobuchi noted 4 cases of subgaleal infection, 1 case of ventriculitis, and 1 case of subdural empyema. Levy et al. reported 23 cases of infection in 17 patients. All but one of these infections was epidural in origin. Of the 23 cases of infection, 17 cases were localized to the scalp at the incision site of electrodes, 3 cases occurred in the chest wall where the external stimulator was placed, and 2 cases occurred in the neck where wire was run through. The final patient was diagnosed with meningitis following the procedure. Kumar et al. reported the highest frequency adverse effect to be headaches; 15 patients suffered migraine-like headaches following the procedure13 These headaches eventually resolved in 14 patients. Kumar et al. report that 1 patient required electrodes to be removed temporarily due to intractable headaches. In addition, 4 patients experienced infection; 3 recovered after administration of antibiotics, but 1 had to have electrodes removed. Although few in number, other adverse effects among these studies included eye movement dysfunction, erosion of hardware, psychosis, and postoperative seizures.13,14,32
Although the advancement of neurologic techniques over the years has led to improvements in postoperative patient care, complications are not unavoidable. In Starr et al.’s recent publication,51 it was reported that of 800 patients treated with stimulation of deep brain structures for their chronic pain, postoperative hemorrhaging per lead was 2.25%, while symptophomatic hemorrhaging per lead was 0.7%. Furthermore, 3% of patients required intervention due to postoperative infection. Starr et al. reported that hemorrhaging can be avoided by the careful placement of electrodes with consideration of proximal vasculature; moreover, patients experiencing infection should be keep under special observation and should receive antibiotic regiments.
Although the hypothalamus is located deep within the cerebrum in respect to the central gray matter and ST, clinical trials conducting stimulation of this structure for the treatment of chronic CH have reported very low occurrences of adverse effects. With their first patient, Leone et al. reported the procedure to be extremely safe.23 Franzini et al. reported similar results in their clinical trial.24 Of the five patients who underwent the procedure, none experienced adverse effects following electrode implantation or afterward during acute or chronic high-frequency stimulation. Schoenen et al. reported that some of their patients expressed mild symptoms of diplopia and dizziness following stimulation intensity above 1.5 V.46 These effects subsided within 24 to 48 hours and were not experienced following lower-intensity stimulation. No other adverse effects were reported in their study. Lastly, Starr et al. reported only one adverse effect in four patients.31 Five minutes after the test stimulation, one patient experienced hemiplegia, ipsilateral to the side the electrode was implanted. The effect subsided after 5 minutes. Remarkably, hypothalamic stimulation for CH pain, thus far, has occurred with minimal to no complications.
Conclusions
Following Heath’s initial induction of analgesia with DBS,1 numerous clinical trials and research studies have successfully utilized the procedure to treat patients suffering from chronic pain. Stimulation of the PAG/PVG matter has provided mild to significant pain relief for patients suffering nociceptive pain, while stimulation of the Vc of the ST has provided similar relief for patients suffering neuropathic pain. Furthermore, while continuing to be heavily researched, stimulation of the hypothalamus for treatment of CH pain has demonstrated highly successful results. Although complications, ranging in severity, have been observed, this procedure remains relatively safe and will only become safer. DBS has proved a novel stereotactical neurosurgical technique for treating chronic pain, as well as a number of other symptoms. In the future, new target regions are anticipated to be found, expanding the number of applications for which DBS can be employed.
Benabid A.L., Henriksen S.J., McGinty J.F., Bloom F.E. Thalamic nucleus ventro-postero-lateralis inhibits nucleus parafascicularis response to noxious stimuli through a non-opioid pathway. Brain Res. 1983;280:217-231.
Bendock B., Levy R.M. Brain stimulation for persistent pain management. New York: McGraw-Hill; 1998.
Bittar R.G., Kar-Purkayastha I., Owen S.L., et al. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci. 2005;12:515-519.
Franzini A., Ferroli P., Leone M., Broggi G. Stimulation of the posterior hypothalamus for treatment of chronic intractable cluster headaches: first reported series. Neurosurgery. 2003;52:1095-1099. discussion 1099-1101
Garonzik I., Samdani A., Ohara S., Lenz F.A. Deep brain stimulation for the control of pain. Epilepsy and Behavior. 2001;2:S55-S60.
Gerhart K.D., Yezierski R.P., Wilcox T.K., Willis W.D. Inhibition of primate spinothalamic tract neurons by stimulation in periaqueductal gray or adjacent midbrain reticular formation. J Neurophysiol. 1984;51:450-466.
