Chapter 100 CyberKnife Radiosurgery for Spinal Neoplasms
The successes of cranial stereotactic radiosurgery (SRS) inspired the development of spinal SRS.1–4 In little more than a decade, spinal SRS has revolutionized the treatment of spinal tumors and vascular malformations. The introduction of CyberKnife™ has allowed the delivery of very large doses of radiation to small lesions while sparing adjacent normal structures. CyberKnife™ outcomes are comparable or superior to those obtained with conventional radiotherapy, frame-based stereotactic systems, or conventional surgery.1–13 Since 1994, the CyberKnife™ has been used to treat over 10,000 spinal lesions at more than 200 sites worldwide.
Technology Overview
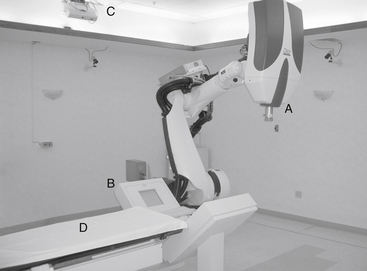
The CyberKnife system consists of a lightweight, 6-megavolt (MV) linear accelerator (LINAC) mounted on an industrial robot, a remotely repositionable treatment couch, orthogonally placed digital x-ray cameras, a treatment-delivery computer, and treatment planning stations (Fig. 100-1). During treatment, numerous images are obtained to optimally locate the target before the delivery of 100 to 150 individual treatment beams.14 The CyberKnife can deliver individual beams to any part of a tumor from nearly any angle and provides highly conformal dosing to complex three-dimensional (3D) targets.15
Treatment Details
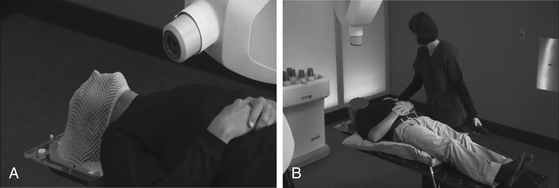
Spinal SRS is image guided and completely frameless. Individually molded masks or cradles are fashioned before the planning scans are completed. The patient rests in the mask or cradle during computed tomography (CT) scanning, magnetic resonance imaging (MRI), 3D angiography or positron emission tomography (PET) imaging, and again during treatment. The devices are comfortable, limit movement, and expedite positioning. At Stanford, we use an Aquaplast mask (WFR Corp., Wyckoff, NJ) for upper cervical lesions (Fig. 100-2A). Lower cervical, thoracic, lumbar, and sacral lesions are treated using an AlphaCradle (Smithers Medical Products, Akron, OH) (Fig. 100-2B). Most patients are treated supine but prone and lateral decubitus positioning is possible.
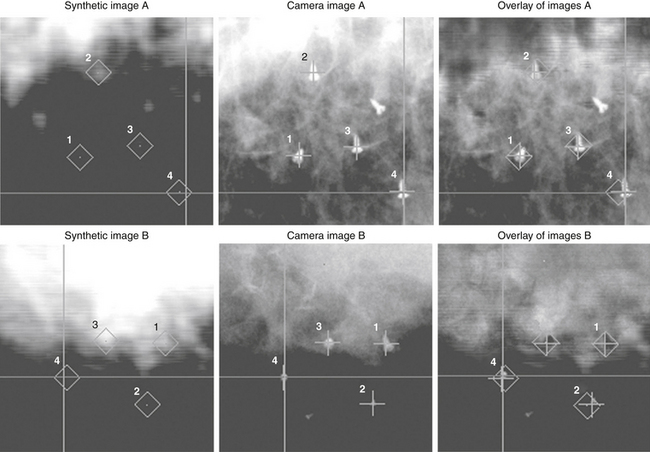
