CHAPTER 18 Decision Making in Meningiomas
INTRODUCTION
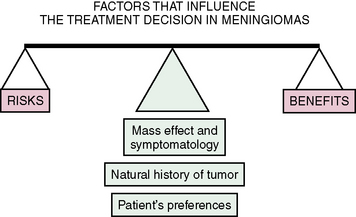
Five factors influence the treatment decision in meningiomas: operative gains, operative risks, tumor biology, mass effect/symptomatology, and the preference of the patient. In short, the balance between the risks and benefits of surgery is evaluated in light of the tumor’s biology, mass effect/symptomatology, and preference of the patient (Fig. 18-1).
MASS EFFECT AND SYMPTOMATOLOGY
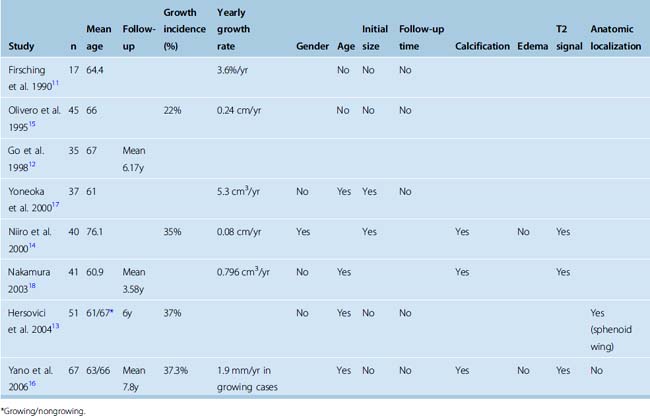
The incidence of asymptomatic meningiomas in the general population ranges from 1% to 1.4% both in noninvasive imaging studies in the general population1,2 and in autopsy series.3–5 The prevalence of meningiomas steadily increases with age;1,2,6–9 in patients older than 60 years of age it is 3%.4 Similarly, the incidence of patients with meningiomas who come to clinical attention is reported to be 3.5 times higher over the age of 70.10 This observation is in line with the hypothesis that oncogenesis in meningiomas is a slow process, which occurs over decades. Most meningiomas remain asymptomatic throughout life, and in fact; half of all meningiomas are discovered only at autopsy.5 Studies that have analyzed the clinical behavior in asymptomatic meningiomas have reported growth rates ranging from 0 to 37.3%,11–17 which indicates that in the short term at least 2/3 of meningiomas do not grow. There are, however, no long-term studies that have evaluated the incidence of growth in asymptomatic meningiomas. Yano and colleagues16 reported their experience with 351 conservatively treated incidental meningiomas. Over a period of 5 years tumor growth was detected in 37.3%, and 16.4% had tumor growth and became symptomatic. Several authors have analyzed factors that may indicate growth potential in meningiomas. A summary of these studies is provided in Table 18-1. As can be seen in the table, there is no general consensus on this issue. The presence of calcifications and a low T2 signal was associated with nongrowth in all studies. In four of six studies age correlated significantly with nongrowth. Many other studies also indicate that, disproportionally with their increasing incidence, the likelihood of a meningioma becoming symptomatic decreases with age. Most meningiomas in the elderly, detected at autopsy, are less than 1 cm in diameter.4 However, the incidence of growth in incidental meningiomas in the elderly is comparable to that in the younger population. Niiro and colleagues14 have reported on the natural history of 40 incidental meningiomas in patients older than the age of 70. During a mean follow-up of 38.4 months, 35% of the tumors showed radiologically documented growth and 12.5% became symptomatic. In contrast, meningiomas in the pediatric population have greater risk of aggressive behavior.10 Several authors have looked for an association between the initial tumor size and the annual growth rate. A higher growth rate was reported in larger tumors by some authors.14,18 Still others have reported very high tumor growth rates in very small tumors.11 A summary of reported growth rates is also presented in Table 18-1.
TUMOR BIOLOGY
Histology
Most meningiomas are slow growing benign tumors that histologically correspond to World Health Organization (WHO) grade I. Rare histologic variants such as clear cell, chordoid, papillary, and rhabdoid meningiomas, as well as atypical (WHO grade II) and anaplastic (WHO grade III) meningiomas can present with more aggressive behavior and relentless growth despite multimodal treatment, and have a high incidence of recurrences and a less favorable prognosis.10
Molecular Biology and Genetics
Molecular genetic analysis of meningiomas has revealed that they carry a wide variety of chromosomal aberrations. In general the complexity of these aberrations correlates with increasing histologic grade.19 Partial loss or monosomy of chromosomes 1, 10, and 14 is associated with aggressive behavior and higher histologic grades.20–23
Angiogenesis
Meningiomas are highly vascular tumors and their angiogenic potential plays an important role in their growth. Studies have shown that an increased expression of vascular endothelial growth factor (VEGF) was correlated with an increased risk of recurrence and higher incidence of peritumoral edema.24–26 Extracellular matrix associated structural proteins and growth factors such as tenascin and matrix metalloprotease MMP-9 were also shown to have a correlation with tumor recurrence.27,28
Hormonal Influences
Hormonal dependence of meningiomas has been suggested by clinical observations such as predominance of female patients, growth during pregnancy, and the luteal phase of pregnancy. Further, the presence of progesterone receptors was associated with benign histology and lower likelihood of recurrences.29–31
Growth Rate of Meningiomas
As is the case for most other parameters on meningiomas, very little is known concerning the growth kinetics of meningiomas. Most meningiomas are slow growing tumors. But there is significant variation between the growth rates of individual meningiomas. Current classification schemes, such as histologic grading, fall short of predicting this growth rate. Jaaskelainen and colleagues32 have shown that the histologic appearance correlated only grossly with the growth rate but did not exactly predict the tumor doubling time. When typical, atypical, and malignant meningiomas were considered there was significant variation within and significant overlap between histologic subgroups.32
In addition to the variability in growth rate between individual tumors, it is also likely that there are temporal changes in the growth rate of individual meningiomas. The assumption that a meningioma will grow at a constant rate is most likely an oversimplification, and most meningiomas do not exhibit a constant growth rate but change in their growth characteristics during their clinical course. Clinical analyses have indicated that they do have variations in the growth characteristics of meningiomas during their growth.33 This is certainly not unexpected. Current evidence indicates that meningiomas, like most other malignancies, develop and progress as a result of accumulating genetic and epigenetic changes; therefore one can easily conclude that the growth rate will change with increasing malignancy.10 As expected, studies have concluded that the growth rate increased with successive recurrences.32,34 This finding was also supported by the demonstration of a decreasing time interval between recurrences.35,36
The tumor doubling time in recurrent benign meningiomas was estimated to be 317 days using computed tomography (CT) volumetrics and bromodeoxyuridine labeling and 415 days using CT planimetry.32,37 Much variation is reported in the tumor doubling times, but it ranged from 138 to 1045 days (mean 415 days) in WHO grade I, 34 to 551 days (mean 178 days) in WHO grade II and 30 to 472 (mean 205 days) in grade III meningiomas.32 The clinical implication of this is that recurrences should be expected late in the course in most meningiomas. Ninety-five percent of meningiomas are either adherent or invasive to the surrounding neurovascular structures, which will preclude a “true” oncologic resection of meningiomas and residual tumor cells will be the source of a recurrence. Jaaskelainen and colleagues have calculated the estimated time to a recurrence detectable by CT, after a seemingly gross total surgical resection to be approximately 8.5 years. This hypothesis is supported by Simpson’s elegant demonstration that up to 10% of meningiomas recurred 10 years after a seemingly complete resection.38 The incidence of detected recurrences increased over time, reaching 20% at the end of the second decade after surgery.36,38,39 This and other studies have shown that up to 50% of all recurrences occurred after 5 years.36,38,39
