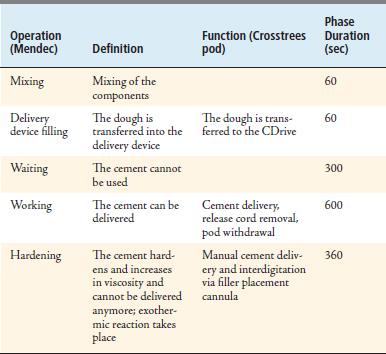
42 Crosstrees Percutaneous Vertebral Augmentation
KEY POINTS
Indications and Contraindications
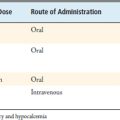
Surgical treatment of VCFs with the Crosstrees PVA pod is indicated when debilitating back pain persists despite nonsurgical therapies (Table 42-1). MRI, the imaging study of choice for diagnosing VCF, typically shows increased signal on short T1 inversion recovery (STIR) sequences when a VCF is acute/subacute or if there is residual bony edema indicating incomplete healing. A nuclear medicine study (bone scan) is particularly useful when a MRI cannot be performed (e.g., when pacemaker is present).
In cases of chronic fracture, vertebral augmentation is not indicated. Other absolute contraindications to Crosstrees or any other PVA technique include pregnancy, coagulopathy, osteomyelitis, spinal instability, known allergy to PMMA, and previous augmentation with PMMA (Table 42-2). Relative contraindications (Table 42-3) include neurologic deficit (i.e., burst fracture with significant bony retropulsion, or fracture extending to the posterior cortical wall) and pathologic vertebral fracture related to primary or metastatic cancer; however, because the Crosstrees pod fully contains the cement during implantation and has a defined shape, it may be used more safely in these cases than current vertebroplasty or kyphoplasty techniques. Another relative contraindication is vertebra plana or greater than 60% loss of height.
TABLE 42-2 Absolute Contraindications
TABLE 42-3 Relative Contraindications
Description of the Device
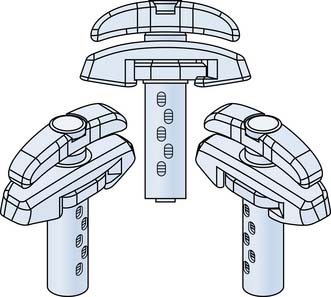
The Crosstrees pod device (Figure 42-1) was developed to provide a percutaneous method of delivering a specific volume of bone cement to the surgical site in orthopedic procedures. The device is designed such that the woven fabric pod is inserted into the intravertebral space, and a predetermined volume of bone cement is delivered into it, thus reducing the likelihood of extravertebral cement leakage. The pod expands to a defined shape with a broad surface area as the cement is injected, elevating the endplates and restoring height to the fractured vertebra. Following delivery of the bone cement, the pod is opened and withdrawn from the vertebra. Additional cement can be delivered to the center of the cement bolus, to provide interdigitation with the bone. PMMA is delivered to the pod by a threaded injection syringe, the Crosstrees CDrive cement dispenser (Figure 42-2), designed to measure and deliver the volume required for the selected pod.
Principles of Procedure
Using a stylet and 5.2-mm-diameter access cannula, the paraspinal musculature is traversed to access the vertebral body via the pedicle. Either a transpedicular or extrapedicular approach can be used. The stylet and cannula can be advanced to the bony site and withdrawn slightly to create a location for placement of the Crosstrees pod within the bony site. As an alternative method, the stylet can be withdrawn from the cannula and a bone drill advanced through the access cannula into the vertebra and then withdrawn creating a space for placement of the Crosstrees pod within the bony site. Often bone fragments that remain on the drill can be sent for pathologic examination.
Background of Scientific Testing and Clinical Outcomes
It is well established in the published medical literature that the effectiveness of vertebral augmentation is evident almost immediately after the procedure. Numerous authors have reported significant pain relief within 24 to 48 hours, with stable results preserved at subsequent follow-up in a majority of patients.1 Studies in vertebral augmentation have generally evaluated efficacy principally based on pain relief, because pain is typically the reason patients seek treatment. Pain relief assessment by VAS is widely reported, with patients reporting significant relief at 24 hours and later following the procedure. Functional outcomes have also been reported using multiple assessment methods, but is typically secondary to evaluation based on pain relief.2–4 Ledlie et al5 showed the stability of outcomes with respect to pain relief post procedure. The study included 117 consecutive subjects undergoing vertebral augmentation procedures. The authors observed rapid relief in pain, with substantial improvement within 1 week postprocedure and relatively stable results from 1 through 24 months postoperative.
In a 2006 review, Hulme et al4 reported that complication rates are in the range of 1% to 2% for osteoporotic fractures and 5% to 10% for metastatic lesions.6 Complications specifically related to cement leakage can include increased local pain, symptomatic pulmonary embolus, radiculopathy, and cord compression and are estimated to occur in approximately 1% to 3% of cases.7 Of the complications potentially associated with vertebroplasty and kyphoplasty, all but new vertebral fractures occur during or immediately following the procedure. Thus the majority of complications can be identified within a very short period following the procedure.
