CHAPTER 45 Cyclophotocoagulation
Introduction
Surgical procedures to lower intraocular pressure (IOP) either improve aqueous outflow or reduce production. In the former, laser trabeculoplasty, goniotomy, trabeculotomy, and filtering surgery are usually effective. However, not all eyes respond well to outflow procedures, even with the adjunctive use of anti-metabolites. Thus techniques that reduce aqueous inflow are also needed. Cyclophotocoagulation uses laser energy to lower IOP by selective destruction of the ciliary body. It can be achieved in three different ways1. The first is the transpupillary technique in which laser photocoagulation is applied directly to visible ciliary processes. As this technique is possible only in aniridic patients or when broad peripheral anterior synechiae cause anterior iris displacement, it has limited indications; unpredictable results minimize practical clinical usage. The second approach is trans-scleral, in which energy is transmitted through the sclera to the ciliary body. Lastly, in the endoscopic technique, an endolaser probe is inserted into the eye with direct photocoagulation of the visualized ciliary processes.
Trans-scleral cyclophotocoagulation
History
In 1961 Weekers et al. used light energy for cyclodestruction2. With trans-scleral applications of the xenon arc, they photocoagulated the ciliary body in rabbit and human eyes, thus lowering IOP. In 1969 Smith et al. and Vucicevic et al. reported the first clinical applications of laser energy for trans-scleral cyclophotocoagulation3,4. In 1985 Wilensky et al. and Beckman and co-workers demonstrated the utility of the Nd:YAG laser for trans-scleral cycloablation5,6. In 1987 Brancato et al. suggested the Nd:YAG laser for contact application through an optic fiber, leading to wide acceptance7. In 1990 diode laser was first introduced for trans-scleral cycloablation, and in 1992 the first clinical trials of diode laser trans-scleral cyclophotocoagulation (TCP) were reported: Hennis et al. used the non-contact technique8, and Carassa et al. and Gaasterland et al. used the contact technique9,10; they achieved good IOP reduction. Therefore, contact trans-scleral cyclophotocoagulation with diode laser (also named cyclodiode) has become the technique of choice for cyclodestruction.
Fundamental principles
Thanks to its easy application and non-invasiveness the trans-scleral approach for cyclophotocoagulation is the most widely used. A laser beam transmitted through the overlying conjunctiva and sclera ablates the ciliary body (Fig. 45.1). Laser energy can be transmitted indirectly through the air (non-contact trans-scleral cyclophotocoagulation, NCTCP), or directly through a fiberoptic placed in contact with the eyeball (contact trans-scleral cyclophotocoagulation, CTCP). Energy absorbed by melanin in the ciliary processes induces thermal coagulation and disrupts the ciliary epithelium and its vessels, as demonstrated in animal and human eyes. The ciliary body disruption is similar with both the contact and non-contact techniques. Nevertheless, the longer exposure time in CTCP results in more thermal damage to the stroma with coagulative necrosis of the ciliary non-pigmented and pigmented epithelia, as opposed to the blister formation seen with NCTCP11,12. There is no clear evidence of damage to the sclera, although areas of compression and hypercellularity have been found histologically. The effect on tissue is similar whether using the Nd:YAG or the diode laser, although the latter causes more damage to the ciliary pigmented structures. The long exposure time combined with the shorter wavelength of the diode laser results in deep coagulation necrosis of the pigmented epithelium, wide disorganization of the collagen in the stroma, and intravascular coagulation in the ciliary vessels. Therefore the diode laser needs less energy to produce ciliary body thermal coagulation (1.2 J versus 4 J for the Nd:YAG laser)13,14.
Preoperative assessment
All preoperative medications are continued. The lids are held open manually or with a wire speculum. In the non-contact technique a specific contact lens can be used to facilitate the procedure15. After administering anesthesia, the patient is placed in an upright position at the laser slit lamp for the non-contact operation and in supine position on the operating table for the contact technique.