Quantification of the proliferative potential is important for predicting future recurrences and planning an optimal management strategy. Several studies have looked for histopathologic findings to evaluate the growth potential of meningiomas. Tumor growth is a function of cell proliferation and death. Cell death has not been systematically studied in meningiomas. Proliferative indices, however, are commonly used. Demonstration of proliferating cell nuclear antigen (PCNA), silver staining for nucleolar organizer regions (AgNOR), immunohistochemistry for MIB-1 (Ki-67), and bromodeoxyuridine (BrdU) labeling are the most common methods for demonstrating the proportion of proliferating cell fraction.10,40,41 There is a well demonstrated correlation between high proliferative indices and recurrences. However, this correlation is not exclusive. Low MIB-1 indices were demonstrated in many recurrent tumors and very high proliferative indices are sometimes demonstrated in typical meningiomas.42
Anatomic Localization
Natural history, symptomatology, and the surgical outcome vary significantly among meningiomas in different intracranial localizations.43 To present a striking example, a small meningioma in the optic canal will come to clinical attention much earlier than a large convexity meningioma. Therefore, clinical correlates of localization should be taken into consideration when planning surgery for meningiomas. Eighty to ninety-six percent of tuberculum sella meningiomas present to clinical attention with progressive visual impairment. Despite wide availability of magnetic resonance imaging (MRI), most anterior fossa meningiomas are quite large at presentation. Seventy-three percent of anterior fossa meningiomas present when larger than 4 cm.44 The same holds true for olfactory groove meningiomas, 65% of which have a diameter larger than 3 cm at presentation.45 Optic nerve sheath meningiomas are associated also with a progressive decrease in vision. Kennerdell and colleagues46 observed 18 patients without treatment, and none of the patients who had normal visual acuity retained it for more than 5 years. Similarly, Keane and colleagues47 and Sarkies48 reported progressive decrease in vision in 20 of 22 patients. Petroclival meningiomas have variable and unpredictable growth patterns. Radiologic growth was reported in 76% of patients at a median follow-up of 85 months, and in 63% this growth was associated with functional deterioration.49
Peritumoral Edema
A significant proportion of meningiomas present with varying degrees of edema in the surrounding brain parenchyma. Different studies have reported incidences ranging from 46% to 92%.31,50 The presence and extent of edema are highly variable and the underlying pathogenesis is not entirely clear. Tumor compression, parasitization of pial vasculature, convexity/middle fossa/anterior fossa localization, irregular tumor–brain interface, hyperintensity on T2-weighted images, brain invasion, high grade, secretory, microcystic and/or angiomatous variants, and high VEGF expression have all been suggested as the cause of edema but none has been proven to date.31 A minor amount of edema may be negligible but significant peritumoral edema may interfere with normal brain function and may be the patient’s presenting symptom.50 Peritumoral edema is also reported to complicate surgery and to be a source of perioperative morbidity.50 Therefore surgical resection is favored in cases with peritumoral edema.50
Invasion of Surrounding Structures
Meningiomas are mesenchymal tumors and have a tendency to invade the surrounding structures including dura, bone, muscle, and brain parenchyma.31 Bone invasion is common in meningiomas, particularly those at the skull base, and presents as hyperostosis and less commonly as bone destruction.51 Hyperostosis is almost invariably associated with tumor invasion. Invaded bone is a source of recurrence and should be removed along with the tumor. The need for bone removal creates a significant challenge in the skull base. Neurovascular invasion is also a significant problem in meningiomas. Such invasion of neighboring brain parenchyma, cranial nerves, and blood vessels is observed in only a minority of meningiomas but in those cases it precludes a gross total resection and is a source of relapse.10 Yasargil52 has reported that 5% of meningiomas were nonadherent, 85% were adherent, and 10% were adhesive so that dissection is impossible without damaging vital structures. Neurovascular invasion may be observed in benign, atypical, and anaplastic meningiomas; however, it signifies a more aggressive biologic behavior so that benign meningiomas with brain invasion have a prognosis similar to that of atypical meningiomas.10 Borovich and colleagues53,54 have demonstrated isolated tumor cell nests detectable at a distance from the main tumor bulk. Preoperative radiologic diagnosis of neural or vascular invasion is not reliable.52
Multiplicity
Multiplicity is observed in 0.9% to 8.9% of meningiomas in clinical series. In 50% of these cases, multiple meningiomas are diagnosed at the initial presentation, excluding the possibility of recurrence, seeding, or metastasis. Autopsy series report a higher incidence of multiplicity.4 Most of these cases are associated with neurofibromatosis type II. Sixty to ninety percent of multiple meningiomas are observed in women. No difference in clinical tumor behavior or outcome has been reported for multiple meningiomas, and the treatment decision does not differ from that for solitary meningioma cases.
Special Conditions
Meningiomas are the second most commonly encountered intracranial tumor in neurofibromatosis type 2 (NF-2) and are seen in approximately 50% of the cases.10 The incidence of this association is clearer in pediatric meningiomas, 25% to 40% of which are seen in association with NF-2. The clinical behavior (their anatomic localization, growth rate, and recurrence rates) of meningiomas associated with NF-2 is not significantly different from sporadic cases. Multiplicity is more commonly observed in meningiomas associated with NF-2.
Meningiomas in children are rare, comprising 1% to 7.7% of all pediatric brain tumors and 2% of all meningiomas.10,55,56 Very little is known regarding the characteristics of meningiomas in children, and the number of reports add up to only a few hundred cases. Studies to date have concluded that pediatric meningioma patients have a poorer prognosis that their adult counterparts due to higher incidence of malignant or aggressive forms of meningioma, increased relative size of the tumors, and their unusual and complicated anatomic localizations.10,55–57 Cases occurring in association with NF-2 (25%–40% of cases) or secondary to radiation are more common than in the adult population and the predominance of female cases documented in adults is not observed.55,56 The incidence of unusual anatomic localizations such as the orbit and the ventricular system, the incidence of cases in the posterior fossa, and the incidence of meningiomas without a dural attachment are higher in the pediatric population.55,56 Pediatric cases almost invariably are symptomatic and present with increased head size in infants, focal neurologic deficits, seizures, and increased intracranial pressure and this justifies a surgical intervention. Drake and Hoffman55 analyzed 207 patients from 11 available series published between 1972 and 1987 and calculated the overall 5-year survival to be 76%. When meningeal sarcomas were excluded, the overall survival increased to 84%. Malignant meningiomas and meningeal sarcomas made up 15% of all childhood meningiomas. A 5-year survival rate of 94% was reported in cases without recurrence and 64% in cases with recurrences.55 The mean operative mortality was 3%.55 In summary, children with typical meningiomas and a gross total surgical resection benefited from surgery. However, those with a papillary histologic subtype, meningeal sarcomas, or subtotally resected tumors and those with recurrences had a poorer prognosis.
OPERATIVE GAINS
Surgical Outcome in Different Anatomic Localizations
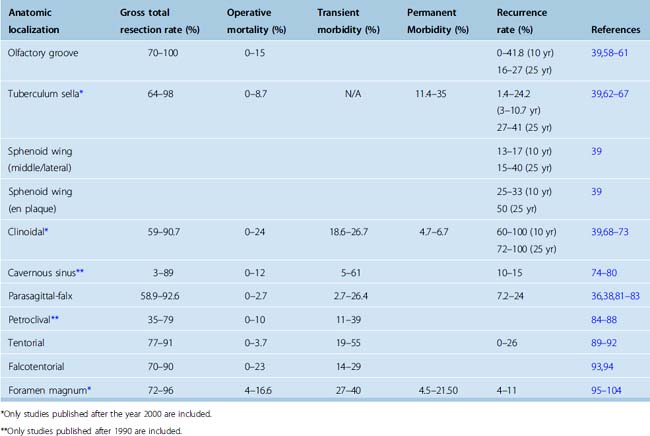
As is the case for their natural history, anatomic localization of the meningioma also strongly influences the surgical outcome. A summary of the surgical outcome in meningiomas at different anatomic localizations is provided in Table 18-2.
