Chapter 57 Cystic neoplasms of the pancreas
Epidemiology, clinical features, assessment, and management
Overview
Cystic neoplasms of the pancreas have become a well-defined radiographic entity over the last decade. With increasing use of cross-sectional imaging, nearly 2.6 cystic lesions per 100 individuals are encountered per year (Brugge et al, 2004a; Gorin & Sackier, 1997; Kimura et al, 1995). These lesions have a broad histologic differential, which has been well characterized by Klöppel and Kosmahl (2001) and others (Table 57.1; Kosmahl et al, 2004). This differential includes benign nonneoplastic pancreatic pseudocysts and also cystic neoplasms, which comprise benign entities such as serous cystadenoma, premalignant cysts such as intraductal papillary mucinous neoplasms (IPMNs), and cystic lesions with invasive carcinoma. The ability to differentiate among neoplastic pancreatic cystic neoplasms without the need for resection continues to evolve. Serous cysts can often be differentiated from mucinous cysts by cross-sectional imaging and endoscopic ultrasound (EUS) with cyst fluid analysis (Allen et al, 2003, 2006; Allen & Brennan, 2007; Brugge et al, 2004b; Carpizo et al, 2008; Ferrone et al, 2009). However, diagnostic dilemmas continue, and the ability to predict future progression to malignancy within the premalignant group is currently limited (Lee et al, 2008).
Table 57.1 Classification of Cystic Neoplasms of the Pancreas
| Neoplastic | Nonneoplastic |
|---|---|
| Epithelial | Epithelial |
| Benign |
Malignant neoplasm (i.e., sarcoma)
IPMN, intraductal papillary mucinous neoplasm; MCN, mucinous cystic neoplasm
Modified from Kosmahl M, et al, 2004: Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch 445(2):168-178.
As shown in Table 57.1, these lesions remain a diagnostic challenge. As their incidence increases, so does the differential diagnosis. With an increasing knowledge of the clinicopathologic variables predictive of malignancy and an improved understanding of the natural history of many of the common cystic lesions, management has changed to one of selective resection. Despite the improvement in diagnostic capability, there still remain a significant number of lesions that are indeterminate; in this setting the clinician must balance the risk of malignancy with the morbidity and mortality associated with pancreatic resection.
Clinicopathologic Variables
The majority of patients with pancreatic cysts will have nonneoplastic inflammatory pseudocysts that develop as a complication of acute pancreatitis (see Chapter 54; Brugge et al, 2004a; Cannon et al, 2009). A pseudocyst is a fluid collection that is devoid of an epithelial lining. Pseudocysts have been reported to develop in up to 50% of patients who have acute pancreatitis (Cannon et al, 2009; Grace & Williamson, 1993; O’Malley et al, 1985). Because of the frequency of pancreatitis, some studies have reported that pseudocysts comprise up to 85% of all cystic pancreatic lesions. Pseudocysts can be managed with observation, endoscopic drainage, or operative drainage (Cannon et al, 2009). The particulars of the management of patients with pancreatic pseudocysts are beyond the scope of this chapter.
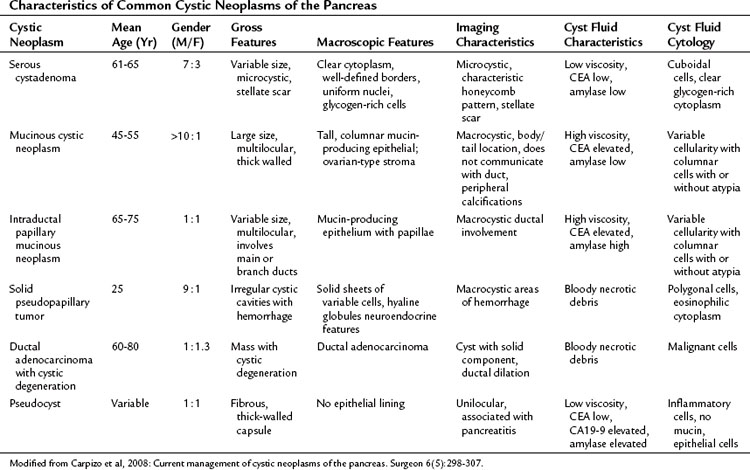
Cystic neoplasms are a heterogeneous group of lesions carefully described by Klöppel and Kosmahl (see Table 57.1; Klöppel & Kosmahl, 2001; Kosmahl et al, 2004). They comprise approximately 10% to 15% of cystic lesions of the pancreas and are a heterogeneous group of lesions that range from benign to malignant. Despite the fact that numerous neoplasms can present as a cystic lesion, the most common cystic neoplasms encountered by surgeons include serous cystadenoma (SCA), mucinous cystic neoplasm (MCN), and intraductal papillary mucinous neoplasm (IPMN). In a study by the French Surgical Association of 372 resected cystic neoplasms from 73 institutions, these three lesions comprised 87% of all lesions (Le Borgne et al, 1999). More recent series of pancreatic cystic neoplasms are outlined in Table 57.2. The clinicopathologic factors associated with the more common cystic neoplasms are shown in Table 57.3.