Bony landmarks are used to target spinal lesions and those in adjacent structures. Spinal fusion hardware does not interfere with treatment. The accuracy of CyberKnife approaches ±0.5 mm.7,16 For lesions not associated with bony landmarks, three or more gold seeds or titanium screws are implanted (Fig. 100-3).13,15
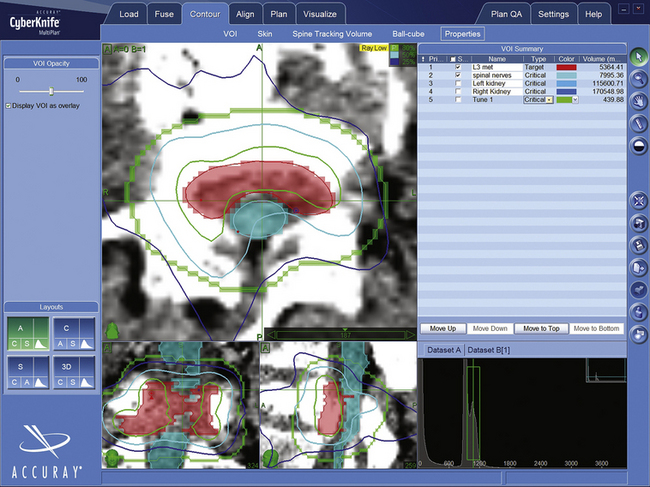
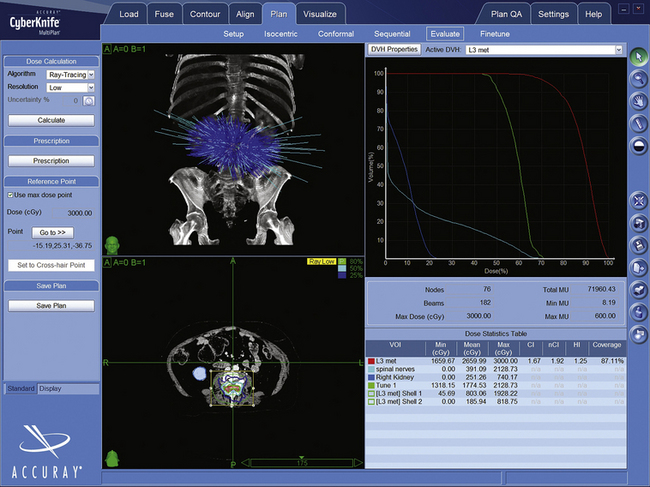
CyberKnife treatment plans use Accuray’s MultiPlan software. BrainLab, Varian, and Elekta systems employ similar programs. After uploading the CT and other images to the planning station, the surgeon and radiotherapist “contour” the target and radiation-sensitive structures by tracing their outlines (Fig. 100-4). The dose and number of sessions, as well as dose limits for adjacent structures, are prescribed by the physicians (Fig. 100-5). Physicists then use the planning system, the contour data, and the dose prescriptions to create a 3D representation of the lesion geometry and define sets of treatment beams. An ideal treatment includes evenly distributed beams that target the dose uniformly while limiting exposure to sensitive structures (Fig. 100-6). The neurosurgeon and/or physicist will iteratively perfect the plan by adding or removing constraints, or re-positioning individual beams. A multidisciplinary team reviews and accepts each treatment plan before delivery.
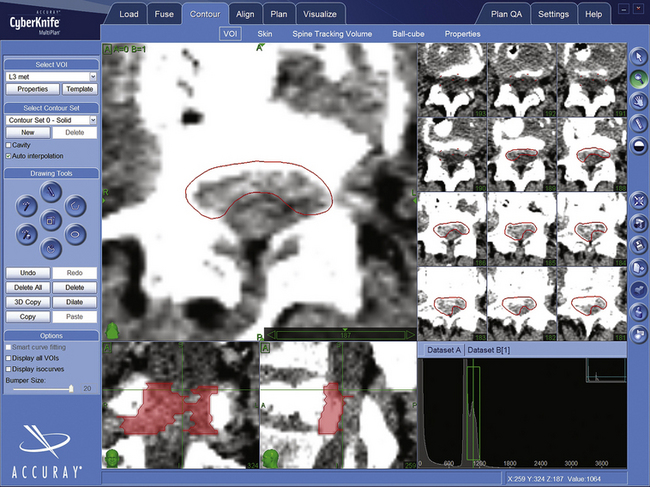
FIGURE 100-4 Contour of L3 metastasis in axial, saggital, and coronal projections. The epidural metastasis is in red.
Indications
It cannot be emphasized enough that the indications for spinal SRS continue to evolve quickly; spinal SRS is a new subdiscipline within neurosurgery and only now are standardized procedures for its application being developed. At our institution, we most frequently treat metastases, small benign tumors, postoperative residuals, lesions that recur following conventional surgery or radiation, vascular malformations, inoperable tumors, and lesions in those who decline surgery (Tables 100-1 and 100-2).3,7,13 Spinal lesions appropriate for CyberKnife™ radiosurgery should be reasonably well circumscribed, clearly visible on CT or MRI, and smaller than approximately 5 cm in diameter. We do not insist on obtaining a biopsy in advance of treatment if the diagnosis is clear from preradiosurgical imaging studies.
