44 Cyanotic Congenital Heart Disease
Clinical Presentation And Evaluation
Transposition of the Great Arteries
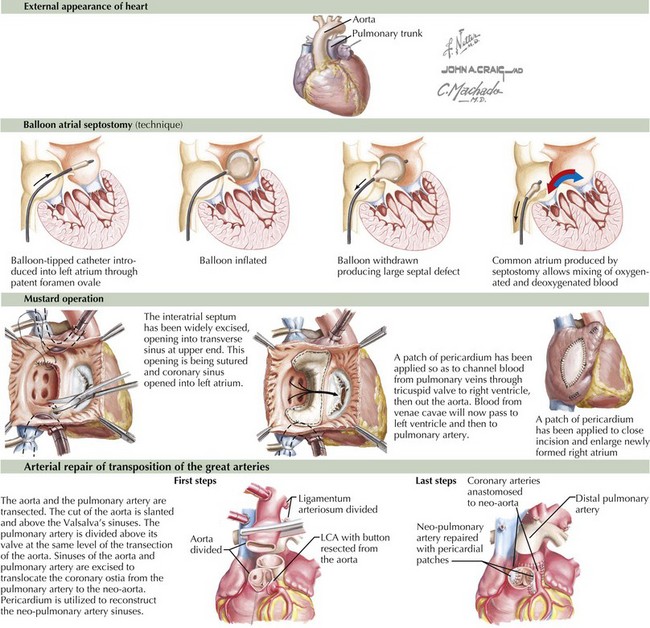
Transposition of the great arteries means that the pulmonary artery arises above the left ventricle and the aorta above the right ventricle (Figure 44-1). It is the most common cardiac cause of cyanosis in the neonatal period (0.2-0.4 in 1000 live births), accounting for 7% of congenital heart defects. Its incidence is increased in infants of diabetic mothers. It is not seen in patients with DiGeorge syndrome. Cyanosis results because the pulmonary and systemic circulations flow parallel to one another with minimal mixing: deoxygenated systemic venous blood returns to the right atrium and right ventricle and out the aorta, and oxygenated pulmonary venous blood returns to the left atrium and left ventricle and exits into the pulmonary arteries. These two parallel circuits are compatible with survival only because there is some point of mixing, usually at the foramen ovale.
Immediate intervention is usually necessary to augment the interatrial shunt. This is accomplished through a Rashkind balloon atrial septostomy (see Figure 44-1). This procedure allows for increased mixing, resulting in tolerable aortic saturations. Surgery remains the definitive therapy. Historically, this was accomplished through an atrial switch operation—Mustard (see Figure 44-1) or Senning procedures—redirecting inflow from the pulmonary veins to the right ventricle and from the venae cavae to the left ventricle. Perioperative mortality was low, but concerns regarding arrhythmia and the durability of the right ventricle (which remains the systemic ventricle) led to a different surgical approach, with a higher degree of technical difficulty but improved physiology. Currently, the preferred method is the arterial switch operation in which (1) the aorta is bisected and the distal aorta is brought beneath the bifurcation of the pulmonary trunk while the pulmonary trunk is displaced anteriorly (the Lecompte maneuver) where the aorta is anastomosed to the former pulmonary trunk and the main pulmonary artery is anastomosed to the former aortic trunk, (2) the coronary arteries are detached from the former aortic trunk and reimplanted on the pulmonary trunk (the new aortic root) with pericardial patches placed on the sites where the coronary arteries were harvested, and (3) the ASD is repaired (see Figure 44-1).
Tetralogy of Fallot
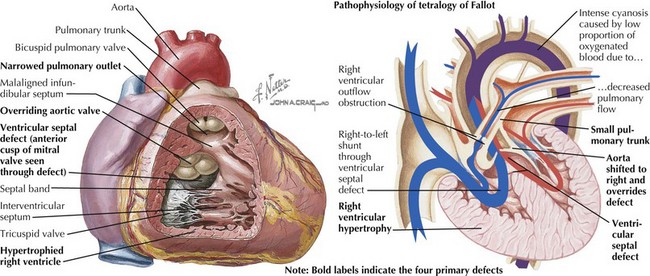
Tetralogy of Fallot is characterized by an abnormally small subpulmonary conus or outflow tract, resulting in anterior and cephalad displacement of the infundibular (outflow tract) septum. This produces the four characteristic findings: (1) subvalvular pulmonic stenosis, (2) VSD caused by malalignment of the infundibular septum relative to the rest of the ventricular septum, (3) “overriding aorta” (i.e., the aortic valve sits above both ventricles), and (4) right ventricular hypertrophy caused by pressure overload from the large VSD (Figure 44-2). It occurs in 0.19 to 0.26 in 1000 live births and represents 8% of congenital cardiac lesions. Tetralogy of Fallot is found in more than 25% of patients with DiGeorge syndrome and chromosome 22q11.2 microdeletion. The incidence of tetralogy of Fallot is higher than the general population in children of mothers with phenylketonuria and occurs more frequently in children with thrombocytopenia absent radii syndrome. Associated cardiac defects include right aortic arch (25% of patients) and ASD (10% of patients).