Clinical literature in vertebral augmentation reports on the incidence of additional fractures as the primary focus of longer term follow-up. Leakage of PMMA from the vertebra has been associated with incidence of new fracture, with average time to new fracture of 48 days for levels adjacent to PMMA extravasation and 98 days absent PMMA extravasation.8 There are reports of the incidence of fracture adjacent to and remote from treated levels with half of new fractures occurring adjacent to treated levels within 3 months follow-up. A majority of subsequent vertebral fractures appear to occur within the first 30 days following a vertebroplasty procedure. Lin et al9 reported that in a series of 38 patients treated with vertebroplasty, new fractures occurred in 14 patients. When cement leakage occurred, the average time to new fracture was 48 days. It was 98 days in patients who did not have any cement leakage.
In addition to evaluation of safety and pain relief, vertebral augmentation studies reported in the literature have often included an assessment of vertebral body morphology. There is disagreement in the literature on the efficacy of current treatments in the restoration of vertebral height and the clinical importance of vertebral height restoration.4,7
Procedural characteristics including the volume of cement used are also reported in the literature. Clinical literature reports variability in the volume of PMMA required for procedure success.8,10 The Crosstrees system delivers an initial bolus of known volume of PMMA, with device size selection determined by the investigator, based on vertebral level, degree of vertebral collapse, and physician assessment of device placement strategy. Pain relief is often immediate and sustained as noted in the literature review noted previously. If complications occur, they should become apparent early in the postoperative period.
Operative Technique
Surgical Procedure for the Crosstrees System
Transpedicular Approach
Make a skin incision slightly lateral and superior (varies per level) to the intersection of the superior and lateral edges of the pedicle as determined under fluoroscopic guidance. Insert the 11-gauge needle into the incision and anchor it in bone, gently tapping it with a mallet if necessary. Confirm its location with fluoroscopy (AP view). Continue tapping the 11-gauge needle into place, confirming the location of the tip periodically with both AP and lateral fluoroscopic views. To avoid the spinal canal, make sure the tip of the needle does not pass medial to the medial border of the pedicle before entering the posterior vertebral cortex. Once the 11-gauge needle has crossed the posterior wall of the vertebral body, remove the inner stylet and replace it with the Indexed Guide Pin. Advance the guide pin anteriorly and medially into the vertebral body. Use the proximal most visible sizing indicator to select the appropriate size pod. With the guide pin approximately halfway across the vertebral body on the lateral view, remove the 11-gauge needle cannula and insert the blunt cannulated assembly over the guide pin. Attach the strike plate to the strike plate extension. Insert the tines of the strike plate into the mating feature of the blunt cannulated stylet and use the mallet to gently tap the stylet until its tip is just past the posterior vertebral wall on the lateral view.
Delivery of PMMA
Insert the filler placement cannula (FPC) through the access cannula, advancing just far enough to place the tip of the FPC into the center of the cement bolus. Use fluoroscopy and the marks on the FPC cannula to confirm position. Manually dispense additional PMMA under continuous fluoroscopic guidance to achieve interdigitation (Table 42-4).
1. Bono C., Kauffman C.P., Garfin S., Herkowitz H., editor. Surgical options and indications: kyphoplasty and vertebroplasty in the lumbar spine. Lippincott Williams, 2004.
2. Mathis J.M. Percutaneous vertebroplasty or kyphoplasty: which one do I choose? Skel. Radiol.. 2006;35:629-631.
3. Gill J.B. Comparing pain reduction following kyphoplasty and vertebroplasty for osteoporotic vertebral compression fractures. Pain Physician. 2007 Jul;10(4):583-590.
4. Hulme P.A. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine. 2001;31(17):1983.
5. Ledlie J.T. Kyphoplasty treatment of vertebral fractures: 2-year outcomes show sustained benefits. Spine. 2006;31(1):57-64.
6. Eicholz K.M., O’Toole J.E., Christie S.D., Fessler R.G. Vertebroplasty and kyphoplasty. Neurosurg Clin N Am. 2006;17:507-518.
7. Talmadge K. Vertebral compression fracture treatments. In: Kurtz S.M., Edidin A.A., editors. Spine technology handbook. Elsevier Academic Press; 2006:371-396.
8. Lin E.P. Vertebroplasty: cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. AJNR Am J Neuroradiol. 2004 Feb;25(2):166-167.
9. Lin E.P., Ekholm S., Hiwatashi A., Westesson P.L. Vertebroplasty: cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. AJNR Am J Neuroradiol. 2004;25:175-180.
10. Frankel B.M. Percutaneous vertebral augmentation: an elevation in adjacent level fracture risk in kyphoplasty as compared with vertebroplasty. Spine J. 2007 Sept-Oct;7(5):575-582.