Table 100-1 Indications and Contraindications for Stereotactic Spinal Radiosurgery
| Indications | Contraindications |
|---|---|
| Progressive but minimal neurologic deficit Postresection or post-RT local irradiation (boost) Disease progression after surgery and/or irradiation Inoperable lesions or high-risk lesion locations Medical comorbidities that preclude surgery Lesions in patients who decline surgery |
Spinal instability (adjunctive treatment only) Neurologic deficit caused by bony compression Severe neurologic deficit due to cord compression Adjacent cord previously radiated to maximum dose Very rare lesions not responsive to ionizing radiation |
Table 100-2 Treated/Treatable Lesions with CyberKnife Radiosurgery
| Tumors |
| Benign |
| Neurofibroma, schwannoma, meningioma, hemangioblastoma, chordoma, paraganglioma, ependymoma, epidermoid |
| Malignant/Metastatic |
| Breast, renal, non–small-cell lung, colon, gastric and prostate metastases; squamous cell (laryngeal, esophageal, and lung) tumors; osteosarcoma; carcinoid; multiple myeloma; clear cell carcinoma; adenoid cystic carcinoma; malignant nerve sheath tumor; endometrial carcinoma; malignant neuroendocrine tumor |
| Vascular Malformations |
| Arteriovenous malformation (types 2 and 3) |
Extradural Metastases
The spine is the most common site for bony metastases, accounting for nearly 40% of osseous tumor spread.17 Forty percent of cancer patients will develop at least 1 spinal metastasis.18 Historically, spinal metastases have been managed with chemotherapy, radiopharmaceuticals, surgery, and external beam irradiation.17,19 Conventional irradiation of spinal metastatic tumors is useful for palliation but its effectiveness is limited by spinal cord tolerance.20 Moreover, relapses are common,21–24 and retreatment with RT is generally impossible.18 SRS enables much larger biologically effective doses to be delivered by utilizing a more highly conformal plan that protects the cord. Multiple courses of spinal SRS can control multiple asynchronous metastases, and SRS may be used to sterilize a vertebral body before vertebroplasty25 or following a debulking procedure. The presence of spinal fusion hardware is not a contraindication.26 SRS is ideal for those with limited life expectancies or those who need other treatment. Most SRS treatments are completed in a single 1-hour session.
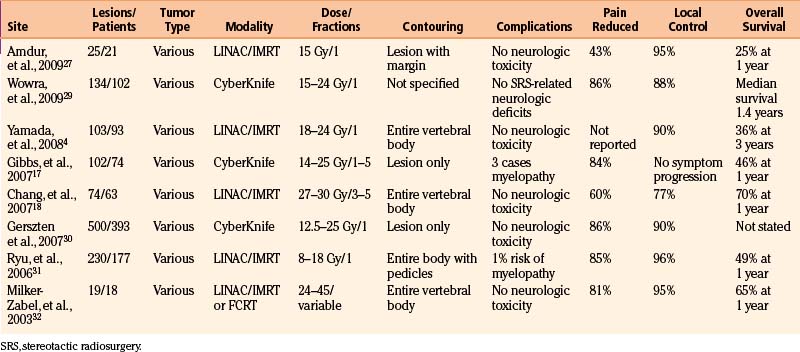
Treatment protocols in the published literature vary greatly and there is significant debate regarding the most appropriate treatment margins.27 For purely bony lesions, Amdur et al.27 recommend treating visible tumor plus a 1-cm margin in bone or a 2-mm volume outside the external cortex. For lesions within the canal, the margins are not extended beyond the visible tumor. Many groups irradiate the entire affected vertebral body including the pedicles. Chang et al.18 recommend treating the pedicles for possible tumor extension, pointing out that 18% of recurrences occurred in the pedicles. At Stanford we typically treat the tumor volume as seen on CT or MRI.17 There are no studies that show a clear benefit of one approach over the other, but those who treat smaller volumes argue that the cord dose is decreased and that recurrences can be retreated. Dose recommendations are variable with single-session prescriptions ranging from 8 to 24 Gy in the published literature.27 At Stanford we have used from 16 to 25 Gy in up to five fractions, but usually opt to treat in the fewest possible sessions as dictated by the length and dose of irradiated spinal cord.17
Overall the efficacy of radiosurgery for spinal metastases appears roughly comparable to that for brain metastases.2,3,17 Tumor growth is arrested by radiosurgery in up to 100% of cases and results are independent of histology (Table 100-3).4,28 Pain relief ranged from 43% to 96% and unlike standard RT, is generally apparent within days of SRS.27
Intradural Metastases
Intramedullary spinal cord metastases, which are rarely seen clinically, are found in 0.9% to 2.1% of autopsies in cancer patients.33 Such lesions comprise 8.5% of central nervous system metastases34 and their frequency will likely increase with longer patient survival. Wowra et al.29 review their results and the literature. They report that 96% of spinal metastases were well controlled with spinal SRS. The risk of myelopathy was less than 1%.