Anterior fossa meningiomas are notorious for remaining asymptomatic for long periods, presenting with large sizes and having a high incidence of recurrence.105 Mathiesen and colleagues106 showed that 10% of these cases recur at 10 years and that 20% of the patients die as a result of these recurrences. Surgical resection of olfactory groove meningiomas is complicated by the fact that they may invade the anterior skull base and the paranasal sinuses.107 Radical resection of the tumor, involved dura, and bone in the anterior fossa or the paranasal sinuses is technically difficult and is associated with a high risk of complications. More conservative resections, in contrast, may result in recurrences as high as 41% at 10 years.36 Bakay108 reported subtotal resection in 17% of his patients, which was associated with blindness in half of these patients due to recurrence. The likelihood of preserving smell after resection of olfactory groove meningiomas is possible in tumors smaller than 3 cm in diameter, whereas ipsilateral smell is lost in all cases.109 In tuberculum sella meningiomas, surgery provides objective, quantifiable, and significant improvement in vision.62,66 The reported likelihood of visual improvement after surgery ranges from 42.4% to 80% in a recent series.62–67 In contrast, some studies reported 10% to 20% incidence of iatrogenic worsening in vision.62–67 The visual outcome in young patients after surgical resection is significantly better than in the elderly62,66,67 Severity and duration of visual symptoms were also shown as predictors of a poor visual outcome.62,65–67,110,111 Size of the tumor,66,112 shape of the tumor,66 extent of tumor removal,62,66 absence of an arachnoid plane,66 binocular involvement,62 optic disc pallor,110 and presence of significant peritumoral edema66 were associated with a worse outcome as reported by some authors and contradicted or not supported by others. Large tumors may involve distal branches of the anterior cerebral artery which will preclude total resection and increase recurrences. Another source of recurrences is small tumor extensions in the optic canals, which can easily be missed when not inspected during surgery. Several authors also have shown that a simple pterional craniotomy is sufficient to achieve high total resection rates with very little morbidity.62,66 Low morbidity rates and good chance of benefit to the patient favor a surgical treatment in tuberculum sella meningiomas. Sphenoid wing meningiomas are classified into pterional, alar, clinoidal, and en plaque subtypes according to their epicenter along the sphenoid ridge.113 The elegant report by Al-Mefty and colleagues68 established clinoidal meningiomas as a new meningioma subtype, distinct from sphenoid wing meningiomas. Clinoidal meningiomas can be differentiated from sphenoid wing meningiomas radiologically, unless the tumor has reached a giant size.71 Visual symptomatology is reported in 45.5% to 91.6% of patients with clinoidal meningiomas.68–73 The most important factor that determines the surgical outcome in clinoidal meningiomas is the anatomic relationship of the tumor to the internal carotid artery and surrounding arachnoid cisterns.68 Large tumor size71 and cavernous sinus invasion68–73 are other factors that have a negative impact on surgical outcome.
Meningiomas of the optic nerve and orbit fall into different categories. Surgery is clearly the treatment of choice in meningiomas invading the optic canal and compressing the optic nerve. This also holds true for ectopic meningiomas within the orbit. However, the results of surgical excision in tumors that arise from the optic sheath and completely surround the optic nerve are dismal and most cases result in blindness. Surgical resection may be performed in patients with intracranial extension, disfiguring proptosis, and in patients with rapid visual deterioration.114 Radiosurgery or fractionated stereotactic radiotherapy is the option in all other cases.
True cavernous sinus meningiomas are among the most difficult meningiomas to resect. Although very conflicting reports can be found in the early literature, it is well established now that gross total resection is possible but is very rarely achieved in meningiomas that involve the cavernous sinus proper.115,116 Meningiomas can invade the cranial nerves inside the cavernous sinus.77 Radical strategies are associated with significant permanent cranial nerve morbidity, which can reach 59%.115 Owing to the disappointing results and high morbidity of radical surgical strategies, most authors to date advocate resection of the extracavernous portion with or without postoperative radiosurgery.80,115,116 A recent study by Sindou and colleagues80 at progression was observed in 13.3% of subtotally resected cases. Small but symptomatic cavernous sinus meningiomas can be successfully treated with radiosurgery alone with adequate short-term tumor growth control and minimal morbidity.116–118 Secondary cavernous sinus involvement may be invaded by tuberculum sella, clinoidal, medial sphenoid wing, and sphenopetroclival meningiomas. Tumors that are localized to the lateral wall (Hirsch grades 0 and 1) can be surgically excised without excess cranial nerve morbidity.119 Less radical treatment strategies paralleling those for cavernous sinus meningiomas are planned for more invasive cases.
Convexity meningiomas are the most easily resected type of meningiomas and therefore have the greatest potential for a “curative” resection. Epilepsy may complicate surgery of convexity meningiomas. Chan and Thompson60 have shown a strong correlation with sacrifice of the surrounding venous structures and postoperative epilepsy. Parasagittal meningiomas are located along the superior sagittal sinus (SSS) in the anterior, middle, or posterior third. Both the surgical treatment and the surgical results are influenced by the extent and localization of sinus invasion. The aim of the surgery is total surgical resection along with the involved dura, bone, and the involved venous sinus. This can be easily accomplished anterior to the Rolandic veins. However, two thirds of parasagittal meningiomas are located posterior to the coronal suture. Resection of a partially occluded sinus caries a high risk of mortality and morbidity. Contrary to general belief, the resection of a totally occluded sinus is not universally safe.120,121 Venous reconstruction has been proposed as a management strategy but even in the hands of the most experienced surgeons, radical surgical resection of meningiomas of the middle third involving venous reconstruction carries substantial risk of morbidity.120 The reported mortality ranged from 0 to 2.7% and surgical morbidity rates ranged from 8.3% to 29%.120–122 An alternative strategy in cases that do not or partially occlude the sinus is to treat the portion that invades the SSS with a gamma-knife after removal of the tumor outside the sinus.123–125 This paradigm has yielded tumor growth control rates of 89% and a reduction in tumor size in approximately 50% of the tumors during follow-up. Surgical series with follow-up in the range of 5 to 10 years have reported recurrence in 7.2% to 24% of parasagittal meningiomas. Bonnal and Brotchi120 have reported a 63% incidence of recurrence even after radical total resection of the tumor and the involved sinus. Meningiomas of the falx are less commonly observed. Involvement of the SSS is far less common in falx meningiomas and surgical removal is less complicated.
Tentorial meningiomas comprise one third of all posterior fossa meningiomas.52,126 Several surgical classification schemes were provided but the one by Yaşargil52 is the most clinically relevant one and differentiates between meningiomas of the free tentorial edge (T1,T2, T3), intermediate tentorial surface (T4), torcular (T5), lateral tentorial (T6, T7), and falcotentorial meningiomas (T8). Difficulty in the management of falcotentorial meningiomas results from their proximity to the brain stem and involvement of the deep venous system and major venous sinuses. Adherence to the vein of Galen is the most common cause for a subtotal resection. Some authors have reported that resection of involved parts of the vein of Galen and the straight sinuses can be performed, but reports have indicated that such a radical resection is associated with significant mortality and morbidity and not necessarily with a decreased recurrence rate.89–92 Meningiomas that arise from the posterior surface of the temporal bone are classified according to their relation to the fifth cranial nerve and fall into two distinct subgroups: cerebellopontine angle (CPA) and petroclival meningiomas. Those that arise laterally are CPA (posterior pyramis) meningiomas, and these tumors can be resected with conventional approaches and have a favorable surgical outcome. Hearing can be preserved in 5% to 8% of vestibular schwannomas, but because meningiomas do not invade, but rather compress the 7th to 8th nerve complex, preoperative nerve function is better preserved in CPA meningiomas and the postoperative cranial nerve outcome is better in meningiomas. Although they are invariably abnormal in vestibular schwannomas, a normal speech audiometry is found in 50% and a normal pure tone audiometry is found in 20% of patients with CPA meningiomas.127 Preservation and even improvement in cranial nerve function can be expected in CPA meningiomas.128 Anatomic preservation of the 7th and 8th cranial nerves was reported in 94% and functional preservation of the 7th and 8th cranial nerves was reported in 86% and 77% of CPA meningiomas, respectively.129,130 The reported outcome was worse in cases that arose in or at the internal auditory canal.131 A report on elderly patients with CPA meningiomas indicated similar good surgical results.131 At 85.7%, the total resection rate was not any different from that of the younger population.131 Elderly patients had higher incidences of cerebrospinal fluid (CSF) fistula and pneumonia but the incidence of other postoperative morbidity was not significantly different.131 In contrast to