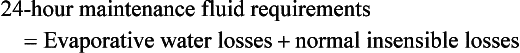Critical Care Management of the Severely Burned Patient
Introduction
This chapter will review the critical care management of burned adults. It will be apparent that in many areas existing literature is neither comprehensive nor rigorously “evidence-based.”1 In providing a rationale for treatment, therefore, it is often necessary to extrapolate from studies in other disorders, particularly trauma. This may or may not be valid, particularly in such “burn-specific” areas as fluid resuscitation, inhalation injury, and nutritional support. These issues will be discussed as they arise, but readers will need to interpret this information for themselves.
Incidence and Survival from Burn Injury
The incidence of burn injury has declined in the United States throughout recent decades. From the 1960s to the early 1990s, reported burns decreased from 10.2 to approximately 4.2 injuries per 1000 Americans annually, or about 1.25 million injuries2; hospitalizations decreased from over 90,000 to approximately 52,000/year; and deaths decreased at least 40%, from 9000/year to 5500/year. These trends are likely to continue.
Simultaneously, survival from burns has improved dramatically. During World War II, burns of 40% total body surface area (TBSA) produced a 50% mortality rate; today a similar mortality rate is seen with burns of over 80% TBSA.3 Survival rate is lower—though still improved—for the elderly, and even higher for children and young adults.4 These accomplishments are due to cumulative advances in fluid resuscitation, critical care, nutrition, surgery, and skin substitutes. However, it has been the organization of specialized burn centers around a consolidated team of experts that has made this success possible.5
Burn survival has been repeatedly shown to correlate with three major factors: patient age, burn size, and the presence of inhalation injury. Pulmonary damage from smoke inhalation is itself a serious injury, and can as much as double the mortality rate from cutaneous burns alone.3,5 Ryan and colleagues found that burn size 40% TBSA or greater, age 60 years or older, and inhalation injury contributed to the mortality rate in a stepwise manner; patients with all three had a mortality rate of 90% (Table 67.1).6
Table 67.1
Risk Factors for Death from Burn Injuries*
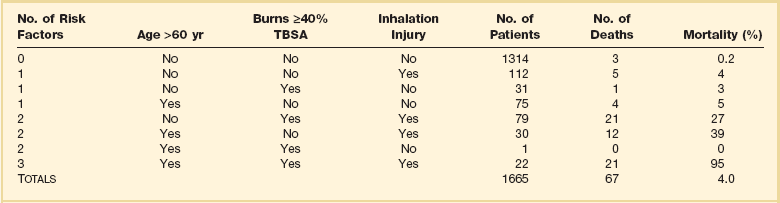
TBSA, total body surface area.
*This table indicates the three risk factors universally accepted to affect burn patient mortality rate, their prevalence, and relative contribution to mortality rate. These data are similar to those from many modern burn centers. Note that overall mortality rate is only 4% and that most patients are relatively young and have limited burn wounds, placing them at little risk of dying.
Adapted with permission from Ryan CM, Schoenfeld DA, Thorpe WP, et al: Objective estimates of the probability of death from burn injuries. N Engl J Med 1998;338(6):362-366. Copyright ©1998, Massachusetts Medical Society. All rights reserved.
This experience has two important implications for current and future burn treatment. First, mortality risk for many patients is now so low that almost no injury is too large to preclude survival. The decision to intentionally withhold treatment is now rare and is based on predicted quality of life rather than predicted fatality itself. Second, it has necessitated unprecedented research and interest on the rehabilitation of patients who increasingly survive catastrophic injuries. Functional outcomes of burn treatment, including quality of life and return to work, are now the most relevant measures of successful care for all patients.7,8
However, the decline in burns has had other unintended consequences, which create challenges to the provision of effective burn care in many areas. As burns have decreased, so has the number of burn centers in the United States. There are now over 25% fewer burn centers than in 19709; only 60 facilities in the United States are currently verified by the American Burn Association (ABA) and American College of Surgeons (ACS).10 This reduced number limits access to specialized burn care for many Americans. For the same reason, the experience of most physicians with even basic burn care has been dramatically reduced; many U.S. doctors—even surgeons—receive little or no burn training. As a result, important errors in initial burn assessment are made commonly by primary care providers,11–13 leading to significant under- or (mostly) overtriage, care that is sometimes inadequate, and further stress on the few remaining centers, which now must cover larger referral areas.14,15
Acute Care of the Burned Patient
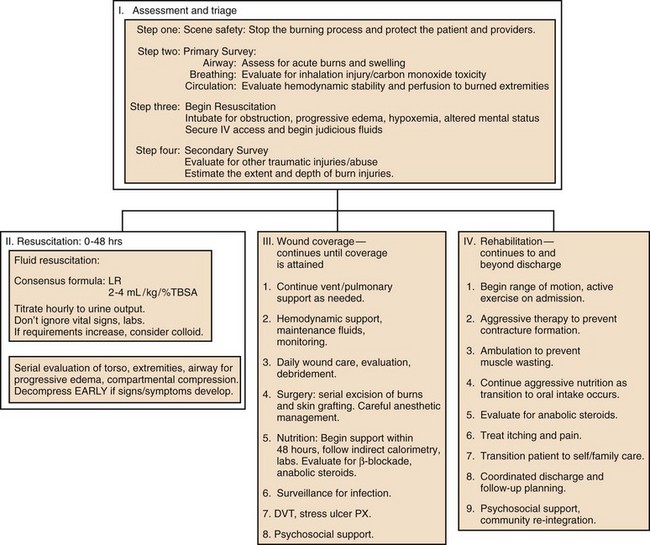
Acute burn treatment can be divided into four phases, which are outlined in Figure 67.1. Initial assessment is the process of stabilizing the acutely injured patient, treating immediate life-threatening injuries, and preparing for inpatient care. The first 48 hours following injury constitute acute resuscitation, focused on initial assessment, airway support, and fluid replacement. The wound coverage phase then ensues, in which the major goal of treatment is surgical excision and closure of burn wounds. Ventilator management, metabolic support, control of infection and pain, physical therapy, and other supportive measures are essential adjuncts during this period. The goals of the final rehabilitation phase include scar control, optimizing function, and return to independent living. This period can last for months and continue long past discharge. Obviously these phases overlap: discoveries made during initial assessment may require prolonged management; many units begin surgical excision even before resuscitation is completed, and several aspects of rehabilitation—physical therapy, psychosocial support, nutrition, etc.––should start essentially at the time of injury.
Initial Assessment
Initial assessment of burn victims should follow the universally accepted protocol of the American College of Surgeons’ Advanced Trauma Life Support (ATLS) course.16 In doing so, attention must be paid to several burn-specific issues, but it must be remembered that other types of trauma or preexisting conditions can always be present, emphasizing the importance of systematic patient evaluation.
The Primary Survey and Inhalation Injury
In burn patients, assessment of airway, breathing, and circulation must include evaluation for signs and symptoms of inhalation injury. Carbon monoxide (CO) poisoning and asphyxia cause the vast majority of scene and emergency room fatalities, and up to 80% of fire-related deaths.17,18 Inhalation injury and its management are covered in Chapter 48.
Inhalation injury should be suspected in all patients exposed to flames or smoke, especially if the patient was unconscious or trapped in a closed space. Physical findings include facial burns, singed nasal hairs or eyebrows, wheezing or stridor, carbonaceous sputum, hoarseness, and anxiety. These findings should alert the examiner to the possible need for airway support.19
Carbon Monoxide Poisoning
CO is a product of incomplete combustion, and its highest concentrations occur in indoor fires, where its effects may be amplified by flame-induced oxygen depletion.20 CO displaces oxygen binding, producing systemic hypoxia despite normal oxygen tension. The most common symptoms are mental status changes, varying from headache to coma, which can present without burn injuries or respiratory distress and be overlooked easily. Classic “cherry red cyanosis” appearance is absent in many victims or can be obscured by soot or burns. Importantly, pulse oximetry is inaccurate in the presence of CO. Direct measurement of carboxyhemoglobin concentration should be performed but should never delay treatment. Patients are at greatest risk on presentation, and immediate application of oxygen is often definitive. As reviewed in Chapter 48, the use of hyperbaric oxygen for acute CO poisoning is controversial; it should probably be reserved for severe cases with neurologic compromise, when its use will not interfere with other essential components of acute burn care.
Smoke contains many other toxic chemicals, including cyanide. Cyanide poisoning has been documented in burn victims and does not always correlate with CO exposure.21 Because blood levels cannot be obtained immediately, empiric treatment using cyanide antidote kits has been advocated in patients with unusually severe acidosis or shock.22 The recent availability of hydroxocobalamin as a cyanide antidote, which has few side effects, has led to both renewed interest in the treatment of cyanide toxicity and aggressive marketing of the drug. However, a recent review points out that confirmed cases of significant cyanide poisoning following smoke exposure are rare.23 At present, treatment of suspected cyanide toxicity in acutely burned patients is controversial, and indications are unclear.24 High-flow oxygen coupled with aggressive resuscitation and cardiovascular support remain the mainstays of treatment.
Upper Airway Injury
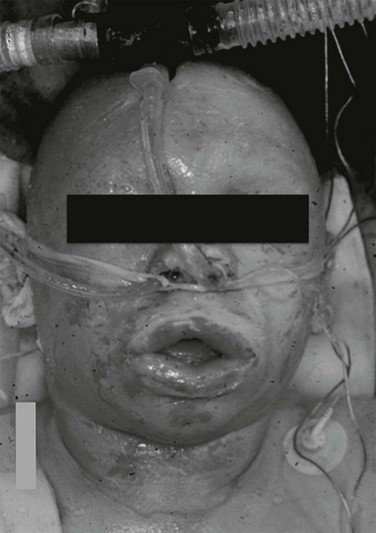
Patients with extensive or deep burns to the face, or who have breathed substantial quantities of hot gases or soot, are at risk of airway occlusion from progressive pharyngeal or supraglottic edema and facial swelling. This swelling can occur even without flame injury in children with scalds or severe chemical burns. Edema formation can be extremely rapid and progress for at least 24 hours. Patients must be followed serially, and intubated early and electively if evidence of progressive airway compromise occurs. Figure 67.2 illustrates such a case.