Serous Cystadenomas
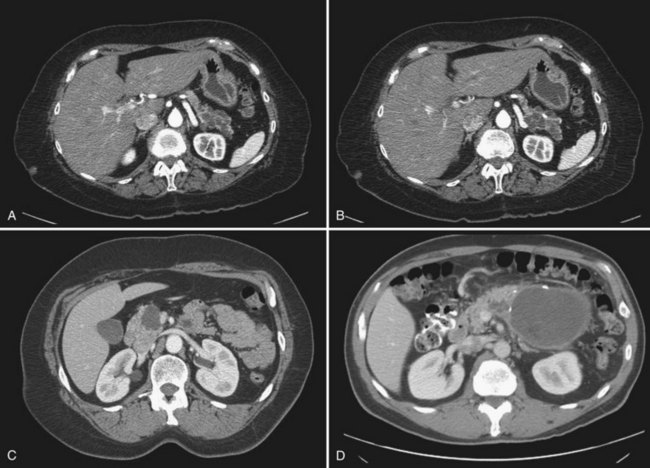
Serous cystadenomas (SCA) were first characterized by Compagno and Oertel in 1978 as microcystic and glycogen rich, and they distinguished these lesions from mucinous cysts (MCNs and IPMNs) (Compagno & Oertel, 1978a). SCAs occur with variable size, and when large (>10 cm) may cause symptoms from local compression. Grossly, these cysts are characterized by septations and thick fibrous walls and have what appear to be innumerable small cysts containing clear, thin fluid. A classic honeycomb appearance is often present, as is a calcified central scar with or without hemorrhage (Fig. 57.1A and B). On imaging, these lesions may appear as a single, large cyst (oligocystic) (Fig. 57.1C and D); if so, they are very difficult to distinguish from mucinous lesions. Because of the fibrous nature of this lesion, a solid component is often visualized; if noted in the presence of the other characteristic findings of SCA, this should not be concerning for malignancy. Histologically these lesions are characterized by a bland, cuboidal epithelial lining without nuclear polymorphism or mitoses. Typically, no mucin is present in the epithelial lining, but glycogen may be detected in the cytoplasm or in the amorphous cellular material.
SCAs are generally considered benign lesions, and only 25 cases of serous “cystadenocarcinoma” have been reported in the world literature as of 2010 (King et al, 2009; Matsumoto et al, 2005). Many of these case reports describe the “malignant” serous cystadenoma as locally invasive, and only 9 (36%) of 25 cases had evidence of metastasis. Despite the presence of metastasis, long-term prognosis remains excellent in these patients; it appears that the true incidence of malignancy within SCA is less than 1%. The institutional database at Memorial Sloan-Kettering Cancer Center (MSKCC) includes approximately 200 patients who have had resection for SCA, and none has developed or presented with metastatic disease. The more common problem caused by these lesions is local invasion that results in symptoms such as early satiety, obstructive jaundice, and pain. It stands to reason that large lesions are more likely to produce symptoms; however, many patients with lesions greater than 10 cm in diameter will have their lesion identified incidentally; for example, with a mean diameter of 11 cm, 71% of patients had symptoms compared with a mean diameter of 5 cm, with which 53% had symptoms (Compagno & Ortel, 1978a; Tseng et al, 2005).
Mucinous Cystic Neoplasms
Mucinous cystic neoplasms (MCNs) are mucin-producing cystic tumors that lack communication with the pancreatic duct and contain mucin-producing columnar epithelium. Ovarian-like stroma surrounding the columnar epithelium is considered a pathognomonic finding and is the presumed reason that MCNs are almost exclusively found in females. The stroma is a characteristic that pancreatic MCNs share with some mucinous cysts of the ovary and the liver. Pancreatic MCNs are most commonly found in the body and tail and can range in size from small (2 cm) to large (25 cm). Despite their distal location, patients are often symptomatic (76% in a review of multiple series) at the time of diagnosis (Goh et al, 2006).
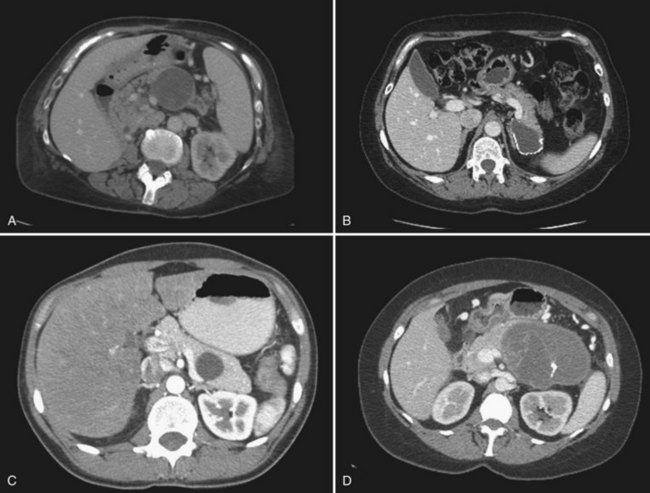
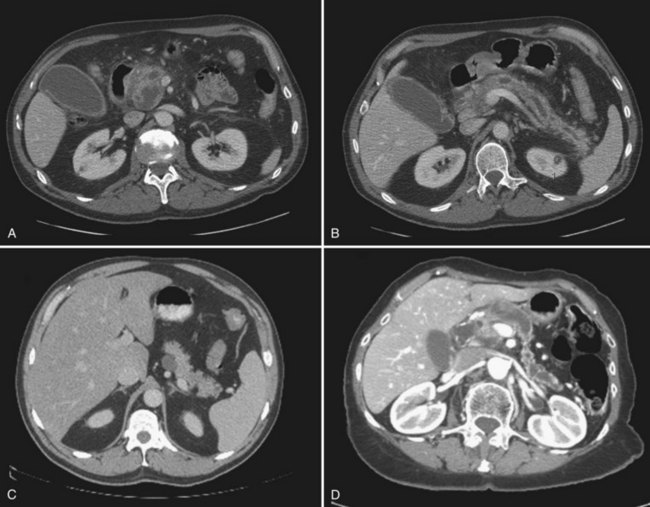
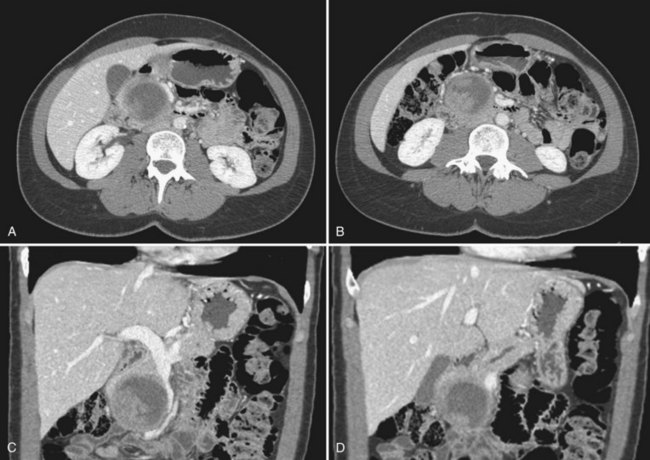
Any macrocystic lesion in the distal pancreas of a female patient should be highly suspicious for MCN. These lesions are often unilocular but may contain septa within the primary lesion (Fig. 57.2A to C). When these lesions are multilocular, they are typically macrocystic, in contrast to serous cystadenomas. These lesions may also have peripheral “eggshell” calcifications. Any evidence of mural nodularity in these lesions should be concerning for invasion (Fig. 57.2D). Upon gross inspection, these tumors are round, with a smooth surface and fibrous pseudocapsule.
