Critically Ill Immunosuppressed Host
This chapter emphasizes the important ways in which immunosuppressed patients differ from immunologically normal individuals in terms of infectious complications. The noninfectious complications of immunosuppression are reviewed in Chapter 80.
Host Defense Mechanisms
The microbial complications that any patient develops in the ICU are determined by general, nonspecific barriers; innate immunity; acquired specific immunity; and environmental exposures. Nonspecific barriers include anatomic barriers such as intact skin and mucous membranes; chemical barriers, such as gastric acidity or urine pH; and flushing mechanisms, such as urinary flow or mucociliary transport in the lungs. Organisms that breach these barriers encounter nonspecific and innate host factors termed the acute phase response. Acute phase responses trigger a cascade of acquired specific immune responses including mononuclear phagocytes and antibodies, which also trigger a cascade of effector molecules and nonspecific inflammatory responses.1
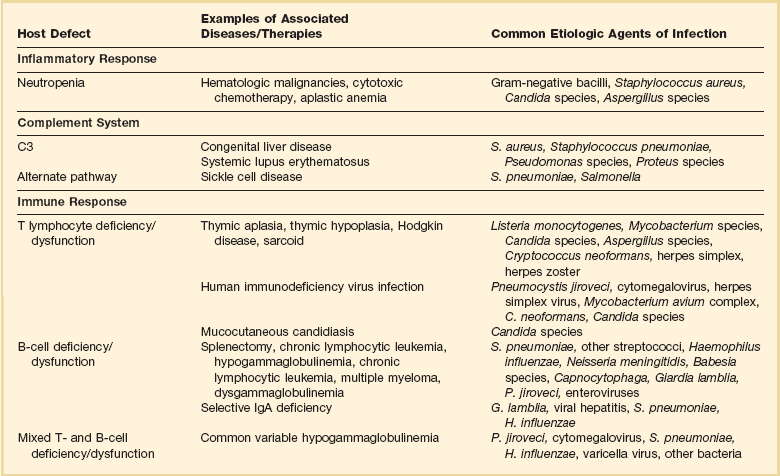
Infections result from normal flora that colonize mucosal or cutaneous surfaces or from abnormal flora that are introduced by surface-to-surface contact, inhalation, ingestion, trauma, or medical procedures. Table 53.1 lists organisms that cause disease when specific anatomic defenses are disrupted in individuals with normal microbial flora. Patients with abnormal flora will develop disease that reflects unique, disease-specific characteristics of the host, the abnormal environment, and modifying factors such as drugs. Infections that result from common defects in the inflammatory or immunologic systems are detailed in Table 53.2.
Table 53.1
Normal Flora That Can Cause Disease When Anatomic Barriers Are Disrupted
| Compromised Host Defense: Anatomic Disruption | Bacteria | Fungi |
| Oral cavity, esophagus | α-Hemolytic streptococci, oral anaerobes | Candida species |
| Lower gastrointestinal tract | Enterococci Enteric organisms Anaerobes |
Candida species |
| Skin | Gram-positive bacilli Staphylococci, streptococci Corynebacterium, Bacillus species Mycobacterium fortuitum, Mycobacterium chelonei |
Candida species Aspergillus |
| Urinary tract | Enterococci Enteric organisms |
Candida species |
Recognition of which host defense mechanisms are disrupted enables the clinician to focus diagnostic, therapeutic, and prophylactic management and optimize patient outcome. For instance, if a patient presents with severe hypoxemia and diffuse pulmonary infiltrates, a health care provider who recognizes a prior splenectomy as the major predisposition to infection would focus the diagnostic evaluation and the empiric therapy on Streptococcus pneumoniae and Haemophilus influenzae.2,3 By contrast, if the patient’s major predisposition to infection was human immunodeficiency virus (HIV) infection with a CD4+ T lymphocyte count below 50 cells/µL, the health care provider would focus on Pneumocystis jiroveci and S. pneumoniae.4,5 However, additional history is also necessary: if the pneumonia occurs during an influenza outbreak, after exposure to a water aerosol (Legionella), or after a seizure (aspiration), the likely cause is plausibly linked to the precipitating event.
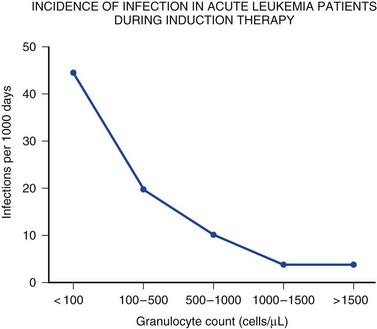
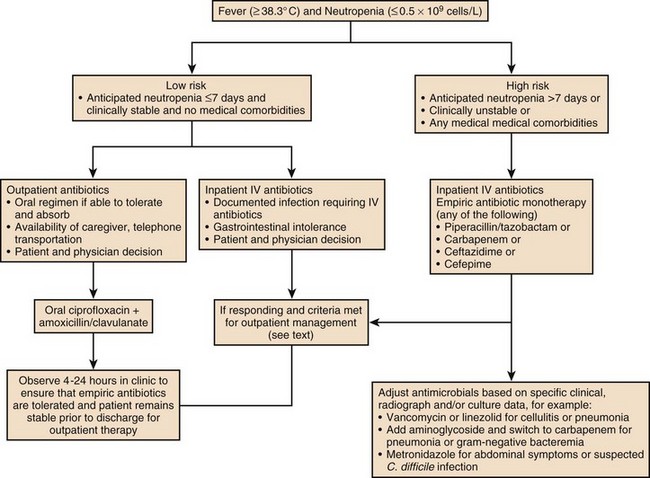
Immune competence should ideally be measurable by objective laboratory parameters. In fact, the risk for opportunistic infection in patients with HIV infection can be assessed by clinical laboratories with a high degree of accuracy by measuring the number of circulating CD4+ T lymphocytes. The susceptibility of cancer patients to opportunistic bacterial and Candida infections can be assessed by measuring the number of circulating neutrophils (Fig. 53.1), and treatment algorithms have been established for managing fever in such patients (Figs. 53.2 to 53.4) and Table 53.3).6 The predisposition of patients with certain congenital immunodeficiencies can be assessed by measuring serum immunoglobulin levels.7 Unfortunately, however, for a large number of immunodeficiencies, such as those associated with antilymphocyte monoclonal antibodies or corticosteroids, no objective laboratory measures have been validated as predicting the risk of infection. Moreover, each parameter must be validated for each specific disease entity: for instance, although CD4+ T-cell counts are excellent predictors of opportunistic infection predisposition for patients with HIV/AIDS (acquired immunodeficiency syndrome) (Fig. 53.5), they are not clinically useful for other immunosuppressive disorders.
Table 53.3
Modification of Standard Empiric Therapy in Patients with Neutropenia
| Clinical Event | Possible Modifications of Standard Empiric Therapy |
| Breakthrough bacteremia | For gram-positive isolate (e.g., Staphylococcus aureus): Add vancomycin or daptomycin or linezolid until susceptibility pattern of isolate is known. For gram-negative isolate: Add two new agents likely to have activity until susceptibility pattern of pathogen is known. |
| Cellulitis or catheter-associated infection | Add vancomycin or daptomycin. |
| Severe necrotizing mucositis or gingivitis | Add specific antianaerobic agent (e.g., metronidazole, meropenem, imipenem, piperacillin-tazobactam) plus agent with activity against streptococci; consider acyclovir. |
| Ulcerative mucositis or gingivitis | Add acyclovir and anaerobic coverage. |
| Esophagitis | Add fluconazole or caspofungin; consider adding acyclovir. |
| Pneumonitis, diffuse or interstitial | Add trimethoprim-sulfamethoxazole and azithromycin or levofloxacin or moxifloxacin (plus broad-spectrum antibiotics if the patient is granulocytopenic). |
| Perianal tenderness | Include anaerobic agents such as metronidazole, imipenem, meropenem, or piperacillin-tazobactam. |
| Abdominal involvement | Add antianaerobic agent (e.g., metronidazole, meropenem, imipenem, piperacillin-tazobactam). |
Clinical series that document the frequency, the timing, and the causative organisms associated with infectious complications are extremely valuable for managing specific populations of immunosuppressed patients. Timelines that depict the time periods of vulnerability following stem cell transplantation (Figs. 53.6 and 53.7) and solid organ transplants (Fig. 53.8) are very useful for clinicians in terms of guiding diagnostic evaluations and for guiding empiric therapy. However, a specific microbial diagnosis should be established for each syndrome that presents in an immunosuppressed patient because the range of possible pathogens is quite broad, and the timelines and laboratory parameters cannot take into account all of the individual patient variables that influence the infectious complications that develop. Although it is useful to narrow the list of likely pathogens by analyzing risk factors, identifying the true causative agents allows therapy to be focused, avoiding unnecessary toxicity and allowing specific therapy to be optimized for efficacy and safety.
General Approach to Management
1. Life-threatening complications often present with subtle symptoms and signs that can easily be overlooked.
2. Fever is not invariably present when patients are infected.
3. Patients are predisposed to deteriorate precipitously.
4. Diagnostic evaluation needs to be prompt and definitive.
5. Infections may be community acquired, nosocomial, or latent, emphasizing the need for a thorough history of the patient’s prior infections and exposures in order to assure the proper diagnostic tests and the optimal empiric therapeutic regimens.
6. Not all infections are related to the underlying disease or immunosuppression.
7. Empiric therapy should be started promptly.
Intensivists are more and more aware of the importance for all patients of “time to antibiotics,” that is, the importance of starting antibiotics sooner rather than later, and including a drug that is active against the pathogen that is ultimately shown to be the causative organism.8,9
8. Empiric therapy should be broad spectrum.
9. Antibiotic therapy should be narrowed when the causative organism is known, and monotherapy is usually adequate.
The development of potent β-lactam and quinolone drugs in the 1980s and 1990s provided single agents that appear to be as effective as combination therapy for the treatment of gram-negative bacillary infections.10–15
10. Foreign bodies and infectious foci should be assessed promptly for drainage or removal.
11. Consideration should be given to reducing the level of immunosuppression.
There is no proven survival benefit to interventions meant to augment or improve the immune or inflammatory response such as granulocyte colony-stimulating factor, neutrophil transfusions, or cytokines. It is plausible to reduce immunosuppression by reducing the dose of corticosteroid or other immunosuppressive agent if that is clinically feasible. Some institutions administer granulocyte infusions or colony-stimulating factors for patients with established infections. There is no documentation that such interventions improve survival, and deleterious effects, especially from granulocyte transfusions, can be life-threatening.16,17
12. The effectiveness and safety of antimicrobial therapy should be monitored regularly.
13. Noninfectious syndromes can masquerade as infections and can be life-threatening.
Management of Specific Patient Populations
Cancer Patients with Neutropenia
General Principles
Cytotoxic therapy–induced neutropenia is a major predisposition to infection.6 Neutrophil counts below 1000 cells/µL (the total absolute number of polymorphonuclear neutrophils plus bands) increase susceptibility to infection in a linear fashion (i.e., the lower the neutrophil count, the greater the degree of susceptibility)18 (see Fig. 53.1). Although most research studies use 500 cells/µL as an arbitrary definition of neutropenia, intensivists must recognize that susceptibility increases as the neutrophil count declines below 500 to 1000 cells/µL. A patient with a neutrophil count of 100 cells/µL is much more vulnerable to infection than a patient with 500 or 1000 cells/µL, and a patient with zero neutrophils is at much higher risk for fulminant infection than a patient with 50 or 100 cells/µL. The trajectory of the neutrophil count is also important: a patient with a neutrophil count of 1500 cells/µL whose counts are dropping precipitously should best be treated like a patient with absolute neutropenia. Similarly, a patient with 500 neutrophils/µL whose counts are rising quickly is not nearly as vulnerable to a poor outcome as a patient with a count of 500 neutrophils/µL that is stable.