Operation technique
Trans-scleral application
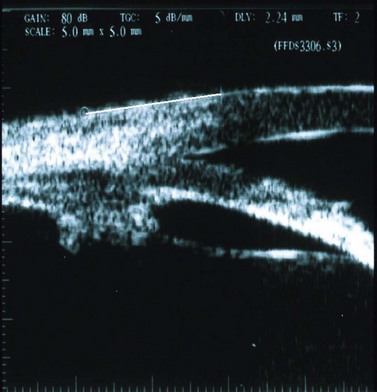
The Nd:YAG laser (1064 nm) and the diode laser (810 nm) are both suitable for trans-scleral applications. Two trans-scleral approaches have been used in cycloablation: non-contact and contact. The diode laser has several advantages, including better absorption by melanin, good portability, and lower cost. Either continuous-wave or pulsed laser systems are available. Continuous-wave laser allows long and sustained energy delivery while a pulsed laser system transmits light energy at short pre-set time intervals. Trans-scleral photocoagulation is a ‘blind’ procedure because the ablative energy is directed toward an invisible target whose position can only be estimated from data obtained experimentally in human autopsy eyes. Exact beam focusing for NCTCP was thus suggested as being 1–1.5 mm posterior to the limbus16, and the optimal location for the center of the probe in CTCP was 1.5–2 mm posterior to the corneo-limbal junction17. Localization of the ciliary body by transillumination should be performed in every eye before surgery. Ultrasound biomicroscopy can help to locate the exact ciliary body position (Fig. 45.2).
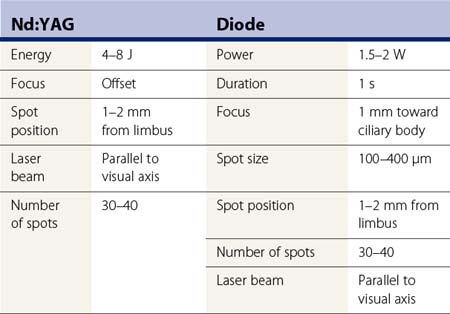
In NCTCP, laser energy is transmitted through the air from a slit-lamp delivery system. The procedure can be carried out using a pulsed Nd:YAG laser such as the Microruptor II, a continuous-wave model such as the Microruptor III (H.S. Meridian, Inc., Mason, OH), or a diode laser such as the DC3000 (Nidek, Inc., Palo Alto, CA), the Microlase (Keeler Instruments, Broomall, PA), or the Oculight SLX (Iris Medical, Mountain View, CA) (Fig. 45.3). The laser beam needs to be focused inside the ciliary body. This is done with the Nd:YAG laser by focusing the HeNe beam on the conjunctiva overlying the ciliary body and selecting the maximum offset18, thus providing a 3.6 mm posterior separation between the HeNe and the therapeutic beam and with the diode laser by defocusing the beam 1 mm toward the ciliary body. The Nd:YAG laser should be set at 4–8 J energy, while the diode laser should be set at 1500–2000 mW power output, 1 second time duration (thus producing 1.5–2 J spots), and 100–400 µm spot size (Table 45.1). The eye is kept in primary position because no significant differences were found between applying laser energy parallel to the visual axis and perpendicular to the sclera18. The beam is focused on the conjunctiva 1–2 mm behind the limbus. The distance is measured with a caliper and a marking pencil, or the slit beam of the biomicroscope. With the latter, the laser spots are placed in the middle of a 3 mm long slit beam, projected perpendicular to the limbus. Thirty to forty applications, evenly spaced over 360°, are placed in a single session, usually sparing the 3 o’clock and 9 o’clock positions to avoid damage to the long posterior ciliary nerves.
The contact technique has several advantages over the non-contact technique, which explains its wider clinical use. Reduced light backscattering and greater scleral transmission mean significantly less energy is needed. Arbitrary beam defocusing is avoided. The longer exposure time results in a predominantly coagulative necrosis of the ciliary processes as opposed to the blister formation found NCTCP. The use of specifically designed, hand-held probes improves precision by allowing easy spot location and by avoiding eye movement. The operation is done in the supine position so patients under general anesthesia can be easily treated. CTCP is carried out using either an Nd:YAG laser or a diode laser. The Nd:YAG lasers are the Microruptor III (H.S. Meridian, Inc., Mason, OH), and the Emerald (Crystal Focus, Viewpoint, Miami, FL; Fig. 45.4). The diode lasers are the Oculight SLX or the IQ810 (Iridex, Mountain View, CA; Fig. 45.5), and the DC-3000 (Nidek Inc., Palo Alto, CA). All systems are coupled with an optic fiber ending either in a sapphire probe (SLT, Microruptor III, Emerald), or in a specifically designed focusing bare quartz tip (Iridex G-Probe). These tips ensure less energy dispersion and easy positioning of their centers 1 to 1.5 mm posterior to the limbus, where the treatment is needed. The Oculight SLX and the IQ810 with their specifically designed G-Probe are the more widely used systems for CTCP.