Unlike serous cystadenomas, MCNs have a risk for harboring malignancy. Rates of invasive disease range from 10% to 50%, which may be an underestimate because of the fact that both benign and malignant epithelium may coexist within the same cyst. Only an extensive pathologic evaluation will detect both entities, which is critical for accurate diagnosis. Clinical factors that increase the risk for malignancy in MCNs are not well characterized, but one study found that older age was more often associated with cancer (Crippa et al, 2008). Factors on imaging associated with malignancy within MCNs include the presence of septations, larger size, and mural nodularity (Allen et al, 2006; Zamboni et al, 1999). Because these lesions are so rare, their natural history is not well defined, although the extent of invasion was demonstrated to be associated with recurrence and death from disease in one study (Allen et al, 2006; Zamboni et al, 1999). In one of the largest studies of MCNs to date, 5-year disease-specific survival for patients with malignancy was 57% (Goh et al, 2006).
Intraductal Papillary Mucinous Neoplasms
Before the first classification system published by the World Health Organization (WHO) (Klöppel et al, 1996), IPMN was described under a variety of names, including mucinous ductal ectasia, papillary carcinoma, and villous adenoma. In contrast to other mucinous and cystic tumors, IPMNs occur equally in men and women and are more often found in older individuals, with the peak incidence between 60 and 70 years of age (Brugge et al, 2004a). Among cystic neoplasms diagnosed in large series, IPMNs represent approximately 15% to 30% of all lesions (see Table 57.2; Allen et al, 2006; Ferrone et al, 2009; Kosmahl et al, 2004; Spinelli et al, 2004). Because of the recent evolution in the understanding of IPMNs, many early reports may include a mixture of patients with IPMNs and MCNs and should be interpreted with caution.
Current nomenclature as defined by the WHO divides IPMN into the categories of adenoma (low-grade dysplasia), borderline (moderate dysplasia), carcinoma in situ (high-grade dysplasia), and carcinoma (Furukawa et al, 2005). As clinical experience with IPMN increases, it is becoming more evident that the underlying process presents as a spectrum of neoplasia with significant variation (Schnelldorfer et al, 2008). The clinical and radiologic presentation, malignant potential, and disease-specific outcomes are highly variable. There also appears to be a progression from adenoma to intraductal carcinoma to invasive carcinoma that can be predicted based on molecular studies.
IPMN is indeed a process that involves the entire pancreas; however, radiographically detectable disease may involve the main duct alone, branch ducts alone, or both (mixed variant). The main duct variant of IPMN is typically seen with diffuse ductal dilation (Fig. 57.3). The presence of a discrete mass or solid component should be concerning for malignancy. Branch duct IPMNs, particularly when smaller than 3 cm, do not have any defining features. They may be unilocular, multilocular, and may be present without dilation of the main pancreatic duct. In the absence of septations, mural nodules, or a solid component, branch duct IPMNs may be indistinguishable from pancreatic retention cysts, MCNs, small cystic endocrine tumors, or even pancreatic pseudocysts.
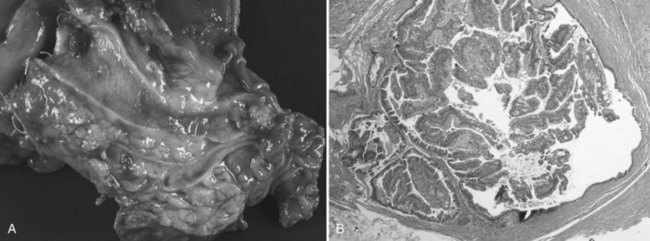
IPMNs are now a well-characterized mucinous cystic tumor of the pancreas evidenced by ductal involvement and mucin production. Recently, a histopathologic consensus conference defined IPMN as a grossly visible, noninvasive, mucin-producing neoplasm arising from either the main or side branches of the pancreatic duct with variable degrees of ductal dilation (Fig. 57.4A; Furukawa et al, 2005). On histologic analysis, IMPN lesions are characterized by papillary projections of columnar lined epithelium with varying degrees of dysplasia. Mucin is typically abundant both within the cytoplasm of the lining epithelial cells as well as within the acellular fluid matrix (Fig. 57.4B).