cerebellopontine angle meningiomas, petroclival meningiomas arise medial to the trigeminal nerve, at the petroclival junction and the upper two thirds of the clivus, and are associated with a progressive natural history and poor surgical outcomes. Sphenopetroclival lesions with involvement of the cavernous sinus and the orbit are the most difficult ones to manage. The treatment strategy for petroclival meningiomas is still evolving. The initial belief that they were “unresectable” was challenged after the introduction of skull-base surgery,84–88 but during the following decades these extensive approaches fell out of favor due to their unacceptably high surgical morbidity and mortality88 and have given way to simpler, more conservative presigmoid132 or retrosigmoid133 approaches. Fibrous tumors with hard consistency; adherence to the brain stem, cranial nerves, and perforating arteries; encasement of major vessels; and cavernous sinus invasion are the most common causes of failure in total resection. The most common morbidity in petroclival meningioma surgery results from iatrogenic cranial nerve palsies, which is reported in 22% to 76% of petroclival meningioma surgeries. Cranial nerve palsies result in a very significant decrease in functionality and quality of life of patients, which is more pronounced in the elderly.131 Therefore most authors favor preservation of function to a more “impressive” postoperative MRI.88,134 With current surgical techniques, petroclival meningiomas can be resected with minimal mortality, and the morbidity is in the range of 30% with radical surgery and less with more conservative approaches.84–88 Tumor progression has been reported in 42% of petroclival meningiomas with a mean time to progression of 36 months and in such cases radiosurgery or radiotherapy provides adequate tumor growth control.86 According to their localization on the axial plane, foramen magnum meningiomas can be classified into ventral, ventrolateral, posterolateral, and posterior groups. Posterior and posterolateral cases can be resected with conventional approaches (or their slight modifications) with low morbidity and good surgical outcome. However, a small percentage of ventral foramen magnum meningiomas are located strictly ventral to the neuraxis or have extradural extensions. These cases present a formidable surgical challenge, require an extreme lateral transcondylar approach, and are associated with high surgical morbidity and poor surgical results. Gross total resection rates of 72% to 96% have been reported.95–104 The surgical outcome of ventral foramen magnum meningiomas is among the poorest of all meningiomas with 4% to 16.6% surgical mortality, and permanent morbidity in up to one fifth of all cases. Use of radiosurgery as an adjunct or adjuvant treatment in case of recurrence or documented growth in residual tumors or in patients with multiple meningiomas has also been reported.135
Tumor Recurrence After Surgery
An oncologic resection is seldom possible for meningiomas, and it is well known that most meningiomas do recur. The risk of this recurrence is dictated by the intrinsic biology of the tumor. However; meningiomas are slow growing tumors and even a subtotal resection can prevent neural compression, and the results may be durable for decades. More complete resections are associated with a lower risk of recurrences. Simpson38 was the first author to report that the risk of recurrence was lower after more complete resections. In his original report Simpson underlined the necessity to address the invasive nature of the tumor and recommended resection of the involved bone and dura and provided a surgical prognostication scheme that has withstood the test of time. His analysis proved that gross total resections, with resection of the involved bone and dura, yielded the most durable long-term results and underlined the fact that a subtotal resection is nothing but a glorified biopsy. In the original report Simpson grade 4 resections had a 39.2% regrowth rate. Others have also supported these findings, reporting recurrence/progression rates of 81% to 85% in 15 to 18 years after subtotal surgery (Simpson grade 4).36,39 A gross total resection is reported in 64% to 97% of operated patients.36,60,81,136,137 However, despite such good surgical results recurrence is observed in 20% to 32% of the patients.36,105,137,138 Borovich and colleagues53,54 have demonstrated isolated meningioma nests around and at a distance from the tumor bulk. In addition, meningiomas also have a propensity to invade the surrounding dura, bone, and even the brain parenchyma, soft tissue and the skin. Borovich and colleagues53 and Al-Mefty and colleagues139 developed a concept of “grade zero removal” for convexity meningiomas, which denoted resection of a dural margin of 2 cm around the tumor in convexity meningiomas. The authors demonstrated a recurrence-free short-term survival; however, their mean follow-up duration was a modest 5.6 years. In addition, such a radical resection is possible only for some convexity meningiomas. The anatomic location of meningioma influences the recurrence risk. Meningiomas that are difficult to resect totally are more likely to recur. However; the lack of prospect for a total resection is not a contraindication for surgery. A gross-total surgical resection is difficult or impossible in certain locations, and a mere subtotal resection or its combination with adjuvant therapies such as radiosurgery may also be an acceptable treatment strategy. As an example meningiomas of the cavernous sinus can rarely be resected totally.74–80 However, the resection of the extracavernous portion of the tumor significantly alters the natural history of the tumor.80,115 Symptomatic regrowth is observed in 10% of patients after gross total resection in 13.3% to 15% of the patients after resection of the intracavernous part.74,80,115
Alternative Strategies in Treatment
Poor surgical results, unacceptably high surgical morbidity in certain meningiomas, or high recurrence rate in others have induced a search for alternative treatment strategies. With current technology, radiosurgery has arisen as an effective, reliable, and safe treatment modality for meningiomas.124,140 The major limiting factors for radiosurgery are the size of the tumor and the radiation damage to neural structures in very close vicinity. The efficiency of gamma-knife radiosurgery is significantly worse for lesions larger than 3 cm in diameter, which results in a limitation. In current practice radiosurgery finds four indications: (1) Primary treatment option for small meningiomas; (2) as a part of combination treatment with surgery in localizations that pose high surgical risks, (3) to prevent or stop growth in residual meningiomas; and last but not least (4) treatment of recurrent meningiomas. Radiosurgery does not result in immediate shrinkage of meningiomas; therefore, favorable outcome is expressed in “tumor growth control rates.” Very high tumor growth control rates are reported in the literature. In a large recent study by Kondziolka and colleagues,124 which involved 972 patients with intracranial meningiomas, an overall growth control rate of 93% was reported for intracranial WHO grade I meningiomas at a median follow-up of 4 years. The outcomes in WHO grade II and III meningiomas are worse, with 50% and 17% tumor growth control rates, respectively.124 The results of gamma-knife surgery are certainly very promising; however, even the largest studies are plagued by small cohorts and short follow-up durations to draw definitive conclusions on efficiency and safety. But we must express again that gamma-knife radiosurgery has provided one of the most significant advancements in the treatment of intracranial meningiomas.
Treatment of cavernous sinus meningiomas is a striking example to the change in treatment protocols. Popularization of skull-base surgery in the 1990s made surgery of cavernous sinus meningiomas possible. Initial reports in the CT era indicated very high total resection rates, however, at the expense of very significant cranial nerve morbidity even in the hands of master surgeons. Use of MRI for postoperative evaluation made it clear that the surgical results were in fact not so brilliant. Again, in the second half of the 1990s, encouraging reports of successful use of gamma-knife radiosurgery in small cavernous sinus meningiomas appeared in the neurosurgical literature.117 However, the efficacy of the gamma-knife was limited by the size of the tumor. These observations led our clinic to an alternative approach of resecting the extracavernous portion of cavernous sinus meningiomas and treating the intracavernous portion with the gamma-knife.116
Conventional radiotherapy and chemotherapy are still used in meningioma treatment, especially in recurrent or inoperable meningiomas.141 In certain cases, such as optic nerve sheath meningiomas, conventional radiotherapy or radiosurgery is the first-line treatment.114 Radiosurgery is also an effective and safe primary treatment option in small meningiomas that have a high morbidity risk, such as small cavernous sinus meningiomas.116,124 In the majority of cases, however, the high complication rate and the seriousness of the side effects of radiation treatment and the low efficiency of chemotherapy exclude them as first-line treatment for meningiomas.
OPERATIVE RISKS
Age and Medical Condition of the Patient
Surgical decision making in meningiomas is far more difficult in the elderly than in the younger population. There are several factors that blend into this problem: The prevalence, size, and multiplicity of meningiomas increase with age. The incidence of intracranial tumors steadily increases with age and reaches its peak in the 8th decade. This is especially true for meningiomas. The likelihood of a meningioma remaining asymptomatic also increases with age.7 To complicate the matters even more, the world population is aging, which results in a steady increase in the elderly population. The decision to treat cannot be made as easily as in a young and symptomatic patient.