Primary Intramedullary Lesions
At Stanford, we have treated 92 hemangioblastomas in 31 patients. Sixteen were spinal intramedullary tumors. Patients were treated with a median radiosurgical dose of 23 Gy, and after a median 34 months of follow-up, 15 of the 16 spinal hemangioblastomas in this series either remained stable or decreased in size. Among all hemangioblastomas, those causing edema and those associated with cysts did less well. None of the patients developed radiation myelopathy.35
Some spinal ependymomas prove difficult to resect for reasons of anatomy or associated medical comorbidities. Although not common, tumor recurrence also occurs. There is very little information available regarding SRS. Ependymomas, which may be controlled with conventional radiation therapy, have responded favorably to spinal SRS in the few cases that have been published.3,36,37 The Stanford experience with CyberKnife radiosurgery has been quite favorable with good local control and no complications (unpublished data). We know even less about the treatment of spinal astrocytomas. For the occasional well-demarcated, biopsy-proven, newly diagnosed or recurrent spinal cord astrocytoma, spinal SRS may be a theoretical alternative if surgery is not possible.
Intradural, Extramedullary Lesions
Meningiomas, schwannomas, neurofibromas, and hemangioblastomas are the benign lesions most frequently considered for treatment with spinal SRS. However, microsurgical resection remains the most appropriate intervention for most patients. Surgical resection is generally curative and in the process, the surgeon both establishes a tissue diagnosis and immediately decompresses the spinal cord.38 Nevertheless, SRS is appropriate for benign lesions that are inaccessible, when lesions are numerous (as in neurofibromatosis or von Hippel-Lindau disease), in patients with significant medical comorbidities, or for patients who decline open surgery.39–41 Moreover, spinal radiosurgery also has the virtue of posing little risk to the parent motor or sensory nerves in cases of nerve sheath tumors.
Short-term control rates for intradural, extramedullary spinal tumors appear comparable to those of similar intracranial lesions. At Stanford we have treated 110 patients with 117 lesions (unpublished data). Greater numbers and longer follow-up periods confirm earlier published observations.42 Following SRS, 56% of schwannomas and meningiomas stabilized and 44% regressed radiographically. Neurofibromas did less well with 11% enlarging radiographically, over 50% causing increased pain, and 80% showing a worsening in at least one examination finding. Even including neurofibromas, most myelopathies and radiculopathies improved with SRS, whereas bowel and bladder dysfunction did not. Two of our patients eventually required surgery for tumor enlargement and three required surgery for persistent or progressing symptoms. Only one patient developed radiation myelopathy. Other centers have reported similar results.39–41
Vascular Malformations
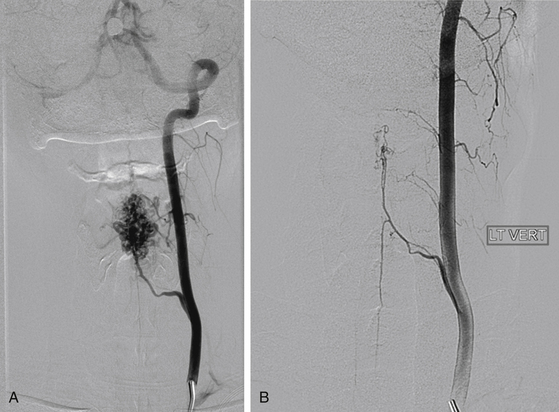
Steinter et al.43 published the first description of SRS for cranial arteriovenous malformations (AVMs) in 1972. The successes in treating the intracranial AVMs inspired the use of SRS for spinal vascular malformations. Spinal AVMs have been described using various systems. Most commonly they have been divided into four types.44 Types I and IV are dural and perimedullary fistulas and are better treated with embolization and resection. Most AVMs treated with SRS are type II, or glomus AVMs, with a well-defined nidus. Some type III, or juvenile AVMs, may be amenable to SRS when well-focal and well-defined (Fig. 100-7). SRS causes endothelial damage that leads to the obliteration of the vascular lumena.45 Low-flow lesions, such as cavernous malformations, are rarely candidates for spinal SRS.46 Of 29 patients treated at Stanford between 1997 and 2009, 22 were followed more than 