IPMN-Associated Malignancy
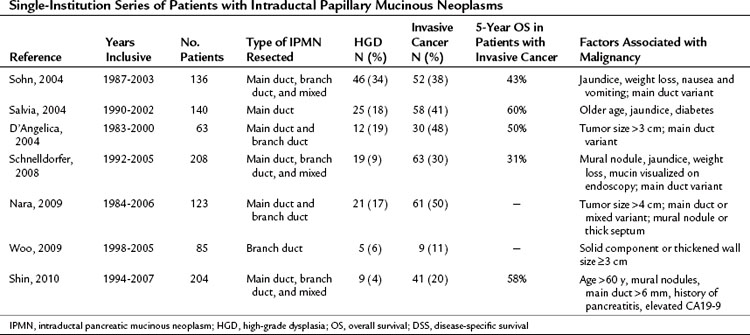
Malignancy arising from IPMN is well documented, and several large series have been reported in the literature (Table 57.4; D’Angelica et al, 2004; Nara et al, 2009; Salvia et al, 2004; Schnelldorfer et al, 2008; Shin et al, 2010; Sohn et al, 2004; Woo et al, 2009). Invasive cancer is found in 20% to 50% of resected IPMN specimens, which is the reason that these lesions are typically resected. Factors shown to be associated with the presence of invasive cancer in large series include symptoms at presentation, large size, mural nodularity, main duct dilation, and elevated levels of carbohydrate antigen 19-9 (CA19-9).
The reported outcome associated with invasive cancer in patients with IPMN of 5-year survival of 43% to 60% is better than that of ductal adenocarcinoma (15%) (see Chapter 58A, Chapter 58B ) following resection. Poultsides and colleagues (2010) reported the Johns Hopkins experience of IPMN-associated invasive cancers and found that compared with patients with ductal adenocarcinoma, IMPN patients had a lower rate of advanced T stage and lymph node metastasis. Patients with ductal adenocarcinoma more often had high-grade lesions and evidence of perineural invasion, vascular invasion, and positive margins. Despite patients with IPMN-associated cancers having an overall better outcome, if any of the adverse tumor-related factors were present, outcome was similar to patients with ductal adenocarcinoma. It can be inferred from this study that the improved outcome of IPMN-associated cancers is a factor of earlier stage of disease at resection.
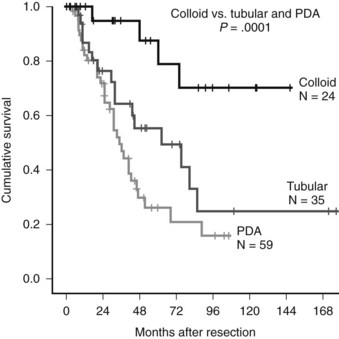
Variants of IPMN-associated cancer also have different prognoses. A matched controlled study from our institution demonstrated that patients with colloid-subtype invasive IPMN had significantly better survival than both matched patients with conventional pancreatic carcinoma and patients with invasive tubular IPMN. Fifty-nine patients with IPMN-associated cancer were matched one-to-one with patients with ductal adenocarcinoma with factors from the MSKCC pancreatic cancer nomogram (unpublished data). Among IPMN-associated cancers, tubular histology (compared with the colloid variant) and lymph node involvement were negative prognostic factors associated with outcome. Patients with conventional adenocarcinoma more often presented with obstructive jaundice and had more specimens with evidence of perineural/vascular invasion and regional lymph node metastasis. Survival among patients with colloid variant carcinoma (87% estimated 5-year survival) was significantly better compared with patients with tubular cancers (55%) and ductal adenocarcinoma (23%) (Fig. 57.5).
Diagnostic Evaluation Of Pancreatic Cystic Neoplasms
High-quality cross-sectional imaging is the key to the diagnostic evaluation of any cystic lesion of the pancreas (see Chapter 16; Sahani et al, 2005). Multidetector computed tomography (CT) allows thin-section imaging of the pancreas and is the most common method at our institution for evaluation of pancreatic cysts. A high-quality CT scan can carefully delineate and characterize the pancreatic parenchyma near a cystic lesion, which is critical in assessing for a radiographically occult malignancy causing adjacent dilation of a pancreatic duct (Allen et al, 2006). In addition, careful visualization of the cystic lesion allows evaluation of septations, mural nodules, and calcifications. Magnetic resonance cholangiopancreatography (MRCP) can also be used to define cyst morphology and may actually be better than CT for determining a communication with the pancreatic duct and to diagnose branch duct IPMN (Koito et al, 1998; Sainani et al, 2009).
An improvement in endoscopic techniques has significantly improved the clinician’s ability to evaluate pancreatic cystic neoplasms (Michael & Gress, 2002). EUS with or without cyst aspiration is a valuable diagnostic tool in the assessment of indeterminate cystic lesions (see Chapter 14). Because this procedure is operator dependent, a gastroenterologist with expertise in performing EUS is highly valuable (Ingram & Arregui, 2004). Careful evaluation with EUS can provide detailed images of the cyst wall and internal cyst architecture. Fine-needle aspiration and biopsy can be performed with EUS and may include both biopsy of the cyst and aspiration of cyst fluid for cytology and analysis for tumor markers (Linder et al, 2006).
