Chapter 119 Deep Brain Stimulation for Intractable Psychiatric Illness
Limited reports of neurosurgical procedures for derangement in behavior and thinking date back to the late 1800s.1 However, the true birth of psychosurgery was not until 1935, when Moniz and Lima developed the frontal leucotomy. Inspired by frontal disconnection studies done at Yale University by Fulton and Jacobsen, which reduced agitation in primates, Moniz and Lima performed a series of bur hole–based frontal lesioning procedures starting in November of that year. This involved placing an instrument through several bur holes and making sweeping motions in roughly a coronal plane to “disconnect” the frontal lobe from the rest of the brain. Shortly thereafter, Freeman, an American neurologist, teamed up with Watts, a neurosurgeon, and in 1936 started performing a similar procedure, which the pair called the frontal lobotomy. These procedures were introduced at a time when there were no medical treatments available; patients were either permanently institutionalized or treated with “shock therapy” using electrical current, insulin, or metrazol. By the end of the 1930s, there were more than 400,000 psychiatric inpatients in the United States, occupying more than 50% of the hospital beds in the country.1 In retrospect, these procedures were crude and imprecise, but given the technology of the day and the desperation of many psychiatrists to find effective treatments for their patients, the frontal lobotomy gained support by many practitioners. Freeman was an outgoing and vocal advocate for the procedure and in some ways was as much a showman as he was a physician. As time went on, he became increasingly convinced that the procedures could be done in an office by non-neurosurgeons; he developed the transorbital approach, or the so-called ice-pick procedure, which led to the dissolution of his professional collaboration with Watts. Even after the introduction of Thorazine (the first truly effective antipsychotic agent) in 1954, Freeman continued to champion what he believed were the merits of frontal lobotomy, despite a clear movement of the rest of his profession away from the procedure over safety concerns. What followed was a backlash against lobotomy in general and Freeman in particular.
Despite this negative sentiment toward surgery, several centers around the world continued to perform and refine lesional procedures. The development of frame-based stereotaxy for human surgery in the 1940s by Spiegel and Wycis,1a along with the development of stereotactic atlases, meant surgeons could make discrete lesions in the brain with far more accuracy. Procedures such as cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leucotomy came into their own right as legitimate treatments for medically refractory illness. At the same time, the field of psychiatry continued to mature, with better characterizations and definitions of the various disorders being treated. The recent resurgence in interest in surgery for psychiatric disorders was brought about largely by the development of DBS in the 1990s. In addition, the development of functional imaging and biochemical analysis allowed researchers to discover brain areas that appear to be involved in diseases like obsessive-compulsive disorder (OCD) and major depression, and as we show, some of this work has translated directly into targets for DBS. Finally, there has been a definite shift in the last decade toward viewing these illnesses as systems-level disorders of specific neuronal circuits, subcortical and cortical in nature. This view is analogous to Parkinson’s disease, where a circuit abnormality leads to movement disorder. Psychiatric disorders appear to also have underlying neuronal circuits, where abnormalities manifest as a limbic disorder. In this vein, the term “limbic disorders surgery” may be a more accurate term for what was previously referred to as “psychosurgery.”
Obsessive-Compulsive Disorder
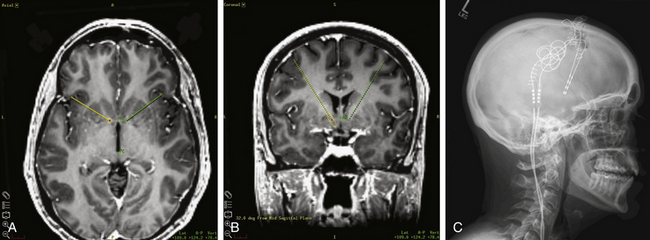
There is a long history of treating OCD with surgical lesioning. Although multiple areas have been explored, including the cingulate gyrus, many have focused on the anterior limb of the internal capsule, the target for the so-called capsulotomy. More recently, the term ventral capsule/ventral striatum (VC/VS) has been coined to describe the region including the ventral anterior internal capsule and the nucleus accumbens (NAcc), which has been implicated as a potential target in all three psychiatric disorders currently treated with DBS. The definition of this region evolved largely because surgical trials in OCD showed that lesioning or stimulating more ventrally and posteriorly in the capsule (or near the NAcc) resulted in better outcomes—in the case of DBS, with lower stimulation amplitudes (Fig. 119-1). The NAcc itself is believed to receive input from multiple areas, including the orbitofrontal cortex, amygdala, and caudate, and have projections to multiple areas, including the cingulate cortex, striatum, and frontal areas.