Indications for Intubation
Awareness of the potential for acute airway compromise following burn injury has led to a liberal attitude toward intubation that is probably appropriate. “When in doubt, intubate” expresses many physicians’ attitude toward this problem. However, this dictum has sometimes led to indiscriminate and unnecessary intubation of patients with even minor facial burns.25 The presence of inhalation injury does not mandate intubation, and airway compromise can be life-threatening even in the absence of inhalation injury. Indications for intubation, as in all trauma patients, are based on symptoms found on initial assessment, including altered mental status, refractory hypoxemia, and signs of impending airway obstruction including wheezing, stridor, dyspnea, and progressive facial swelling. Emergency tracheostomy or cricothyroidotomy, which should rarely be needed if intubation is performed in a timely manner, can be an extremely challenging procedure in the setting of massive head and neck swelling.
Pulmonary (“True”) Inhalation Injury
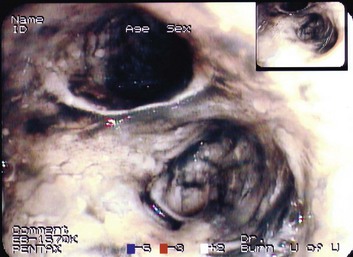
Exposure of the bronchi and small airways to toxic smoke causes chemical injury to the epithelium, which can progress to mucosal sloughing, mucous plugging, bronchiectasis, hypoxemia, and pneumonia. Clinical signs of this problem may be absent for up to 72 hours after exposure, and initial chest radiographs are usually normal.26 Patients with immediate respiratory distress, stridor, or hypoxemia are much more likely to have upper airway injury. Even in the absence of symptoms, patients suspected of having inhalation injury require hospital admission and close observation. Fiberoptic bronchoscopy demonstrating carbonaceous debris, erythema, or mucosal sloughing is highly sensitive for diagnosis of inhalation injury and can facilitate immediate intubation if injury is confirmed27 (Fig. 67.3). Inhalation injury is discussed further in Chapter 48.
Begin Resuscitation
Following quick initial assessment, intravenous resuscitation and other support should be initiated.
The Secondary Survey
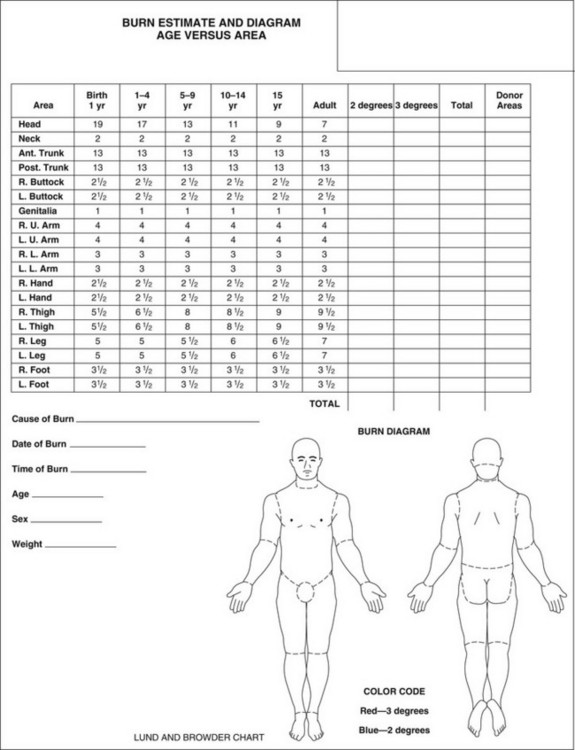
As resuscitation is beginning, a thorough head-to-toe examination is performed. Burn victims frequently suffer other trauma, which can be obscured by overlying burns (see later). Much of the subsequent care of the burn patient is based on assessment of the extent and depth of injury, including fluid resuscitation, nutritional requirements, surgery, and in extreme cases, the decision to provide aggressive treatment. For these reasons, the burn wound should be washed and thoroughly debrided and then documented as accurately as possible, preferably by an experienced clinician. Rough estimates of burn size are often made using the “rule of nines”; more accurate detailed documentation can be done using the Lund and Browder chart (Fig. 67.4). Computerized programs are also becoming popular, such as the Sage diagram (www.sagediagram.com). In evaluating smaller injuries, another useful rule is that the area of the patient’s palm (with fingers) equals about 1% of his/her TBSA.
Burns and Multiple Trauma
The many mishaps that cause burns often cause other trauma as well. These situations can include explosions (blasts, falls, projectile wounds), electrocutions (tetany-induced fractures, falls), fires in motor vehicle or airplane crashes, and escaping fires (lacerations, falls). Combined burn/trauma injuries are particularly likely following assault or child abuse and should increase suspicion for such causes.28
The combined mortality rates for burns and trauma appear to be at least additive compared to either injury alone.29,30 Thorough evaluation of all potential injuries according to ATLS guidelines is imperative and can be challenging in burn patients: the discoloration, pain, and swelling of burn wounds, for example, can conceal underlying fractures31; and burns of the torso or acute inhalation injury can obscure a pneumothorax or other injuries. For this reason, it is important not to focus too heavily on burn injuries in performing the secondary survey.
In addition, care should be provided by a multispecialty team with expertise in managing all injuries. Treatment of many traumatic injuries takes priority over definitive burn care and mandates immediate operation. Because fresh burn wounds are initially—and briefly—free of bacteria, laparotomy, craniotomy, repair of lacerations, and operative fixation of fractures should be performed immediately, and no later than 12 to 24 hours after burn.32,33 This approach will minimize risk of infections, permit early mobilization, and facilitate access to burn wounds for dressings and surgery. These interventions can be performed safely provided essential components of burn care—including airway support, temperature control, and aggressive fluid resuscitation—are instituted immediately and continued through surgery. Close coordination between the burn center and trauma center will optimize outcome for these patients.
The Resuscitation Phase
Burn Shock
Following initial evaluation, the primary focus of early burn treatment is fluid resuscitation. The goals of resuscitation are to support organ function while avoiding complications of over- or underadministration of fluid.34 This task can be challenging given the huge fluid shifts and cardiovascular effects of acute burns. Development of effective protocols for resuscitation represents one of the major advancements in burn care in this century.
The magnitude of fluid shifts following a major burn can exceed those of any other injury. At the cellular level, burn injury immediately impairs membrane adenosine triphosphatase (ATPase) activity and reduces transmembrane potential,35,36 resulting in increased intracellular sodium and extracellular potassium concentrations, cellular swelling, and acidosis. Fluid resuscitation only partially corrects these abnormalities, which may require several days to resolve completely as local inflammation subsides.
These events present clinically as a profound inflammatory response, causing increased capillary permeability and massive tissue edema at the expense of intravascular volume. Immediate release of histamine and serotonin increase local perfusion, initiate capillary leakage, and potentiate vasoconstriction caused by massive catecholamine secretion.36–38 Tumor necrosis factor (TNF)-α produces myocardial depression and activates a number of other vasoactive mediators.39 Prostaglandins, leukotrienes, oxygen radicals, products of platelet activation and coagulation, and a cascade of cytokines also contribute to these abnormalities.40,41 Alterations in local blood flow increase arteriolar tone and pressure and dilate postcapillary venules, causing local capillary perfusion to become less selective and increased, favoring extensive edema formation.
The forces that control transcapillary fluid flux are summarized in Starling’s equation42; their alterations in burn injury have been reviewed by Demling43 (Table 67.2). The greatest edema formation occurs almost immediately within the wound, caused by near-total permeability to even very large (35 nm) molecules,44 and permitting protein-rich plasma to pour into the interstitium.45 Maximum protein extravasation occurs within the first hour of injury, and both its duration and magnitude are proportional to burn size.46,47
Table 67.2
Starling Forces and Capillary Permeability*
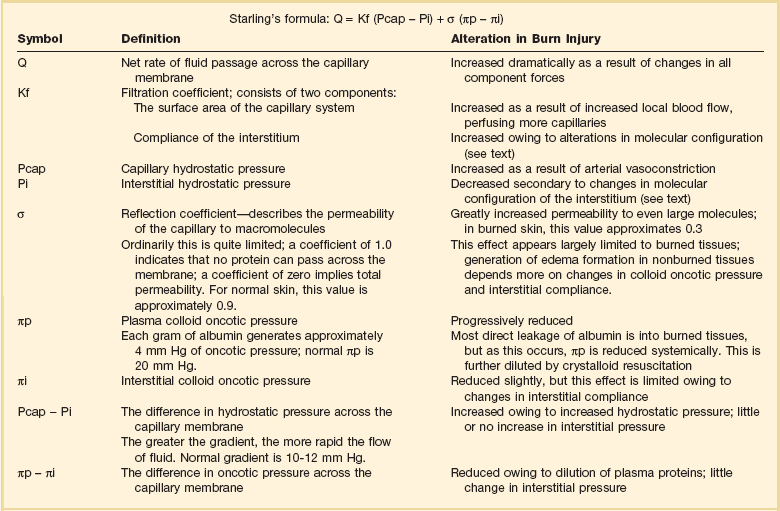
*See discussion in Demling RH: The burn edema process: Current concepts. J Burn Care Rehabil 2005;26:207-227.
Adapted with permission from Ryan CM, Schoenfeld DA, Thorpe WP, et al: Objective estimates of the probability of death from burn injuries. N Engl J Med 1998;338:362-366. Copyright ©1998, Massachusetts Medical Society. All rights reserved.
Even after local capillary integrity normalizes within 8 to 12 hours, edema formation continues in unburned as well as burned tissues.48 Depletion of intravascular proteins, further diluted by crystalloid resuscitation,49 eliminates the oncotic pressure gradient (see πp − πi in Table 67.2), which maintains intravascular volume. Hypoproteinemia alone can mimic burn edema, and infusions of colloid can almost completely prevent edema in unburned tissues.50,51 Progressive edema also alters the configuration of interstitial collagen and hyaluronic acid, which are normally densely coiled to limit fluid influx.52 Burn injury disrupts this “safety valve” configuration, increasing compliance, producing osmotically active molecular fragments, and generating negative (“sucking”) interstitial pressure53 and extremely rapid fluid sequestion.48 Though this gradient is neutralized within a few hours, compliance continues to increase as interstitial gel is hydrated, allowing liters of fluid to accumulate with little change in hydrostatic pressure54 and permitting edema to persist for weeks following injury.