24 months (unpublished data). Most had glomus AVMs and one had a type III lesion. Sixteen patients presented with hemorrhage and 8 had more than one bleed. Twelve lesions were cervical, 8 were thoracic, and 3 were in the conus. The usual treatment dose was 16 Gy in one session or 20 Gy in two sessions (with 10 Gy delivered to adjacent spinal cord). Ten patients (43%) were previously embolized. All postoperative MRIs in treated patients showed a reduction in volume. Of the 8 patients who had post-SRS angiography, 3 had complete obliteration. None of the treated patients suffered a rebleed, including those where the AVM was not obliterated by MRI or angiography. There was no mortality. Symptoms improved in over 50% and only 3 patients reported worsening of symptoms. There was only 1 case of radiation myelopathy (3%).
Treatment Failures and Complications
Treatment failures fall into several groups. “In-field failures” refer to tumor regrowth within the treated volume and may be related to inadequate dosing. “Marginal failures” involve regrowth at the edges of the treated volume and may be related to poor imaging, underestimation of the tumor volume, or inaccuracies in position or setup. “Distant failures” are not complications but rather involve new lesions in untreated areas. For vertebral metastases, the chance of an asynchronous metastasis in an adjacent level is only 5%.47 Furthermore, neurologic damage can be divided into three groups: (1) acute complications that occur within 1 month are usually due to edema, are transient, and are treated with steroids; (2) subacute complications that occur 3 to 6 months after treatment, may be secondary to demyelination, and usually recover; and (3) radiation myelopathy, which is the most feared complication and usually occurs after 6 months. The latter risk more than any other limits the radiosurgical dose used for most paraspinal lesions.48 In conventional radiotherapy, it is generally believed that treatment with 45 Gy in 22 to 25 fractions is associated with an incidence of myelopathy of only 0.2%. Meanwhile, among the first 1000 patients treated with the CyberKnife for spinal lesions at both Stanford and the University of Pittsburgh over the past decade, only 6 developed myelopathy (0.6%). In large part due to this 10-year track record with radiosurgery, a re-evaluation of the conventional wisdom and long-standing radiotherapy guidelines pertaining to spinal cord tolerance is taking place. Nevertheless, at Stanford we generally seek to avoid exposing more than 1 cm3 of spinal cord to greater than 10 Gy in single-session plans.49
Summary
While the complexity of spinal lesions and their close association with the cord make operative treatment difficult, it also makes them ideal candidates for spinal SRS. SRS, although a recent development, is supported by a rapidly expanding literature. For many lesions of the vertebral bodies, and some intradural, extramedullary lesions, CyberKnife radiosurgery is clearly both safe and effective. For vascular lesions, the treatment is superior to embolization and surgery for AVMs. Early results show that treatment of selected intramedullary lesions is also possible. It is likely that the indications will expand and the quality of the results will improve as our experience increases.
Amdur R.J., Bennett J., Olivier K., et al. A prospective phase II study demonstrating the potential value and limitation of radiosurgery for spine metastases. Am J Clin Oncol. 2009;32:1-6.
Bhatnagar A.K., Gerszten P.C., Ozhasaglu C., et al. CyberKnife radiosurgery for the treatment of extracranial benign tumors. Technol Cancer Res Treat. 2005;4:571-576.
Chang S.D., Le Q.T., Martin D.P., et al. The CyberKnife. In: Fehlings M.G., Gokaslan Z.L., Dickman C.A. Spinal Cord and Spinal Column Tumors: Principles and Practice. New York: Thieme, 2006.
Chang E.L., Shiu A.S., Mendel E., et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7:151-160.