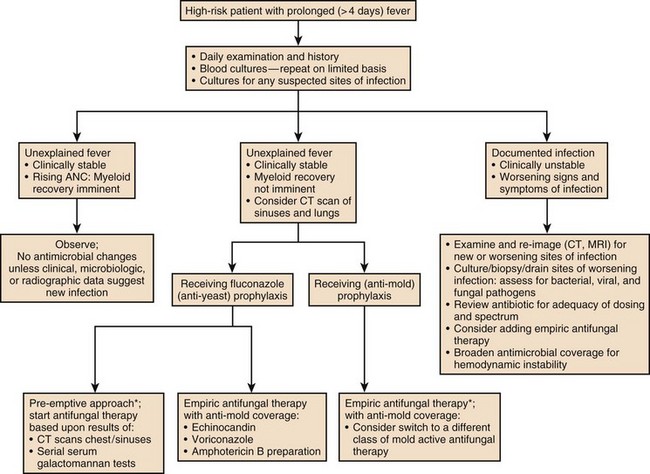
Patients with neutropenia are generally divided into high-risk and low-risk patients based on their likelihood of developing severe infectious complications. Markers for high risk include neutropenia for more than 7 days’ duration and neutrophil count less than 100 cells/µL, as well as obvious signs of a life-threatening process such as hypotension, obtundation, pneumonia, or severe abdominal pain. As Figures 53.2 to 53.4 outline, this risk assessment is used in designating empiric regimens.
In the 1960s and 1970s, aerobic gram-negative bacilli such as Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa predominated as pathogens in neutropenic patients. In the 1990s the spectrum of causative pathogens in neutropenic patients shifted from a predominance of gram-negative bacilli to a majority of gram-positive cocci including streptococci, staphylococci (including oxacillin-resistant Staphylococcus aureus), and enterococci (including vancomycin-resistant enterococci).10,12–15,19–21 Candida species have also become more frequent as pathogens, especially as patients are on broad-spectrum antibacterials and have long-term venous access devices in place.
More recently, highly resistant gram-negative bacilli have become major threats for nosocomial transmission. Clinicians must consider the possibility that a patient may be colonized and then infected with a Stenotrophomonas, a Burkholderia, or a carbapenemase-producing gram-negative Enterobacteriaceae such as a Klebsiella, an Enterobacter, or an E. coli that has developed mechanisms that evade currently marketed drugs.22–26
The management of febrile, neutropenic fever is reviewed in a guideline that is widely used to direct care in North America.6 Figures 53.2 to 53.4 summarize important aspects of management. Table 53.3 also provides a summary of useful management information. Table 53.4 outlines common prevention strategies that will modify the spectrum of causative pathogens. Box 53.1 summarizes the organisms that most often cause disease in neutropenic patients.
Table 53.4
Prevention of Infectious Complications in Compromised Patients
| Method/Agent to Prevent Acquisition of, Suppress, or Eliminate Microbial Flora | Description/Example |
| Isolation | Total protective isolation with high-efficiency particulate air filters and absorbable or nonabsorbable antibiotics for bone marrow transplant recipient |
| Prophylactic antibacterial drugs | |
| Ciprofloxacin | Reduce bacterial infections in neutropenic patients |
| Trimethoprim-sulfamethoxazole | Suppress flora in patients with chronic bronchitis |
| Penicillin | Reduce frequency of streptococcal infections after splenectomy or in rheumatic valvular disease or graft-versus-host disease |
| Clarithromycin | Prevention of Mycobacterium avium complex infection in patients with advanced HIV disease |
| Isoniazid | Prevention of tuberculosis in PPD-positive individuals |
| Nonabsorbable broad-spectrum agents (i.e., aminoglycoside, plus bacitracin) | Gut decontamination for neutropenic patients |
| Prophylactic antiviral drugs | |
| Oral acyclovir or valganciclovir, or IV ganciclovir | Reduce frequency of CMV disease after transplantation |
| Rimantadine, oseltamivir | Prevent influenza |
| Prophylactic antifungal drugs | |
| Fluconazole | Prevent recurrent candidiasis |
| Liposomal amphotericin B or voriconazole or caspofungin | Prevent Candida or mold infections |
| Trimethoprim-sulfamethoxazole | Prevent Pneumocystis pneumonia |
| Prophylactic antiprotozoal/anthelmintic drugs | |
| Albendazole or ivermectin | Prevent disseminated strongyloidiasis in high-risk patients |
| Augmentation of host defenses | |
| Immunization | Pneumococcal and Haemophilus vaccine for patients before splenectomy |
| Immune serum globulin | Augment levels in deficient patients (e.g., common variable immunodeficiency) |
| Fresh frozen plasma | Augment complement levels in deficient patients |
| Neutrophil transfusions | Augment inflammatory response in neutropenic patients or patients with chronic functional neutrophil disorders |
| Lymphocyte or other mononuclear cell transfusions | Experimental therapies for tumors, various immunodeficiencies |
| Bone marrow or stem cell transplantion | Reconstitute patients with congenital immunodeficiencies or certain acquired cytopenias |
| Bone marrow human stem cell stimulation | G-CSF or GM-CSF to increase neutrophil or mononuclear cell quantity and function |
| Gene therapy | Replace genes to allow normal function |
As noted earlier, the initial regimen should not be parsimonious in terms of spectrum. Because this population of patients is susceptible to a wide variety of bacterial and fungal pathogens, a very wide broad-spectrum regimen should be used. There are many potential regimens, each of which must be tailored to the local experience with the patient population and the hospital, specific patient factors such as evidence of prior colonization or recent antimicrobial therapy, and clinical manifestations suggesting infection. Popular regimens would include (1) meropenem or cefepime or piperacillin-tazobactam for broad-spectrum antibacterial activity plus (2) vancomycin or daptomycin for staphylococcal infections27,28 plus (3) ciprofloxacin or moxifloxacin or aztreonam for broader gram-negative bacillus coverage. Many experienced clinicians would add an echinocandin for anti-Candida activity given the frequency of intravascular catheter-associated infections due to Candida species.29–33 Intensivists need to work closely with their infectious disease consultants, microbiology laboratories, and referring teams to develop regimens that are optimal for their hospital environment, for the patient population involved, and for the specific, unique patient who is being managed.
As noted previously, there is no documented reason, even in this population, to use combination therapy to treat a specific pathogen in most situations, although combination therapy is needed for the empiric approach for the duration of neutropenia (Box 53.2). In rare situations, if the causative organism is not susceptible to agents with well-documented efficacy, combination therapy may be an appropriate strategy out of desperation. As an example, for treating enteric carbapenemase-producing organisms, a combination of tigecycline plus colistin plus an aminoglycoside might be desirable given the high minimum inhibitory concentrations for all antibiotics for these organisms and the dreadful clinical results with any therapeutic intervention.34–38
If a neutropenic patient is started on antibacterial therapy without fungal therapy, and defervescence has not occurred by days 3 to 5, an antifungal agent should be added (Figs. 53.3 and 53.4). The choice of antifungal agent depends on the patient population and the patient’s specific history. In the current era many patients have been receiving short- or long-term prophylaxis with fluconazole, voriconazole, or posaconazole. Although in general clinicians could add fluconazole, an echinocandin (e.g., caspofungin or micafungin or anidulofungin) or liposomal amphotericin B can also be used. An echinocandin is often a preferred choice if the patient has been on long-term azole prophylaxis and if a mold infection is not suspected.39–42
As patients receive chemoprophylaxis with quinolones or azoles during periods of intense neutropenia or immunosuppression, breakthrough pathogens are more and more likely to be resistant to the prophylactic agents.40,42 Thus empiric regimens must be chosen with keen attention to the drugs that patients have received in the recent past, as well as pathogens they have previously been colonized or infected with.
Diagnostic Approach
Some of these approaches, despite their promising initial reports, are not yet clinically practical because of their level of sensitivity, specificity, or the cost or expertise required to perform them adequately. For instance, the PCR test for Pneumocystis is so sensitive that there is no clear separation of patients who are colonized with Pneumocystis (and whose pulmonary dysfunction is due to another process), and the serum β-glucan antigen detection system is so nonspecific that some clinicians are not confidant that the test provides useful information.43–45 Similarly, the PCR test for respiratory syncytial virus (RSV) or influenza or parainfluenza is so sensitive that immunosuppressed patients may shed small quantities of virus for many weeks after acute infection, confusing the diagnosis of the new pulmonary processes that occur after the acute viral infection is over, and at a time when another process is causing fever or pulmonary manifestations. Thus, these new tests must be interpreted with caution.
Empiric and Specific Antimicrobial Therapy
Patients in the ICU are by definition either unstable hemodynamically or medically fragile due to concurrent disease. In such situations, many clinicians would expand antibacterial coverage with a second broad-spectrum drug (e.g., a quinolone such as ciprofloxacin), aztreonam, or an aminoglycoside (e.g., gentamicin or tobramycin).20,46–51
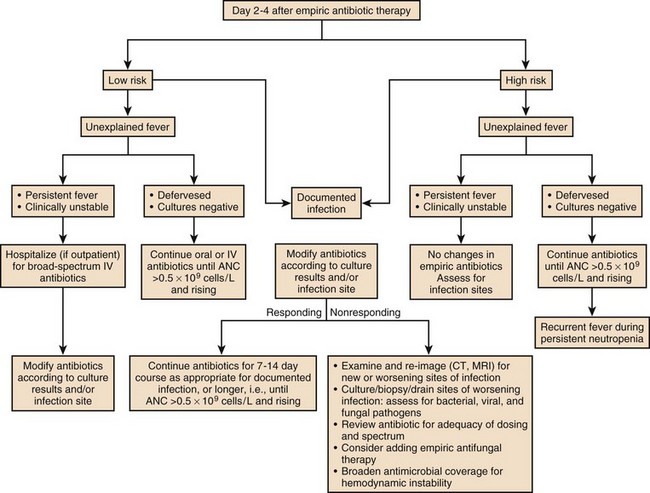
A substantial number of febrile, neutropenic patients fail to improve in terms of fever or other manifestations. Once febrile and neutropenic patients are started on empiric therapy, if no causative process or organism is detected, one of the first three scenarios listed here is likely to be encountered. Figures 53.2 to 53.4 list some of the therapeutic options for such patients.