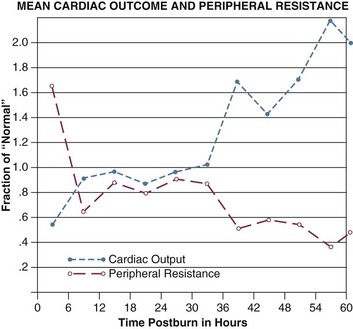
Cardiac output falls within minutes of injury, reaching 50% to 60% of normal within an hour, although systemic vascular resistance (SVR) increases dramatically. Both changes occur far faster than the depletion of intravascular volume45,55 and regardless of fluid resuscitation. Release of catecholamines and other vasoactive mediators clearly increases SVR, but the decrease in cardiac output is harder to explain. Researchers have long postulated the existence of a “myocardial depressant factor,”56 though no such substance has been isolated. It seems more likely that cardiac output is impaired by the combination of increased SVR, volume depletion, and actions of TNF-α, endotoxin, and other chemicals. One clinically important side effect of capillary permeability is hemoconcentration—a marked rise in hematocrit as plasma is sieved out of the bloodstream into the interstitium—which increases blood viscosity and further impairs cardiac output.
Cardiac output begins to recover within a few hours of injury and requires 12 to 24 hours to normalize, even with vigorous resuscitation. SVR follows an opposite course, peaking quickly and then declining to near-normal within 24 to 48 hours (Fig. 67.5). Following these initial changes a chronic hyperdynamic circulation is maintained, with cardiac output persisting well above normal, and marked vasodilation and decreased SVR often persisting even after wound coverage is attained.
Fluid Resuscitation of Burn Patients
Before World War II, patients with even moderate burns usually died within a few days because of progressive shock and renal failure. In 1942, surgeons Oliver Cope and Francis Moore designed the first formal resuscitation regimen to treat victims of the Cocoanut Grove nightclub fire, with substantial improvements in survival.36 With continued refinements, today almost all patients can be resuscitated successfully, and renal failure complicating burn injury is rare. A host of formulas have been developed, almost all based on body weight and burn size, and utilizing various combinations of crystalloid and colloid solutions. The archetype and most widely used of such regimens is the Parkland formula, designed by Baxter.35 He demonstrated that a volume of lactated Ringer’s (LR) solution equal to 4 mL per kilogram body weight for each percent TBSA burned (4 mL/kg/%TBSA), given in the first 24 hours after injury, would maintain urine output of 50 to 70 mL/hour, replete blood volume, and restore cellular transmembrane potential and cardiac output in most patients. Half this calculated volume is given in the first 8 hours after burn, the remainder over the next 16 hours. The intravenous rate is adjusted hourly to maintain urine output and is decreased gradually until a “maintenance” rate is reached at approximately 24 hours.
Maintenance requirements for burn patients are also increased due to ongoing evaporative and metabolic losses, and continued fluid support is essential even after resuscitation is complete. A popular formula to estimate these requirements is that developed by Warden:57
In this equation,
This experience forms the basis of modern burn resuscitation. But despite widespread acceptance of the principles of fluid replacement, disagreement persists over almost every aspect of practical management, and the practice of resuscitation varies significantly among units. One example exists in defining the end points of resuscitation. Successful resuscitation requires meticulous monitoring and adjustment of resuscitation based on patient response. Traditional resuscitation relies almost exclusively on hourly urine output for this purpose, so the amount of fluid required for resuscitation depends partly on the urine output targeted. Baxter used an output of 50 to 70 mL/hour; several other formulas that accept outputs of 0.5 mL/kg/hour (1.0 mL/kg/hour in children) call for LR infusion rates between 2 and 3 mL/kg/%TBSA, and are also in widespread use.34,36
The Phenomenon of “Fluid Creep”
Clinicians have long accepted the consensus that patients should receive as little fluid as possible to maintain organ perfusion.58 But several patient groups routinely require more resuscitation fluid than predicted by the Parkland formula, including patients with inhalation injury,59 those with multiple trauma and electrical burns, patients in whom resuscitation is substantially delayed,4 and patients with alcohol or drug abuse.60 Inexperienced clinicians often make substantial errors in estimating burn size, which can result in significant under- or overestimation of fluid requirements.11 Careful monitoring of resuscitation and individualized fluid therapy can often obviate these problems.
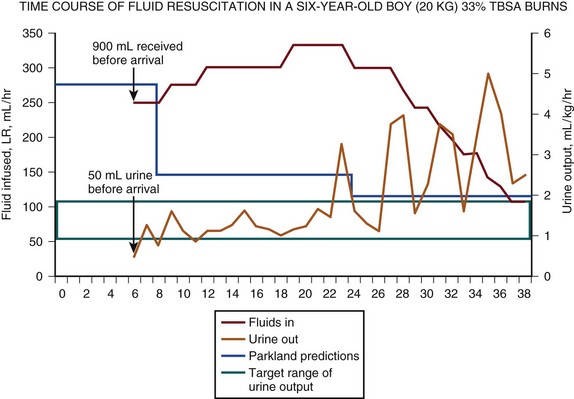
But in recent years a widespread tendency for patients to receive increasing quantities of resuscitation fluid has been observed, a phenomenon dubbed “fluid creep” by Pruitt.61 Cancio and associates found that 63% of their recent patients required a mean of 6.1 ± 0.22 mL/kg/%TBSA for resuscitation despite very modest urine output.62 Others have documented such increased fluid requirements in up to 100% of patients.63–65 The potential complications of fluid creep are far from benign, including extremity and abdominal compartment syndromes, worsening pulmonary dysfunction, and more frequent and prolonged endotracheal intubation. Figure 67.6 illustrates the actual resuscitation of a child who required an excessive amount of crystalloid to achieve successful resuscitation and maintain adequate urine output.
This departure from tradition is probably due to several influences. First, the successful resuscitation of patients with massive burns appears to routinely require increased fluid volumes.62 In Baxter’s initial report of 277 patients, 89% of those with burns 60% TBSA or greater died.45 However, modern survival is much better and owes some of its success to persisting with aggressive fluid resuscitation beyond the confines of the Parkland formula.
Second, it appears that fluid creep perpetuates itself. Overly zealous initial fluid administration—often performed in the field—increases serum protein depletion, generating a vicious circle of reduced oncotic pressure and enhanced edema, which in turn increases crystalloid requirements. This mechanism also helps explain the occurrence of resuscitation failure, in which fluid requirements actually escalate despite adequate—or excessive—crystalloid administration.66 The use of colloid-containing fluids can arrest this cycle and restore oncotic pressure but adds to overall fluid requirements as well.
Fluid creep has probably also been encouraged by resuscitation trends in other ICU populations. Traditional burn resuscitation formulas permit significant deficits in vascular volume and cardiac output to persist until the end of 24 hours.45 The routine observation of hematocrits as high as 70% during resuscitation is consistent with this concept. Both lactic acid (LA) and base deficit (BD) remain elevated throughout traditional resuscitation,67 which also fails to reflect changes in cardiac output and oxygen delivery (VO2).68 In contrast, resuscitation in other shock states has been increasingly directed at normalizing LA and BD, and optimizing cardiac output, VO2, and pulmonary wedge pressure.69,70 As this concept gained acceptance in critical care, burn clinicians have become more inclined to monitor these parameters and to respond to abnormalities by increasing fluid support.
However, this approach may not be valid in burns. Although it is accepted that patients’ ability to achieve optimal values of cardiac output and VO2 correlates strongly with outcome, it is less clear whether exaggerated fluid infusion, invasive monitoring, and inotropic support can change nonresponders into responders and thus improve survival.71 Optimizing VO2 and improved survival in one group of patients with severe burns,72 but both Kaups and colleagues64 and Choi and coworkers65 were unable to determine if resuscitation aimed at normalizing elevated BD was effective or beneficial. In other studies of goal-directed therapy in burns, attaining target values of cardiac output, VO2, or wedge pressure routinely required more fluid—as much as four times Parkland calculations—without obvious improvement in survival,73,74 and potential increase in edema-related complications, which appear directly related to the fluid volume administered.75,76
Hypertonic Resuscitation
Crystalloid solutions remain the mainstay of all resuscitation regimens as they are inexpensive, well tolerated, readily available, and easily mobilized and excreted. The effective component of crystalloid is sodium,77 which has led to the use of hypertonic saline solutions to satisfy capillary and cellular leakage while minimizing fluid volume and edema.78,79 Solutions containing as much sodium as 300 mEq/L result in delivery of almost identical quantities of sodium as LR solution with substantially less volume.80 Hypertonic saline resuscitation has been advocated for children81 and the elderly,82 who tolerate under- and overresuscitation poorly, as well as for patients with head injuries,83 and for field and combat resuscitation when the weight of available solutions must be minimized.84,85
Hypertonic saline resuscitation carries the risk of significant hypernatremia, hyperchloremia, acidosis, and hyperosmolarity, and requires careful monitoring. In addition, savings in initial fluid requirements may be balanced by increased free water retention later.86 Hypertonic saline has been associated with increased mortality risk in at least one study, possibly related to increased renal failure.86 However, it is still used selectively in some burn centers79,87 and remains an option in acute burn management.