Heath R.G. Studies in schizophrenia: a multidisciplinary approach to mind–brain relationships. Cambridge: Harvard University Press; 1954.
Hosobuchi Y. Subcortical electrical stimulation for control of intractable pain in humans. Report of 122 cases (1970-1984). J Neurosurg. 1986;64:543-553.
Hosobuchi Y., Adams J.E., Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977;197:183-186.
Hosobuchi Y., Rossier J., Bloom F.E., Guillemin R. Stimulation of human periaqueductal gray for pain relief increases immunoreactive beta-endorphin in ventricular fluid. Science. 1979;203:279-281.
Kumar K., Toth C., Nath R.K. Deep brain stimulation for intractable pain: a 15-year experience. Neurosurgery. 1997;40:736-746. discussion 746-737
Lenz C., Rebel A., van Ackern K., et al. Local cerebral blood flow, local cerebral glucose utilization, and flow-metabolism coupling during sevoflurane versus isoflurane anesthesia in rats. Anesthesiology. 1998;89:1480-1488.
Leone M., Franzini A., Bussone G. Stereotactic stimulation of posterior hypothalamic gray matter in a patient with intractable cluster headache. N Engl J Med. 2001;345:1428-1429.
Levy R.M., Lamb S., Adams J.E. Treatment of chronic pain by deep brain stimulation: long term follow-up and review of the literature. Neurosurgery. 1987;21:885-893.
Liebeskind J.C., Guilbaud G., Besson J.M., Oliveras J.L. Analgesia from electrical stimulation of the periaqueductal gray matter in the cat: behavioral observations and inhibitory effects on spinal cord interneurons. Brain Res. 1973;50:441-446.
May A., Ashburner J., Buchel C., et al. Correlation between structural and functional changes in brain in an idiopathic headache syndrome. Nat Med. 1999;5:836-838.
May A., Bahra A., Buchel C., et al. PET and MRA findings in cluster headache and MRA in experimental pain. Neurology. 2000;55:1328-1335.
Mayer D.J., Wolfle T.L., Akil H., et al. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971;174:1351-1354.
Rezai A.R., Lozano A.M., Crawley A.P., et al. Thalamic stimulation and functional magnetic resonance imaging: localization of cortical and subcortical activation with implanted electrodes. Technical note. J Neurosurg. 1999;90:583-590.
Richardson D.E., Akil H. Pain reduction by electrical brain stimulation in man. 1. Acute administration in periaqueductal and periventricular sites. J Neurosurg. 1977;47:178-183.
Schoenen J., Di Clemente L., Vandenheede M., et al. Hypothalamic stimulation in chronic cluster headache: a pilot study of efficacy and mode of action. Brain. 2005;128:940-947.
Starr P.A., Barbaro N.M., Larson P.S. Neurosurgical Operative Atlas, 2nd ed. New York: Thieme Medical Publishers; 2008.
Starr P.A., Barbaro N.M., Raskin N.H., Ostrem J.L. Chronic stimulation of the posterior hypothalamic region for cluster headache: technique and 1-year results in four patients. J Neurosurg. 2007;106:999-1005.
Turk D.C. Combining somatic and psychosocial treatment for chronic pain patients: perhaps 1 + 1 does = 3. Clin J Pain. 2001;17:281-283.
Turnbull I.M., Shulman R., Woodhurst W.B. Thalamic stimulation for neuropathic pain. J Neurosurg. 1980;52:486-493.
Young R.F., Chambi V.I. Pain relief by electrical stimulation of the periaqueductal and periventricular gray matter. Evidence for a non-opioid mechanism. J Neurosurg. 1987;66:364-371.
Young R.F., Kroening R., Fulton W., et al. Electrical stimulation of the brain in treatment of chronic pain. Experience over 5 years. J Neurosurg. 1985;62:389-396.
1. Heath R.G. Studies in schizophrenia: a multidisciplinary approach to mind–brain relationships. Cambridge: Harvard University Press; 1954.
3. Ervin FR X. Effects of opioids on electrical activity of deep structures in the human brain. Res Publ Assoc Res Nerv Ment Dis. 1968;46:150-156.
4. Gol A. Relief of pain by electrical stimulation of the septal area. J Neurol Sci. 1967;5:115-120.
5. Pool J.L., Clark K.W., Hudson P., Lombardo M. laboratory and clinical assessment. In Hypothalamic–hypophysial dysfunction in man. Springfield: Thomas; 1956.