1. The patient defervesces and remains stable but the source remains unknown. In this case the empiric regimen is usually continued for a minimum of 7 to 10 days, and must be continued until the neutrophil count is over 500 to 1000 cells/µL unless no end is likely with the neutropenia.
2. The patient remains febrile and stable but the source remains unknown. Failure to improve may result from poor immune response, a need for drainage or necessity to remove foreign bodies, the use of drugs without activity against the causative organism, or a noninfectious process including drug allergy (i.e., fever resulting from a drug such as phenytoin or an antimicrobial agent). The potential causative processes need to be aggressively reassessed on a regular basis by physical examination, history, cultures, and imaging techniques. Most centers add antifungal therapy empirically at day 4 to day 7 of therapy if patients remain febrile.6,40,52 Fluconazole, liposomal amphotericin B, caspofungin, or voriconazole may be used: In some situations fluconazole would be less attractive either because the patient has received fluconazole prophylaxis or because molds are suspected.41 The toxicity profile of amphotericin B, even in its liposomal form, has led many clinicians to prefer voriconazole or one of the echinocandins (i.e., caspofungin, micafungin, or anidulafungin).32,53–56
3. The patient deteriorates clinically but the source remains unknown. In this case continued evaluation for infectious and noninfectious sources of the infection should be pursued, and further empiric changes to the antimicrobial regimen should be considered.
4. The source of the infection is identified. The drug and the duration of therapy depend on the causative syndrome and microorganism. Table 53.3 lists some common scenarios. Rarely should the therapy be discontinued while the patient is neutropenic. Rarely is combination therapy necessary unless the causative organisms are multiple (suspected or confirmed) or (as described earlier) the causative organism is not highly susceptible to available antimicrobial agents.12–16,18–21,49
A common problem in febrile, neutropenic patients is managing indwelling intravascular lines.57,58 In general, these lines may be left in place initially if examination of the site reveals no indication of infection and the patient is hemodynamically stable. Blood cultures should be drawn through the catheter. Although some experts advocate drawing a culture through each port of each catheter, obtaining this many blood cultures is often not feasible because of time, cost, and volume of blood. If a patient is hemodynamically unstable and fails to respond promptly to fluid administration, it is prudent to remove the line in case an infected catheter is the source of the sepsis. Failure to remove the foreign body in this situation probably increases the likelihood of an unfavorable outcome. Should blood cultures become positive and should the suspicion be high that the catheter is the source, antibacterial therapy may be successful in some settings (e.g., if the pathogen is a bacterium that is relatively sensitive to antibacterial therapy), thus avoiding the need to remove the catheter. Situations suggesting that catheter removal is necessary include hemodynamic instability despite aggressive fluid resuscitation, tunnel infection, or infections resulting from fungi or relatively antibiotic-resistant bacteria such as P. aeruginosa.
Granulocyte transfusions have not been proved in randomized trials to improve survival in clinical settings probably because of the inability to administer a large number of cells with adequate frequency.16,17 However, many clinicians are convinced that matched white blood cell transfusions are helpful in managing life-threatening infections when patients are neutropenic and will use them when such cells are available. The manipulation of immune response with cytokines, cytokine inhibitors, or immunoglobulins is the subject of considerable investigation: Such interventions may reduce the duration of fever or the incidence of infections when used empirically, but in no setting have they been clearly shown to improve survival when administered after an infection has been documented. Algorithms for managing fever in neutropenic patients are provided in Figures 53.2 to 53.4. Table 53.3 suggests modifications of standard empiric regimens in certain common clinical scenarios.
Prevention of Infection
Given the experience with frequent and severe infectious complications in cancer patients with neutropenia, it has been logical to attempt to prevent infection. Most microorganisms causing disease in this patient population arise from endogenous gastrointestinal, cutaneous, or respiratory flora. Total protected environments probably reduce frequency of infection, but this approach is expensive and inconvenient. Trying to prove a consistent beneficial impact on survival has been difficult, and thus such isolation is rarely used anymore. Some experts are enthusiastic about placing patients in positive-pressure rooms so that pathogens do not enter via particles and droplets from outside the room. This type of isolation has not clearly improved outcome, however, and is not a standard of care.59,60 In Europe, there is more enthusiasm for such an approach than in the United States. Controversies over interpretation of data and concern that such antibiotic pressure will encourage the development of drug-resistant bacteria and fungi have diminished widespread acceptance in the United States.
Systemic antibacterial prophylaxis and systemic antifungal prophylaxis have been shown in some studies to reduce the number of infections, but their lack of effect on patient survival, their cost, and their impact on the emergence of resistance have made many clinicians reluctant to use them. Anti-Pneumocystis prophylaxis is, in contrast, highly effective in susceptible populations. Prophylaxis for CMV is rarely used unless the patient has received a solid organ or human stem cell transplant. Table 53.4 summarizes general strategies of infection prevention in immunosuppressed patients including patients with neutropenia.
Patients with HIV/AIDS
Opportunistic infections continue to occur in three groups of HIV-infected patients: (1) those who are unaware of their HIV status until they develop an opportunistic infection or tumor such as Pneumocystis pneumonia or Toxoplasma encephalitis or Kaposi sarcoma; (2) those who are unable or unwilling to receive appropriate therapy; and (3) those who fail antiretroviral therapy and opportunistic infection prophylaxis.4,61–65 In the United States, only about 20% to 40% of patients with HIV infection have a viral load under 50 copies/µL; thus, the majority of patients are not aware of their infection, not linked to care, or not able to adhere to an effective regimen.66 It is notable that half of all HIV-infected patients are located in 12 large cities: in those areas, patients frequently come to emergency rooms and ICUs with opportunistic infections that are preventable with earlier antiretroviral therapy plus anti-infective chemoprophylaxis if these patients were successfully engaged in care.67
Patients with HIV infection who are well controlled by antiretroviral drugs do not develop the classic complications of immunosuppression because their immunosuppression is subtle once their viral load is less than 50 copies/µL and their CD4 cell count rises, especially if it is greater than 200 cells/µL. Patients with CD4 counts greater than 200 cells/µL and viral loads less than 50 copies/µL may be seen in ICUs because of medical or surgical issues unrelated to their HIV infection. Such patients may also develop accelerated “processes of aging,” which include accelerated coronary artery disease, stroke, renal disease, or hepatic disease, but these processes appear to be related to enhanced chronic inflammation and not to immunosuppression.68–70
Management of Antiretroviral Drugs
For any HIV-infected patient in the ICU, clinicians must be cognizant of the need for careful management of antiretroviral drugs.71 If patients are not receiving antiretroviral drugs at the time of ICU admission, the ICU is not a desirable setting for initiating them: patient commitment to long-term adherence is difficult to assess when patients are critically ill, and drug toxicities and interactions will be hard to assess.72,73 There is virtually no indication to start antiretroviral therapy acutely in the ICU following the diagnosis of an acute opportunistic infection with the rare exception of rapidly progressive forms of untreatable diseases such as JC virus encephalitis or cryptosporidiosis.
Clinicians should refer to the Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents and the Guidelines for Antiretrovial Therapy in Adults and Adolescents for more detailed discussion on when to initiate antiretroviral therapy in the setting of a specific opportunistic infection.74
Diagnosis of Opportunistic Infections
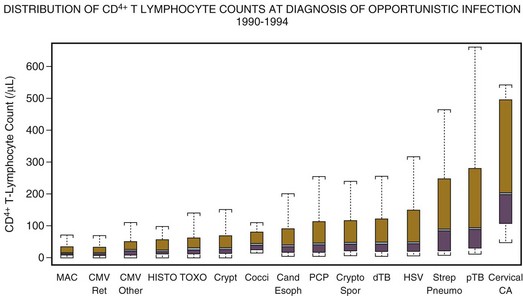
The CD4+ T lymphocyte cell number continues to be a useful marker for predicting the occurrence of opportunistic infections in patients with HIV infection.5 This relationship of CD4+ T lymphocyte count to the occurrence of opportunistic infection continues to be as valid in the era of antiretroviral therapy as it was before the licensing of the first antiretroviral agent, zidovudine, in 1987.75 Figure 53.5 demonstrates the typical relationship of CD4+ T lymphocyte counts to the occurrence of opportunistic infections. Knowledge of this relationship permits the focusing of diagnostic, therapeutic, and prophylactic management.
Keeping in mind that CD4+ T lymphocyte counts are useful predictors of susceptibility to infection is important, but they are not perfect. Occasionally, patients will develop opportunistic infections at “uncharacteristically” high CD4+ T lymphocyte counts. For instance, 5% to 10% of cases of pneumocystosis occur at CD4+ T lymphocyte counts greater than 200 cells/µL.76 Clinical parameters can provide additional clues; for example, oral candidiasis, a previous opportunistic infection, a prior episode of pneumonia, or high viral load are independent risk factors for the occurrence of Pneumocystis jiroveci pneumonia (PCP), and logically for other infections as well.