Crystalloid Versus Colloid
Because of the indiscriminate nature of early postburn capillary leakage, colloids are not superior to crystalloids in initial burn resuscitation.55 For this and other reasons, colloid use in resuscitation of all types has been condemned.88 However, capillary integrity is largely restored by the end of 24 hours after injury, and colloids given at this time can effectively restore oncotic pressure, expand plasma volume, and reduce secondary edema in unburned tissue,35,51 including possible prevention of abdominal compartment syndrome.89
Colloids used in burn resuscitation have included albumin, plasma protein fraction (Plasmanate), fresh frozen plasma, and the synthetic colloids dextran and hetastarch. Routine albumin administration is a component of the traditional Evans and Brooke formulas36,55 and was originally used at the end of Parkland resuscitation.35 In a widely quoted randomized trial, Goodwin and associates found that albumin-based resuscitation required less total fluid than solution alone but was associated with a sustained increase in extravascular lung water.90 Critics have also pointed out that routine albumin supplementation has no apparent benefits.91 Nonetheless, albumin remains a popular colloid; its current use varies among burn centers from routine administration within 8 to 12 hours of injury,50,57 to selective use as “rescue” in problem resuscitations, to near-total interdiction. Fresh frozen plasma has also been used successfully92; its colloid effect probably explains much of the efficacy of plasma exchange therapy in complex burn resuscitations.93 Both low-molecular-weight dextran and hetastarch have been used in burn resuscitation and appear as effective as albumin in maintaining oncotic pressure and limiting edema in unburned tissues.51,94,95 They offer advantages of long shelf life, availability, freedom from disease transmission, and lower cost. However, hetastarch has been associated with rare anaphylaxis and dose-dependent coagulopathy and bleeding, which limits is use in large volumes.96
Colloid is probably unnecessary for resuscitation of most patients with uncomplicated injuries. With increasing burn size, the benefits of colloid administration, restricted to use after the first 8 to 12 hours after burn, may be significant in reducing total fluid requirements and edema-related complications. Many centers now utilize LR solution for the first hours after injury, followed by hypertonic saline, colloid, or both to reduce edema as resuscitation is completed.57 The patient illustrated in Figure 67.6 would be a very appropriate candidate for this form of “rescue” therapy, which would likely have reduced his ongoing fluid requirements. This area of resuscitation is extremely controversial and will benefit from randomized multicenter trials.
Pharmacologic Manipulation of Resuscitation
Improved understanding of the pathophysiology of burn shock has led investigators to attempt to reduce its severity by blocking some of its specific chemical mediators. These efforts have included use of vasodilators such as hydralazine, histamine blockade using cimetidine, the serotonin antagonist ketanserin, and anti-inflammatory drugs such as hydrocortisone and ibuprofen.43,55,97–99 Large doses of the antioxidant vitamin C has been shown to decrease fluid requirements in clinical burn resuscitation.100 Many of these efforts have shown modest benefits, though none has found its way into widespread clinical use. The possibility of developing an effective “cocktail” to ameliorate the effects of burn shock holds promise for the future.
Practicing Effective Resuscitation
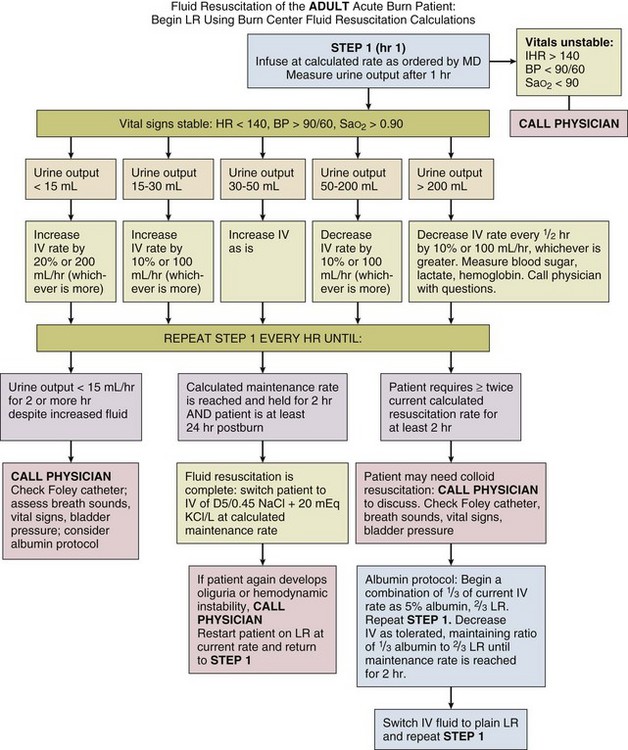
An example of the protocol used at the University of Utah is included in Figure 67.7. The protocol is based on the Parkland formula, but contains an option for the use of LR/albumin to arrest escalating fluid requirements. It requires nurses to adjust infusions based on hourly urine output, but mandates that physicians be contacted if worrisome parameters develop. We have found this protocol effective in resuscitating the vast majority of patients without frequent input from physicians. Similar protocols are in use in a number of burn centers.
Complications of Edema
Facial Swelling
Full-thickness facial burns can swell massively and produce complete airway obstruction within an hour or 2 of injury, as illustrated in Figure 67.2. As discussed under initial assessment, endotracheal intubation is essential in this setting and should be performed early and electively.
Ocular Swelling
Elevated intraocular pressures (IOPs) have been documented in burn patients and appear to correlate with resuscitation volume. No data exist on the consequences of this phenomenon, but the risk of optic nerve ischemia and permanent visual loss may exist. Lateral canthotomy has been recommended for patients with persistently high IOPs.101,102 Measurement of IOP during acute burn resuscitation may be warranted in patients with severe facial injuries and eyelid edema.
Extremity Compartment Syndromes
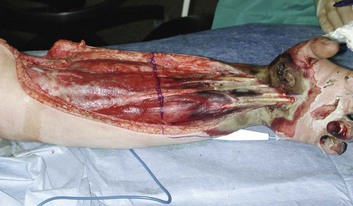
Swollen extremities should be initially treated with elevation and avoidance of constricting dressings. Classic findings of pain, paralysis, pallor, paresthesia, pulselessness (the “five Ps”) may be difficult beneath burn injuries or in intubated patients, and may not develop until irreversible ischemia has occurred. Similarly, limb-threatening compression can exist despite persistent palpable or Doppler pulses.103 For these reasons, some authorities perform escharotomies based on a general impression of extremity swelling, or even prophylactically. Alternatively, many clinicians monitor intramuscular pressure to determine the need for decompression. This procedure is easily done by inserting a large-bore (18-gauge) needle attached to a pressure transducer through the eschar into the underlying muscle. Pressures that exceed 30 cm H2O in the relaxed extremity indicate compromised capillary perfusion and mandate decompression.104 Measurements should be repeated following escharotomy; pressures that remain elevated indicate the need to deepen or extend incisions or to proceed with fasciotomy. This may be particularly likely in patients with very deep burns, associated trauma, or electrical injury (Fig. 67.8).
Escharotomies can often be done at the bedside using electrocautery and appropriate analgesia. Longitudinal incisions should run the length of the burn wound, be placed medially and laterally on the supinated limb to avoid major nerves and vessels, and facilitate resurfacing with skin grafts. Incisions must penetrate through the burn until the wound edges “pop open,” and pressure is clearly relieved. Incisions in burned hands should extend at least to the metacarpophalangeal joints; performance of individual digit escharotomies has some support105 but is often omitted. As an alternative, the use of enzymatic debriding agents to provide chemical escharotomy is popular in some centers.106
Torso Compartment Syndromes
Recent awareness of massive abdominal swelling as a cause of cardiorespiratory compromise has led to protocols for monitoring intra-abdominal pressure and performing decompressive laparotomy in many ICU populations. In burn patients, occurrence of abdominal compartment syndrome has accompanied reports of fluid creep, as discussed earlier,75 though it can clearly develop in the absence of excessive resuscitation. Patients with major (≥25% TBSA) burns and extensive torso injuries or who require large resuscitation volumes (≥500 mL/hour76) should have routine monitoring of bladder pressures.76,107 The finding of intra-abdominal hypertension in the absence of clinical symptoms should prompt other measures to reduce abdominal pressure.108 There is evidence that both hypertonic saline and colloid-based resuscitation can prevent the development of abdominal compartment syndrome79,89; removal of peritoneal fluid with dialysis catheters has also been effective.109 Unfortunately, many patients with abdominal compartment syndrome present with diffuse retroperitoneal swelling rather than free peritoneal fluid; diagnostic ultrasound can make this decision and guide catheter placement if free fluid is seen.
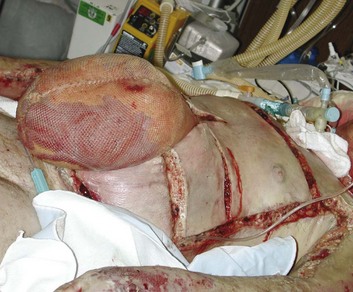
Like other edema-related complications, abdominal hypertension can develop insidiously and then present precipitously with oliguria and critical hypotension and respiratory embarrassment. In patients with deep burns of the chest or abdomen, performance of torso escharotomy—sometimes repeatedly—will often improve ventilation and obviate laparotomy. Patients in whom other measures fail or symptoms progress rapidly should undergo immediate decompressive laparotomy, at the bedside if necessary. Delayed decompression may worsen shock and even lead to intestinal necrosis, although prompt decompression will both improve survival and reduce ongoing resuscitation requirements.110 Containment of the exposed viscera by mesh or plastic silos may be needed following laparotomy, as widely used “vac-pack” dressings111 may be impossible to secure over burn eschar. As edema resolves, every effort should be made to reduce abdominal contents and obtain fascial closure through repeated wound revisions. Figure 67.9 illustrates a patient in whom numerous complications of edema—massive facial swelling, extremity compartment syndromes, and abdominal compartment syndrome—have occurred simultaneously. Although the mortality rate of abdominal compartment syndrome in burn patients is high,112 this likely reflects the severity of the underlying burn more than this complication itself.
Acute Renal Failure
The development of effective protocols for fluid resuscitation has greatly reduced the incidence of acute renal failure (ARF) during initial burn treatment. ARF may still develop if resuscitation is delayed or inadequate,113 particularly in patients with high-voltage electrical injuries or extremely deep burns, in whom pigment-induced nephropathy from myoglobin/hemoglobin is possible. Patients with visibly red or black urine should have resuscitation increased to produce urine outputs of 50 to 100 mL/hour. A one-time initial dose of an osmotic diuretic such as mannitol to stimulate urine production, and alkalinization of the urine by adding bicarbonate to intravenous fluids, is also widely practiced.114,115
ARF in burn patients is now most often a late complication of infection or dehydration and continues to have a high mortality rate.116 Clearly its prevention is the best management strategy through meticulous fluid management and infection control. When ARF develops, patients should be treated with dialysis according to standard indications. Continuous renal replacement therapy can be particularly effective in managing patients who tolerate intermittent dialysis poorly.117 Nutritional support should not be reduced in this setting; rather, patients should continue to receive the calories and protein they require and undergo dialysis as needed.