Cyst fluid analysis has been extensively studied as a diagnostic tool in assessment of cystic lesions of the pancreas (Allen et al, 2009; Brugge et al, 2004b; Hammel et al, 1995, 1997; Sand et al, 1996). Numerous markers have been evaluated including CA19-9, CEA, CA15-3, MUC proteins, K-Ras, and amylase. In a landmark study by Brugge and colleagues (2004b), cyst fluid CEA greater than 192 ng/mL was the best predictor of a mucinous lesion, which accurately defined these lesions in 79% of patients. An elevation of cyst fluid CEA in combination with the presence of extracellular mucin has been shown to have a positive predictive value as high as 85% in some series. The ability to predict the presence of invasive disease, however, has not been demonstrated by measurement of cyst fluid CEA. SCA and pancreatic retention cysts uniformly have undetectable levels of CEA (Lewandrowski et al, 1993; van der Waaij et al, 2005).
Treatment Of Pancreatic Cystic Neoplasms
Serous Cystadenoma
Tseng and colleagues (2005) radiographically followed 24 patients with SCA for a median of 23 months and reported a 0.6 cm per year median growth rate for these lesions and noted that larger lesions (≥4 cm) had a higher growth rate (1.98 cm/year) compared with lesions smaller than 4 cm (0.12 cm/year). The authors recommended that all patients with SCAs larger than 4 cm be resected, regardless of symptoms. At MSKCC, we have noted a growth rate for SCA of approximately 0.5 cm per year but have not found an association between larger size and faster growth (Allen et al, 2006). It is our current practice to observe asymptomatic patients, regardless of lesion size, with the possible exception of those patients who have large lesions that are marginally resectable at presentation, where tumor growth would preclude the ability to perform resection. However, even in the latter circumstance, resection of asymptomatic SCA is difficult to justify given its benign nature.
Intraductal Papillary Mucinous Neoplasms
Resection is reserved for selected patients with IPMNs. When radiographic or endoscopic studies identify dilation of the main pancreatic duct and/or any other concerning features, the standard approach is resection. Concerning radiographic features in the setting of branch duct IPMN include a solid component or mural nodule, septations, or size greater than 3 cm (Allen et al, 2006). Lesions in the head of the pancreas are resected with pancreatoduodenectomy, and in the tail, distal pancreatectomy with or without splenectomy is used. In centers where pancreatectomy is commonplace, central pancreatectomy may be considered for lesions in the body of the pancreas. Total pancreatectomy is reserved for fit patients with diffuse IPMN; however, this procedure should be reserved for highly selected patients, in whom surveillance of the affected remnant duct would not be feasible.
The goal of resection of IPMN is to achieve a complete resection. One of the controversies that exist is the extent of pancreatic resection (Lai & Lau, 2005). IPMN is considered a diffuse process; therefore some concern has been raised that removal of only part of the pancreas would be inadequate. The clinical significance of a glandular recurrence in a patient with invasive IPMN is small because invasive IPMN is a disease characterized more by distant recurrence, similar to that of ductal adenocarcinoma. However, patients who undergo resection of noninvasive IPMN are at risk for recurrence in the remnant pancreas. In a recent report from MSKCC, White and colleagues (2007) reviewed 78 patients who had a resection for noninvasive IPMN; at a median follow-up of 40 months, a recurrence rate in the remnant pancreas of 8% was reported. Only 2% of patients (1 in 50) with a negative margin had recurrence, but 17% of those (4 of 23) with a margin positive for IPMN had recurrence (P = .02). Because 92% of this cohort of patients did not have a recurrence, it would seem that the risk associated with total pancreatectomy outweighs the risk for recurrence. However, routine surveillance of these patients with cross-sectional imaging is recommended. In this study, recurrences occurred at a median of 22 months (range, 8 to 62 months) from the time of initial resection and were noninvasive. Completion pancreatectomy is indicated for treatment of any detected recurrence.
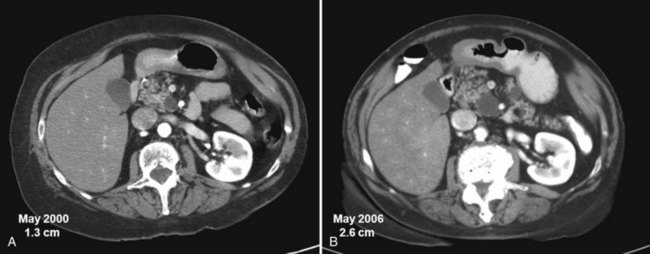
One of the challenges of managing patients with IPMN is that many patients are young and are seen with small cysts in the head of the pancreas. Our institution and others have demonstrated the exceedingly rare incidence of invasive malignancy in cysts smaller than 3 cm (Allen et al, 2006; Lee et al, 2008; Sahani et al, 2006). We have not seen invasive IPMN less than 3 cm in the setting of isolated branch duct disease without concerning radiographic features. Because of the low risk of invasive cancer in small mucinous cysts, our current approach to these lesions is selective. Resection is indicated for patients with symptoms, which are infrequently present in patients with cystic neoplasms. A second indication for resection is for cysts with concerning features, such as a solid component or increasing size (Fig. 57.6; Allen et al, 2006). If the decision is made for nonoperative management, our current surveillance protocol includes high-quality cross-sectional imaging with multidetector CT scan or magnetic resonance imaging (MRI) every 6 months for 2 years (Landa et al, 2009). After 2 years of demonstrable stability, patients can graduate to annual imaging. Indications for resection in patients undergoing surveillance include significant tumor growth or development of other features concerning for malignancy.