The first published series of OCD patients treated with DBS was in 2003 by a group of researchers from Belgium, who stimulated the anterior limb of the internal capsule in six patients using a target similar to that used for capsulotomy.2 In stereotactic space relative to the midpoint between the anterior commissure (AC) and the posterior commissure (PC), the target was 13 to 14 mm lateral, 3.5 mm anterior in the anteroposterior dimension, and at a depth of 0 mm (at the AC-PC plane). A widely spaced electrode array was used to span the length of the capsule (Medtronic model 3391, 3-mm-long electrodes with 4-mm interelectrode spacing). All patients entered a screening phase for several weeks to several months after surgery to determine optimal stimulation parameters. Four of the patients went on to a blinded crossover evaluation phase; two other patients formally enrolled, and two anecdotal patients were described in an addendum to the original paper.2 The mean Y-BOCS score with stimulation was 19.8 ± 8.0 (considered “moderate severity”), compared to 32.3 ± 3.9 (“extreme severity”) without stimulation. Relatively high stimulation parameters were needed to achieve benefit, with pulse generator replacements required every 5 to 12 months. A concern related to the short battery life was a severe, acute recurrence of OCD symptoms if there was an abrupt cessation of stimulation. Prophylactic battery changes were recommended to avoid this complication. Several groups believed that the high stimulation parameters and use of ventral active contacts in this study might be stimulating the NAcc.3,4 A subsequent multicenter study in the United States implanted 10 patients in the VC/VS.4 Eight patients completed the study with a 36-month follow-up. Two patients had less than a 25% improvement in their Y-BOCS scores and were considered nonresponders, but the six others were responders, with two patients showing improvement between 25% and 35% and four showing improvement greater than 35%.
The combined European and American experience with DBS in the VC/VS led to a Humanitarian Device Exemption (HDE) approval by the U.S. Food and Drug Administration in early 2009. Although the HDE requires that surgeons obtain Institutional Review Board approval from the hospital where surgeries will be performed and the approval does not guarantee that insurance carriers will reimburse for the procedures, the approval was nevertheless an important event in modern-day limbic disorders surgery in the United States. The HDE approval is specific to the VC/VS, and although this is the most described target in the literature, the optimal target for intervention in OCD is still not known. For example, a double-blind, crossover study in France of associative-limbic STN territory stimulation versus sham stimulation in 16 OCD patients showed lower Y-BOCS scores with active treatment (19 ± 8) than with sham (28 ± 7) stimulation (P = 0.01)—and at significantly lower stimulation amplitude (2.0 ± 0.8 vs. 7.6V in Nuttin’s original series).5 Further investigation is needed to determine the relative merits of each target. One interesting observation is that patients with hoarding typically do not respond nearly as well to DBS. Some have questioned whether hoarding is a distinct subtype of OCD, and this symptom’s differential response to DBS may support this. The issues of how to address hoarding and whether these patients should be treated with DBS are still under debate.
Treatment-Resistant Depression
Major depression is by far the most common of the psychiatric disorders, affecting an estimated 14.8 million American adults in a given year.6 It is believed that up to 20% of those patients fail to improve with traditional antidepressant agents and/or psychotherapy and therefore fall into the diagnosis of treatment-resistant depression (TRD). These patients typically go on to be placed on combination therapy with multiple agents, and a subset of them eventually requires electroconvulsive therapy (ECT). ECT has been and continues to be the mainstay of therapy for patients with severe TRD, as well as some patients with acute suicidality, psychosis, and mania. Although it is effective in the acute setting, remission rates are only 30% to 47%, and the relapse rate was found to be 64% within 6 months.7 Moreover, it is generally accepted that repeated use can have long-term effects on memory and cognition. There are two widely accepted clinical rating scales for patients with depression, the Hamilton Depression Rating Scale (HDRS; alternatively, the Hamilton Rating Scale for Depression, referred to as HRSD or HAM-D) and the slightly more recent Montgomery-Asberg Depression Rating Scale (MADRS).