In evaluating the differential diagnosis of infectious syndromes in patients with HIV (and in every other patient population as well), geography is an important part of the history. Tuberculosis is always a concern because of the extraordinary susceptibility of HIV-infected patients for developing active disease once they have been exposed.77–79 It is notable, however, that although HIV and tuberculosis overlap in many patients in much of the developing world, in the United States only 10% of cases of tuberculosis occur in HIV-infected patients.80 In many urban settings in the United States, each pulmonary evaluation should include smears and cultures for M. tuberculosis, both to diagnose the appropriate cause of the pulmonary dysfunction and to assist in determining which respiratory precautions are appropriate. However, in the United States where only about 11,000 cases of new tuberculosis occur per year, and where 50% of cases occur in immigrants, the likelihood of tuberculosis in a U.S. native with no known exposure is quite low, in contrast to a recent immigrant from a highly endemic area.80
Clinical Syndromes
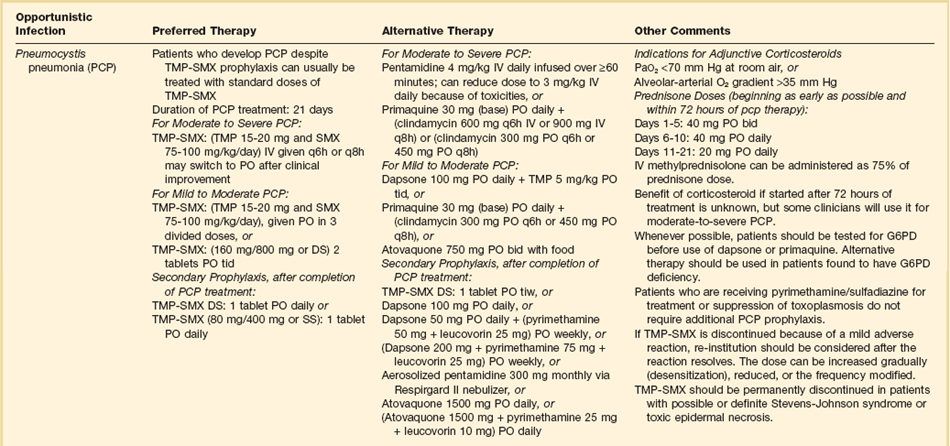
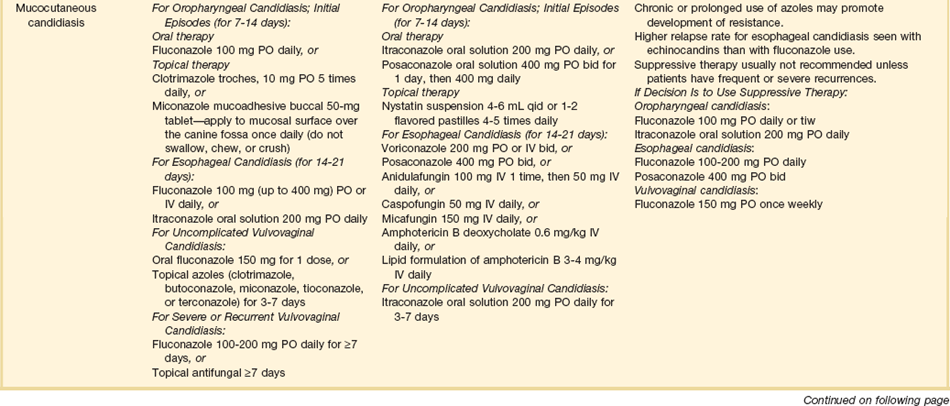
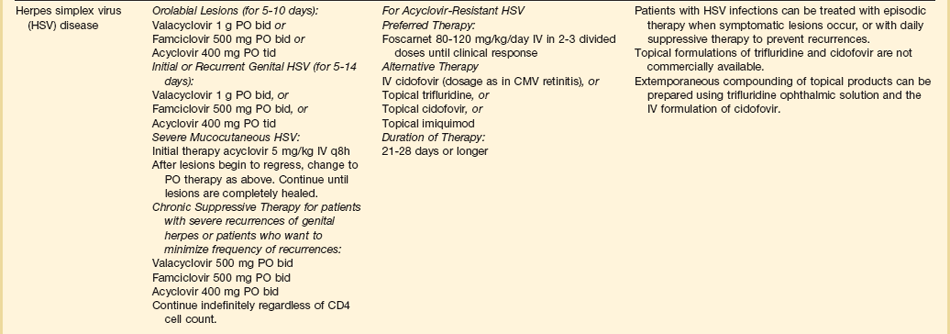
The therapies for specific opportunistic pathogens are summarized in Table 53.5.
Respiratory Insufficiency
Patients with HIV infection can develop severe pulmonary dysfunction because of common community-acquired pathogens such as S. pneumonia, Legionella, Mycoplasma, and Chlamydia; adenovirus; influenza; or respiratory syncytial virus, as well as other opportunistic viruses and fungi. Thus the diagnostic evaluation needs to be comprehensive, emphasizing direct smears of sputum or bronchoalveolar lavage. It is important to recognize that the clinical presentations produced by many causative agents can be similar. For instance, histoplasmosis, tuberculosis, and nonspecific interstitial pneumonitis can present identically to PCP.76,77,81,82 Thus although empiric diagnosis and empiric therapy may be reasonable as initial approaches to some patients with HIV infection and mild pneumonitis, such an approach is usually not appropriate for patients in an ICU.
Evaluation of induced sputum is the first step in the diagnostic approach to PCP. Sensitivity can be as high as 80% to 95% at many hospitals (at some institutions the yield is considerably lower).83 Specificity should be 100% in an experienced laboratory. Other pathogens, including mycobacteria, fungi, and routine bacteria, can be identified in sputum as well. For intubated patients, respiratory secretions obtained by deep intratracheal suctioning are also likely to be useful, although they have not been as carefully studied as induced sputum. Should the diagnosis not be established by evaluation of sputum or intratracheal secretions, bronchoscopy should be performed. Bronchoalveolar lavage should diagnose almost 100% of cases of PCP, even if patients have already received 7 to 10 days of empiric therapy at the time of the diagnostic procedure.76 A diagnosis of PCP is established by visualizing one or more clusters of organisms. Some laboratories are now using PCR to diagnose PCP, but this test is not standardized and is likely to be highly sensitive but not highly specific for identifying Pneumocystis as the cause of the pulmonary dysfunction.44,84
Diagnostic criteria for other opportunistic infections are reviewed in Chapters 12 and 42. CMV merits special mention. CMV pneumonia almost never occurs in patients with HIV infection, as opposed to patients with solid organ or stem cell transplants. CMV should be considered the cause only if other causative processes have been ruled out, and there is convincing histologic or cytologic evidence. Culture of sputum or bronchoalveolar lavage for CMV does not provide useful information: in particular, patients with CD4+ T lymphocyte counts below 100 cells/µL will predictably have CMV present in their secretion independent of whether or not pulmonary disease is present.85 A diagnosis of CMV pneumonia in this patient population is suggested by cytologic test and confirmed by the presence of multiple inclusion bodies in lung tissue obtained by transbronchial or open lung biopsy.
Therapy of opportunistic infections is summarized in Table 53.5.74 While awaiting a specific diagnosis, it is reasonable to initiate empiric therapy in patients ill enough to merit admission to an ICU. For patients with a CD4+ T lymphocyte count greater than 250 to 300 cells/µL, levofloxacin or moxifloxacin and ceftriaxone or azithromycin and ampicillin-sulbactam would be reasonable choices. For patients with CD4+ T lymphocyte counts below 200 to 250 cells/µL, levofloxacin or moxifloxacin plus trimethoprim-sulfamethoxazole or pentamidine plus levofloxacin or moxifloxacin would be potential regimens.
If PCP is documented, trimethoprim-sulfamethoxazole is always the drug of choice in patients who can tolerate it. Table 53.5 lists alternatives for sulfa-intolerant individuals. Regardless of which specific anti-Pneumocystis regimen is used, corticosteroid therapy is indicated for any patient who presents with an oxygen pressure (PO2) below 70 mm Hg or an alveolar-arterial gradient higher than 30 mm Hg.86–88 Patients with an initial PO2 lower than 70 mm Hg are the subgroup with substantial mortality risk for whom corticosteroids have been shown to provide a survival benefit. Corticosteroids also provide more rapid resolution of pulmonary manifestations in patients who present with better pulmonary function, but survival in this population is so high that clinical trials have not been able to show survival benefit and thus corticosteroids are not conventionally recommended for patients who present with a room air PO2 greater than 70 mm Hg. Some experts are concerned that corticosteroid use will be associated with reactivation of latent infections such as CMV or tuberculosis. However, reactivation of life-threatening infections has not been associated with this corticosteroid regimen.
Should patients with AIDS-related PCP be intubated and provided with mechanical ventilation? The mortality rate for such patient populations was 70% to 80% in several series in the early 1980s.89,90 Since that era, supportive care has improved, and treatment modalities for concurrent infectious and noninfectious processes have become more effective. Patient selection for ventilatory support is probably also improving. Patients who have multiple active opportunistic infections, substantial weight loss, and no response to 14 days of therapy have a worse prognosis than previously ambulatory patients who develop respiratory failure during the first few days of therapy. Thus decisions about ICU support for patients with HIV infection and respiratory failure need to be individualized on the basis of a realistic assessment of prognosis, the availability of resources, and the preference of the individual patient.
As indicated earlier, the ICU is not an ideal setting for initiating antiretroviral therapy. It should be noted, however, that for PCP, early initiation of antiretroviral therapy is generally associated with increased survival and thus therapy generally should not be delayed.73 However, the studies that have been done had very few patients with life-threatening manifestations of their acute opportunistic infection. Thus, for patients ill enough to be in the ICU, the initiation of antiretroviral therapy might be associated with an immune reconstitution syndrome that could make respiratory support difficult or impossible. Moreover, patients in the ICU may not be able to absorb oral antiretroviral therapy, or might have complicated drug interactions with other necessary drugs. Thus, decisions to start antiretroviral therapy in the ICU require considerable thought and do not lend themselves to simple algorithms.