Solid Pseudopapillary Tumor and Other Cystic Pancreatic Neoplasms
Solid pseudopapillary tumors (SPTs) are also known as Frantz tumors or Hamoudi tumors after the pathologists who first described this unique entity (Frantz, 1959; Hamoudi et al, 1970). SPTs are rare tumors that account for up to 2.5% of resected pancreatic neoplasms. They predominate in young women and are often discovered when patients have symptoms. In a review of SPTs, Papavramidis and Papavramidis (2005) described the clinicopathologic factors of 718 patients collected from the English literature. The female/male ratio was 9.8 : 1, and the mean age was 22 years. Only 15% of the patients in the review were asymptomatic at presentation; 47% had pain, and 35% had a mass. Tumors were often large, and 34% were greater than 10 cm in diameter. The most common location for SPT was the tail (36%) and head (34%) of the pancreas, and metastases were present in approximately 20% of patients.
Cross-sectional imaging characteristically reveals encapsulated lesions with irregular areas of hypodensity secondary to necrosis or hemorrhage (Fig. 57.7). The wall often reveals calcifications. Because of the cystic degeneration exhibited by these lesions, they are often difficult to differentiate from pseudocysts or other pancreatic cystic neoplasms. What differentiates these lesions from other cystic tumors is a combination of solid and cystic components with gradual degenerative changes resulting in pseudopapillae formation (Tang et al, 2005). SPTs are distinct from the normal pancreatic parenchyma, and like other cystic neoplasms, they rarely cause ductal dilation because of the lack of invasion.
Grossly, tumors appear tan or yellow and are well circumscribed and smooth on palpation. When opened, the tumors show irregular cystic cavities with some areas of necrosis or hemorrhage (Fig. 57.8). Most SPT can be diagnosed with routine histologic evaluation by the presence of polygonal epithelial cells arranged in a discohesive pattern; the boundaries resemble a mass effect as opposed to being invasive. Immunohistochemical staining for vimentin, keratin, neuron-specific enolase, CD10, and progesterone is common (Martin et al, 2002). These lesions rarely express chromogranin or estrogen. In contrast to ductal adenocarcinoma, abnormal β-catenin expression has been shown to be present in SPTs, but KRAS, TP53, and SMAD4 appear to be unaltered (Abraham et al, 2002). Aggressive phenotypes of SPT have been demonstrated and histologically exhibit a high mitotic rate, nuclear atypia, spindling of tumor cells, and anaplastic giant tumor cells consistent with sarcomatoid carcinoma (Tang et al, 2005).
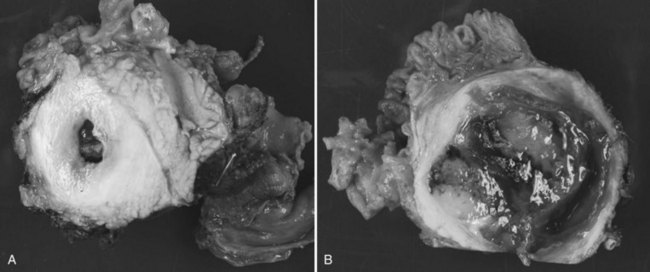
FIGURE 57.8 Gross appearance of solid pseudopapillary tumor (resected specimen from patient in Fig. 57.7).
Clinically, SPTs are indolent and are typically cured with complete surgical extirpation. Many series have demonstrated long-term survival after resection (Lam et al, 1999; Martin et al, 2002; Reddy et al, 2009). Significant vascular invasion is the most common reason for the inability to resect these lesions, and metastatic disease has been noted to be present in up to 15% of patients. Criteria for defining SPTs as “malignant” may be locally advanced disease with vascular invasion precluding resection or lymph node or hepatic metastasis. Long-term survival is often possible despite the presence of malignant factors, such as metastatic disease, and should not preclude resection in selected cases (Lam et al, 1999; Martin et al, 2002; Reddy et al, 2009).
Indeterminate Cystic Neoplasm
The spectrum of cystic lesions discussed above represents many of the incidental lesions seen on cross-sectional imaging. However, a significant number of patients present with lesions that lack diagnostic certainty. Because of the inability to determine the exact histologic diagnosis without pancreatectomy, and because of the risk for malignancy of some cyst subtypes, some authors advocate a routine resection of pancreatic cysts (Horvath & Chabot, 1999; Ooi et al, 1998; Siech et al, 1998; Spinelli et al, 2004). Advocates of this approach argue that the clinical distinction between benign and malignant lesions is unreliable, and that there is a risk for the development of cancer; therefore all medically fit patients should undergo pancreatic resection. Despite the fact that this approach allows definitive treatment of all malignant lesions, it exposes patients with benign lesions to the risk associated with pancreatectomy without known benefit.