6. Reynolds D.V. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444-445.
7. Akil H., Liebeskind J.C. Monoaminergic mechanisms of stimulation-produced analgesia. Brain Res. 1975;94:279-296.
8. Akil H., Mayer D.J., Liebeskind J.C. Comparison in the rat between analgesia induced by stimulation of periaqueducal gray matter and morphine analgesia. C R Acad Sci Hebd Seances Acad Sci D. 1972;274:3603-3605.
9. Mayer D.J., Liebeskind J.C. Pain reduction by focal electrical stimulation of the brain: an anatomical and behavioral analysis. Brain Res. 1974;68:73-93.
10. Mayer D.J., Wolfle T.L., Akil H., et al. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971;174:1351-1354.
11. Liebeskind J.C., Guilbaud G., Besson J.M., Oliveras J.L. Analgesia from electrical stimulation of the periaqueductal gray matter in the cat: behavioral observations and inhibitory effects on spinal cord interneurons. Brain Res. 1973;50:441-446.
12. Bittar R.G., Kar-Purkayastha I., Owen S.L., et al. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci. 2005;12:515-519.
13. Kumar K., Toth C., Nath R.K. Deep brain stimulation for intractable pain: a 15-year experience. Neurosurgery. 1997;40:736-746. discussion 746-737
14. Levy R.M., Lamb S., Adams J.E. Treatment of chronic pain by deep brain stimulation: long term follow-up and review of the literature. Neurosurgery. 1987;21:885-893.
15. Young R.F., Kroening R., Fulton W., et al. Electrical stimulation of the brain in treatment of chronic pain. Experience over 5 years. J Neurosurg. 1985;62:389-396.
16. Mazars G., Merienne L., Ciolocca C. Intermittent analgesic thalamic stimulation. Preliminary note. Rev Neurol (Paris). 1973;128:273-279.
17. Richardson D.E., Akil H. Pain reduction by electrical brain stimulation in man. Part 1: Acute administration in periaqueductal and periventricular sites. J Neurosurg. 1977;47:178-183.
18. Hosobuchi Y., Adams J.E., Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977;197:183-186.
19. May A., Leone M. Update on cluster headache. Curr Opin Neurol. 2003;16:333-340.
20. May A., Ashburner J., Buchel C., et al. Correlation between structural and functional changes in brain in an idiopathic headache syndrome. Nat Med. 1999;5:836-838.
21. May A., Bahra A., Buchel C., et al. Hypothalamic activation in cluster headache attacks. Lancet. 1998;352:275-278.
22. May A., Bahra A., Buchel C., et al. PET and MRA findings in cluster headache and MRA in experimental pain. Neurology. 2000;55:1328-1335.
23. Leone M., Franzini A., Bussone G. Stereotactic stimulation of posterior hypothalamic gray matter in a patient with intractable cluster headache. N Engl J Med. 2001;345:1428-1429.
24. Franzini A., Ferroli P., Leone M., Broggi G. Stimulation of the posterior hypothalamus for treatment of chronic intractable cluster headaches: first reported series. Neurosurgery. 2003;52:1095-1099. discussion 1099-1101
25. Turk D.C. Combining somatic and psychosocial treatment for chronic pain patients: perhaps 1 + 1 does = 3. Clin J Pain. 2001;17:281-283.
26. Almay B.G., Johansson F., Von Knorring L., et al. Endorphins in chronic pain. 1. Differences in CSF endorphin levels between organic and psychogenic pain syndromes. Pain. 1978;5:153-162.
27. Garonzik I., Samdani A., Ohara S., Lenz F.A. Deep brain stimulation for the control of pain. Epilepsy and Behavior. 2001;2:S55-S60.
28. Loewy A., Spyer M. Central regulation of autonomic functions US. Oxford University Press; 1990.
29. Rasche C. Neuromorphic excitable maps for visual processing. IEEE Trans Neural Netw. 2007;18:520-529.
30. Peters A., Jones E., Diamond I. Cerebral Cortex: The Barrel Cortex of Rodents. Springer. Vol 11, 1995.
31. Starr P.A., Barbaro N.M., Raskin N.H., Ostrem J.L. Chronic stimulation of the posterior hypothalamic region for cluster headache: technique and 1-year results in four patients. J Neurosurg. 2007;106:999-1005.