Interestingly, the majority of patients enrolled in the U.S. pilot study of DBS for OCD also had comorbid depression, and with stimulation in VC/VS, their HDRS scores improved dramatically. This led to a separate pilot trial to study DBS in the same target to treat TRD.8 in This open-label trial, 15 patients were implanted: 14 had major depression, and 1 had recurrent bipolar depression. As a group, the patients had averaged more than six medication monotherapy trials and more than six combination medication trials and had at least 30 ECT sessions. Both the HDRS and the MADRS were used as endpoints, with minimum follow-up of 6 months and the average approaching 2 years. Significant improvement was seen in both rating scales, with the mean HDRS declining from a mean of 33.1 at baseline to 17.5 at 6 months and 14.3 at last follow-up. “Response” was defined as more than 50% improvement in either the HDRS or the MADRS, and “remission” was defined as an absolute score of 10 on either scale. At last follow-up, the response rate in the trial was 53.3% on both scales, with a remission rate of 40% on the HDRS and 33.3% on the MADRS. This pilot study has led to a multicenter trial study in the United States that is under way at the time of this writing. The targeting methodology is the same as that for VC/VS stimulation in OCD (Fig. 119-1). A German group also published a series of three patients implanted in the ventral striatum in 2007.9 They reported similar improvements, although with a double-blind design and short assessment period; after only 1 week of stimulation, the baseline HDRS average dropped from 33.7 ± 3.8 to 19.7 ± 6.7. After another week of blinded withdrawal of stimulation, the average rose again to 29.3 ± 5.5.
A second target for TRD currently being studied is the subgenual cingulate (Cg25 or “area 25”), a cortical region in the inferior medial frontal lobe. Positron emission tomography studies of this area in patients with clinical depression demonstrated high activity; a decrease in Cg25 activity is associated with clinical improvement using multiple treatment modalities for depression, including medical therapy, ECT, transcranial magnetic stimulation, and ablative surgery. These observations provided the rationale for treating depression by modulating the activity of Cg25 directly using DBS.10,11 The pilot study included six patients implanted bilaterally in an open-label design. Cg25 is a cortical area and does not lend itself to AC-PC–based targeting; rather, targeting is performed relative to the rostral corpus callosum, AC, and base of the frontal lobe (Fig. 119-2).12 Response was defined as a decrease in the HDRS of 50% or more from baseline. Two months following device programming, five of the patients were responders, although in one patient the benefit was not sustained to the end of the study. At the 6-month endpoint, four patients showed sustained benefit and met criteria for response. A multicenter study is also under way.
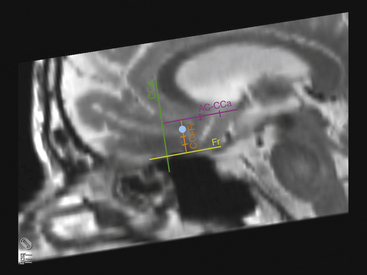
FIGURE 119-2 Sagittal magnetic resonance images reformatted in the AC-PC plane showing a method for targeting Cg25 as originally described by Hamani et al. in Fig. 4 of their paper.12 First, on a sagittal slice in or close to the midline, draw a line at the base of the frontal lobe parallel to the AC-PC plane (Fr line, yellow). Second, draw a line perpendicular to this that abuts the anterior border of the corpus callosum (CCa line, green). Third, draw a line starting at the AC and extend it forward until it hits the CCa line (AC-CCa line, purple); it should be parallel to the Fr and perpendicular to the CCa. Divide this AC-CCa line into quarters. At approximately 25% to 30% of the distance from the CCa to the AC, draw a fourth line (CCi-Fr, orange) down to the Fr line. Divide this CCi-Fr line into quarters. Place the initial target 25% to 30% down the CCi-Fr line, then modify the target on a coronal view to correspond to the gray/white junction of the subcallosal cingulate gyrus just off the midline (blue dot).