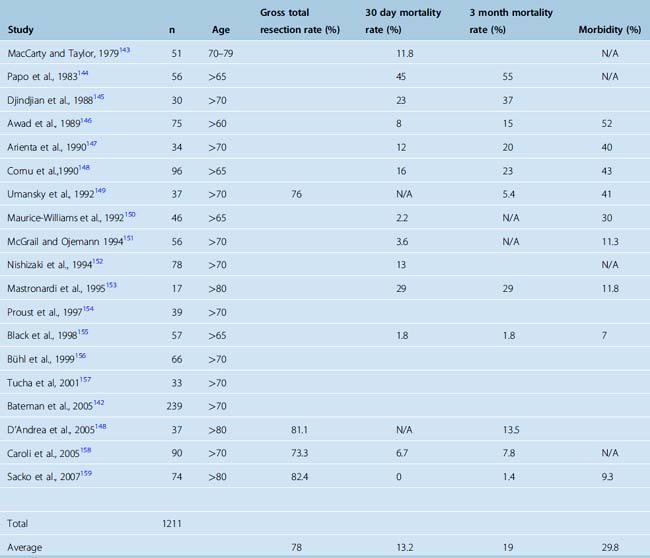
Although old age is not a contraindication for surgery, the outcome of meningioma surgery in the elderly is less satisfactory and the complication rate is higher than in younger patients. Yano and colleagues16 reported their experience with a large cohort of meningioma patients consisting of 603 asymptomatic and 831 symptomatic meningiomas. When 213 surgically treated asymptomatic meningiomas were considered, a perioperative morbidity of 4.4% was observed in patients younger than 70 years and 9.1% in patients older than 70 years. Bateman and colleagues142 reported surgical results of meningioma resection in 8861 patients, and 26% of their cases were 70 years or older. These authors indicated a five times higher mortality (1.4% vs. 7%) in patients older than 80 years when compared to those between 50 and 59 years and also a five times higher morbidity in patients who were older than 70 years.142 A summary of articles on the outcome of surgery for meningiomas in the elderly is provided in Table 18-3. Well over a thousand cases have been reported in the literature, and most studies concluded that poor preoperative general health and severe neurologic findings, large tumor size, critical locations, and marked peritumoral edema were indicators of poor outcome.142–150,152–159 Preoperative health was assessed with the American Society of Anesthesiology (ASA) classification scheme and functionality with the Karnofsky performance scoring. ASA classes of 3 and 4 and KPS of 60 or less were associated with higher mortality and morbidity. Interestingly, female sex was also associated with poor outcome in several studies.158 In patients with good functional status and no associated systemic morbidity surgery is a safe and effective treatment.
PREFERENCE OF THE PATIENT
The diagnosis of an intracranial tumor is a significant psychological burden for the patient.160 Immediately after diagnosis the patient will go through questions as: What is this thing in my head? What is the risk that it will grow? How fast will it grow? What will it cause? Will I die or become crippled? Is there any treatment? What are the treatment options? Can it be operated on? Will the surgery get rid of the tumor? Will it grow again? If it does so, how fast will it grow again? Will I have any options then? What are the risks of surgery? What if I don’t want to be operated? Often, the experienced surgeon does not have all the answers either. Therefore, when possible, the treatment decision must be taken together with the patient. The discussion with the patient related to informed consent should include a brief description of the natural history of the condition and its presumed course without treatment and the treatment alternatives, with their possible complications and benefits. Every patient has a different potential of understanding the proposed treatment and its risks, but it is he or she who will live through the treatment and afterwards; therefore the surgeon should make every attempt to empathetically share his or her thoughts with the patient.
[1] Kuratsu J., Kochi M., Ushio Y. Incidence and clinical features of asymptomatic meningiomas. J Neurosurg. 2000;92:766-770.
[2] Vernooij M.W., Ikram M.A., Tanghe H.L., et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821-1828.
[3] Annegers J.F., Schoenberg B.S., Okazaki H., Kurland L.T. Epidemiologic study of primary intracranial neoplasms. Arch Neurol. 1981;38:217-219.
[4] Nakasu S., Hirano A., Shimura T., Llena J.F. Incidental meningiomas in autopsy study. Surg Neurol. 1987;27:319-322.
[5] Staneczek W., Janisch W. Epidemiologic data on meningiomas in East Germany 1961–1986: incidence, localization, age and sex distribution. Clin Neuropathol. 1992;11:135-141.
[6] Christensen H.C., Kosteljanetz M., Johansen C. Incidences of gliomas and meningiomas in Denmark, 1943 to 1997. Neurosurgery. 2003;52:1327-1333. discussion 1333–1324
[7] Claus E.B., Bondy M.L., Schildkraut J.M., et al. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088-1095. discussion 1088–1095
[8] Elia-Pasquet S., Provost D., Jaffre A., et al. Incidence of central nervous system tumors in Gironde, France. Neuroepidemiology. 2004;23:110-117.
[9] Rausing A., Ybo W., Stenflo J. Intracranial meningioma–a population study of ten years. Acta Neurol Scand. 1970;46:102-110.
[10] Louis D.N., Scheithauer B.W., Budka H., et al. Meninigomas. Kleihues P., Cavenee W.K., editors. The WHO classification of tumors of the nervous system. 2002;176-184. Lyon
[11] Firsching R.P., Fischer A., Peters R., et al. Growth rate of incidental meningiomas. J Neurosurg. 1990;73:545-547.
[12] Go R.S., Taylor B.V., Kimmel D.W. The natural history of asymptomatic meningiomas in Olmsted County, Minnesota. Neurology. 1998;51:1718-1720.
[13] Herscovici Z., Rappaport Z., Sulkes J., et al. Natural history of conservatively treated meningiomas. Neurology. 2004;63:1133-1134.
[14] Niiro M., Yatsushiro K., Nakamura K., et al. Natural history of elderly patients with asymptomatic meningiomas. J Neurol Neurosurg Psychiatry. 2000;68:25-28.
[15] Olivero W.C., Lister J.R., Elwood P.W. The natural history and growth rate of asymptomatic meningiomas: a review of 60 patients. J Neurosurg. 1995;83:222-224.
[16] Yano S., Kuratsu J. Indications for surgery in patients with asymptomatic meningiomas based on an extensive experience. J Neurosurg. 2006;105:538-543.
[17] Yoneoka Y., Fujii Y., Tanaka R. Growth of incidental meningiomas. Acta Neurochir (Wien). 2000;142:507-511.
[18] Nakamura M., Roser F., Michel J., et al. The natural history of incidental meningiomas. Neurosurgery. 2003;53:62-70. discussion 70–61
[19] Weber R.G., Bostrom J., Wolter M., et al. Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: toward a genetic model of meningioma progression. Proc Natl Acad Sci USA. 1997;94:14719-14724.
[20] Ishino S., Hashimoto N., Fushiki S., et al. Loss of material from chromosome arm 1p during malignant progression of meningioma revealed by fluorescent in situ hybridization. Cancer. 1998;83:360-366.
[21] Krayenbuhl N., Pravdenkova S., Al-Mefty O. De novo versus transformed atypical and anaplastic meningiomas: comparisons of clinical course, cytogenetics, cytokinetics, and outcome. Neurosurgery. 2007;61:495-503. discussion 503–494
[22] Menon A.G., Rutter J.L., von Sattel J.P., et al. Frequent loss of chromosome 14 in atypical and malignant meningioma: identification of a putative ’tumor progression’ locus. Oncogene. 1997;14:611-616.
[23] Wrobel G., Roerig P., Kokocinski F., et al. Microarray-based gene expression profiling of benign, atypical and anaplastic meningiomas identifies novel genes associated with meningioma progression. Int J Cancer. 2005;114:249-256.
[24] Bitzer M., Wockel L., Morgalla M., et al. Peritumoural brain oedema in intracranial meningiomas: influence of tumour size, location and histology. Acta Neurochir (Wien). 1997;139:1136-1142.
[25] Yamasaki F., Yoshioka H., Hama S., et al. Recurrence of meningiomas. Cancer. 2000;89:1102-1110.
[26] Yoshioka H., Hama S., Taniguchi E., et al. Peritumoral brain edema associated with meningioma: influence of vascular endothelial growth factor expression and vascular blood supply. Cancer. 1999;85:936-944.