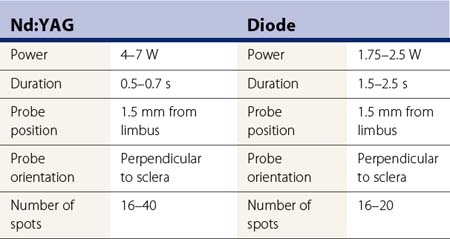
The Nd:YAG laser is set at 4–7 W of power and 0.5–0.7 seconds duration while the diode laser is set at 1.75–2.6 W and 1.5–2.5 seconds (Table 45.2). The wide range in settings is partly related to the different lasers and, mostly, to the different sizes of the optic fiber used. The Oculight SLX and IQ810 are set at 1.8–2.0 W and 2 seconds duration. Considering the delicacy of the laser system and of the optic fibers and sapphire probes, a fiber transmission check is advisable in order to verify the total energy being delivered by the system19. The G-probe is intended to be used for just one treatment, even though different studies showed its safety for multiple use. The probe is placed in contact with the conjunctiva, positioning its center 1.2–1.5 mm posterior to the surgical limbus (Fig. 45.6). Particular care should be taken to keep the probe perpendicular to the scleral surface: an orientation as little as 15° off the perpendicular reduces the photocoagulative effect. A firm indentation is always needed in order to increase the energy transmission through the sclera and to avoid eye movement. The G-probe is specifically designed to facilitate its positioning. By placing the heel of its footplate adjacent to the limbus, the sapphire tip will be located 1.2 mm posterior to the limbus over the ciliary body and will have the correct angle on the scleral surface. Moreover, the laser tip, which protrudes 0.7 mm, is providing the required indentation (Fig. 45.7); 16–40 spots (Nd:YAG laser) or 16–20 spots (diode laser) are then applied over 360°, sparing the 3 o’clock and 9 o’clock positions in order to avoid damage to the long posterior ciliary nerves. In patients considered at high risk of hypotony, the treatment may be reduced to 180°. The energy must be adjusted in order to avoid audible ‘pops’ caused by tissue disruption secondary to overtreatment. The Oculight SLX and the IQ810 are initially set at 2 seconds and 1750 mW. The power is then increased in 250 mW increments to a maximum of 2500 mW until a pop is heard, then the power is decreased 250 mW.
Intraoperative complications
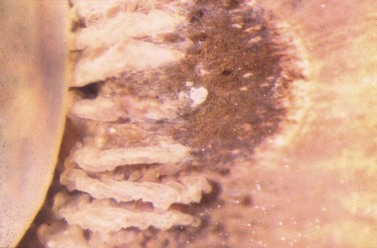
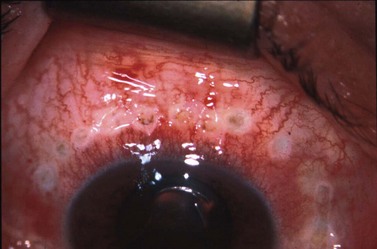
Both non-contact and contact techniques are safe procedures. However, a wrong spot position can lead to damage to the iris or lens. Overtreatment with multiple audible ‘pops’ can end in vitreous hemorrhage due to bleeding from the disrupted ciliary body. During the contact technique, conjunctival burns can occur in up to 33% of cases (Fig. 45.8). Frequent irrigation of the eye with BSS is suggested in order to avoid this complication. Perforation of the sclera has been reported in few cases in eyes with a thin sclera, areas of scleromalacia, or by the use of damaged probes with carbonized tip.