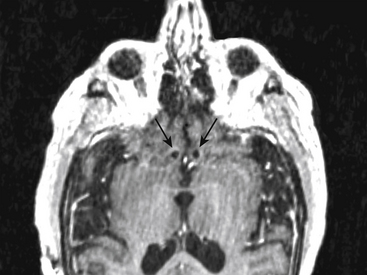
These two areas, VC/VS and Cg25, represent the most studied targets to date for treatment of TRD, although other targets have been explored or proposed, including the inferior thalamic peduncle and the habenula.13–15 DBS in bipolar patients has not been well studied but may present special considerations for target choice. The initial VC/VS trial included one bipolar patient, who had the most variable response of all subjects. Although this patient improved in depressive symptoms for periods of several weeks, the patient then went into hypomania, which required cessation of stimulation. This pattern was seen several times, and each time the hypomania resolved promptly with removal of stimulation.8 Anecdotal reports of Cg25-implanted patients have not shown an obvious propensity for depressed patients to become hypomanic. Our own group has experience with a severe, medically refractory bipolar patient who required weekly ECT for more than a year. He was beginning to suffer from significant cognitive side effects from such frequent ECT. After consultation with all practitioners involved and with permission of the medical center, he was implanted in Cg25 bilaterally (Fig. 119-3). Eight months after initial programming, he was significantly improved and had not required any further ECT. This patient had one brief episode of hypomania several weeks after activating his DBS that was managed with medication adjustments only; stimulation was continued during this event. He had some recurrence of depressive symptoms 6 months after surgery that has so far responded to an increase in his stimulation parameters with no evidence of hypomania. It is not yet clear whether the risk of stimulation-induced exacerbation of manic symptoms is different for Cg25 compared with VC/VS.
Tourette Syndrome
TS presents in children and is characterized by vocal tics, motor tics, or both. While often mild, tics can become severe and significantly disrupt the patient’s ability to function at school or in social situations. The mainstay of treatment is medical and includes neuroleptics and the newer generation of antipsychotics. The tics subside or remit by the time most patients grow into their late teens or early 20s. In some cases, tics may continue into adulthood, when they can continue to be disabling. Medical treatment is not always effective, and increasing doses of such medications can be complicated by the development of tardive dyskinesias. TS is commonly associated with OCD and attention-deficit/hyperactivity disorder; there is some evidence that these disorders are different points along a disease spectrum, which may eventually have some implications on how we treat these disorders surgically when they occur together.16
There is a relatively rich history of treating TS with lesioning procedures. Perhaps the best known series was performed by Hassler and Dieckmann in the middle part of last century.17–19 Targets for lesioning included regions of the frontal lobes, cingulate gyrus, cerebellum, and multiple targets in the thalamus. The lesions placed in the medial thalamus served as inspiration for a Dutch-Flemish group to perform DBS on three TS patients, published in 2003; subsequently, the medial thalamus has become the most commonly reported target in the literature.20 The Dutch-Flemish group implanted bilaterally standard wide-spaced DBS electrodes (Medtronic model 3387) using a trajectory that placed the stimulating contacts across the nucleus ventro-oralis internus, centromedian nucleus, and substantia periventricularis. Their target was lateral at 5 mm, was anteroposterior at −4 mm, and had a depth of 0 mm. At long-term follow-up, all three patients showed a striking reduction in the number of tics counted by blinded observers of 86.2% (at 8 months), 72.2% (at 1 year), and 90.1% (at 5 years). In this original patient series, the more ventral contacts in the vicinity of centromedian nucleus and substantia periventricularis seemed to provide the most benefit. Other studies have subsequently been published that support various regions of the medial thalamus as potential targets to treat TS.21,22
Other targets for DBS within the basal ganglia and subcortex have been explored. The globus pallidus internus (GPi) has been implanted, both the traditional motor territory (the region targeted for movement disorders) and the limbic territory of the GPi, which is more anterior and medial. One case study reported a mean postoperative tic reduction of 73% over a 14-month period with stimulation of motor GPi, although other centers have had less success with this target.23 Other groups have implanted multiple targets, including the medial thalamus (centromedian nucleus and parafascicular nucleus) plus limbic GPi and medial thalamus plus motor GPi.24,25 Comparisons were made within patients of thalamic stimulation, pallidal stimulation, or both, and each scenario seemed to produce relatively equivalent tic reductions.