Electrolyte Abnormalities
Hypokalemia may occur immediately after burn related to epinephrine release from injury,118 although hyperkalemia can result from cellular injury and acidosis. Both are usually short-lived and rarely require treatment. Thereafter, potassium levels often drop below normal, and patients may require substantial supplementation. Elevated aldosterone secretion following burn injury contributes to chronic hypokalemia and alkalosis.119 Magnesium levels also commonly drop, and supplementation of both potassium and magnesium is required as part of the “refeeding” effect of aggressive nutritional support.
Hypophosphatemia is also extremely common from multiple causes including catecholamine secretion, metabolic alkalosis, impaired renal phosphate absorption, and increased excretion during postresuscitation diuresis.120 Phosphate levels typically reach a nadir 2 to 5 days after injury, and afterward rebound slowly. Significant hypophosphatemia can lead to cardiac dysfunction, reduced red blood cell survival, and neurologic abnormalities, particularly during refeeding. Substantial phosphate supplementation may be needed throughout the wound coverage phase of burn treatment. Routine monitoring and replacement of all electrolytes should be performed at least until patients no longer require intravenous fluids.
The Wound Coverage Phase
Surgical Treatment of Burn Patients
Burn eschar left in place eventually separates from underlying tissue through the action of leukocyte enzymes. Bacterial infection facilitates this process, and burn eschar is an ideal medium for bacterial growth. In the era before routine excision, many patients died from sepsis while awaiting eschar separation while experiencing unremitting pain and prolonged muscle wasting. Surgical excision of burns was first practiced in the early twentieth century but produced high mortality rates from blood loss and anesthesia. Beginning in the 1970s, improved support has permitted aggressive excision of major burns with increased survival and decreased length of stay.121,122–124 Early excision is now considered a standard of care. A detailed discussion of these techniques is beyond the scope of this chapter. However, intensivists must be aware of the principles of surgical treatment and be able to support patients pre- and postoperatively.125
Indications and Timing of Surgery
The larger the burn injury, the more acute the need for early excision. Patients with small (<10-15% TBSA) burns can be followed for up to 14 days to evaluate healing, but when burn size exceeds 25% to 30% TBSA, a widely accepted goal is removal of essentially all of the burn wound within 7 days of injury. Muller and Herndon excise the entire wound in a single procedure even while fluid resuscitation is ongoing,126 but most units practice a staged approach, removing 15% to 20% TBSA at each operation.
Unless covered, excised wounds will desiccate and develop a new layer of necrotic tissue—in essence, a second eschar—with attendant metabolic stress, pain, and infection. The ultimate goal of excision is autografting—coverage with the patient’s own skin. Burns excised within 7 to 10 days of injury are usually quite clean, and are often autografted immediately, providing prompt wound closure and facilitating early mobility. If donor sites are limited, autografts can be meshed to expand and cover more area. Excised wounds can be covered with antibiotic-soaked dressings to maintain tissue viability and combat infection for a few days. In recent years, a variety of skin substitutes have been utilized when donors are not available or if burns appear infected or poorly vascularized. The most widely used material is cadaver allograft skin obtained from tissue banks. Like autograft, allograft permits vascular ingrowth and take—sometimes for weeks—though this tissue is always eventually rejected. Allograft placement reduces bacterial colonization and pain, retards wound contraction, creates a clean, vascular bed, and can serve as a test for subsequent autografting. Cultured epithelial autografts (cultured skin) can be used to cover extensive areas but are expensive, fragile, and prone to loss from infection. They are best used over a scaffolding of intact or allograft dermis, and meticulous wound care is essential for success. A variety of synthetic and processed skin are also available, including synthetic dermis (Integra), acellular dermal matrix (Alloderm), amniotic membrane, collagen-bound polymer membranes (Biobrane, Transcyte), and others. Products still in development combine synthetic dermis with cultured epidermis, providing the potential for future one-step complete skin replacement.127 All of these products require experience and skill to utilize successfully.
Hemodynamic Support
In acutely burned patients blood pressure is sustained by elevated catecholamine levels, though blood volume may remain below normal and hematocrit artificially high. With induction of anesthesia, adrenergic blockade and vasodilation can produce sudden hypotension as these hidden hypovolemia and anemia are revealed. In addition, surgical excision of old or infected burn wounds may stimulate bacteremia and potentiate hemodynamic instability.128 The surgical team should prepare for these contingencies by continuing liberal fluid infusions during surgery and anticipating blood loss. Major burn excisions produce significant bleeding, which is often underestimated by surgeons. The blood loss from excision of 1% of the body surface area of an adult has been estimated at approximately 100 mL.129 Use of tourniquets for excision, performance of staged excisions, and use of subdermal clysis of epinephrine-containing solutions—the Pitkin procedure—can all help reduce intraoperative bleeding.130
Burn Pharmacology and Anesthesia
Depolarizing muscle relaxants (succinylcholine) should not be used in patients with acute injuries,131 but burn patients are also relatively resistant to nondepolarizing agents and frequently require increased dosage. Induction agents that produce vasodilation, such as propofol, may potentiate hypotension, and should be used with caution. Ketamine maintains hemodynamic stability, and can be used effectively in surgery or for bedside procedures and dressing changes.132 Pharmacokinetics and dynamics of many other drugs are also significantly altered in burn patients.133 Increased volume of distribution, decreased albumin binding, and increased renal blood flow necessitate monitoring and individualized dosing of many drugs.134
Pain Control
Pain management is notoriously difficult in burn patients, who often require remarkable quantities of narcotics and sedatives throughout their hospital course. Although altered pharmacology undoubtedly plays a role in this process, extreme levels of sustained pain are typical, and tachyphylaxis often develops during long-term administration of opioids. Patient-controlled analgesia (PCA) can be effective, but use may be limited by impaired consciousness and hand function.135 Nonopioid analgesics such as ibuprofen and toradol can be useful adjuncts in patients with cutaneous injuries. Finally, behavior modifications such as relaxation therapy, hypnosis, and virtual reality can be extremely helpful in overcoming the anxiety of dressing changes and physical therapy. Careful assessment of pain and anxiety and individualized dosing regimens are essential for successful management of burn patients.136
Pulmonary Management
Chapter 48 provides a detailed discussion of the pathophysiology and treatment of the pulmonary complications of burn injury. Recent evidence suggests that inadequate fluid resuscitation may actually accentuate pulmonary damage137; burn patients with inhalation injuries require increased resuscitation volumes59 and should be resuscitated at least as aggressively as other burn patients. Pulmonary artery catheterization may be helpful in patients with severe lung injuries who require high ventilator pressures. Although colloid-based resuscitation may be associated with persistently increased extravascular lung water,90 this does not appear to be the case with crystalloid, even in patients who require substantially more fluid than Parkland requirements.74
A number of reports have focused on techniques to improve oxygenation in burn patients with severe acute lung injury, including extracorporeal membrane oxygenation,138 inhaled nitric oxide,139 and novel ventilator strategies such as high-frequency volume diffusive,140 oscillation,141 or percussive ventilation.142 However, these modalities are limited in their availability and usefulness, and clear-cut indications for each have not been developed. The use of permissive hypercapnia may be beneficial,143 though other protective lung strategies have not been evaluated in burns. The vast majority of burn patients can be managed effectively with conventional ventilation techniques, and death purely from hypoxia is rare.
Multiple organ failure is the most common cause of death in burn patients,144,145 very frequently triggered by ongoing pulmonary infection, which can be severe and persistent.146 This pneumonia is usually ventilator associated; intubation facilitates contamination of the lower airways already primed for infection by the mucosal sloughing, mucous plugging, and atelectasis caused by inhalation injury. Frequent cultures and aggressive antibiotic use are necessary for successful treatment. The use of ventilator bundles, including frequent suctioning and chest physiotherapy, elevation of the head of the bed, closed suction techniques, postpyloric feeding, and frequent oral care, may also be helpful.147 The use of heparin and acetylcysteine aerosols—sometimes combined with bronchodilators—to liquefy and mobilize casts and debris has shown some promise in pediatric patients.148 Improved mobilization of secretions may also be the mechanism by which percussive or oscillatory ventilation is beneficial.142 Fiberoptic bronchoscopy and bronchoalveolar lavage can be used both for diagnosis of pneumonia and as a therapeutic maneuver to clear tenacious plugs and improve ventilation.19 Based on limited evidence, neither corticosteroids nor prophylactic antibiotics appear to be helpful in treating respiratory failure or preventing pneumonia.149 The performance of tracheostomy in patients who fail initial attempts at extubation may or may not reduce infections150 but unquestionably provides a more comfortable and secure airway for long-term ventilator support.
Cardiovascular Complications and Care
As mentioned previously, the immediate response to burn injury consists of reduced cardiac output and increased peripheral resistance, followed by gradual return of both values to near-normal at the end of resuscitation, after which markedly elevated cardiac output and reduced peripheral resistance persists through wound coverage and into rehabilitation (see Fig. 67.5). This response is so characteristic that invasive hemodynamic monitoring is rarely needed during resuscitation.
Catecholamine stimulation of cardiac function often results in hypertension, which can be difficult to manage, as well as sustained tachycardia and tachyarrhythmias. Beta blockade is useful in treating these problems, and may be indicated for routine administration as well. Beta blockers are tolerated well in this situation, and may even contribute to improved outcomes.151
Adrenal Insufficiency
Recent studies have demonstrated a surprisingly high frequency of absolute or relative adrenal insufficiency in critical care patients, including burns, which may increase mortality risk from shock and sepsis.152,153 However, the quoted incidence of this problem has varied greatly, depending on the diagnostic criteria used and the populations studied.154–157 No systematic evaluation of adrenal insufficiency has been conducted in burn patients. The incidence of this complication remains unknown,158 but may be more common than previously suspected, and should be considered in burn patients with refractory hypotension.