32. Hosobuchi Y. Subcortical electrical stimulation for control of intractable pain in humans. Report of 122 cases (1970-1984). J Neurosurg. 1986;64:543-553.
33. Akil H., Richardson D.E., Hughes J., Barchas J.D. Enkephalin-like material elevated in ventricular cerebrospinal fluid of pain patients after analgetic focal stimulation. Science. 1978;201:463-465.
34. Hosobuchi Y., Rossier J., Bloom F.E., Guillemin R. Stimulation of human periaqueductal gray for pain relief increases immunoreactive beta-endorphin in ventricular fluid. Science. 1979;203:279-281.
35. Meyerson B., Lind G., Linderoth B. Treatment of spasticity using an intrathecal infusion of baclofen. Lakartidningen. 1989;86:4125-4128.
36. Rezai A.R., Lozano A.M., Crawley A.P., et al. Thalamic stimulation and functional magnetic resonance imaging: localization of cortical and subcortical activation with implanted electrodes. Technical note. J Neurosurg. 1999;90:583-590.
37. Benabid A.L., Henriksen S.J., McGinty J.F., Bloom F.E. Thalamic nucleus ventro-postero-lateralis inhibits nucleus parafascicularis response to noxious stimuli through a non-opioid pathway. Brain Res. 1983;280:217-231.
38. Cassinari V., Dorizzi A., Monolo L. Thrombosis of the internal carotid artery and its branches caused by closed injuries of the head. Riv Patol Nerv Ment. 1969;90:127-170.
39. Garcin R., Rondot P., Guiot G. Rhythmic myoclonus of the right arm as the presenting symptom of a cervical cord tumour. Brain. 1968;91:75-84.
40. Gerhart K.D., Yezierski R.P., Wilcox T.K., Willis W.D. Inhibition of primate spinothalamic tract neurons by stimulation in periaqueductal gray or adjacent midbrain reticular formation. J Neurophysiol. 1984;51:450-466.
41. Sorkin L.S., McAdoo D.J., Willis W.D. Stimulation in the ventral posterior lateral nucleus of the primate thalamus leads to release of serotonin in the lumbar spinal cord. Brain Res. 1992;581:307-310.
42. Lenz C., Rebel A., van Ackern K., Kuschinsky W., Waschke K.F. Local cerebral blood flow, local cerebral glucose utilization, and flow-metabolism coupling during sevoflurane versus isoflurane anesthesia in rats. Anesthesiology. 1998;89:1480-1488.
43. Lenz F.A., Garonzik I.M., Zirh T.A., Dougherty P.M. Neuronal activity in the region of the thalamic principal sensory nucleus (ventralis caudalis) in patients with pain following amputations. Neuroscience. 1998;86:1065-1081.
44. Lenz F.A., Gracely R.H., Hope E.J., et al. The sensation of angina can be evoked by stimulation of the human thalamus. Pain. 1994;59:119-125.
45. Swaab D.F. Neuropeptides in hypothalamic neuronal disorders. Int Rev Cytol. 2004;240:305-375.
46. Schoenen J., Di Clemente L., Vandenheede M., et al. Hypothalamic stimulation in chronic cluster headache: a pilot study of efficacy and mode of action. Brain. 2005;128:940-947.
47. Young R.F., Chambi V.I. Pain relief by electrical stimulation of the periaqueductal and periventricular gray matter. Evidence for a non-opioid mechanism. J Neurosurg. 1987;66:364-371.
48. Tasker R.R., Tsuda T., Hawrylyshyn P. Clinical neurophysiological investigation of deafferentation pain. Advanced Pain Restoration Therapy. 1983;5:713-738.
49. Schaltenbrand G., Bailey P. Introduction to Stereotaxis with an Atlas of the Human Brain. New York: Grune & Stratton; 1959. vol 2
50. Richardson D.E., Akil H. Pain reduction by electrical brain stimulation in man. Part 2: Chronic self-administration in the periventricular gray matter. J Neurosurg. 1977;47:184-194.
51. Starr P.A., Barbaro N.M., Larson P.S. Neurosurgical Operative Atlas, Second Edition. New York: Thieme Medical Publishers, Inc; 2008.
52. Bendock B., Levy R.M. Brain stimulation for persistent pain management. New York: McGraw-Hill; 1998.
53. Turnbull I.M., Shulman R., Woodhurst W.B. Thalamic stimulation for neuropathic pain. J Neurosurg. 1980;52:486-493.