Central Nervous System Dysfunction
Meningitis
In HIV-infected patients with CD4+ counts greater than 200 cells/µL, the causes of meningitis do not differ markedly from those in the normal population: S. pneumonia and Neisseria meningitidis are the most common causes. For patients with CD4+ counts lower than 100 cells/µL, Cryptococcus neoformans is common.91
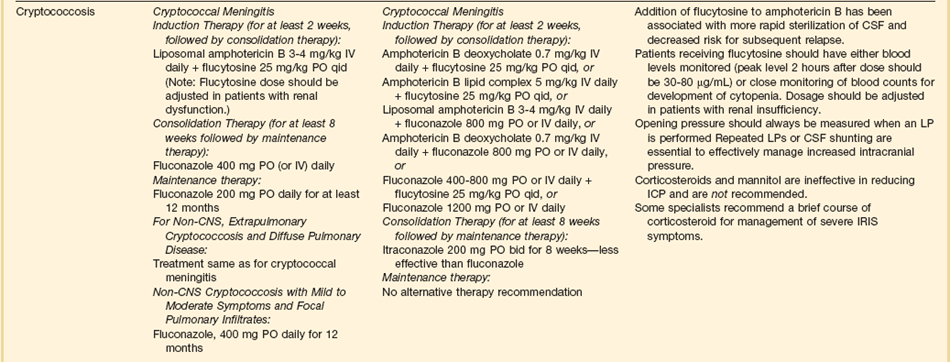
The therapy of choice for cryptococcal meningitis is liposomal amphotericin B for at least 2 weeks, plus flucytosine. Fluconazole should not be used for initial therapy, although it can be used after the initial 2 weeks if patients have a good clinical response.92
Some patients with cryptococcal meningitis have symptomatic elevations of CSF pressure. Such patients should have therapeutic lumbar punctures to remove enough CSF to reduce the pressure to 20 to 25 cm H2O. Multiple lumbar punctures may be needed. If after multiple lumbar punctures the patient still have symptomatic elevation of CSF pressure greater than 20 to 25 cm H2O, the insertion of a ventricular shunt should be considered, although there are no specific guidelines regarding when to place such a shunt. Corticosteroids should not generally be used to treat elevated intracranial pressure in this situation.93–95
Focal Central Nervous System Lesions
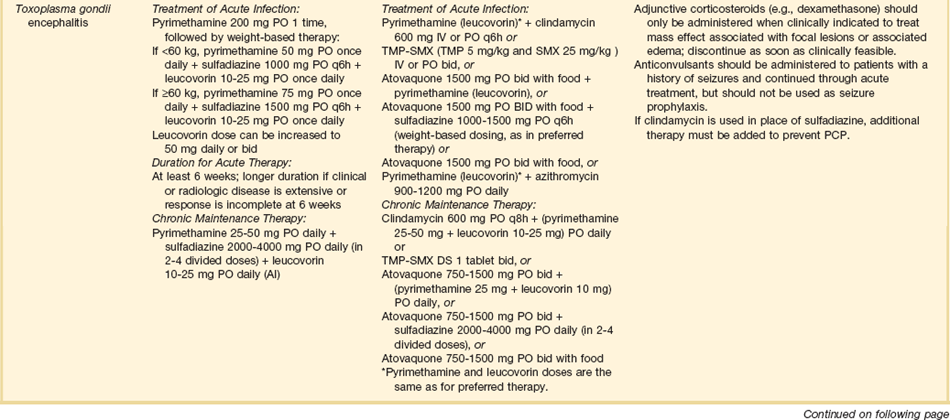
Patients with HIV infection and CD4 counts less than 100 cells/µL may present with focal motor lesions, altered mental status, or seizures. Although the differential diagnosis is extensive, the major considerations are toxoplasmosis and lymphoma.96,97
All patients with CNS toxoplasmosis are IgG seropositive for toxoplasmosis using sensitive assays. However, some laboratories use less sensitive assays and thus some patients may appear to be seronegative. If lumbar puncture can be performed, a PCR for toxoplasmosis is positive in about 50% of cases, although there is no standardization of these Toxoplasma PCRs, making results variable from laboratory to laboratory.98 The standard practice for HIV-infected patients with CD4 counts less than 200 cells/µL is to undergo an empiric trial of oral sulfadiazine plus pyrimethamine plus leucovorin. Most patients who have toxoplasmosis will show clinical and radiologic improvement within 2 weeks. If such improvement has not been documented, and the diagnosis is in doubt, a needle biopsy of the intracranial lesion should be considered.99–101
CNS lymphoma can present identically to CNS lymphoma. A negative serum IgG for toxoplasmosis, and a negative CSF toxoplasma PCR should suggest lymphoma.102 Most patients with CNS lymphoma will have a positive CSF PCR for EBV (Epstein-Barr virus), although this test is neither 100% sensitive nor 100% specific for lymphoma. Patients who are CSF EBV PCR negative or in whom the diagnosis is uncertain may require a brain biopsy to document the lymphoma.
Focal White Matter Lesions
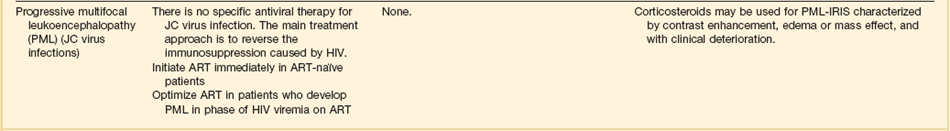
In patients with HIV, JC virus encephalitis (also known as progressive multifocal encephalopathy) causes focal white matter lesions that are associated with progressive motor, sensory, and neurocognitive dysfunction.103 A small fraction of patients can present with rapidly progressive cognitive decline.
The CT scan or MRI images of JC virus encephalitis are characteristic of this disease. Diagnosis is usually confirmed by CSF PCR for JC virus. There is no specific therapy. Patients may stabilize or improve if antiretroviral therapy results in immune reconstitution.104
Diffuse Encephalopathy
CMV encephalitis, in contrast, is rapid in progression and has a neutrophilic pleocytosis in the CSF and a positive CSF PCR for CMV.105,106 Imaging shows either periventricular enhancement or focal nodules. Therapy is typically not successful in reversing the cerebral dysfunction. Either ganciclovir, foscarnet, or a combination of both drugs can be used in addition to the institution of antiretroviral therapy.
Hypotension
Patients with HIV infection develop hypotension resulting from the same types of disorders as with non-HIV-infected individuals—sepsis from a primary infection or a wound or device (especially an intravascular access device), fluid depletion from vomiting or diarrhea, and hemorrhage from a gastrointestinal lesion are examples of common causes. The evaluation of hypotension in a patient with HIV infection must take into account factors particular to this patient population: HIV-infected patients are susceptible to opportunistic infections; they undergo many procedures that can be associated with infectious complications; and they receive an array of drugs, some of which have cardiovascular effects. Thus, evaluating hypotension in this patient population requires a comprehensive and thorough approach. A differential diagnosis of the major causes is shown in Table 53.6. Adrenal function always deserves special attention because several viral processes, fungal and mycobacterial diseases, HIV, and drugs can suppress the adrenal axis and either cause hypotension or exacerbate it.
Prevention of Opportunistic Infection
All patients with HIV infection and CD4+ T lymphocyte counts below 200 cells/µL typically receive anti-Pneumocystis prophylaxis. Trimethoprim-sulfamethoxazole is the regimen of choice. Patients who actually take this drug have very few breakthroughs of PCP and receive considerable protection against toxoplasmosis and certain routine bacterial infections. Alternative regimens include monthly dapsone, weekly dapsone-pyrimethamine, or daily aerosol pentamidine. Prophylaxis against M. avium complex is recommended for patients with CD4+ T lymphocyte counts under 100 cells/µL; clarithromycin and azithromycin are currently the drugs of choice.74
HIV Transmission in the Intensive Care Unit
Transmission of HIV is an issue that requires attention in the ICU.107–109 No evidence exists that HIV-infected health care professionals can infect patients, regardless of what procedure they perform, with the exception of two unusual and unexplained events. Intensivists should realize that although there are no federal policies defining how HIV-infected practitioners should be credentialed and monitored, many hospitals have policies and procedures. Some are modeled after guidelines from the Society of Healthcare Epidemiology.108,110
HIV patients pose a risk to health care professionals.107–110 This risk can be substantially reduced by staff education, by strict monitoring for compliance with universal precautions, and by having proper equipment. Almost all HIV transmission has occurred in an occupational setting as a result of injuries involving sharp instruments (e.g., needles, scalpels). The risk of such injuries is about one case of HIV transmission per 250 injuries, but the likelihood of transmission in an individual accident depends on the amount of viremia at the time of the accident (late-stage patients generally have more circulating virus than do early-stage patients) and the nature of the accident. Most authorities recommend immediate prophylaxis if a significant injury occurs involving an HIV-infected patient. Considerable debate exists over the optimal choice of drugs and the optimal duration of therapy, but it is clear that initiating therapy within a period of hours rather than days is best. Many authorities now advocate an antiretroviral regimen for any situation when the patient and health care provider determine that therapy is appropriate, and continue that for 4 to 6 weeks. If providers have questions about appropriate medical treatment for occupational exposures, 24-hour assistance is available from the Clinicians’ Post Exposure Prophylaxis Hotline (PEPline) at 1-888-448-4911 (http://www.nccc.ucsf.edu).111
Human Stem Cell, Bone Marrow, and Solid Organ Transplant Recipients
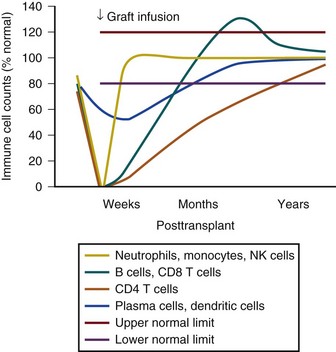
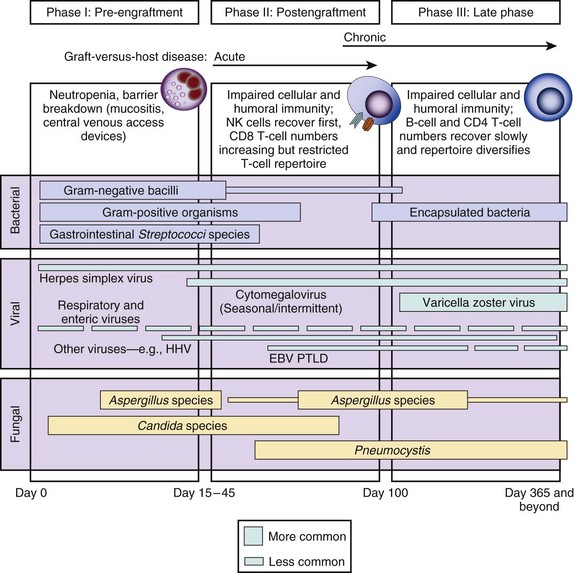
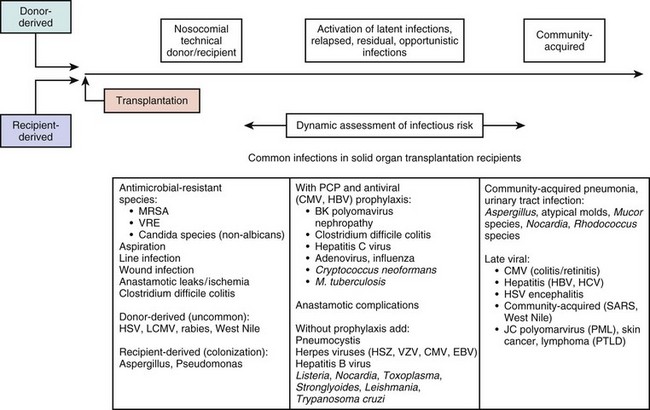
Timelines are useful to provide clinicians with a general understanding of when infectious and noninfectious complications occur after a transplant procedure. Figure 53.6 demonstrates the typical return of cells and cell function after a stem cell transplant. Figures 53.7 and 53.8 demonstrate timelines typical for stem cell and solid organ transplant infections. However, the occurrence of complications will vary depending on the details of transplant management that may be unique to an institution or a specific protocol, so that these figures should not be considered as inviolable.