Postoperative complications
The most common complications affecting all patients after cyclophotocoagulation are mild pain lasting less than 1 day (although severe pain has been reported in up to 13.5% of cases), mild uveitis, easily managed by topical steroids within the first postoperative weeks, and transient conjunctival burns and edema. An IOP increase higher than baseline occurs in up to 30% of eyes within a few hours of treatment. Visual reduction of two or more lines occurs in up to 47% of patients, with those with neovascular glaucomas most commonly affected. Complete loss of vision during the follow-up period occurred in up to 18% of eyes20. This is the most important complication in all cyclodestructive procedures, including CTCP. The cause is still unclear, although hypotony, cystoid macular edema, and neovascular glaucoma appear to be the major risk factors. Visual loss might be less after CTCP than after NCTCP21. Hypotony has been reported in up to 26% with the non-contact technique and up to 17.6% after cyclodiode, mostly in neovascular glaucoma, and as late as 36 months postoperatively. The disaster of phthisis bulbi was found in 0.5–2% of the eyes after the contact technique and in 8.6–10.7% of the eyes after the non-contact approach22. Rare complications such as vitreous hemorrhage, suprachoroidal serous effusion, cystoid macular edema, focal scleral thinning, necrotizing scleritis, and malignant glaucoma have also been reported. One very worrying complication is sympathetic ophthalmia, which has been reported in five cases treated with Nd:YAG-NCTCP and in four cases after Nd:YAG-CTCP23–28. Most of these eyes had previous multiple penetrating surgeries and all were treated with multiple high doses of energy. The relationship between cyclophotocoagulation and sympathetic ophthalmia remains uncertain. Patients must be informed of it as a possibility, both from their underlying eye disease, as well as from the treatment itself.
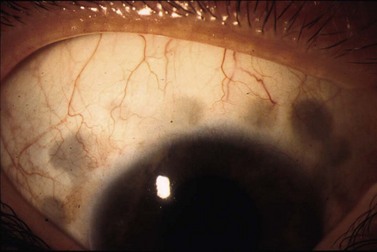
After contact procedures, dark scleral spots in the previously treated area have been described (Fig. 45.9)29. Ultrasound biomicroscopy excluded the presence of tissue thinning, suggesting a reactive focal hyperpigmentation.
Results of surgery
NCTCP done with the Nd:YAG laser significantly reduced IOP in refractory glaucomatous eyes by an average of 44–68%. IOP below 22 mmHg is obtained in 46–87% of the cases, with 21–48% requiring multiple treatments30,31. In the long-term follow-up of 115 patients the success rate (IOP < 22 mmHg, with or without medications, absence of phthisis bulbi, no further procedures) was 65% at 1 year, 49.8% at 3 years, and 34.8% at 6 years32. Diode-NCTCP is similarly efficient for glaucoma treatment, reducing IOP below 21 mmHg in 56–70% of the eyes33,34.
Several studies aimed to define the relationship between success and demographic or clinical characteristics. In one study, Caucasian patients had better IOP control than black, but age, preoperative IOP level, and gender were not related to outcome35. Good IOP control was obtained in eyes with penetrating keratoplasty (with a risk of graft failure ranging from 11% to 57%)36,37 and in inflammatory glaucomas38.
Contact TCP with the Nd:YAG and the diode laser are also effective at reducing IOP in refractory glaucomatous eyes. With the Nd:YAG laser, IOP below 22 mmHg was achieved in 41–62% of the eyes, with IOP reduction ranging from 24% to 48%, but with 11–57% of eyes requiring multiple treatments. Schuman et al. presented the largest series on 140 eyes followed up to 36 months, with a success rate (IOP < 22 mmHg) of 65%, with 27% requiring multiple procedures, and a mean IOP of 46.8%20. In a 10-year report, Lin et al. showed an IOP < 21 mmHg in 48.5% of the eyes after one single Nd:YAG CTCP treatment39.
Diode laser CTCP was successful in all clinical reports with IOP below 20–22 mmHg in 50–84.9% of the cases, with 28–70% requiring retreatments. Bloom and co-workers presented the largest series of cases treated with diode laser CTCP: on 210 eyes followed for a mean of 10 months (range 3–30 months), 66% achieved a final IOP below 22 mmHg with 49% requiring multiple treatments (mean 1.75, range 2–5 treatments). Mean final IOP was 20.1 mmHg, and total medical therapy was reduced from 2.3 to 1.7 treatments40. Two studies were aimed at comparing Nd:YAG CTCP with diode CTCP. In one randomized controlled trial on 95 eyes followed for a mean of 10.4 months, the same results were achieved with both treatments41, although diode laser CTCP was shown to be more successful in a retrospective analysis on 39 eyes with 3-year follow-up42. Another prospective study comparing CTCP with the Ahmed implant in neovascular glaucomas showed similar results between the two groups with success rates at 2 years of 61.2% and 59.3% respectively43.