Dodd R.L., Ryu M.R., Kamnerdsupaphon P., et al. CyberKnife radiosurgery for benign intradural extramedullary spinal tumors. Neurosurgery. 2006;58:674-685.
Gibbs I.C., Kamnerdsupaphon P., Ryu M.R., et al. Image-guided robotic radiosurgry for spinal metastases. Radiother Oncol. 2007;82:185-189.
Gerszten P.C., Burton S.A., Ozhasoglu C., et al. Radiosurgery for benign intradural spinal tumors. Neurosurgery. 2008;62:887-895.
Gerszten P.C., Burton S.A., Ozhasoglu C., Welch W.C. Radiosurgery for spinal metastases. Clinical experience in 500 cases from a single institution. Spine. 2007;32:193-199.
Gerszten P.C., Germanwala A., Burton S.A., et al. Combination kyphoplasty and spinal radiosurgery: a new treatment paradigm for pathological fractures. Neurosurg Focus. 2005;18:E8.
Gerszten P.C., Ozhasoglu C., Burton S.A., et al. CyberKnife frameless real-time image-guided stereotactic radiosurgery for the treatment of spinal lesions. Int J Radiat Oncol Biol Phys. 2003;30:S370-S371.
Gibbs I.C., Patil C., Gerszten P.C., et al. Delayed radiation-induced myelopathy after spinal radiosurgery. Neurosurgery. 2009;64:A67-A72.
Kim L.J., Spetzler R.F. Classification and surgical management of spinal arteriovenous lesions: arteriovenous fistulae and arteriovenous malformations. Neurosurgery. 2006;59:195-201.
Moss J.M., Choi C.Y., Adler J.R., et al. Stereotactic radiosurgical treatment of cranial and spinal hemangioblastomas. Neurosurgery. 2009;65:79-85.
Muacevic A., Staehler M., Drexler C., et al. Technical description, phantom accuracy, and clinical feasibility for fiducial free frameless real-time image-guided spinal radiosurgery. J Neurosurg Spine. 2006;5:303-312.
Murovic J.A., Gibbs I.C., Chang S.D., et al. Foraminal nerve sheath tumors: intermediate follow-up after CyberKnife radiosurgery. Neurosurgery. 2009;64(Suppl):A33-A43.
Murphy M.J., Cox R.S. The accuracy of dose localization for an image-guided frameless radiosurgery system. Med Physics. 1996;23:2043-2049.
Rampling R., Symonds P. Radiation myelopathy. Curr Opin Neurol. 1998;11:627-632.
Ryu S.I., Chang S.D., Kim D.H., et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery. 2001;49:838-846.
Ryu S., Jian-Yue J., Ryan J., et al. Partial volume tolerance of the spinal cord and complicationsof single-dose radiosurgery. Cancer. 2006;109:628-636.
Ryu S., Kim D.H., Chang S.D. Stereotactic radiosurgery for hemangioblastomas and ependymomas of the spinal cord. Neurosurgery Focus. 2003;15:1-5.
Ryu S., Rock J., Rosenblum M., et al. Pattern of failure after single dose radiosurgery for spinal metastasis. Neurosurgery. 2004;101:402-405.
Selch M.T., Lin K., Agazaryan N., et al. Initial clinical experience with image-guided linear accelerator-based spinal radiosurgery for treatment of benign nerve sheath tumors. Surg Neurol. 2009;72:668-674.
Sinclair J., Chang S.D., Gibbs I.C., Adler J.R. Multisession CyberKnife radiosurgery for intramedullary spinal cord arteriovenous malformations. Neurosurgery. 2006;58:1081-1089.
Yamada Y., Bilsky M.H., Lovelock D.M., et al. High-dose, single-fraction intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484-490.
Yamada Y., Lovelock D.M., Yenice K.M., et al. Multifractionated image-guided and stereotactic intensity-modulated radiotherapy of paraspinal tumors: a preliminary report. Int J Radiat Oncol Biol Phys. 2005;62:53-61.
1. Leksell L. The stereotactic method and radiosurgery of the brain. Acta Chir Scand. 1959;102:316-319.
2. Schultheiss T.E., Kun L.E., Ang K.K., Stephens L.C. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995;22:1093-1112.
3. Ryu S., Fang Y.F., Rock J., et al. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97:2013-2018.
4. Yamada Y., Bilsky M.H., Lovelock D.M., et al. High-dose, single-fraction intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484-490.