Tricuspid Atresia
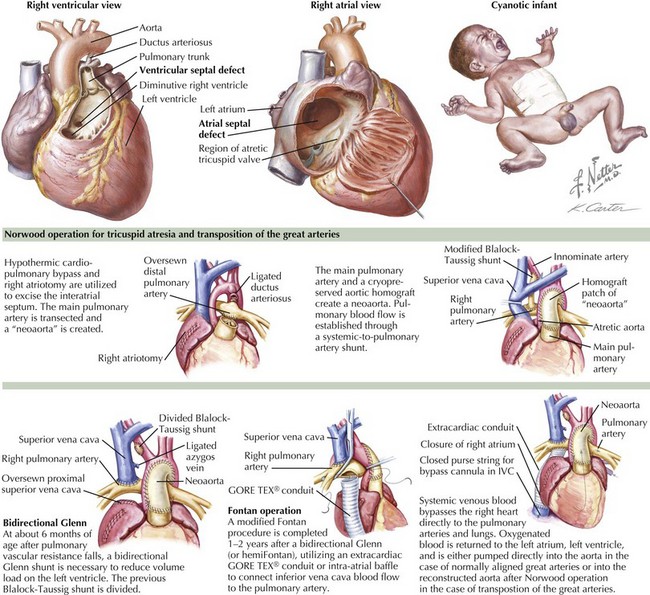
Tricuspid atresia is absence of communication between the right atrium and either ventricle. All blood returning to the right atrium flows across a patent foramen ovale or ASD to the left atrium, left ventricle, and usually through a VSD to the remnant of the right ventricle. In cases with normally aligned great arteries, the size of the VSD affects the amount of pulmonary blood flow and therefore the degree of cyanosis (Figure 44-3). Because many patients have muscular VSDs, it may be large early in life with relatively high pulmonary blood flow and mild cyanosis. Over time, the VSD may become smaller, resulting in decreasing pulmonary blood flow and increasing cyanosis. Rarely, there is no VSD, and pulmonary blood flow is exclusively from a ductus arteriosus. In cases with transposition of the great arteries, the aorta arises from the small remnant of the right ventricle. These patients usually have high pulmonary blood flow and therefore mild cyanosis. When the VSD is relatively small, there may be additional coarctation of the aorta. These VSDs are rarely muscular and therefore tend to stay about the same relative size over time. High pulmonary blood flow often results in heart failure and, if not surgically addressed, can eventually cause pulmonary vascular disease. In patients with transposition and a restrictive VSD, a Damus-Kaye-Stansel or a Norwood operation is required to effectively bypass the subaortic obstruction by using the pulmonary valve (arising unobstructed from the left ventricle) as an additional (or only) systemic outlet. These operations involve transection of the pulmonary artery above the valve and amalgamation of the pulmonary stump with the ascending aorta and aortic arch, either with (Norwood) (see Figure 44-3) or without (Damus-Kaye-Stansel) supplementary graft material depending on the size difference between the two vessels. The distal pulmonary arteries are then supplied by a systemic-to-pulmonary shunt.
Because all patients with tricuspid atresia, regardless of associated abnormalities, have a functional single ventricle, the ultimate treatment is a Fontan operation, in which all systemic venous return goes directly to the pulmonary arteries (without passing through a ventricle) and pulmonary venous return goes to the left atrium and left ventricle and out the aorta (normally aligned great arteries) or through the VSD to the right ventricular remnant to the aorta (transposition). Because the Fontan operation depends on low pulmonary resistance to allow systemic venous blood to flow without a pump into the pulmonary arteries, it cannot be carried out until the high pulmonary resistance of the normal newborn has resolved. An intermediary operation, superior cavopulmonary anastomosis (bidirectional Glenn [see Figure 44-3] or hemi-Fontan), is typically performed at 4 to 6 months of age. In this operation, the superior vena cava is connected to the right pulmonary artery so that all venous drainage from the upper body goes to the lungs but inferior vena caval blood mixes with pulmonary venous return and goes to the body. One or 2 years later, the inferior vena cava is connected to the pulmonary arteries by way of an intraatrial baffle or extracardiac conduit, completing the Fontan circuit (see Figure 44-3).