Issues and Future Directions
The clinical trials of DBS are encouraging, even though we are far from having a “standard treatment” for any of these disorders. Strict inclusion and exclusion criteria have been used, and multidisciplinary groups have published guidelines for patient selection, inclusion/exclusion criteria, pre- and postoperative evaluations, and target selection for TS and OCD. Although typical complications such as infection and lead fractures have been seen, there have been few cases of stimulation-induced adverse events, which is in some ways remarkable given how little we know about the circuits we are modulating. However, the work so far does have significant drawbacks. The sample sizes are low, and most studies are open label. Blinding can be a significant challenge because a severe rebound in symptoms can occur with cessation of stimulation.2,20 Although the studies discussed here were done in the context of clinical trials with institutional oversight, an increasing number of “off label” cases are being done at community hospitals in the United States, particularly for TS. This highlights a potential concern that DBS has become a familiar treatment modality for some surgeons and, given the perception that it is safer than lesioning, it may be used more confidently to treat disorders that we are really not familiar with. The fact is that patients with intractable psychiatric illness are complex and require the ongoing expertise of a specialized team with an experienced psychiatrist.
Ackermans L., Temel Y., Cath D., et al. Deep brain stimulation in Tourette’s syndrome: two targets? Mov Disord. 2006;21(5):709-713.
Coffey B.J., Miguel E.C., Biederman J., et al. Tourette’s disorder with and without obsessive-compulsive disorder in adults: are they different? J Nerv Ment Dis. 1998;186(4):201-206.
Diederich N.J., Kalteis K., Stamenkovic M., et al. Efficient internal pallidal stimulation in Gilles de la Tourette syndrome: a case report. Mov Disord. 2005;20(11):1496-1499.
Greenberg B.D., Malone D.A., Friehs G.M., et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384-2393.
Hamani C., Mayberg H., Snyder B., et al. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg. 2009;111(6):1209-1215.
Hassler R., Dieckmann G. Stereotaxic treatment of tics and inarticulate cries or coprolalia considered as motor obsessional phenomena in Gilles de la Tourette’s disease. Rev Neurol (Paris). 1970;123(2):89-100.
Houeto J.L., Karachi C., Mallet L., et al. Tourette’s syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry. 2005;76(7):992-995.
Jimenez F., Velasco F., Salín-Pascual R., et al. Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl. 2007;97(Pt 2):393-398.
Kessler R.C., Chiu W.T., Demler O., et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
Maciunas R.J., Maddux B.N., Riley D.E., et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007;107(5):1004-1014.
Mallet L., Polosan M., Jaafari N., et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359(20):2121-2134.
Malone D.A.Jr., Dougherty D.D., Rezai A.R., et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267-275.
Mashour G.A., Walker E.E., Martuza R.L. Psychosurgery: past, present, and future. Brain Res Brain Res Rev. 2005;48(3):409-419.
Mayberg H.S. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193-207.
Mayberg H.S., Lozano A.M., Voon V., et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
Nuttin B.J., Gabriëls L.A., Cosyns P.R., et al. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2003;52(6):1263-1272. discussion 1272-4
Porta M., Brambilla A., Cavanna A.E., et al. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology. 2009;73(17):1375-1380.
Prudic J., Olfson M., Marcus S.C., et al. Effectiveness of electroconvulsive therapy in community settings. Biol Psychiatry. 2004;55(3):301-312.
Sartorius A., Kiening K.L., Kirsch P., et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67(2):e9-e11.