Endoscopic (intraocular) cyclophotocoagulation
History
Described by Shields et al. in 1985, the endoscopic technique was first used clinically in 1992 when Uram reported promising results after intraocular cyclophotocoagulation with a diode laser attached to an endoscope with a television monitor44.
Fundamental principles
In the intraocular technique, a diode laser attached to an endoscope with a television is inserted into the eyeball and is used to photocoagulate the ciliary body epithelium45. This approach, compared with the trans-scleral cyclophotocoagulation, has the advantage of the direct observation of treatment on the target tissue. It is, however, an invasive procedure, which requires extensive training and practice, and, in phakic eyes, has the risk of damaging the lens.
Operation technique
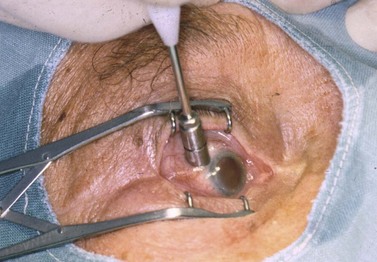
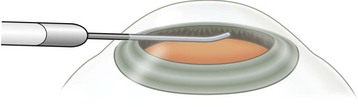
In the limbal approach, after pupil dilation, a paracentesis is made to fill the anterior chamber with viscoelastic in order to balloon the iris away from the lens or posterior chamber implant. The use of iris hooks, as a safer alternative to viscoelastic, was recently proposed46. A 2.2 mm keratome is then used to enter the anterior chamber at the limbus, and the 18-G or 20-G probe is then inserted through the incision and positioned in the posterior chamber (Fig. 45.10). In order to maintain orientation, the handpiece should be rotated so that images on the video monitor are upright. The laser is set in continuous-wave with 300 mW power to produce a visible whitening and shrinkage of the ciliary processes without tissue explosion. Approximately 180° span of ciliary processes are photocoagulated. If necessary, a second limbal incision can be made in order increase the angle of treatment. The two site approach may result in lower IOPs when compared with a one site technique47. It is important to remove the sodium hyaluronate from the eye after the procedure to prevent postoperative IOP spikes from this.
Results of surgery
Endoscopic cyclophotocoagulation is effective in reducing IOP in refractory glaucomatous eyes with a success rate up to 82.2%48. The largest report, on 68 eyes, showed a mean IOP decrease of 34% at 1 year. An IOP below 22 mmHg was found in 94% and 82% of the eyes at 1 and 2 years respectively49. The procedure was shown moderately effective in the pediatric population with a success rate of 43% at 2 years50. One prospective study showed comparable results between endocyclophotocoagulation and the Ahmed drainage implant, with an IOP < 21 mmHg obtained in 73.5% and 70.6% of the cases respectively51.
1 Pastor SA, Singh K, Lee DA, et al. Cyclophotocoagulation. A report by the American Academy of Ophthalmology. Ophthalmology. 2001;108:2130-2138.
2 Weekers R, Lavergne G, Watillon M, et al. Effects of photocoagulation of ciliary body upon ocular tension. Am J Ophthalmol. 1961;52:156.
3 Smith RS, Stein MN. Ocular hazards of transscleral laser radiation: II intraocular injury produced by ruby and neodymium lasers. Am J Ophthalmol. 1969;67:100.
4 Vucicevic ZM, Tsou KZ, Nazarian IH, et al. A cytochemical approach to the laser coagulation of the ciliary body. Bibl Ophthalmol. 1969;79:467.
5 Wilensky JT, Welch D, Mirolovich M. Transscleral cyclocoagulation using a neodymium:YAG laser. Ophthalmic Surg. 1985;16:95.
6 Beckman H. Transscleral laser cyclocoagulation. Trans New Orleans Acad Ophthalmol. 1985;33:158.
7 Brancato R, Leoni G, Trabucchi E, et al. Transscleral contact cyclophotocoagulation with CW Nd:YAG laser: experimental study on rabbit eyes. Int J Tissue React. 1987;6:493.
8 Hennis HL, Steward WC. Semiconductor diode laser transscleral cyclophotocoagulation in patients with glaucoma. Am J Ophthalmol. 1992;113:81.
9 Carassa RG, Trabucchi G, Bettin P, et al. Contact transscleral cyclophotocoagulation (CTCP) with diode laser: a pilot clinical study. Invest Ophthalmol Vis Sci. 1992;33(suppl):1019.