Infection Control
Burn Wound Infections
Historically overwhelming burn wound sepsis was a major cause of death in patients who survived resuscitation. Over the past 50 years, the use of systemic and topical antibiotics coupled with surgical excision and skin grafting has dramatically decreased this problem. However, infection in burn wounds remains an important source of morbidity and mortality risks. As with other infections, the development of increasingly effective antibiotics has been matched by rapid adaptation of microbial pathogens. The initial use of antibiotics in World War II controlled Streptococcus and Staphylococcus infections, but these were supplanted by gram-negative organisms, particularly Pseudomonas. In the 1960s and 1970s, development of silver nitrate solution, mafenide acetate (Sulfamylon) and silver sulfadiazine (Silvadene, Thermazene) were effective in combating these infections, but microflora continued to evolve. Today, burn wound infections are increasingly caused by multiply resistant gram-negative organisms, Acinetobacter, methicillin-resistant Staphylococcus, Candida, and fungi.159 Individual patients may display a similar progression of wound colonization as therapy and antibiotic administration continue.
Established principles of infection control remain a critical component of burn care. Essentially every open wound should be treated with topical agents until healed. The time-tested agents sulfadiazene and mafenide remain effective against a wide range of bacteria, and still constitute the first line for acute burn treatment despite some limitations. Silver sulfadiazine has been associated with neutropenia, though this is usually a transient phenomenon in the early postburn period (see later). Mafenide is a carbonic anhydrase inhibitor that can generate significant metabolic acidosis if used on large areas and has also been linked to the emergence of fungal infections.160 A number of newer topical agents are now available, including silver-containing dressings (Acticoat, Acquacel-Ag), cerium-silver nitrate, mupirocin (Bactroban), chlorhexidine, betadine, and others. The choice of agent and technique should be adjusted based on culture results and wound appearance, and can often be reduced as wounds heal. Silver-containing agents and topical antifungals such as nystatin are effective against yeast and mold infections, and antibiotic solutions including mafenide and silver nitrate are widely used to soak fresh skin grafts and some skin substitutes. The so-called melting graft syndrome has been attributed to chronic infection with Staphylococcus species161; topical mupirocin may be particularly effective in this setting.
Surface cultures of burn wounds will identify dominant organisms, but cannot distinguish between invasive infection and colonization. Burn wound biopsies can be obtained for quantitative culture; bacterial counts of 105 per gram of tissue or more has been used as a criterion for invasive burn wound sepsis. However, false-positive results are frequent; wounds may be heavily colonized without bacterial invasion, and biopsies must be obtained in exactly the right place.162 Histologic demonstration of organisms penetrating into viable tissue and capillaries remains a valid diagnostic finding.159 However, many centers lack an experienced pathologist to read these samples accurately, so routine biopsies are now used infrequently. Daily examination of wounds and clinical suspicion remain the most important tools for prompt diagnosis of burn wound infection. Once detected, burn wound sepsis is best treated by aggressive, total wound excision, broad-spectrum systemic coverage, and topical antibiotic soaks in preparation for allograft or autograft placement. Burn wound sepsis is an extremely serious complication and should involve every member of the burn team.
Other Infections
Modern methods of infection prevention and control have contributed to decreased rates of many types of infections in burn patients. With the decline in burn wound infections, pneumonia has emerged as the most common infection in burn patients. Central venous catheter infections—particularly from Candida—also remain a constant threat163 and can contribute to bacterial endocarditis, particularly if lines are allowed to reach the atrium or ventricle.164 Neither routine catheter changes nor rewires have been shown to decrease infectious risks.165 The current use of peripherally inserted central catheters (PICC lines) may or may not reduce the risks of catheter-related infection,166 but regardless of the line or site used, adherence to accepted principles of catheter placement and care167 and prompt removal of catheters when possible should be practiced. Improved wound management, nutrition, and hygiene have all but eliminated such historically important infections as suppurative thrombophlebitis, chondritis of the ear, and suppurative parotitis as clinical problems.
Diagnosis of infection can be difficult in burn patients. Fever, leukocytosis, and tachycardia are all normal consequences of acute burn injury, and standard criteria for the diagnosis of systemic inflammatory response syndrome (SIRS) and sepsis are difficult to interpret.168 Low-grade fever may be sustained during acute treatment, but any increase in temperature above 38.5° C, especially when accompanied by increases in white blood cells (WBCs) or heart rate, should prompt an evaluation for infection, including appropriate cultures. The once-common policy of obtaining routine surveillance cultures of blood, urine, or wounds has been shown to have relatively low yield and a high incidence of false-positive results.169 Some units use swab cultures to detect colonization and to track dominant flora for epidemiologic reasons. The value of measuring specific biomarkers to detect infection, including C-reactive protein and procalcitonin, has been inconsistent and remains controversial.170,171
Systemic prophylactic antibiotics have not been shown to reduce burn wound or other infections and should not be used.172,173 As in all ICU patients, antibiotic use should be guided by the clinical situation and culture results. As noted previously, burn patients often exhibit abnormal drug kinetics and require increased antibiotic dosing and careful monitoring of antibiotic efficacy. The clinical pharmacist is an essential member of the burn team for this and other reasons. The isolation of burn patients through universal precautions and the separation of infected patients by cohort nursing should be practiced as routine components of infection control. The practice of selective digestive decontamination in burn patients is controversial; although some centers report benefits,174 and some no effect,175 from this time-consuming technique, it is not routinely practiced in U.S. burn centers.
Metabolic Support and Gastrointestinal Management
The Hypermetabolism of Burn Injury
Burns induce the greatest hypermetabolic response of any injury. This response typically follows the ebb and flow pattern described over 70 years ago,176 in which initial (24-72 hours) reduced energy expenditure is followed by increasing metabolism, which peaks 10 to 14 days after injury and then tapers slowly as wound healing progresses. The degree of hypermetabolism correlates with burn size but appears to level off with burns of 40% to 50% TBSA, above which no further increase is seen.177 This response is sustained by the secretion of catecholamines, cortisol, and glucagon that persists until after wounds are closed. Together these hormones accelerate catabolism through breakdown of skeletal muscle, reduced uptake of fats, and opposition to the effects of insulin.178,179 The result is obligatory muscle wasting to support gluconeogenesis; lipids have very little protein-sparing effect, and even exogenous glucose is limited in its ability to prevent protein wasting.180
In the 1970s burn patients frequently demonstrated prolonged calorie and protein consumption that exceeded twice normal and caused fatal inanition within a few weeks of injury.181–184 Modern burn treatment—aggressive excision and wound coverage, control of sepsis and pain, mechanical ventilation, and improved environmental control—have all helped reduce both the duration and magnitude of this response.177,185,186 Nonetheless, significant hypermetabolism persists well after burn wounds are closed, and careful nutritional support is a requirement for the successful care of burn patients.187
Route and Timing of Nutritional Support
The superiority of enteral nutrition is widely accepted,188 and may be especially true for burn patients. Enteral nutrition nourishes bowel mucosa, preserves blood supply, reduces permeability, and improves associated immune function.189–191 In trauma and ICU patients, early and aggressive enteral nutrition appears to decrease infectious complications, but total parenteral nutrition (TPN) has been associated with an increased mortality rate in burn patients,192,193 and it also promotes development of fatty liver.194 It may even be preferable to withhold nutrition entirely for limited periods, rather than use TPN.
Enteral feeding should begin as quickly as practical following injury, especially if patients will not be able to eat within 5 to 7 days. In contrast to early studies,195 immediate enteral feedings have not been consistently shown to reduce hypermetabolism,196 but do decrease calorie deficit and improve nitrogen balance.197,198 Feedings can be started within a few hours of injury, and continued even through surgical procedures.199 The routine use of promotility agents has not been studied in burn patients200; it is also unclear whether small intestinal feedings are superior to gastric feedings; both can have complications, and both require careful monitoring.
Energy Requirements
Dozens of regimens have been used to estimate the caloric needs of burn patients, including the famous Curreri formula.201 However, static formulas cannot accommodate the major fluctuations that occur over time and between individuals of different ages and burn sizes.202 In addition, because modern burn treatment has reduced hypermetabolism, older formulas overestimate requirements significantly.203 Current recommended caloric intake for adults with major burns is 120% to 150% of Harris-Benedict estimates of basal needs.204 Many centers also use indirect calorimetry to measure energy expenditure and to detect significant under- or overfeeding.205 Regardless, support should still be increased during periods of peak energy expenditure to avoid underfeeding, and reduced later to avoid overfeeding.
Whatever regimen is chosen, the practical difficulties of delivering nutritional support are substantial.206,207 Interruptions in feedings, fluctuations in energy consumption caused by fever and activity, and delivery of empty calories in glucose solutions all frustrate attempts to tailor individual nutrition exactly, which may explain why the superiority of indirect calorimetry-based nutrition has not been proved.202 Involvement of the team—including a dietician—in implementing, assessing, and adjusting feedings is probably more important than adherence to predetermined estimations.208
Enteral Formulas
Chapter 82 provides details on the composition of many commercially available enteral formulas which can be used in burn patients. Specific components of effective enteral formulas include the following:
Carbohydrates and Glucose Control
The catabolism of burn injury makes carbohydrates the preferred energy source, but also worsens hyperglycemia. Recent studies have demonstrated the value of meticulous glucose control in ICU populations in reducing organ failure, length of stay, and inflammation.209,210 These benefits are likely seen in burn patients as well,211 but hormonally mediated resistance to insulin—the so-called diabetes of injury—makes attaining control a major challenge. Many burn units use hyperglycemia protocols, which require frequent (or continuous) dosing of insulin and careful monitoring. Oral hypoglycemics appear helpful in this effort212; in addition, giving limited calories as fat helps reduce the glucose burden required by these patients.