Sartorius A., Henn F.A. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med Hypotheses. 2007;69(6):1305-1308.
Schlaepfer T.E., Cohen M.X., Frick C., et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368-377.
Sturm V., Lenartz D., Koulousakis A., et al. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive and anxiety disorders. J Chem Neuroanat. 2003;26(4):293-299.
Temel Y., Visser-Vandewalle V. Surgery in Tourette syndrome. Mov Disord. 2004;19(1):3-14.
Vandewalle V., van der Linden C., Groenewegen H.J., Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999;353(9154):724.
Visser-Vandewalle V., Temel Y., Boon P., et al. Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. Report of three cases. J Neurosurg. 2003;99(6):1094-1100.
1. Mashour G.A., Walker E.E., Martuza R.L. Psychosurgery: past, present, and future. Brain Res Brain Res Rev. 2005;48(3):409-419.
1a. Spiegel E.A., Wycis G.T., Marks M., Lee A.J. Stereotactic apparatus for operations on the human brain. Science. 1947;106:349-350.
2. Nuttin B.J., Gabriëls L.A., Cosyns P.R., et al. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2003;52(6):1263-1272. discussion 1272-4
3. Sturm V., Lenartz D., Koulousakis A., et al. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive and anxiety disorders. J Chem Neuroanat. 2003;26(4):293-299.
4. Greenberg B.D., Malone D.A., Friehs G.M., et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384-2393.
5. Mallet L., Polosan M., Jaafari N., et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359(20):2121-2134.
6. Kessler R.C., Chiu W.T., Demler O., et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
7. Prudic J., Olfson M., Marcus S.C., et al. Effectiveness of electroconvulsive therapy in community settings. Biol Psychiatry. 2004;55(3):301-312.
8. Malone D.A.Jr., Dougherty D.D., Rezai A.R., et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267-275.
9. Schlaepfer T.E., Cohen M.X., Frick C., et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368-377.
10. Mayberg H.S. Modulating dysfunctional limbic–cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193-207.
11. Mayberg H.S., Lozano A.M., Voon V., et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
12. Hamani C., Mayberg H., Snyder B., et al. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg. 2009;111(6):1209-1215.
13. Jiménez F., Velasco F., Salín-Pascual R., et al. Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl. 2007;97(Pt 2):393-398.
14. Sartorius A., Henn F.A. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med Hypotheses. 2007;69(6):1305-1308.
15. Sartorius A., Kiening K.L., Kirsch P., et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67(2):e9-e11.
16. Coffey B.J., Miguel E.C., Biederman J., et al. Tourette’s disorder with and without obsessive-compulsive disorder in adults: are they different? J Nerv Ment Dis. 1998;186(4):201-206.
17. Hassler R., Dieckmann G. Stereotaxic treatment of tics and inarticulate cries or coprolalia considered as motor obsessional phenomena in Gilles de la Tourette’s disease. Rev Neurol (Paris). 1970;123(2):89-100.
18. Vandewalle V., van der Linden C., Groenewegen H.J., Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999;353(9154):724.
19. Temel Y., Visser-Vandewalle V. Surgery in Tourette syndrome. Mov Disord. 2004;19(1):3-14.
20. Visser-Vandewalle V., Temel Y., Boon P., et al. Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. Report of three cases. J Neurosurg. 2003;99(6):1094-1100.
21. Porta M., Brambilla A., Cavanna A.E., et al. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology. 2009;73(17):1375-1380.
22. Maciunas R.J., Maddux B.N., Riley D.E., et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007;107(5):1004-1014.
23. Diederich N.J., Kalteis K., Stamenkovic M., et al. Efficient internal pallidal stimulation in Gilles de la Tourette syndrome: a case report. Mov Disord. 2005;20(11):1496-1499.
24. Houeto J.L., Karachi C., Mallet L., et al. Tourette’s syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry. 2005;76(7):992-995.
25. Ackermans L., Temel Y., Cath D., et al. Deep brain stimulation in Tourette’s syndrome: two targets? Mov Disord. 2006;21(5):709-713.