Critical Pulmonic Stenosis
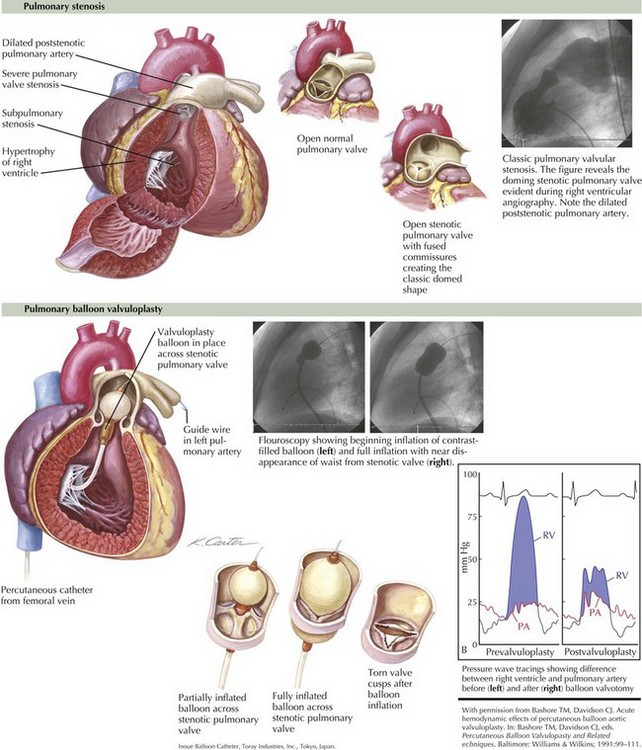
Infants with extreme narrowing of the pulmonic valve orifice (critical pulmonic stenosis) (Figure 44-4) can present with hepatomegaly and cyanosis from right-to-left shunting across the foramen ovale because of right ventricular failure or low right ventricular compliance.
Treatment is aimed at relieving obstruction at the pulmonary valve. This is usually accomplished through balloon valvuloplasty in the interventional catheterization laboratory (see Figure 44-4). Before this, patients are often palliated with prostaglandin E1 infusion to maintain ductal patency to increase pulmonary blood flow and reduce cyanosis. If the right ventricular compliance is sufficiently low because of severe hypertrophy or hypoplasia, valvotomy may not be sufficient to provide adequate pulmonary blood flow, and a temporary systemic–pulmonary shunt may be necessary until compliance improves.
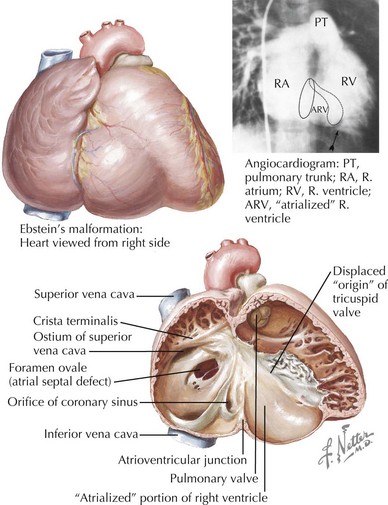
Ebstein’s Anomaly of the Tricuspid Valve
Downward displacement of the tricuspid valve partitions the right ventricle into an apical right ventricular portion and a proximal atrialized right ventricle (Figure 44-5). The tricuspid valve anterior leaflet becomes large redundant and “sail-like” with variable tricuspid regurgitation. These patients have a high incidence (20%-30%) of preexcitation with accessory pathway.