Fat
A certain (small) quantity of dietary fat is an essential nutrient. Lipolysis is suppressed in burn patients, as is the ability to utilize exogenous fat as an energy source. Fat intake should be restricted to less than 30% of total nonprotein calories, or about 1 g/kg/day213 or less214,215; withholding fat entirely from TPN for substantial periods may be beneficial.188,216 In addition, products high in ω-3 free fatty acids (FFAs) and correspondingly low in ω-6 FFAs may improve glucose control and reduce infections.217,218 Most clinical experience has been obtained with the use of immune-enhancing diets (IEDs) (see later) in which the effects of individual components have been difficult to assess.
Protein
Accelerated proteolysis is often the most important characteristic of burn-induced hypermetabolism. A major goal of nutrition is to reduce or replace protein losses, which can exceed 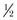 lb of lean body mass per day. Providing adequate calories alone will not reduce muscle breakdown,219 so increased protein must be provided as well. Protein requirements increase with burn size220,221; appropriate provision of dietary protein has resulted in reduced infections and a lower mortality rate in patients with major injuries.222 Current recommendations are 1.5 to 2.0 g of protein/kg/day (up to 3.0 g/kg/day in children),205,208,223 which corresponds to a nonprotein calorie/nitrogen ratio of 100 : 1 or less. Nitrogen balance should be monitored regularly.
lb of lean body mass per day. Providing adequate calories alone will not reduce muscle breakdown,219 so increased protein must be provided as well. Protein requirements increase with burn size220,221; appropriate provision of dietary protein has resulted in reduced infections and a lower mortality rate in patients with major injuries.222 Current recommendations are 1.5 to 2.0 g of protein/kg/day (up to 3.0 g/kg/day in children),205,208,223 which corresponds to a nonprotein calorie/nitrogen ratio of 100 : 1 or less. Nitrogen balance should be monitored regularly.
Specific Amino Acids
The amino acids arginine (Arg) and glutamine (Glu) play enhanced roles in critical illness. Both are depleted rapidly in burn patients, and are considered conditionally essential.219,224 Glutamine is important in energy transport, as a precursor of glutathione225 and as a nutrient for enterocytes, helping to preserve bowel integrity and limit permeability.226–228 In burn patients provision of up to 25 g Glu/day, given parenterally229 or enterally,230 has been associated with improved visceral protein levels and reduced infectious mortality rate and length of stay. Glutamine supplementation has been recommended188 but is not routinely practiced. Glutamine is almost totally absent from TPN formulas, which may explain some of the benefits of enteral nutrition in burn patients.
Arginine enhances natural killer cell function and nitric oxide (NO) synthesis, promoting inflammation and resistance to infection.231,232 Specific studies of arginine supplementation have not been performed in burn patients, but arginine has been incorporated into complex immune-enhancing diets (IEDs) containing ω-3 FFAs, arginine, glutamine, and RNA, among other components.233 These diets have shown benefits in some surgical populations,234 but deleterious or no effects in patients with sepsis or pneumonia,235 and their widespread use has been questioned.188 Few data are available on IEDs in burn patients,236 but the mixed results in other groups suggest that components should be studied separately before incorporating them into cocktails with even more unpredictable effects. Most burn centers simply use high-protein diets rather than these specialized and expensive formulas.
Other Nutrients
A number of micronutrients—including vitamins A, C, and D, iron, zinc, and others—are important in wound healing and may be depleted following burn injury,237 and some supplementation has been recommended.238 Many centers simply administer multivitamins, which is probably adequate.178,239 Commercial enteral formulas also contain far more than the recommended daily dietary allowances of many vitamins and trace elements.
Monitoring Nutritional Support
Because burn injury alters many parameters of nutritional status, monitoring nutrition can be as difficult as providing it. For example, substantial weight gain invariably accompanies fluid resuscitation,240 although overfeeding can increase body fat even as protein stores are depleted.241,242 As a result, patients have often lost more lean body mass than is reflected by weight alone.243 Nitrogen balance studies can be useful, but sedation and bed rest make attaining positive nitrogen balance difficult,244 emphasizing the need to continue physical therapy even during acute care. Serum protein markers are often distorted; levels of albumin,245 prealbumin, and transferrin205,246 fall quickly following injury, and recover only very slowly even with appropriate nutrition. Perhaps the best marker of nutritional adequacy in burn patients is their overall status, including vital signs, wound healing, and functional improvement. Body weight, nitrogen balance, and protein markers may be most useful in tracking trends in individuals.247
Modulation of Hypermetabolism
Burn-related hormonal changes complicate the provision of nutritional support, but also provide mechanisms by which hypermetabolism can be manipulated. Recent studies have shown promising results, and suggest that manipulation of the metabolic response to injury may become routine in the future. A variety of approaches have been utilized, including beta blockade with propranolol, low-dose insulin infusions, use of counterregulatory hormones such as insulin-like growth factor-1 (IGF-1), or anabolic agents such as testosterone and oxandrolone.248 The use of propranolol appears to be a safe, low-cost way to ameliorate both the cardiovascular response and hypermetabolic muscle wasting that occur during acute burn care.249 Administration of the synthetic oral androgen oxandrolone has been shown to reduce muscle breakdown, speed rehabilitation, and lead to decreased length of stay in hospitalized patients.250,251 Although none of these therapies is now routine, they are becoming more widespread. Recommendations for their routine use will need to await larger controlled trials to demonstrate efficacy.
Gastrointestinal Complications in Burn Patients
Acute Cholecystitis
Cholecystitis—usually acalculous—can have a number of causes, including gallbladder ischemia from shock and dehydration,252 biliary stasis from narcotics and TPN, bile pigment loads from hemolysis and transfusion, and bacterial seeding from sepsis. With more effective control of these factors, acute cholecystitis is now rare; a recent review documented just 20 cases among 10,762 acutely burned patients (0.18%), with progressive declines in recent years.253 Fever, leukocytosis, feeding intolerance, and abdominal pain are characteristic findings, though they may be difficult to sort out in acutely ill patients. Ultrasound and computed tomography (CT) scanning are preferred for confirmation of diagnosis; biliary scintigraphy can have a high false-positive rate. Treatment has traditionally consisted of prompt cholecystectomy, though placement of cholecystostomy tubes under ultrasound guidance can provide definitive treatment in many patients.254 With prompt diagnosis, the mortality rate from this complication should be low.
Hepatic Enzyme Elevation
Immediately following burn injury, hemolysis and ileus can contribute to a cholestatic picture, which should resolve within a few days. Some degree of chronic fatty infiltration of the liver may be inevitable in patients with major burns, and mild enzyme elevations are often seen, which is exacerbated by overfeeding and the use of TPN.194 Severe hepatic steatosis may be both a cause and consequence of sepsis, and correlates with increased mortality rate.
Pancreatitis
Pancreatitis can also result from systemic hypoperfusion and secondary sepsis. In contrast to cholecystitis, the incidence of pancreatitis may be increasing as more severely injured patients now survive burn shock. Ryan and colleagues documented hyperamylasemia in 40% of patients with large burns, with an associated increased mortality rate and length of stay,255 although pancreatic pseudocysts and abscesses were quite rare. Pancreatic enzymes should be measured in patients with feeding intolerance, abdominal pain, or nausea; in many cases, transient bowel rest and fluid support will be effective treatment. Use of TPN should be avoided unless pancreatitis is unusually severe and prolonged.
Gastrointestinal Bleeding
Although the time-honored eponym Curling’s ulcer has sometimes been considered a unique entity, burn patients develop the stress ulcerations typical among acutely ill patients. Mucosal erosions and atrophy can occur within 72 hours of injury and progress to large (often multiple) ulcerations of the prepyloric area or duodenum. Their primary cause is thought to be gastric mucosal hypoperfusion from shock or sepsis; the role of Helicobacter pylori infection is unclear.256,257 Modern treatment strategies including aggressive fluid resuscitation, suppression or neutralization of gastric acid, and early enteral feeding have resulted in a dramatic decline in the incidence of this complication from as high as 86% in early postmortem studies258 to less than 2% today. Bleeding is more frequent with large injuries and in patients with coagulopathy, prolonged mechanical ventilation, and sepsis. Aggressive evaluation of any observed GI bleeding should be undertaken. Most patients can be controlled with endoscopic maneuvers and continuous proton pump inhibitors. Surgery for acute upper GI bleeding is now rarely required.259
Ileus and Intestinal Necrosis
Ileus occurs routinely following acute burns, and oral intake should be withheld at least until resuscitation is completed. Impaired intestinal motility can be aggravated by narcotics, infection, and ventilatory support. Severe colonic ileus—Ogilvie’s syndrome—can occur in this setting,260 and overly aggressive enteral feeding can rarely lead to bowel distention, ischemia, necrosis, perforation, and death.261,262 For this reason, feedings should be monitored carefully, and held if obstipation, distention, or pain becomes significant. CT scanning can confirm bowel dilatation or free air, in which case immediate laparotomy is mandatory.
Diarrhea
Diarrhea is extremely common with enteral feedings, and can be a major problem in management. The cause is often multifactorial,263 including high osmotic loads, overfeeding, enteral medications, and infections including Cytomegalovirus and Clostridium difficile.264 Treatment options include enteral opiates such as Imodium or paregoric or the use of fiber-containing products. However, fiber can clog small-diameter tubes and has been blamed for intestinal distention and necrosis,265 and slowing intestinal transit can potentiate infectious diarrhea. Often simply holding feedings for a few hours will suffice; diarrhea unresponsive to simple measures should prompt a search for infectious causes, inside or outside the bowel.266
Hematologic Considerations
Red Blood Cells
Hemoconcentration, which accompanies burn shock, can result in hematocrits of 70% or higher. This will theoretically produce sludging and increased vascular resistance, though clinical problems with thrombosis are rare. As resuscitation proceeds hematocrits fall progressively, and are usually below normal by the end of 48 hours. Thereafter, anemia often persists or worsens until wound coverage is obtained. Several mechanisms contribute to this finding.267 First, burn injury destroys red blood cells directly; significant hemolysis can occur within minutes of injury and produce hemoglobinuria or jaundice. Burn-induced alterations in red blood cell metabolism result in spherocytosis, increased membrane fragility, and shortened red blood cell survival. Erythropoietic response is blunted, apparently at the stem cell level,268 and erythropoietin supplementation is not helpful.269 Acute hemorrhage from surgery, and more gradual blood loss from wound debridements add to these effects.