5. Adler J.R., Chang S.D., Murphy M.J., et al. The CyberKnife: a frameless robotic system for radiosurgery. Funct Neurosurg. 1997;69:124-128.
6. Chang S.D., Murphy M., Geis P., et al. Clinical experience with image-guided robotic radiosurgery (the CyberKnife) in the treatment of brain and spinal cord tumors. Neurol Med Chir (Tokyo). 1998;38:780-783.
7. Ryu S.I., Chang S.D., Kim D.H., et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery. 2001;49:838-846.
8. Chang S.D., Meisel J.A., Hancock S.L., et al. Treatment of hemangioblastomas in von Hippel-Lindau disease with linear accelerator-based radiosurgery. Neurosurgery. 1998;43:28-35.
9. Chang S.D., Murphy M.J., Martin D.P., et al. Image-guided robotic radiosurgery: clinical and radiographic results with the CyberKnife. In: Kondziolka D., editor. Radiosurgery. Basel: Karger, 1999.
10. Chang S.D., Murphy M.J., Martin D.P., et al. Frameless stereotactic neurosurgery. In: Brady I.W., Apuzzo M.L., Petrovich Z., et al. Combined Modality Therapy of Central Nervous System Tumors. New York: Springer, 2000.
11. Murphy M.J., Chang S.D., Gibbs I.C., et al. Image-guided radiosurgery in the treatment of spinal metastases. Neurosurg Focus. 2001;11:1-7.
12. Murphy M.J., Martin D., Whyte R., et al. The effectiveness of breath-holding to stabilize lung and pancreas tumors during radiosurgery. Int J Radiat Oncol Biol Phys. 2002;29:475-482.
13. Gerszten P.C., Ozhasoglu C., Burton S.A., et al. CyberKnife frameless real-time image-guided stereotactic radiosurgery for the treatment of spinal lesions. Int J Radiat Oncol Biol Phys. 2003;30:S370-S371.
14. Murphy M.J., Chang S.D., Gibbs I.C., et al. Patterns of patient movement during frameless image-guided radiosurgery. Int J Radiat Oncol Biol Phys. 2003;31:1400-1408.
15. Chang S.D., Le Q.T., Martin D.P., et al. The CyberKnife. In: Fehlings M.G., Gokaslan Z.L., Dickman C.A. Spinal Cord and Spinal Column Tumors: Principles and Practice. New York: Thieme, 2006.
16. Murphy M.J., Cox R.S. The accuracy of dose localization for an image-guided frameless radiosurgery system. Med Physics. 1996;23:2043-2049.
17. Gibbs I.C., Kamnerdsupaphon P., Ryu M.R., et al. Image-guided robotic radiosurgry for spinal metastases. Radiother Oncol. 2007;82:185-189.
18. Chang E.L., Shiu A.S., Mendel E., et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7:151-160.
19. Loblaw D.A., Laperriere N.J. Emergency treatment of malignant extradural spinal cord compression: an evidence-based guideline. J Clin Oncol. 1998;16:1613-1624.
20. Kopelson G., Linggood R., Kleinmann G. Management of intramedulary spinal cord tumors. Radiol. 1980;135:473-479.
21. McCuniff A.J., Liang M.J. Radiation tolerance of the cervical spinal cord. Int J Radiat Oncol Biol Phys. 1989;16:675-678.
22. Marcus R.B., Million R.R. The incidence of myelitis after irradiation of the cervical spinal cord. Int J Radiat Oncol Biol Phys. 1990;17:3-8.
23. van der Kogel A.V. Radiation njury in the central nervous system. In: Lundsford D., editor. Stereotactic Radiosurgery. New York: McGraw Hill, 1993.
24. Schultheiss T.E., Kun L.E., Ang K.K., Stephens L.C. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995;22:1093-1112.
25. Gerszten P.C., Germanwala A., Burton S.A., et al. Combination kyphoplasty and spinal radiosurgery: a new treatment paradigm for pathological fractures. Neurosurg Focus. 2005;18:E8.
26. Muacevic A., Staehler M., Drexler C., et al. Technical description, phantom accuracy, and clinical feasibility for fiducial free frameless real-time image-guided spinal radiosurgery. J Neurosurg Spine. 2006;5:303-312.
27. Amdur R.J., Bennett J., Olivier K., et al. A prospective phase II study demonstrating the potential value and limitation of radiosurgery for spine metastases. Am J Clin Oncol. 2009;32:1-6.