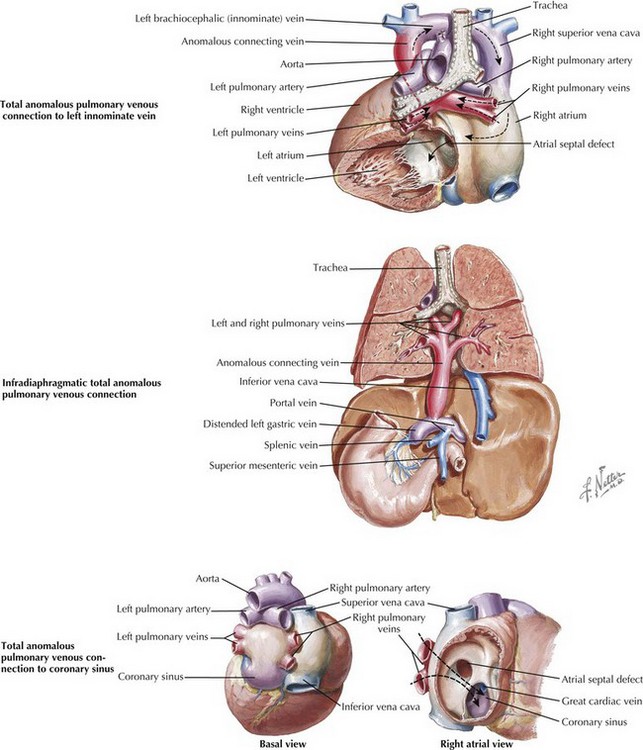
Total Anomalous Pulmonary Venous Connection
Total anomalous pulmonary venous connection means connection of all pulmonary veins to somewhere other than the left atrium. Connections to the innominate vein, the portal system of the liver, and the coronary sinus are representative of the main categories noted below (Figure 44-6). This defect comprises 1% to 3% of congenital cardiac lesions. Anatomically, patients are divided among those whose pulmonary veins connect above the diaphragm (70%), below the diaphragm (25%), or in mixed fashion (i.e., to more than one connection; 5%). Those connecting above the diaphragm are further divided into those connecting to a vein, most often the innominate vein, and those connecting to the heart, either the coronary sinus or the right atrium. The majority of these are unobstructed. Connection below the diaphragm is almost always to the portal vein or one of its branches or to the ductus venosus; hence, they become obstructed when the ductus venosus closes in the first few days of life.
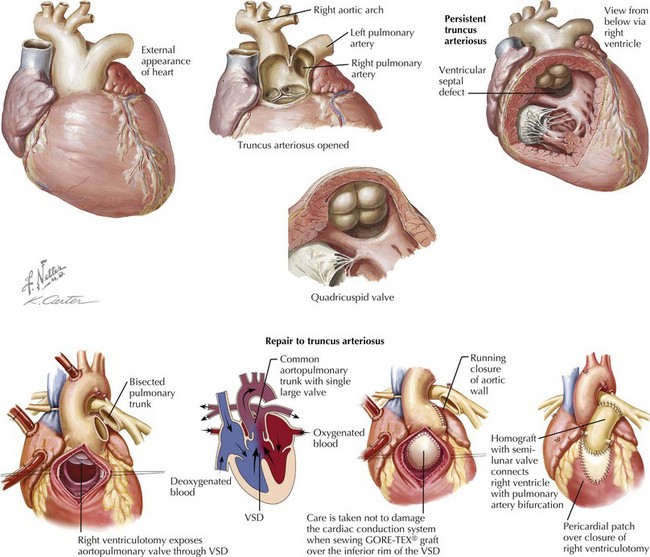
Truncus Arteriosus Communis
Truncus arteriosus communis denotes a single arterial trunk that serves as the common origin of the aorta, pulmonary artery, and coronary arteries (Figure 44-7). It comprises 2% to 2.8% of congenital cardiac lesions. This condition is almost always associated with a VSD. The truncus is fed by a single truncal valve, most commonly with three cusps, but may have two to five cusps, often myxomatous and asymmetric. This truncal valve typically sits over the VSD but in rare cases arises predominantly above the right ventricle. Associated cardiac defects include right aortic arch (33%) and anomalous coronary artery origins. Extracardiac anomalies associated with truncus include 22q11 microdeletion. The physiology of truncus is usually that of high pulmonary blood flow with minimal cyanosis but commonly tachypnea and respiratory distress. Congestive heart failure is exaggerated by poor coronary perfusion secondary to low diastolic pressures from runoff into the pulmonary arteries.
Treatment is surgical separation of aorta and pulmonary artery, placement of an extracardiac right ventricle to pulmonary artery conduit, and closing the VSD by baffling the left ventricle to the aorta (see Figure 44-7).
Ballweg JA, Wernovsky G, Gaynor JW. Neurodevelopmental outcomes following congenital heart surgery. Pediatr Cardiol. 2007;28(2):126-133.
Beghetti M, Galie N. Eisenmenger syndrome a clinical perspective in a new therapeutic era of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;53(9):733-740.
Cohen MS, Frommelt MA. Does fetal diagnosis make a difference? Clin Perinatol. 2005;32(4):877-890.
Crean A. Cardiovascular MR and CT in congenital heart disease. Heart. 2007;93(12):1637-1647.
Dorfman AT, Marino BS, Wernovsky G, et al. Critical heart disease in the neonate: presentation and outcome at a tertiary care center. Pediatr Crit Care Med. 2008;9(2):193-202.
Marino BS, Tomlinson RS, Drotar D, et al. Quality-of-life concerns differ among patients, parents, and medical providers in children and adolescents with congenital and acquired heart disease. Pediatrics. 2009;123(4):e708-e715.