A selective approach to resection of pancreatic cystic neoplasms has been advocated by several reports, including those from our institution (Allen et al, 2003, 2006; Fernandez-del Castillo et al, 2003). Advocates of this approach argue that with improved knowledge of specific histologic subtypes and with the advances in diagnostics, a group of patients can be identified who are at very low risk (<1%) of harboring malignancy. Most authors who have reported their experience with this approach have recommended radiologic surveillance over resection for small, incidentally discovered cysts that lack a solid component or that have other concerning features of malignancy. Although this approach avoids the risks associated with pancreatectomy in patients with benign lesions, it does not guarantee that a cancer is being mistakenly observed. Operative morbidity following pancreatectomy is consistently as high as 45%, and mortality rates range from 2% to 10% (Büchler et al, 2000; Mezhir et al, 2009; Muscari et al, 2006; Winter et al, 2006). Published data suggest that with current imaging and endoscopic techniques, a group of patients with pancreatic cysts can be identified with a malignancy rate of less than 3% (Allen et al, 2006). It is in this select group of patients that the risk of death from pancreatectomy approaches or exceeds the risk of the lesion harboring a malignancy.
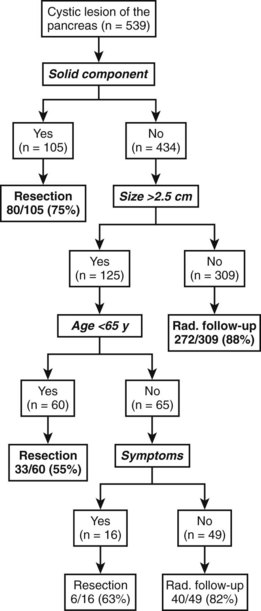
Despite the well-characterized lesions described above, many patients are seen with a cystic neoplasm that does not fit a specific diagnosis. In these patients, a nonoperative histologic diagnosis may not be made; therefore treatment recommendations for these patients are primarily based on imaging findings. The majority of patients who fit into this category have small, asymptomatic lesions, and their management is controversial. Our approach to such patients has been published in recent reports (Allen et al, 2003, 2006). Allen and colleagues (2006) reviewed 539 patients, the majority of which (369 [68%]) were followed up without operative intervention. Compared with patients treated with resection, observed patients were more likely to have small (median diameter, 2.4 cm) asymptomatic cysts without a solid component. At a median follow-up of 24 months, the average change in diameter of the observed lesions was 0.2 cm. Changes leading to resection developed in 29 patients (8% of 369 initially observed), and no patients with malignant mucinous tumors were reported in this group. Eight patients (2%) who did not undergo immediate resection developed malignancy, which is lower than the mortality rate associated with pancreatic resection, even in high-volume centers. Of note, in five of the eight patients who developed malignancy in the observed group, the cysts appeared to have represented retention cysts that developed adjacent to a radiographically undetectable malignancy. The decision-tree analysis illustrating the approach used is shown in Fig. 57.9.
Abraham SC, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002;160(4):1361-1369.
Allen PJ, Brennan MF. The management of cystic lesions of the pancreas. Adv Surg. 2007;41:211-228.
Allen PJ, et al. Cystic lesions of the pancreas: selection criteria for operative and nonoperative management in 209 patients. J Gastrointest Surg. 2003;7(8):970-977.
Allen PJ, et al. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg. 2006;244(4):572-582.
Allen PJ, et al. Pancreatic cyst fluid protein expression profiling for discriminating between serous cystadenoma and intraductal papillary mucinous neoplasm. Ann Surg. 2009;250(5):754-760.
Brugge WR, et al. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351(12):1218-1226.
Brugge WR, et al. Diagnosis of pancreatic cystic neoplasms: a report of the Cooperative Pancreatic Cyst Study. Gastroenterology. 2004;126(5):1330-1336.
Büchler MW, et al. Pancreatic fistula after pancreatic head resection. Br J Surg. 2000;87(7):883-889.
Cannon JW, et al. Diagnosis and management of pancreatic pseudocysts: what is the evidence? J Am Coll Surg. 2009;209(3):385-393.
Carpizo DR, et al. Current management of cystic neoplasms of the pancreas. Surgeon. 2008;6(5):298-307.
Compagno J, Oertel JE. Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): a clinicopathologic study of 34 cases. Am J Clin Pathol. 1978;69(3):289-298.
Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma): a clinicopathologic study of 41 cases. Am J Clin Pathol. 1978;69(6):573-580.
Crippa S, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247(4):571-579.
D’Angelica M, et al. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239(3):400-408.
Fernandez-del Castillo C, et al. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138(4):427-433.
Ferrone CR, et al. Current trends in pancreatic cystic neoplasms. Arch Surg. 2009;144(5):448-454.
Frantz V. Tumors of the pancreas. In: Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology; 1959.
Furukawa T, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447(5):794-799.
Goh BK, et al. A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: clinicopathological features of 344 patients. World J Surg. 2006;30(12):2236-2245.
Gorin AD, Sackier JM. Incidental detection of cystic neoplasms of the pancreas. Md Med J. 1997;46(2):79-82.
Grace PA, Williamson RC. Modern management of pancreatic pseudocysts. Br J Surg. 1993;80(5):573-581.
Hammel P, et al. Preoperative cyst fluid analysis is useful for the differential diagnosis of cystic lesions of the pancreas. Gastroenterology. 1995;108(4):1230-1235.
Hammel PR, et al. Detection of gastric mucins (M1 antigens) in cyst fluid for the diagnosis of cystic lesions of the pancreas. Int J Cancer. 1997;74(3):286-290.
Hamoudi AB, et al. Papillary epithelial neoplasm of pancreas in a child: report of a case with electron microscopy. Cancer. 1970;26(5):1126-1134.
Horvath KD, Chabot JA. An aggressive resectional approach to cystic neoplasms of the pancreas. Am J Surg. 1999;178(4):269-274.