28. Yamada Y., Lovelock D.M., Yenice K.M., et al. Multifractionated image-guided and stereotactic intensity-modulated radiotherapy of paraspinal tumors: a preliminary report. Int J Radiat Oncol Biol Phys. 2005;62:53-61.
29. Wowra B., Zausinger S., Muacevic A., Tonn J- C. Radiosurgery for spinal malignant tumors. Dtsch Arztel Int. 2009;106:106-112.
30. Gerszten P.C., Burton S.A., Ozhasoglu C., Welch W.C. Radiosurgery for spinal metastases. Clinical experience in 500 cases from a single institution. Spine. 2007;32:193-199.
31. Ryu S., Jian-Yue J., Ryan J., et al. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2006;109:628-636.
32. Milker-Zabel S., Zabel A., Thilmann C., et al. Clinial results of retreatment of vertebral bone metastases by stereotactic conformal radiotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55:162-167.
33. Chamberlain M.C., Eaton K.D., Fink J.R., Tredway T. Intradural intramedullary spinal cord metastasis due to mesothelioma. J Neurooncol. 2010;97:133-136.
34. Parikh S., Heron D.E. Fractionated radiosurgical management of intramedullary spinal cord metastasis: a case report and review of the literature. Clin Neurol Neurosurg. 2009;111:858-861.
35. Moss J.M., Choi C.Y., Adler J.R., et al. Stereotactic radiosurgical treatment of cranial and spinal hemangioblastomas. Neurosurgery. 2009;65:79-85.
36. Ryu S., Kim D.H., Chang S.D. Stereotactic radiosurgery for hemangioblastomas and ependymomas of the spinal cord. Neurosurgery Focus. 2003;15:1-5.
37. Bhatnagar A.K., Gerszten P.C., Ozhasaglu C., et al. CyberKnife radiosurgery for the treatment of extracranial benign tumors. Technol Cancer Res Treat. 2005;4:571-576.
38. Cherqui A., Kim D.H., Kim S.H., et al. Surgical approaches to paraspinal nerve sheath tumors. Neurosurg Focus. 2007;22:E9.
39. Gerszten P.C., Burton S.A., Ozhasoglu C., et al. Radiosurgery for benign intradural spinal tumors. Neurosurgery. 2008;62:887-895.
40. Murovic J.A., Gibbs I.C., Chang S.D., et al. Foraminal nerve sheath tumors: intermediate follow-up after CyberKnife radiosurgery. Neurosurgery. 2009;64(Suppl):A33-A43.
41. Selch M.T., Lin K., Agazaryan N., et al. Initial clinical experience with image-guided linear accelerator-based spinal radiosurgery for treatment of benign nerve sheath tumors. Surg Neurol. 2009;72:668-674.
42. Dodd R.L., Ryu M.R., Kamnerdsupaphon P., et al. CyberKnife radiosurgery for benign intradural extramedullary spinal tumors. Neurosurgery. 2006;58:674-685.
43. Steinter L., Leksell L., Greitz T., et al. Stereotaxic radiosurgery for cerebral arteriovenous malformations: report of a case. Acta Chir Scand. 1972;138:459-464.
44. Kim L.J., Spetzler R.F. Classification and surgical management of spinal arteriovenous lesions: arteriovenous fistulae and arteriovenous malformations. Neurosurgery. 2006;59:195-201.
45. Chang S.D., Shuster D.L., Steinberg G.K., et al. Stereotactic radiosurgery of arteriovenous maformation: pathologic changes in resected tissue. Clin Neuropathol. 1997;16:111-116.
46. Sinclair J., Chang S.D., Gibbs I.C., Adler J.R. Multisession CyberKnife radiosurgery for intramedullary spinal cord arteriovenous malformations. Neurosurgery. 2006;58:1081-1089.
47. Ryu S., Rock J., Rosenblum M., et al. Pattern of failure after single dose radiosurgery for spinal metastasis. Neurosurgery. 2004;101:402-405.
48. Rampling R., Symonds P. Radiation myelopathy. Curr Opin Neurol. 1998;11:627-632.
49. Gibbs I.C., Patil C., Gerszten P.C., et al. Delayed radiation-induced myelopathy after spinal radiosurgery. Neurosurgery. 2009;64:A67-A72.