[27] Kilic T., Bayri Y., Ozduman K., et al. Tenascin in meningioma: expression is correlated with anaplasia, vascular endothelial growth factor expression, and peritumoral edema but not with tumor border shape. Neurosurgery. 2002;51:183-192. discussion 192–183
[28] Okada M., Miyake K., Matsumoto Y., et al. Matrix metalloproteinase-2 and matrix metalloproteinase-9 expressions correlate with the recurrence of intracranial meningiomas. J Neurooncol. 2004;66:29-37.
[29] Fewings P.E., Battersby R.D., Timperley W.R. Long-term follow up of progesterone receptor status in benign meningioma: a prognostic indicator of recurrence? J Neurosurg. 2000;92:401-405.
[30] Hsu D.W., Efird J.T., Hedley-Whyte E.T. Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg. 1997;86:113-120.
[31] Perry A., Gutmann D.H., Reifenberger G. Molecular pathogenesis of meningiomas. J Neurooncol. 2004;70:183-202.
[32] Jaaskelainen J., Haltia M., Laasonen E., et al. The growth rate of intracranial meningiomas and its relation to histology. An analysis of 43 patients. Surg Neurol. 1985;24:165-172.
[33] Nakamura M., Roser F., Michel J., et al. Volumetric analysis of the growth rate of incompletely resected intracranial meningiomas. Zentralbl Neurochir. 2005;66:17-23.
[34] Jellinger K., Slowik F. Histological subtypes and prognostic problems in meningiomas. J Neurol. 1975;208:279-298.
[35] Boker D.K., Meurer H., Gullotta F. Recurring intracranial meningiomas. Evaluation of some factors predisposing for tumor recurrence. J Neurosurg Sci. 1985;29:11-17.
[36] Mirimanoff R.O., Dosoretz D.E., Linggood R.M., et al. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18-24.
[37] Cho K.G., Hoshino T., Nagashima T., et al. Prediction of tumor doubling time in recurrent meningiomas. Cell kinetics studies with bromodeoxyuridine labeling. J Neurosurg. 1986;65:790-794.
[38] Simpson D. Recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22-39.
[39] Mathiesen T., Lindquist C., Kihlstrom L., Karlsson B. Recurrence of cranial base meningiomas. Neurosurgery. 1996;39:2-7. discussion 8–9
[40] Demirtas E., Yilmaz F., Ovul I., Oner K. Recurrence of meningiomas versus proliferating cell nuclear antigen (PCNA) positivity and AgNOR counting. Acta Neurochir (Wien). 1996;138:1456-1463.
[41] Langford L.A., Cooksley C.S., DeMonte F. Comparison of MIB-1 (Ki-67) antigen and bromodeoxyuridine proliferation indices in meningiomas. Hum Pathol. 1996;27:350-354.
[42] Roser F., Samii M., Ostertag H., Bellinzona M. The Ki-67 proliferation antigen in meningiomas. Experience in 600 cases. Acta Neurochir (Wien). 2004;146:37-44. discussion 44
[43] Drummond K.J., Zhu J.J., Black P.M. Meningiomas: updating basic science, management, and outcome. Neurologist. 2004;10:113-130.
[44] Rubin G., Ben David U., Gornish M., Rappaport Z.H. Meningiomas of the anterior cranial fossa floor. Review of 67 cases. Acta Neurochir (Wien). 1994;129:26-30.
[45] Ojemann R. Olfactory groove meningiomas. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press, 1991.
[46] Kennerdell J.S., Maroon J.C., Malton M., Warren F.A. The management of optic nerve sheath meningiomas. Am J Ophthalmol. 1988;106:450-457.
[47] Keane W.M., Bilaniuk L.T., Zimmerman R.A., Lowry L.D. Cranal computed tomography: application to otolaryngology. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol. 1977;84:ORL859-ORL865.
[48] Sarkies N.J. Optic nerve sheath meningioma: diagnostic features and therapeutic alternatives. Eye. 1987;1(Pt 5):597-602.
[49] Van Havenbergh T., Carvalho G., Tatagiba M., et al. Natural history of petroclival meningiomas. Neurosurgery. 2003;52:55-62. discussion 62–54
[50] Maiuri F., Gangemi M., Cirillo S., et al. Cerebral edema associated with meningiomas. Surg Neurol. 1987;27:64-68.
[51] Pieper D.R., Al-Mefty O., Hanada Y., Buechner D. Hyperostosis associated with meningioma of the cranial base: secondary changes or tumor invasion. Neurosurgery. 1999;44:742-746.
[52] Yasargil M. Microneurosurgery, vol. 4b. Stuttgart-NewYork: Thieme Verlag, 1996.
[53] Borovich B., Doron Y. Recurrence of intracranial meningiomas: the role played by regional multicentricity. J Neurosurg. 1986;64:58-63.
[54] Borovich B., Doron Y., Braun J., et al. Recurrence of intracranial meningiomas: the role played by regional multicentricity. Part 2: Clinical and radiological aspects. J Neurosurg. 1986;65:168-171.
[55] Drake J.M., Hoffmann H.J. Meningiomas in children. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press; 1991:145-152.
[56] Greene S., Nair N., Ojemann J.G., et al. Meningiomas in children. Pediatr Neurosurg. 2008;44:9-13.
[57] Crouse S.K., Berg B.O. Intracranial meningiomas in childhood and adolescence. Neurology. 1972;22:135-141.
[58] Obeid F., Al-Mefty O. Recurrence of olfactory groove meningiomas. Neurosurgery. 2003;53:534-542. discussion 542–533
[59] Tsikoudas A., Martin-Hirsch D.P. Olfactory groove meningiomas. Clin Otolaryngol Allied Sci. 1999;24:507-509.
[60] Chan R.C., Thompson G.B. Morbidity, mortality, and quality of life following surgery for intracranial meningiomas. A retrospective study in 257 cases. J Neurosurg. 1984;60:52-60.
[61] Nakamura M., Struck M., Roser F., et al. Olfactory groove meningiomas: clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery. 2007;60:844-852. discussion 844–852
[62] Fahlbusch R., Schott W. Pterional surgery of meningiomas of the tuberculum sellae and planum sphenoidale: surgical results with special consideration of ophthalmological and endocrinological outcomes. J Neurosurg. 2002;96:235-243.
[63] Goel A., Muzumdar D., Desai K. Tuberculum sellae meningioma: a report on management on the basis of a surgical experience with 70 patients. Neurosurgery. 2002;51:1358-1364.
[64] Jallo G.I., Benjamin V. Tuberculum sellae meningiomas: microsurgical anatomy and surgical technique. Neurosurgery. 2002;51:1432-1439. discussion 1439–1440
[65] Ohta K., Yasuo K., Morikawa M., et al. Treatment of tuberculum sella Meningiomas: a long-term follow-up study. J Clin Neurosci. 2001;8:26-31.
[66] Pamir M.N., Ozduman K., Belirgen M., et al. Outcome determinants of pterional surgery for tuberculum sellae meningiomas. Acta Neurochir (Wien). 2005;147:1121-1130. discussion 1130
[67] Zevgaridis D., Medele R.J., Mueller A., et al. Meningiomas of the sellar region presenting with visual impairment: impact of various prognostic factors on surgical outcome in 62 patients. Acta Neurochir Suppl. 2001;143:471-476.
[68] Al-Mefty O. Clinoidal meningiomas. J Neurosurg. 1990;73:840-849.
[69] Goel A., Gupta S., Desai K. New grading system to predict resectability of anterior clinoid meningiomas. Neurol Med Chir (Tokyo). 2000;40:610-616. discussion 616–617
[70] Lee J.H., Jeun S.S., Evans J., Kosmorsky G. Surgical management of clinoidal meningiomas. Neurosurgery. 2001;48:1012-1019. discussion 1019–1021
[71] Pamir M.N., Belirgen M., Ozduman K., et al. Anterior clinoidal meningiomas: analysis of 43 consecutive surgically treated cases. Acta Neurochir (Wien). 2008.
[72] Puzzilli F., Ruggeri A., Mastronardi L., et al. Anterior clinoidal meningiomas: report of a series of 33 patients operated on through the pterional approach. Neuro-oncol. 1999;1:188-195.
[73] Risi P., Uske A., de Tribolet N. Meningiomas involving the anterior clinoid process. Br J Neurosurg. 1994;8:295-305.