Ingram M, Arregui ME. Endoscopic ultrasonography. Surg Clin North Am. 2004;84(4):1035-1059. vi
Kimura W, et al. Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995;18(3):197-206.
King JC, et al. Pancreatic serous cystadenocarcinoma: a case report and review of the literature. J Gastrointest Surg. 2009;13(10):1864-1868.
Klöppel G, Kosmahl M. Cystic lesions and neoplasms of the pancreas: the features are becoming clearer. Pancreatology. 2001;1(6):648-655.
Klöppel G, Solcia E, Longnecker D. World Health Organization International Classification of Tumors. Berlin: Springer; 1996.
Koito K, et al. Mucin-producing pancreatic tumors: comparison of MR cholangiopancreatography with endoscopic retrograde cholangiopancreatography. Radiology. 1998;208(1):231-237.
Lai EC, Lau WY. Intraductal papillary mucinous neoplasms of the pancreas. Surgeon. 2005;3(5):317-324.
Lam KY, et al. Pancreatic solid-cystic-papillary tumor: clinicopathologic features in eight patients from Hong Kong and review of the literature. World J Surg. 1999;23(10):1045-1050.
Landa J, et al. Recurrence patterns of intraductal papillary mucinous neoplasms of the pancreas on enhanced computed tomography. J Comput Assist Tomogr. 2009;33(6):838-843.
Le Borgne J, et al. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230(2):152-161.
Lee CJ, et al. Risk of malignancy in resected cystic tumors of the pancreas < or = 3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg. 2008;12(2):234-242.
Lewandrowski KB, et al. Cyst fluid analysis in the differential diagnosis of pancreatic cysts: a comparison of pseudocysts, serous cystadenomas, mucinous cystic neoplasms, and mucinous cystadenocarcinoma. Ann Surg. 1993;217(1):41-47.
Linder JD, et al. Cyst fluid analysis obtained by EUS-guided FNA in the evaluation of discrete cystic neoplasms of the pancreas: a prospective single-center experience. Gastrointest Endosc. 2006;64(5):697-702.
Martin RC, et al. Solid-pseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol. 2002;9(1):35-40.
Matsumoto T, et al. Malignant serous cystic neoplasm of the pancreas: report of a case and review of the literature. J Clin Gastroenterol. 2005;39(3):253-256.
Mezhir JJ, et al. A matched case-control study of preoperative biliary drainage in patients with pancreatic adenocarcinoma: routine drainage is not justified. J Gastrointest Surg. 2009;13(12):2163-2169.
Michael H, Gress F. Diagnosis of cystic neoplasms with endoscopic ultrasound. Gastrointest Endosc Clin N Am. 2002;12(4):719-733.
Muscari F, et al. Risk factors for mortality and intra-abdominal complications after pancreatoduodenectomy: multivariate analysis in 300 patients. Surgery. 2006;139(5):591-598.
Nara S, et al. Preoperative evaluation of invasive and noninvasive intraductal papillary-mucinous neoplasms of the pancreas: clinical, radiological, and pathological analysis of 123 cases. Pancreas. 2009;38(1):8-16.
O’Malley VP, et al. Pancreatic pseudocysts: cause, therapy, and results. Am J Surg. 1985;150(6):680-682.
Ooi LL, et al. Cystic tumours of the pancreas: a diagnostic dilemma. Aust N Z J Surg. 1998;68(12):844-846.
Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200(6):965-972.
Poultsides GA, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm–associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251(3):470-476.
Reddy S, et al. Surgical management of solid-pseudopapillary neoplasms of the pancreas (Franz or Hamoudi tumors): a large single-institutional series. J Am Coll Surg. 2009;208(5):950-957. discussion 957-959
Sahani DV, et al. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005;25(6):1471-1484.
Sahani DV, et al. Pancreatic cysts 3 cm or smaller: how aggressive should treatment be? Radiology. 2006;238(3):912-919.
Sainani NI, et al. Comparative performance of MDCT and MRI with MR cholangiopancreatography in characterizing small pancreatic cysts. AJR Am J Roentgenol. 2009;193(3):722-731.
Salvia R, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239(5):678-685. discussion 685-677
Sand JA, et al. Clinical assessment compared with cyst fluid analysis in the differential diagnosis of cystic lesions in the pancreas. Surgery. 1996;119(3):275-280.
Schnelldorfer T, et al. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143(7):639-646. discussion 646
Shin SH, et al. Validating a simple scoring system to predict malignancy and invasiveness of intraductal papillary mucinous neoplasms of the pancreas. World J Surg. 2010;34(4):776-783.
Siech M, et al. Cystic tumours of the pancreas: diagnostic accuracy, pathologic observations and surgical consequences. Langenbecks Arch Surg. 1998;383(1):56-61.
Sohn TA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239(6):788-797. discussion 797-789
Spinelli KS, et al. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239(5):651-657. discussion 657-659
Tang LH, et al. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol. 2005;29(4):512-519.
Tseng JF, et al. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005;242(3):413-419. discussion 419-421
van der Waaij LA, et al. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. 2005;62(3):383-389.
White R, et al. Fate of the remnant pancreas after resection of noninvasive intraductal papillary mucinous neoplasm. J Am Coll Surg. 2007;204(5):987-993. discussion 993-985
Winter JM, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10(9):1199-1210. discussion 1210-1191
Woo SM, et al. Branch duct intraductal papillary mucinous neoplasms in a retrospective series of 190 patients. Br J Surg. 2009;96(4):405-411.
Zamboni G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23(4):410-422.