• The risk of infection is related to the “net state of immunosuppression,” which is related to the function of the patient’s pretransplant immune status, conditioning regimens, antirejection chemotherapy, as well as severity of illness and nutritional status. Net immunosuppression cannot be measured easily: it involves the number and function of neutrophils, T lymphocytes, and B lymphocytes. Although neutrophil number is a highly reliable surrogate for susceptibility to bacterial diseases, measures of cellular immune function, which are relevant to many viral and fungal pathogens, are not reliable surrogates in terms of sensitivity or specificity for predicting disease. Thus, susceptibility to viral and fungal pathogens is more difficult to predict.
• The risk of noninfectious complications is dependent on the organ transplanted and the immunosuppressive regimen used.
• Distinguishing infectious from noninfectious complications can be challenging, emphasizing the need for a broad consideration of causes of fever, hypotension, or organ-related syndromes, and the institution of prompt diagnostic tests that focus on both infectious and noninfectious causes.
• With solid organ transplants or stem cell transplants, the donor is a source of infection if the donor had an unrecognized transmissible infection at the time of organ donation, or if the donor had a latent infection that was transmitted with the donated organ.
• With solid organ transplants, complications of the surgical procedure must be considered when postoperative complications occur.
• Organ transplant recipients characteristically have extensive contact with health care environments and may have been receiving antimicrobial prophylaxis: these factors influence the spectrum of likely causative organisms.
• Diagnostic studies for infections rely more and more on molecular testing, although tissue biopsy and cultures of blood and suspicious body fluids and anatomic areas remain important. Tissue biopsies can be especially useful for differentiating infectious and noninfectious causes.
• Drug levels and microbial markers of infection must be carefully monitored to enhance the likelihood of effective therapy and minimize the likelihood of drug-related toxicities.
Infectious Complications of Transplantation
Pneumocystis Pneumonia
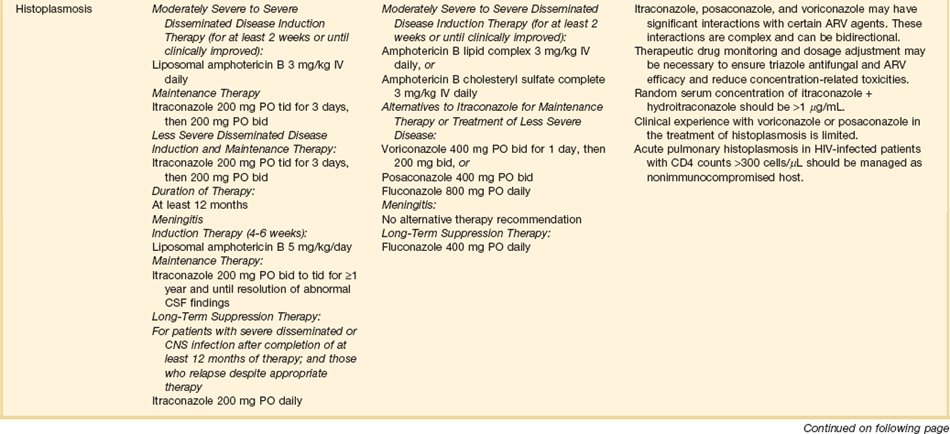
PCP has been reported in recipients of most types of organ transplants. Most organ transplant programs use PCP prophylaxis during the period of perceived susceptibility, although there can be underappreciation of the duration of true risk, leading to premature discontinuation of prophylaxis.30,112 Trimethoprim-sulfamethoxazole is usually the prophylactic agent of choice because it is more effective than other agents, is well tolerated, and reduces the frequency of urinary tract infections and other potential complications (e.g., disease resulting from Nocardia, S. pneumoniae, and Haemophilus organisms). However, trimethoprim-sulfamethoxazole is moderately immunosuppressive, and thus some human stem cell transplant programs prefer aerosolized pentamidine or oral atovaquone for prophylaxis.
Fungal Infections
The spectrum of causative fungal organisms is changing because of changes in antifungal prophylactic regimens.113,114 With the use of fluconazole prophylaxis, Candida albicans infections became less common and molds, especially Aspergillus, became more important pathogens, as did fluconazole-resistant Candida. Some programs are now using voriconazole prophylaxis, which has resulted in the development of disease due to voriconazole-resistant molds such as Mucor and certain non-albicans Candida. Thus, clinicians must know which antifungal prophylaxis has been used in order to anticipate which complications will occur.
Mold infections are best documented by tissue biopsy, although such biopsies are not always feasible due to the anatomic location of the suspicious lesion, the severity of patient illness, or the presence of a severe coagulopathy. Serologic tests are not yet highly sensitive and specific. Although some clinicians are enthusiastic about using the galactomannan test in the bronchoalveolar lavage or blood, this test is not optimally sensitive for molds, especially when used on serum.115–118 Many clinicians are less enthusiastic about the serum β-glucan test, which was popular as a test for fungal disease, but which is increasingly seen as insensitive, nonspecific, and subject to laboratory variation.63,119–123
Respiratory Viruses Including Respiratory Syncytial Virus
RSV is one respiratory virus that is opportunistic, however, in stem cell transplant recipients. Although RSV can, like other community-acquired viruses, cause disease in any patient population, it is especially lethal in those with stem cell transplants. Thus, RSV must be specifically sought in this patient population, as well as their visitors and health care providers, so that it does not spread to highly susceptible patients. RSV is best diagnosed by nasopharyngeal washes and molecular testing or by molecular testing of bronchoalveolar lavage.124–126
Many clinicians treat RSV pneumonia in transplant recipients with aerosolized ribavirin, monoclonal antibody against RSV, or both, but the efficacy of these regimens is controversial.127,128
Noninfectious Complications in Human Stem Cell Transplant Recipients
Toxicities of Immunosuppressive Drugs
Diagnosis and therapy of opportunistic infections and nosocomial infections should follow the guidelines given in Chapters 51 to 53 and 55. In choosing therapies, attention must be focused on the toxicities of antimicrobial agents and how they influence the outcome of the transplanted organ. In addition, drug interactions are important, especially with cyclosporine. Drugs that alter hepatic metabolism, such as rifampin, rifabutin, and fluconazole, can have substantial influence on cyclosporine levels and thus need to be used with careful pharmacologic attention. Finally, clinicians must recognize that new immunosuppressive regimens and changing prophylactic regimens are changing the spectrum of infectious complications. As mentioned earlier, fungal infections are increasingly likely to be caused by species other than C. albicans: non-albicans Candida, Fusarium, and Rhizopus are recognized with increasing frequency. Similarly, prophylaxis with valganciclovir is reducing CMV disease and pushing disease that does occur later and later in relation to the transplant procedure. Viruses such as HHV-6 and BK virus are causing disease. Thus, clinicians need to look for a changing spectrum of pathogens, as well as changing manifestations if the morbidity and death caused by infection are to be managed optimally.
References
1. Dieffenbach, CW, Tramont, EC, Plaeger, S. Innate (general or nonspecific) host defense mechanisms. In Mandell GL, Bennett JE, Dolin R, eds. : Principles and Practices of Infectious Diseases, 7th ed, Philadelphia: Elsevier Churchill Livingstone, 2010.
2. Davies, JM, Barnes, R, Milligan, D. Update of guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen. Clin Med. 2002; 2(5):440–443.
3. Di Sabatino, A, Carsetti, R, Corazza, GR. Post-splenectomy and hyposplenic states. Lancet. 2011; 378(9785):86–97.
4. Kaplan, JE, Benson, C, Holmes, KH, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009; 58(RR-4):1–207.
5. Masur, H, Ognibene, FP, Yarchoan, R, et al. CD4 counts as predictors of opportunistic pneumonias in human immunodeficiency virus (HIV) infection. Ann Intern Med. 1989; 111(3):223–231.
6. Freifeld, AG, Bow, EJ, Sepkowitz, KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011; 52(4):e56–93.
7. Orange, JS, Hossny, EM, Weiler, CR, et al. Use of intravenous immunoglobulin in human disease: A review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006; 117(4 Suppl):S525–S553.
8. Kumar, A, Roberts, D, Wood, KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006; 34(6):1589–1596.
9. Zubert, S, Funk, DJ, Kumar, A. Antibiotics in sepsis and septic shock: Like everything else in life, timing is everything. Crit Care Med. 2010; 38(4):1211–1212.
10. De Jongh, CA, Joshi, JH, Newman, KA, et al. Antibiotic synergism and response in gram-negative bacteremia in granulocytopenic cancer patients. Am J Med. 1986; 80(5C):96–100.
11. Kontoyiannis, DP, Ratanatharathorn, V, Young, JA, et al. Micafungin alone or in combination with other systemic antifungal therapies in hematopoietic stem cell transplant recipients with invasive aspergillosis. Transplant Infect Dis. 2009; 11(1):89–93.
12. Paul, M, Silbiger, I, Grozinsky, S, et al. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev. (1):2006.
13. Paul, M, Soares-Weiser, K, Grozinsky, S, Leibovici, L. Beta-lactam versus beta-lactam-aminoglycoside combination therapy in cancer patients with neutropaenia. Cochrane Database Syst Rev. (2):2002.
14. Paul, M, Soares-Weiser, K, Grozinsky, S, Leibovici, L. Beta-lactam versus beta-lactam-aminoglycoside combination therapy in cancer patients with neutropaenia. Cochrane Database Syst Rev. (3):2003.
15. Paul, M, Soares-Weiser, K, Leibovici, L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for fever with neutropenia: Systematic review and meta-analysis. BMJ. 2003; 326(7399):1111.
16. Massey, E, Paulus, U, Doree, C, Stanworth, S. Granulocyte transfusions for preventing infections in patients with neutropenia or neutrophil dysfunction. Cochrane Database Syst Rev. (1):2009.
17. Smith, TJ, Khatcheressian, J, Lyman, GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. J Clin Oncol. 2006; 24(19):3187–3205.
18. Bodey, GP, Buckley, M, Sathe, YS, Freireich, EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966; 64(2):328–340.
19. Murray, BE. Vancomycin-resistant enterococcal infections. N Engl J Med. 2000; 161:397.
20. Paul, M, Borok, S, Fraser, A, et al. Additional anti-gram-positive antibiotic treatment for febrile neutropenic cancer patients. Cochrane Database Syst Rev. (3):2005.
21. Wade, JC, Schimpff, SC, Newman, KA, Wiernik, PH. Staphylococcus epidermidis: An increasing cause of infection in patients with granulocytopenia. Ann Intern Med. 1982; 97(4):503–508.
22. Centers for Disease Control and Prevention (CDC). Update: Detection of a verona integron-encoded metallo-beta-lactamase in Klebsiella pneumoniae—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010; 59(37):1212.
23. Deshpande, P, Shetty, A, Kapadia, F, et al. New Delhi metallo 1: Have carbapenems met their doom? Clin Infect Dis. 2010; 51(10):1222.