[74] De Jesus O., Sekhar L.N., Parikh H.K., et al. Long-term follow-up of patients with meningiomas involving the cavernous sinus: recurrence, progression, and quality of life. Neurosurgery. 1996;39:915-919. discussion 919–920
[75] DeMonte F., Smith H.K., al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81:245-251.
[76] Hirsch W.L., Sekhar L.N., Lanzino G., et al. Meningiomas involving the cavernous sinus: value of imaging for predicting surgical complications. AJR Am J Roentgenol. 1993;160:1083-1088.
[77] Larson J.J., van Loveren H.R., Balko M.G., Tew J.M.Jr. Evidence of meningioma infiltration into cranial nerves: clinical implications for cavernous sinus meningiomas. J Neurosurg. 1995;83:596-599.
[78] Sekhar L.N., Babu R.P., Wright D.C. Surgical resection of cranial base meningiomas. Neurosurg Clin N Am. 1994;5:299-330.
[79] Sekhar L.N., Pomeranz S., Sen C.N. Management of tumours involving the cavernous sinus. Acta Neurochir Suppl (Wien). 1991;53:101-112.
[80] Sindou M., Wydh E., Jouanneau E., et al. Long-term follow-up of meningiomas of the cavernous sinus after surgical treatment alone. J Neurosurg. 2007;107:937-944.
[81] Giombini S., Solero C.L., Morello G. Late outcome of operations for supratentorial convexity meningiomas. Report on 207 cases. Surg Neurol. 1984;22:588-594.
[82] Philippon J. Les recidives des meningiomes sus-tentoriels. Neurochirurgie (Suppl.). 1986;32:6-53.
[83] Yamashita J., Handa H., Iwaki K., Abe M. Recurrence of intracranial meningiomas, with special reference to radiotherapy. Surg Neurol. 1980;14:33-40.
[84] Bricolo A.P., Turazzi S., Talacchi A., Cristofori L. Microsurgical removal of petroclival meningiomas: a report of 33 patients. Neurosurgery. 1992;31:813-828. discussion 828
[85] Couldwell W.T., Fukushima T., Giannotta S.L., Weiss M.H. Petroclival meningiomas: surgical experience in 109 cases. J Neurosurg. 1996;84:20-28.
[86] Jung H.W., Yoo H., Paek S.H., Choi K.S. Long-term outcome and growth rate of subtotally resected petroclival meningiomas: experience with 38 cases. Neurosurgery. 2000;46:567-574. discussion 574–565
[87] Sekhar L.N., Swamy N.K., Jaiswal V., et al. Surgical excision of meningiomas involving the clivus: preoperative and intraoperative features as predictors of postoperative functional deterioration. J Neurosurg. 1994;81:860-868.
[88] Zentner J., Meyer B., Vieweg U., et al. Petroclival meningiomas: is radical resection always the best option? J Neurol Neurosurg Psychiatry. 1997;62:341-345.
[89] Bret P., Guyotat J., Madarassy G., et al. Tentorial meningiomas. Report on twenty-seven cases. Acta Neurochir (Wien). 2000;142:513-526.
[90] Colli B.O., Assirati J.A.Jr, Deriggi D.J., et al. Tentorial meningiomas: follow-up review. Neurosurg Rev. 2008;31:421-430.
[91] Gokalp H.Z., Arasil E., Erdogan A., et al. Tentorial meningiomas. Neurosurgery. 1995;36:46-51. discussion 51
[92] Samii M., Carvalho G.A., Tatagiba M., et al. Meningiomas of the tentorial notch: surgical anatomy and management. J Neurosurg. 1996;84:375-381.
[93] Asari S., Maeshiro T., Tomita S., et al. Meningiomas arising from the falcotentorial junction. Clinical features, neuroimaging studies, and surgical treatment. J Neurosurg. 1995;82:726-738.
[94] Goto T., Ohata K., Morino M., et al. Falcotentorial meningioma: surgical outcome in 14 patients. J Neurosurg. 2006;104:47-53.
[95] Arnautovic K.I., Al-Mefty O., Husain M. Ventral foramen magnum meninigiomas. J Neurosurg. 2000;92:71-80.
[96] Bassiouni H., Ntoukas V., Asgari S., et al. Foramen magnum meningiomas: clinical outcome after microsurgical resection via a posterolateral suboccipital retrocondylar approach. Neurosurgery. 2006;59:1177-1185. discussion 1185–1177
[97] Boulton M.R., Cusimano M.D. Foramen magnum meningiomas: concepts, classifications, and nuances. Neurosurg Focus. 2003;14:e10.
[98] Goel A., Desai K., Muzumdar D. Surgery on anterior foramen magnum meningiomas using a conventional posterior suboccipital approach: a report on an experience with 17 cases. Neurosurgery. 2001;49:102-106. discussion 106–107
[99] Margalit N.S., Lesser J.B., Singer M., Sen C. Lateral approach to anterolateral tumors at the foramen magnum: factors determining surgical procedure. Neurosurgery. 2005;56:324-336. discussion 324–336
[100] Marin Sanabria E.A., Ehara K., Tamaki N. Surgical experience with skull base approaches for foramen magnum meningioma. Neurol Med Chir (Tokyo). 2002;42:472-478. discussion 479–480
[101] Nanda A., Vincent D.A., Vannemreddy P.S., et al. Far-lateral approach to intradural lesions of the foramen magnum without resection of the occipital condyle. J Neurosurg. 2002;96:302-309.
[102] Pamir M.N., Kılıç T., Özduman K., Türe U. Experience of a single institution treating foramen magnum meningiomas. J Clin Neurosci. 2004;11:863-867.
[103] Parlato C., Tessitore E., Schonauer C., Moraci A. Management of benign craniovertebral junction tumors. Acta Neurochir (Wien). 2003;145:31-36.
[104] Roberti F., Sekhar L.N., Kalavakonda C., Wright D.C. Posterior fossa meningiomas: surgical experience in 161 cases. Surg Neurol. 2001;56:8-20. discussion 20–21
[105] Jaaskelainen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol. 1986;26:461-469.
[106] Mathiesen T., Kihlstrom L. Visual outcome of tuberculum sellae meningiomas after extradural optic nerve decompression. Neurosurgery. 2006;59:570-576. discussion 570–576
[107] Derome P.J., Guiot G. Bone problems in meningiomas invading the base of the skull. Clin Neurosurg. 1978;25:435-451.
[108] Bakay L. Olfactory meningiomas. The missed diagnosis. Jama. 1984;251:53-55.
[109] Welge-Luessen A., Temmel A., Quint C., et al. Olfactory function in patients with olfactory groove meningioma. J Neurol Neurosurg Psychiatry. 2001;70:218-221.
[110] Rosenstein J., Symon L. Surgical management of suprasellar meningioma. Part 2: Prognosis for visual function following craniotomy. J Neurosurg. 1984;61:642-648.
[111] Symon L., Rosenstein J. Surgical management of suprasellar meningioma. Part 1: The influence of tumor size, duration of symptoms, and microsurgery on surgical outcome in 101 consecutive cases. J Neurosurg. 1984;61:633-641.
[112] Andrews B.T., Wilson C.B. Suprasellar meningiomas: the effect of tumor location on postoperative visual outcome. J Neurosurg. 1988;69:523-528.
[113] Bonnal J., Thibaut A., Brotchi J., Born J. Invading meningiomas of the sphenoid ridge. J Neurosurg. 1980;53:587-599.
[114] Roser F., Nakamura M., Martini-Thomas R., et al. The role of surgery in meningiomas involving the optic nerve sheath. Clin Neurol Neurosurg. 2006;108:470-476.
[115] O’Sullivan M.G., van Loveren H.R., Tew J.M.Jr. The surgical resectability of meningiomas of the cavernous sinus. Neurosurgery. 1997;40:238-244. discussion 245–237
[116] Pamir M.N., Kilic T., Bayrakli F., Peker S. Changing treatment strategy of cavernous sinus meningiomas: experience of a single institution. Surg Neurol. 2005;64(Suppl. 2):S58-S66.
[117] Duma C.M., Lunsford L.D., Kondziolka D., et al. Stereotactic radiosurgery of cavernous sinus meningiomas as an addition or alternative to microsurgery. Neurosurgery. 1993;32:699-704. discussion 704–695
[118] Lee J.Y., Niranjan A., McInerney J., et al. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg. 2002;97:65-72.