10 Gaasterland DE, Abrams DA, Belcher CD, et al. A multicentric study of contact diode laser transscleral cyclophotocoagulation in glaucoma patients. Invest Ophthalmol Vis Sci. 1992;33(suppl):1019.
11 Schubert HD, Federman JL. The role of inflammation in CW Nd:YAG contact trans-scleral photocoagulation and cryopexy. Invest Ophthalmol Vis Sci. 1989;30:543.
12 Latina MA, Patel S, de Kater AW, et al. Trans-scleral cyclophotocoagulation using a contact laser probe: a histologic and clinical study in rabbits. Laser Surg Med. 1989;9:437.
13 Brancato R, Leoni G, Trabucchi G, et al. Histopathology of continuous wave neodymium:yttrium aluminum garnet and diode laser contact trans-scleral lesions in rabbit ciliary body. A comparative study. Invest Ophthalmol Vis Sci. 1991;32:1586.
14 Assia EI, Hennis HL, Stewart WC. A comparison of neodymium:yttrium aluminum garnet and diode laser trans-scleral cyclophotocoagulation and cyclocryotherapy. Invest Ophthalmol Vis Sci. 1991;32:2774.
15 Shields MB, Blasini M, Simmons R. A contact lens for trans-scleral Nd:YAG cyclophotocoagulation. Am J Ophthalmol. 1989;108:457.
16 Hennis HL, Assia E, Steward W, et al. Trans-scleral cyclophotocoagulation using semiconductor diode laser in cadaver eyes. Ophthalmic Surg. 1991;22:274.
17 Brancato R, Leoni G, Trabucchi G, et al. Probe placement and energy levels in CW Nd:YAG contact trans-scleral cyclophotocoagulation. Arch Ophthalmol. 1990;108:679.
18 Hardten DR, Brown JD. Trans-scleral neodymium:YAG cyclophotocoagulation: comparison of 180-degree and 360-degree initial treatments. Ophthalmic Surg. 1993;24:181.
19 Zeyen T, Vandenberghe K. Miscalibration and severe complications after diode laser cyclophotocoagulation: two case reports. Bull Soc Belge Ophtalmol. 2004;292:27-30.
20 Schuman JS, Bellows AR, Shingleton BJ, et al. Contact trans-scleral Nd:YAG laser cyclophotocoagulation. Midterm results. Ophthalmology. 1992;99:1089.
21 Schuman JS, Puliafito CA, Allingham RR, et al. Contact trans-scleral continuous wave neodymium:YAG laser cyclophotocoagulation. Ophthalmology. 1990;97:571.
22 Trope GE, Ma S. Mid-term effects of Nd:YAG transcleral cyclophotocoagulation in glaucoma. Ophthalmology. 1990;97:73.
23 Brown SVL, Higginbotham E, Tessler H. Sympathetic ophthalmia following Nd:YAG cyclotherapy. Ophthalmic Surg. 1990;21:736.
24 Lam S, Tessler HH, Lam BL, et al. High incidence of sympathetic ophthalmia after contact and noncontact neodymium:YAG cyclotherapy. Ophthalmology. 1992;99:1818.
25 Pastor SA, Iwach A, Nozik RA, et al. Presumed sympathetic ophthalmia following Nd:YAG trans-scleral cyclophotocoagulation. J Glaucoma. 1993;2:30.
26 Edward DP, Brown SVL, Higginbotham E, et al. Sympathetic ophthalmia following neodymium:YAG cyclotherapy. Ophthalmic Surg. 1989;20:544.
27 Lam S, Tessler HH, Lam BL, et al. High incidence of sympathetic ophthalmia after contact and noncontact neodymium:YAG cyclotherapy. Ophthalmology. 1992;99:1818.
28 Kumar N, Chang A, Beaumont P. Sympathetic ophthalmia following ciliary body laser photocoagulation for rubeotic glaucoma. Clin Exp Ophthalmol. 2004;32:196-198.
29 Verdi M, Carassa RG, Bettin P, et al. Does diode laser contact trans-scleral cyclophotocoagulation produce sclera thinning? An ultrasound biomicroscopical in vivo study on human eyes. Invest Ophthalmol Vis Sci. 1995;36:S565.