24. Herbert, S, Halvorsen, DS, Leong, T, et al. Large outbreak of infection and colonization with gram-negative pathogens carrying the metallo-beta-lactamase gene blaIMP-4 at a 320-bed tertiary hospital in Australia. Infect Control Hosp Epidemiol. 2007; 28(1):98–101.
25. Sidjabat, H, Nimmo, GR, Walsh, TR, et al. Carbapenem resistance in Klebsiella pneumoniae due to the New Delhi metallo-beta-lactamase. Clin Infect Dis. 2011; 52(4):481–484.
26. Yong, D, Toleman, MA, Giske, CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009; 53(12):5046–5054.
27. Dugdale, DC, Ramsey, PG. Staphylococcus aureus bacteremia in patients with Hickman catheters. Am J Med. 1990; 89(2):137–141.
28. Fowler, VG, Jr., Sanders, LL, Sexton, DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: Experience with 244 patients. Clin Infect Dis. 1998; 27(3):478–486.
29. Bucaneve, G, Micozzi, A, Menichetti, F, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med. 2005; 353(10):977–987.
30. Centers for Disease Control and Prevention. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Recommendations of CDC, the Infectious Disease Society of America, and the American Society of Blood and Marrow Transplantation. MMWR. 2000; 49(RR10):1–128.
31. Klastersky, J. Antifungal therapy in patients with fever and neutropenia—More rational and less empirical? N Engl J Med. 2004; 351(14):1445–1447.
32. Walsh, TJ, Teppler, H, Donowitz, GR, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med. 2004; 351(14):1391–1402.
33. Wingard, JR, White, MH, Anaissie, E, et al. A randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. L Amph/ABLC Collaborative Study Group. Clin Infect Dis. 2000; 31(5):1155–1163.
34. Bergamasco, MD, Barroso Barbosa, M, de Oliveira Garcia, D, et al. Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae in solid organ transplantation. Transplant Infect Dis. 2012; 14(2):198–205.
35. Bush, K. Bench-to-bedside review: The role of beta-lactamases in antibiotic-resistant gram-negative infections. Crit Care. 2010; 14(3):224.
36. Gupta, N, Limbago, BM, Patel, JB, Kallen, AJ. Carbapenem-resistant Enterobacteriaceae: Epidemiology and prevention. Clin Infect Dis. 2011; 53(1):60–67.
37. Qureshi, ZA, Paterson, DL, Potoski, BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: Superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012; 56(4):2108–2113.
38. Tumbarello, M, Viale, P, Viscoli, C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae: Importance of combination therapy. Clin Infect Dis. 2012; 55(7):943–950.
39. Center for International Blood and Marrow Transplant Research (CIBMTR), National Marrow Donor Program (NMDP), European Blood and Marrow Transplant Group (EBMT), et al. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: A global perspective. Bone Marrow Transplant. 2009; 44(8):453–558.
40. Madureira, A, Bergeron, A, Lacroix, C, et al. Breakthrough invasive aspergillosis in allogeneic haematopoietic stem cell transplant recipients treated with caspofungin. Int J Antimicrob Agents. 2007; 30(6):551–554.
41. Marty, FM, Cosimi, LA, Baden, LR. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stem-cell transplants. N Engl J Med. 2004; 350(9):950–952.
42. Segal, BH, Almyroudis, NG, Battiwalla, M, et al. Prevention and early treatment of invasive fungal infection in patients with cancer and neutropenia and in stem cell transplant recipients in the era of newer broad-spectrum antifungal agents and diagnostic adjuncts. Clin Infect Dis. 2007; 44(3):402–409.
43. Botterel, F, Cabaret, O, Foulet, F, et al. Clinical significance of quantifying Pneumocystis jiroveci DNA by using real-time PCR in bronchoalveolar lavage fluid from immunocompromised patients. J Clin Microbiol. 2012; 50(2):227–231.
44. Larsen, HH, Huang, L, Kovacs, JA, et al. A prospective, blinded study of quantitative touch-down polymerase chain reaction using oral-wash samples for diagnosis of Pneumocystis pneumonia in HIV-infected patients. J Infect Dis. 2004; 189(9):1679–1683.
45. Samuel, CM, Whitelaw, A, Corcoran, C, et al. Improved detection of Pneumocystis jiroveci in upper and lower respiratory tract specimens from children with suspected Pneumocystis pneumonia using real-time PCR: A prospective study. BMC Infect Dis. 2011; 11:329.
46. Freifeld, AG, Walsh, T, Marshall, D, et al. Monotherapy for fever and neutropenia in cancer patients: A randomized comparison of ceftazidime versus imipenem. J Clin Oncol. 1995; 13(1):165–176.
47. Herbrecht, R, Denning, DW, Patterson, TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002; 347(6):408–415.
48. Jaksic, B, Martinelli, G, Perez-Oteyza, J, et al. Efficacy and safety of linezolid compared with vancomycin in a randomized, double-blind study of febrile neutropenic patients with cancer. Clin Infect Dis. 2006; 42(5):597–607.
49. Paul, M, Borok, S, Fraser, A, et al. Empirical antibiotics against gram-positive infections for febrile neutropenia: Systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2005; 55(4):436–444.
50. Vardakas, KZ, Samonis, G, Chrysanthopoulou, SA, et al. Role of glycopeptides as part of initial empirical treatment of febrile neutropenic patients: A meta-analysis of randomised controlled trials. Lancet Infect Dis. 2005; 5(7):431–439.
51. Vazquez, JA. Anidulafungin: A new echinocandin with a novel profile. Clin Ther. 2005; 27(6):657–673.
52. Marr, KA, Boeckh, M, Carter, RA, et al. Combination antifungal therapy for invasive aspergillosis. Clin Infect Dis. 2004; 39(6):797–802.
53. Toubai, T, Tanaka, J, Ota, S, et al. Efficacy and safety of micafungin in febrile neutropenic patients treated for hematological malignancies. Intern Med. 2007; 46(1):3–9.
54. Walsh, TJ, Finberg, RW, Arndt, C, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med. 1999; 340(10):764–771.
55. Walsh, TJ, Pappas, P, Winston, DJ, et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med. 2002; 346(4):225–234.
56. Yanada, M, Kiyoi, H, Murata, M, et al. Micafungin, a novel antifungal agent, as empirical therapy in acute leukemia patients with febrile neutropenia. Intern Med. 2006; 45(5):259–264.
57. Mermel, LA, Farr, BM, Sherertz, RJ, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001; 32(9):1249–1272.
58. O’Grady, NP, Alexander, M, Burns, LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011; 39(4 Suppl 1):S1–34.
59. Oostdijk, EA, de Smet, AM, Kesecioglu, J, Bonten, MJ. The role of intestinal colonization with gram-negative bacteria as a source for intensive care unit-acquired bacteremia. Crit Care Med. 2011; 39(5):961–966.
60. Quigley, EM. Passing the bug—translocation, bacteremia, and sepsis in the intensive care unit patient: Is intestinal decontamination the answer? Crit Care Med. 2011; 39(5):1202–1203.
61. Crothers, K, Huang, L, Goulet, JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011; 183(3):388–395.
62. Huang, L, Quartin, A, Jones, D, Havlir, DV. Intensive care of patients with HIV infection. N Engl J Med. 2006; 355(2):173–181.
63. Morris, A, Masur, H, Huang, L. Current issues in critical care of the human immunodeficiency virus-infected patient. Crit Care Med. 2006; 34(1):42–49.
64. Palella, FJ, Jr., Delaney, KM, Moorman, AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998; 338(13):853–860.
65. Rosen, MJ, Narasimhan, M. Critical care of immunocompromised patients: Human immunodeficiency virus. Crit Care Med. 2006; 34(9 Suppl):S245–S250.
66. Mugavero, MJ, Norton, WE, Saag, MS. Health care system and policy factors influencing engagement in HIV medical care: Piecing together the fragments of a fractured health care delivery system. Clin Infect Dis. 2011; 52(Suppl 2):S238–S246.
67. Hall, HI, Espinoza, L, Benbow, N, Hu, YW. Epidemiology of HIV infection in large urban areas in the United States. PLoS One. 5(9), 2010.
68. Guaraldi, G, Zona, S, Alexopoulos, N, et al. Coronary aging in HIV-infected patients. Clin Infect Dis. 2009; 49(11):1756–1762.
69. Kalyani, R, Thej, MJ, Prabhakar, K, Kiran, J. Accelerated atherosclerosis in a human immunodeficiency virus infected patient not on highly active anti-retroviral therapy: An autopsy case report. J Cardiovasc Dis Res. 2011; 2(4):241–243.
70. Worm, SW, Kamara, DA, Reiss, P, et al. Evaluation of HIV protease inhibitor use and the risk of sudden death or nonhemorrhagic stroke. J Infect Dis. 2012; 205(4):535–539.
71. Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Washington, DC, 2012. http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf
72. Grant, PM, Zolopa, AR. When to start ART in the setting of acute AIDS-related opportunistic infections: The time is now!. Curr HIV/AIDS Rep. 2012; 9(3):251–258.
73. Zolopa, A, Andersen, J, Powderly, W, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: A multicenter randomized strategy trial. PLoS One. 4(5), 2009.
74. Kaplan, J, Benson, C, Holmes, K, et al. Guidelines for the management of opportunistic infections among HIV-infected adolescents and adults. www.aidsinfo.nih.gov, 2012. [Accessed on June 1, 2013 available at].
75. Miller, V, Mocroft, A, Reiss, P, et al. Relations among CD4 lymphocyte count nadir, antiretroviral therapy, and HIV-1 disease progression: results from the EuroSIDA study. Ann Intern Med. 1999; 130(7):570–577.
76. Kovacs, JA, Masur, H. Prophylaxis against opportunistic infections in patients with human immunodeficiency virus infection. N Engl J Med. 2000; 342(19):1416–1429.
77. Barnes, PF, Steele, MA, Young, SM, Vachon, LA. Tuberculosis in patients with human immunodeficiency virus infection. How often does it mimic Pneumocystis carinii pneumonia? Chest. 1992; 102(2):428–432.
78. Havlir, DV, Getahun, H, Sanne, I, Nunn, P. Opportunities and challenges for HIV care in overlapping HIV and TB epidemics. JAMA. 2008; 300(4):423–430.
79. Swaminathan, S, Padmapriyadarsini, C, Narendran, G. HIV-associated tuberculosis: Clinical update. Clin Infect Dis. 2010; 50(10):1377–1386.
80. Gordin, FM, Masur, H. Current approaches to tuberculosis in the United States. JAMA. 2012; 308(3):283–289.
81. Havlir, DV, Barnes, PF. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1999; 340(5):367–373.
82. Wheat, LJ, Connolly-Stringfield, PA, Baker, RL, et al. Disseminated histoplasmosis in the acquired immune deficiency syndrome: Clinical findings, diagnosis and treatment, and review of the literature. Medicine (Baltimore). 1990; 69(6):361–374.