[119] Abdel-Aziz K.M., Froelich S.C., Dagnew E., et al. Large sphenoid wing meningiomas involving the cavernous sinus: conservative surgical strategies for better functional outcomes. Neurosurgery. 2004;54:1375-1383. discussion 1383–1374
[120] Bonnal J., Brotchi J. Surgery of the superior sagittal sinus in parasagittal meningiomas. J Neurosurg. 1978;48:935-945.
[121] Sindou M.P., Alvernia J.E. Results of attempted radical tumor removal and venous repair in 100 consecutive meningiomas involving the major dural sinuses. J Neurosurg. 2006;105:514-525.
[122] Hakuba A. Reconstruction of dural sinus involved in meningiomas. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press; 1991:371-382.
[123] Kondziolka D., Lunsford L.D., Coffey R.J., et al. Gamma knife radiosurgery of meningiomas. Stereotact Funct Neurosurg. 1991;57:11-21.
[124] Kondziolka D., Mathieu D., Lunsford L.D., et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62:53-58. discussion 58–60
[125] Pamir M.N., Peker S., Kilic T., Sengoz M. Efficacy of Gamma-Knife surgery for treating meningiomas that involve the superior sagittal sinus. Zentralbl Neurochir. 2007;68:73-78.
[126] Yasargil M., Mortara R., Curcic M. Meningiomas of basal posterior cranial fossa. Krayenbühl H., editor. Advances and Technical Standards in Neurosurgery, vol. 7. Springer-Verlag, Wien, 1980;1-115.
[127] Baguley D.M., Beynon G.J., Grey P.L., et al. Audio-vestibular findings in meningioma of the cerebello-pontine angle: a retrospective review. J Laryngol Otol. 1997;111:1022-1026.
[128] Matthies C., Carvalho G., Tatagiba M., et al. Meningiomas of the cerebellopontine angle. Acta Neurochir Suppl. 1996;65:86-91.
[129] Maniglia A.J., Fenstermaker R.A., Ratcheson R.A. Preservation of hearing in the surgical removal of cerebellopontine angle tumors. Otolaryngol Clin North Am. 1989;22:211-232.
[130] Roser F., Nakamura M., Dormiani M., et al. Meningiomas of the cerebellopontine angle with extension into the internal auditory canal. J Neurosurg. 2005;102:17-23.
[131] Nakamura M., Roser F., Dormiani M., et al. Surgical treatment of cerebellopontine angle meningiomas in elderly patients. Acta Neurochir (Wien). 2005;147:603-609. discussion 609–610
[132] Ture U., Pamir M.N. Small petrosal approach to the middle portion of the mediobasal temporal region: technical case report. Surg Neurol. 2004;61:60-67.
[133] Samii M., Tatagiba M., Carvalho G.A. Resection of large petroclival meningiomas by the simple retrosigmoid route. J Clin Neurosci. 1999;6:27-30.
[134] Samii M., Gerganov V.M. Surgery of extra-axial tumors of the cerebral base. Neurosurgery. 2008;62:1153-1166. discussion 1166–1158
[135] Muthukumar N., Kondziolka D., Lunsford L.D., Flickinger J.C. Stereotactic radiosurgery for anterior foramen magnum meningiomas. Surg Neurol. 1999;51:268-273.
[136] Giombini S., Solero C.L., Lasio G., Morello G. Immediate and late outcome of operations for Parasagittal and falx meningiomas. Report of 342 cases. Surg Neurol. 1984;21:427-435.
[137] Stafford S.L., Perry A., Suman V.J., et al. Primarily resected meningiomas: outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin Proc. 1998;73:936-942.
[138] Adegbite A.B., Khan M.I., Paine K.W., Tan L.K. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58:51-56.
[139] Kinjo T., al-Mefty O., Kanaan I. Grade zero removal of supratentorial convexity meningiomas. Neurosurgery. 1993;33:394-399. discussion 399
[140] Lee J.Y., Kondziolka D., Flickinger J.C., Lunsford L.D. Radiosurgery for intracranial meningiomas. Prog Neurol Surg. 2007;20:142-149.
[141] Milker-Zabel S., Zabel-du Bois A., Huber P., et al. Intensity-modulated radiotherapy for complex-shaped meningioma of the skull base: long-term experience of a single institution. Int J Radiat Oncol Biol Phys. 2007;68:858-863.
[142] Bateman B.T., Pile-Spellman J., Gutin P.H., Berman M.F. Meningioma resection in the elderly: nationwide inpatient sample, 1998–2002. Neurosurgery. 2005;57:866-872. discussion 866–872
[143] MacCarty C.S., Taylor W.F. Intracranial meningiomas: experiences at the Mayo Clinic. Neurol Med Chir (Tokyo). 1979;19:569-574.
[144] Papo I. Intracranial meningiomas in the elderly in the CT scan era. Acta Neurochir (Wien). 1983;67:195-204.
[145] Djindjian M., Caron J.P., Athayde A.A., Fevrier M.J. Intracranial meningiomas in the elderly (over 70 years old). A retrospective study of 30 surgical cases. Acta Neurochir (Wien). 1988;90:121-123.
[146] Awad I.A., Kalfas I., Hahn J.F., Little J.R. Intracranial meningiomas in the aged: surgical outcome in the era of computed tomography. Neurosurgery. 1989;24:557-560.
[147] Arienta C., Caroli M., Crotti F., Villani R. Treatment of intracranial meningiomas in patients over 70 years old. Acta Neurochir (Wien). 1990;107:47-55.
[148] D’Andrea G., Roperto R., Caroli E., et al. Thirty-seven cases of intracranial meningiomas in the ninth decade of life: our experience and review of the literature. Neurosurgery. 2005;56:956-961. discussion 956–961
[149] Umansky F., Ashkenazi E., Gertel M., Shalit M.N. Surgical outcome in an elderly population with intracranial meningioma. J Neurol Neurosurg Psychiatry. 1992;55:481-485.
[150] Maurice-Williams R.S., Kitchen N.D. Intracranial tumours in the elderly: the effect of age on the outcome of first time surgery for meningiomas. Br J Neurosurg. 1992;6:131-137.
[151] McGrail K.M., Ojemann R.G. The surgical management of benign intracranial meningiomas and acoustic neuromas in patients 70 years of age and older. Surg Neurol. 1994;42:2-7.
[152] Nishizaki T., Kamiryo T., Fujisawa H., et al. Prognostic implications of meningiomas in the elderly (over 70 years old) in the era of magnetic resonance imaging. Acta Neurochir (Wien). 1994;126:59-62.
[153] Mastronardi L., Ferrante L., Qasho R., et al. Intracranial meningiomas in the 9th decade of life: a retrospective study of 17 surgical cases. Neurosurgery. 1995;36:270-274.
[154] Proust F., Verdure L., Toussaint P., et al. [Intracranial meningioma in the elderly. Postoperative mortality, morbidity and quality of life in a series of 39 patients over 70 years of age]. Neurochirurgie. 1997;43:15-20.
[155] Black P., Kathiresan S., Chung W. Meningioma surgery in the elderly: a case-control study assessing morbidity and mortality. Acta Neurochir (Wien). 1998;140:1013-1016. discussion 1016–1017
[156] Buhl R., Hasan A., Behnke A., Mehdorn H.M. Results in the operative treatment of elderly patients with intracranial meningioma. Neurosurg Rev. 2000;23:25-29.
[157] Tucha O., Smely C., Lange K.W. Effects of surgery on cognitive functioning of elderly patients with intracranial meningioma. Br J Neurosurg. 2001;15:184-188.
[158] Caroli M., Locatelli M., Prada F., et al. Surgery for intracranial meningiomas in the elderly: a clinical-radiological grading system as a predictor of outcome. J Neurosurg. 2005;102:290-294.
[159] Sacko O., Sesay M., Roux F.E., et al. Intracranial meningioma surgery in the ninth decade of life. Neurosurgery. 2007;61:950-954. discussion 955
[160] D’Angelo C., Mirijello A., Leggio L., et al. State and trait anxiety and depression in patients with primary brain tumors before and after surgery: 1–year longitudinal study. J Neurosurg. 2008;108:281-286.