30 Noureddin BN, Wilson-Holt N, Lavin M, et al. Advanced uncontrolled glaucoma. Nd:YAG cyclophotocoagulation or tube surgery. Ophthalmology. 1992;99:430.
31 Simmons RB, Shields MB, Blasini M, et al. Trans-scleral Nd:YAG laser cyclophotocoagulation with a contact lens. Am J Ophthalmol. 1991;112:671.
32 Delgado MF, Dickens CJ, Iwach AG, et al. Long-term results of noncontact neodymium:yttrium-aluminium-garnet cyclophotocoagulation in neovascular glaucoma. Ophthalmology. 2003;110:895-899.
33 Hennis HL, Steward WC. Semiconductor diode laser trans-scleral cyclophotocoagulation in patients with glaucoma. Am J Ophthalmol. 1992;113:81.
34 Hamard P, Kopel J, Valtot F, et al. Traitement des glaucomes refractaires par cyclophotocoagulation au laser semiconducteur a diode. J Fr Ophthalmol. 1995;18:447.
35 Simmons RB, Shields MB, Blasini M, et al. Trans-scleral Nd:YAG laser cyclophotocoagulation with a contact lens. Am J Ophthalmol. 1991;112:671.
36 Thofts RA, Gordon JM, Dohlman CH. Glaucoma following keratoplasty. Trans Am Acad Ophthalmol Otolaryngol. 1974;78:352.
37 Threlkeld AB, Shields MB. Non-contact trans-scleral Nd:YAG cyclophotocoagulation for glaucoma after penetrating keratoplasty. Am J Ophthalmol. 1995;120:569.
38 Zaidman GW, Wandel T. Trans-scleral YAG laser photocoagulation for uncontrolled glaucoma in corneal patients. Cornea. 1988;7:112.
39 Lin P, Wollstein G, Glavas IP, et al. Contact transcleral neodymium:yttrium-aluminium-garnet laser cyclophotocoagulation. Long term outcome. Ophthalmology. 2004;111:2137-2143.
40 Bloom PA, Tsai JC, Sharma K, et al. ‘Cyclodiode’. Trans-scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology. 1997;104:1508.
41 Youn J, Cox TA, Herndon LW, et al. A clinical comparison of trans-scleral cyclophotocoagulation with neodymium:YAG and semiconductor diode lasers. Am J Ophthalmol. 1998;126:640.
42 Oguri A, Takahashi E, Tomita G, et al. Trans-scleral cyclophotocoagulation with the diode laser for neovascular glaucoma. Ophthalmic Surg Lasers. 1998;29:722.
43 Yildirim N, Yalvac IS, Sahin A, et al. A comparative study between diode laser cyclophotocoagulation and Ahmed glaucoma valve implant in neovascular glaucoma: a long-term follow-up. J Glaucoma. 2009;18:192-196.
44 Shields MB, Chandler DB, Hickingbotham C, et al. Intraocular cyclophotocoagulation. Histopathologic evaluation in primates. Arch Ophthalmol. 1985;103:1731.
45 Lin SC. Endoscopic and transcleral cyclophotocoagulation for the treatment of refractory glaucoma. J Glaucoma. 2008;17:238-247.
46 Kahook MY, Schuman JS, Noecker RJ. Endoscopic cyclophotocoagulation using iris hooks versus viscoelastic devices. Ophthalmic Surg Lasers Imaging. 2007;38:170-172.
47 Kahook MY, Lathrop KL, Noecker RJ. One-site versus two-site endoscopic cyclophotocoagulation. J Glaucoma. 2007;6:527-530.
48 Murthy GJ, Murthy PR, Murthy KR, et al. A study of the efficacy of endoscopic cyclophotocoagulation for the treatment of refractory glaucomas. Indian J Ophthalmol. 2009;57:127-132.
49 Chen J, Cohn RA, Lin SC, et al. Endoscopic photocoagulation of the ciliary body for treatment of refractory glaucomas. Am J Ophthalmol. 1997;124:787-796.
50 Neely DE, Plager DA. Endocyclophotocoagulation for management of difficult pediatric glaucomas. J AAPOS. 2001;4:221-229.
51 Lima FE, Magacho L, Carvalho DM, et al. A prospective, comparative study between endoscopic cyclophotocoagulation and the Ahmed drainage implant in refractory glaucoma. J Glaucoma. 2004;14:233-237.