83. Kovacs, JA, Ng, VL, Masur, H, et al. Diagnosis of Pneumocystis carinii pneumonia: Improved detection in sputum with use of monoclonal antibodies. N Engl J Med. 1988; 318(10):589–593.
84. Hauser, PM, Bille, J, Lass-Florl, C, et al. Multicenter, prospective clinical evaluation of respiratory samples from subjects at risk for Pneumocystis jiroveci infection by use of a commercial real-time PCR assay. J Clin Microbiol. 2011; 49(5):1872–1878.
85. Zurlo, JJ, O’Neill, D, Polis, MA, et al. Lack of clinical utility of cytomegalovirus blood and urine cultures in patients with HIV infection. Ann Intern Med. 1993; 118(1):12–17.
86. Bozzette, SA, Sattler, FR, Chiu, J, et al. A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. California Collaborative Treatment Group. N Engl J Med. 1990; 323(21):1451–1457.
87. Gagnon, S, Boota, AM, Fischl, MA, et al. Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A double-blind, placebo-controlled trial. N Engl J Med. 1990; 323(21):1444–1450.
88. Montaner, JS, Lawson, LM, Levitt, N, et al. Corticosteroids prevent early deterioration in patients with moderately severe Pneumocystis carinii pneumonia and the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1990; 113(1):14–20.
89. Wachter, RM, Luce, JM, Hopewell, PC. Critical care of patients with AIDS. JAMA. 1992; 267(4):541–547.
90. Wachter, RM, Russi, MB, Bloch, DA, et al. Pneumocystis carinii pneumonia and respiratory failure in AIDS. Improved outcomes and increased use of intensive care units. Am Rev Respir Dis. 1991; 143(2):251–256.
91. Park, BJ, Wannemuehler, KA, Marston, BJ, et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009; 23(4):525–530.
92. Jarvis, JN, Harrison, TS. HIV-associated cryptococcal meningitis. AIDS. 2007; 21(16):2119–2129.
93. Bach, MC, Tally, PW, Godofsky, EW. Use of cerebrospinal fluid shunts in patients having acquired immunodeficiency syndrome with cryptococcal meningitis and uncontrollable intracranial hypertension. Neurosurgery. 1997; 41(6):1280–1282.
94. Claus, JJ, Portegies, P. Reversible blindness in AIDS-related cryptococcal meningitis. Clin Neurol Neurosurg. 1998; 100(1):51–52.
95. Fessler, RD, Sobel, J, Guyot, L, et al. Management of elevated intracranial pressure in patients with cryptococcal meningitis. J Acquir Immune Defic Syndr Hum Retrovirol. 1998; 17(2):137–142.
96. d’Arminio Monforte, A, Cinque, P, Mocroft, A, et al. Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann Neurol. 2004; 55(3):320–328.
97. Gray, F, Chretien, F, Vallat-Decouvelaere, AV, Scaravilli, F. The changing pattern of HIV neuropathology in the HAART era. J Neuropathol Exp Neurol. 2003; 62(5):429–440.
98. Mesquita, RT, Ziegler, AP, Hiramoto, RM, et al. Real-time quantitative PCR in cerebral toxoplasmosis diagnosis of Brazilian human immunodeficiency virus-infected patients. J Med Microbiol. 2010; 59(Pt 6):641–647.
99. Dannemann, B, McCutchan, JA, Israelski, D, et al. Treatment of toxoplasmic encephalitis in patients with AIDS. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. The California Collaborative Treatment Group. Ann Intern Med. 1992; 116(1):33–43.
100. Katlama, C, De Wit, S, O’Doherty, E, et al. Pyrimethamine-clindamycin vs. pyrimethamine-sulfadiazine as acute and long-term therapy for toxoplasmic encephalitis in patients with AIDS. Clin Infect Dis. 1996; 22(2):268–275.
101. Nath, A, Sinai, AP. Cerebral toxoplasmosis. Curr Treat Options Neurol. 2003; 5(1):3–12.
102. Ivers, LC, Kim, AY, Sax, PE. Predictive value of polymerase chain reaction of cerebrospinal fluid for detection of Epstein-Barr virus to establish the diagnosis of HIV-related primary central nervous system lymphoma. Clin Infect Dis. 2004; 38(11):1629–1632.
103. Padgett, BL, Walker, DL, ZuRhein, GM, et al. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971; 1(7712):1257–1260.
104. Engsig, FN, Hansen, AB, Omland, LH, et al. Incidence, clinical presentation, and outcome of progressive multifocal leukoencephalopathy in HIV-infected patients during the highly active antiretroviral therapy era: A nationwide cohort study. J Infect Dis. 2009; 199(1):77–83.
105. Miller, RF, Lucas, SB, Hall-Craggs, MA, et al. Comparison of magnetic resonance imaging with neuropathological findings in the diagnosis of HIV and CMV associated CNS disease in AIDS. J Neurol Neurosurg Psychiatry. 1997; 62(4):346–351.
106. Moulignier, A, Mikol, J, Gonzalez-Canali, G, et al. AIDS-associated cytomegalovirus infection mimicking central nervous system tumors: A diagnostic challenge. Clin Infect Dis. 1996; 22(4):626–631.
107. Baggaley, RF, Boily, MC, White, RG, Alary, M. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: A systematic review and meta-analysis. AIDS. 2006; 20(6):805–812.
108. Henderson, DK, Dembry, L, Fishman, NO, et al. SHEA guideline for management of healthcare workers who are infected with hepatitis B virus, hepatitis C virus, and/or human immunodeficiency virus. Infect Control Hosp Epidemiol. 2010; 31(3):203–232.
109. Henderson, DK, Fahey, BJ, Willy, M, et al. Risk for occupational transmission of human immunodeficiency virus type 1 (HIV-1) associated with clinical exposures. A prospective evaluation. Ann Intern Med. 1990; 113(10):740–746.
110. Saag, MS, Squires, KE, Aberg, JA, Bardeguez, A. Letter in response to the new SHEA guideline for healthcare workers with hepatitis B virus, hepatitis C virus, and/or human immunodeficiency virus. Infect Control Hosp Epidemiol. 2010; 31(10):1092–1093.
111. University of California San Francisco. PEPline: National Clinicians’ Post-Exposure Prophylaxis Hotline. http://www.nccc.ucsf.edu/, 2010. [Accessed on August 20, 2012 available at].
112. Gordon, SM, LaRosa, SP, Kalmadi, S, et al. Should prophylaxis for Pneumocystis carinii pneumonia in solid organ transplant recipients ever be discontinued? Clin Infect Dis. 1999; 28(2):240–246.
113. MacMillan, ML, Goodman, JL, DeFor, TE, Weisdorf, DJ. Fluconazole to prevent yeast infections in bone marrow transplantation patients: A randomized trial of high versus reduced dose, and determination of the value of maintenance therapy. Am J Med. 2002; 112(5):369–379.
114. Marr, KA, Seidel, K, Slavin, MA, et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: Long-term follow-up of a randomized, placebo-controlled trial. Blood. 2000; 96(6):2055–2061.
115. Marr, KA, Laverdiere, M, Gugel, A, Leisenring, W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin Infect Dis. 2005; 40(12):1762–1769.
116. Marr, KA, Leisenring, W. Design issues in studies evaluating diagnostic tests for aspergillosis. Clin Infect Dis. 2005; 41(Suppl 6):S381–S386.
117. Mennink-Kersten, MA, Donnelly, JP, Verweij, PE. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect Dis. 2004; 4(6):349–357.
118. Pfeiffer, CD, Fine, JP, Safdar, N. Diagnosis of invasive aspergillosis using a galactomannan assay: A meta-analysis. Clin Infect Dis. 2006; 42(10):1417–1427.
119. Azoulay, E, Bergeron, A, Chevret, S, et al. Polymerase chain reaction for diagnosing Pneumocystis pneumonia in non-HIV immunocompromised patients with pulmonary infiltrates. Chest. 2009; 135(3):655–661.
120. Desmet, S, Van Wijngaerden, E, Maertens, J, et al. Serum (1-3)-beta-D-glucan as a tool for diagnosis of Pneumocystis jiroveci pneumonia in patients with human immunodeficiency virus infection or hematological malignancy. J Clin Microbiol. 2009; 47(12):3871–3874.
121. Marty, FM, Koo, S, Bryar, J, Baden, LR. (1->3)beta-D-glucan assay positivity in patients with Pneumocystis (carinii) jiroveci pneumonia. Ann Intern Med. 2007; 147(1):70–72.
122. Sax, PE, Komarow, L, Finkelman, MA, et al. Blood (1->3)-beta-D-glucan as a diagnostic test for HIV-related Pneumocystis jiroveci pneumonia. Clin Infect Dis. 2011; 53(2):197–202.
123. Tasaka, S, Hasegawa, N, Kobayashi, S, et al. Serum indicators for the diagnosis of pneumocystis pneumonia. Chest. 2007; 131(4):1173–1180.
124. Casiano-Colon, AE, Hulbert, BB, Mayer, TK, et al. Lack of sensitivity of rapid antigen tests for the diagnosis of respiratory syncytial virus infection in adults. J Clin Virol. 2003; 28(2):169–174.
125. Moore, C, Valappil, M, Corden, S, Westmoreland, D. Enhanced clinical utility of the NucliSens EasyQ RSV A+B Assay for rapid detection of respiratory syncytial virus in clinical samples. Eur J Clin Microbiol Infect Dis. 2006; 25(3):167–174.
126. Puppe, W, Weigl, JA, Aron, G, et al. Evaluation of a multiplex reverse transcriptase PCR ELISA for the detection of nine respiratory tract pathogens. J Clin Virol. 2004; 30(2):165–174.
127. Fuller, H, Del Mar, C. Immunoglobulin treatment for respiratory syncytial virus infection. Cochrane Database Syst Rev. (4):2006.
128. Glanville, AR, Scott, AI, Morton, JM, et al. Intravenous ribavirin is a safe and cost-effective treatment for respiratory syncytial virus infection after lung transplantation. J Heart Lung Transplant. 2005; 24(12):2114–2119.
129. Tomblyn, M, Chiller, T, Einsele, H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: A global perspective. Biol Blood Marrow Transplant. 2009; 15(10):1143–1238.
130. Fishman, JA. Infection in the solid organ transplant recipient. In: Basow DS, ed. UpToDate. Waltham, MA: UpToDate, 2012.
131. Kaplan, J, Benson, C, Holmes, K, et al. Guidelines for the management of opportunistic infections among HIV-infected adolescents and adults. www.aidsinfo.nih.gov, 2013. [available at].