The issue of appropriate transfusion trigger is controversial in burn patients. No systematic trial of transfusion thresholds has been performed, and no standard is universally accepted,270 though limited evidence supports the practice of conservative transfusion in burn patients as in other ICU populations. In a multicenter review, Palmieri and coworkers found that transfusions correlated with both mortality risk and infectious episodes in major burns,271 which underscores that transfusions should be based on physiologic assessment and not given routinely.
Platelets
Platelets and coagulation factors are also consumed in the early postburn period; platelet counts below 100,000/µL and coagulopathy are common for the first few days, and supplementation may be needed if major excisions are anticipated. Subsequently, both rebound to supranormal levels. During wound coverage, patients may demonstrate platelet counts in excess of 1,000,000/µL, and fibrinogen levels may exceed twice normal. These are reactive abnormalities and do not require treatment.272 Platelet counts are a sensitive indicator of infection; a falling platelet count occurring after the first few days should suggest sepsis, and is associated with a poor prognosis.273
Thromboembolism
Systemic inflammation, a hypercoagulable state, and frequent immobilization might all suggest that thromboembolic complications are common in burn patients. In fact, reported incidences of clinically apparent deep venous thrombosis (DVT) and pulmonary embolism (PE) are low.274 However, prospective evaluation of burn patients suggests an incidence of DVT/PE similar to that of other moderate- to high-risk populations.275 At present, no consensus exists on the issue of DVT prophylaxis in burn patients. Some centers use it routinely, but many centers provide it for high-risk patients, including the obese and patients with large burns, lower extremity burns, or femoral venous lines.
The Rehabilitation Phase
Although the focus of this chapter is the acute care of burn patients, it must be remembered that rehabilitation begins at the time of injury. The physical therapist is an essential member of the acute burn team, and therapy should begin before resuscitation is completed. Physical therapy, mobilization, and prevention of skin breakdown and muscle wasting can have profound effects on the overall success of acute burn care. Immobility and inactivity result in rapid depletion of muscle mass and function; other complications include demineralization of bone and pathologic fractures,276 myositis ossificans, and extensive contracture formation. Therapeutic exercise regimens, beginning during acute care and continuing beyond hospital discharge, can result in improved function and well-being, and increased chances of return to independent living.277
References
1. Saffle, JR. Practice guidelines for burn care. (ed). J Burn Care Rehabil. 2001; 22:S1–69.
2. Brigham, PA, McLoughlin, E. Burn incidence and medical care use in the United States: Estimates, trends, and data sources. J Burn Care Rehabil. 1996; 17:95–107.
3. Saffle, JR, Davis, B, Williams, P. Recent outcomes in the treatment of burn injury in the United States: A report from the American Burn Association Patient Registry. J Burn Care Rehabil. 1995; 16:219–232.
4. Wolf, SE, Rose, JK, Desai, MH, et al. Mortality determinants in massive pediatric burns. An analysis of 103 children with > or = 80% TBSA burns (> or = 70% full-thickness). Ann Surg. 1997; 225:554–569.
5. Shirani, KZ, Pruitt, BA, Jr., Mason, AD, Jr. The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987; 205:82–87.
6. Ryan, CM, Schoenfeld, DA, Thorpe, WP, et al. Objective estimates of the probability of death from burn injuries. N Engl J Med. 1998; 338:362–366.
7. Salisbury, R. Burn rehabilitation: Our unanswered challenge. The 1992 presidential address to the American Burn Association. J Burn Care Rehabil. 1992; 13:495–505.
8. Pereira, C, Murphy, K, Herndon, D. Outcome measures in burn care. Is mortality dead? Burns. 2004; 30:761–771.
9. Saffle, JR. The 2002 presidential address: N. P. D. G. B. and other surgical sayings. J Burn Care Rehabil. 2002; 23:375–384.
10. American Burn Association. Burn Care Resource Directory. Available at ameriburn.org.
11. Saffle, JR, Edelman, L, Morris, SE. Regional air transport of burn patients: A case for telemedicine? J Trauma. 2004; 57:57–64.
12. Hammond, JS, Ward, CG. Transfers from emergency room to burn center: Errors in burn size estimate. J Trauma. 1987; 27:1161–1165.
13. Freiburg, C, Igneri, P, Sartorelli, K, Rogers, F. Effects of differences in percent total body surface area estimation on fluid resuscitation of transferred burn patients. J Burn Care Res. 2007; 28:42–48.
14. Vercruysse, GA, Ingram, WL, Feliciano, DV. The demographics of modern burn care: Should most burns be cared for by non-burn surgeons? Am J Surg. 2011; 201:91–96.
15. Klein, MB, Kramer, CB, Nelson, J, et al. Geographic access to burn center hospitals. JAMA. 2009; 302:1774–1781.
16. Initial assessment and management. In: Manual of Advanced Trauma Life Support. Chicago: American College of Surgeons; 2004:1–10.
17. Birky, MM, Clarke, FB. Inhalation of toxic products from fires. Bull N Y Acad Med. 1981; 57:997–1013.
18. Zikria, BA, Weston, GC, Chodoff, M, Ferrer, JM. Smoke and carbon monoxide poisoning in fire victims. J Trauma. 1972; 12:641–645.
19. Fitzpatrick, J, Cioffi, W. Diagnosis and treatment of inhalation injury. In: Herndon D, ed. Total Burn Care. Philadelphia: WB Saunders; 2002:232–241.
20. Fein, A, Leff, A, Hopewell, PC. Pathophysiology and management of the complications resulting from fire and the inhaled products of combustion: Review of the literature. Crit Care Med. 1980; 8:94–98.
21. Silverman, SH, Purdue, GF, Hunt, JL, Bost, RO. Cyanide toxicity in burned patients. J Trauma. 1988; 28:171–176.
22. Kirk, MA, Gerace, R, Kulig, KW. Cyanide and methemoglobin kinetics in smoke inhalation victims treated with the cyanide antidote kit. Ann Emerg Med. 1993; 22:1413–1418.
23. Barillo, DJ. Diagnosis and treatment of cyanide toxicity. J Burn Care Res. 2009; 30:148–152.
24. Erdman, AR. Is hydroxocobalamin safe and effective for smoke inhalation? Searching for guidance in the haze. Ann Emerg Med. 2007; 49:814–816.
25. Mackie, DP, van Dehn, F, Knape, P, et al. Increase in early mechanical ventilation of burn patients: An effect of current emergency trauma management? J Trauma. 2011; 70:611–615.
26. Wittram, C, Kenny, JB. The admission chest radiograph after acute inhalation injury and burns. Br J Radiol. 1994; 67:751–754.
27. Masanes, MJ, Legendre, C, Lioret, N, et al. Fiberoptic bronchoscopy for the early diagnosis of subglottal inhalation injury: Comparative value in the assessment of prognosis. J Trauma. 1994; 36:59–67.
28. Varghese, TK, Kim, AW, Kowal-Vern, A, Latenser, BA. Frequency of burn-trauma patients in an urban setting. Arch Surg. 2003; 138:1292–1296.
29. Hawkins, A, Maclennan, PA, McGwin, G, Jr., et al. The impact of combined trauma and burns on patient mortality. J Trauma. 2005; 58:284–288.
30. Santaniello, JM, Luchette, FA, Esposito, TJ, et al. Ten year experience of burn, trauma, and combined burn/trauma injuries comparing outcomes. J Trauma. 2004; 57:696–700.
31. Chang, CH, Holmes, JF, Mower, WR, Panacek, EA. Distracting injuries in patients with vertebral injuries. J Emerg Med. 2005; 28:147–152.
32. Saffle, JR, Schnelby, A, Hofmann, A, Warden, GD. The management of fractures in thermally injured patients. J Trauma. 1983; 23:902–910.
33. Purdue, GF, Hunt, JL. Multiple trauma and the burn patient. Am J Surg. 1989; 158:536–539.
34. Shock and fluid resuscitation. In: Manual of Advanced Burn life Support. Chicago: American Burn Association; 2001.
35. Baxter, C. Fluid volume and electrolyte changes in the early post-burn period. Clin Plast Surg. 1974; 1:693–703.
36. Warden, G. Fluid resuscitation and early management. In: Herndon D, ed. Total Burn Care. Philadelphia: WB Saunders; 2002:88–97.
37. Carvajal, HF, Brouhard, BH, Linares, HA. Effect of antihistamine-antiserotonin and ganglionic blocking agents upon increased capillary permeability following burn trauma. J Trauma. 1975; 15:969–975.
38. Friedl, HP, Till, GO, Trentz, O, Ward, PA. Roles of histamine, complement and xanthine oxidase in thermal injury of skin. Am J Pathol. 1989; 135:203–217.
39. Sherwood, E, Traber, D. The systemic inflammatory response syndrome. In: Herndon D, ed. Total Burn Care. Philadelphia: WB Saunders; 2002:257–270.
40. Kramer, G, Lund, T, Herndon, D. Pathophysiology of burn shock and burn edema. In: Herndon D, ed. Total Burn Care. Philadelphia: WB Saunders; 2002:78–85.
41. Youn, YK, LaLonde, C, Demling, R. The role of mediators in the response to thermal injury. World J Surg. 1992; 16:30–36.
42. Starling, E. On the absorption of fluids from the connective tissue spaces. J Physiol (Lond). 1986; 19:312–326.
43. Demling, RH. The burn edema process: Current concepts. J Burn Care Rehabil. 2005; 26:207–227.
44. Arturson, G. Microvascular permeability to macromolecules in thermal injury. Acta Physiol Scand Suppl. 1979; 463:111–122.
45. Baxter, CR, Shires, T. Physiological response to crystalloid resuscitation of severe burns. Ann N Y Acad Sci. 1968; 150:874–894.
46. Birke, G, Liljedahl, SO, Plantin, LO. Distribution and losses of plasma proteins during the early stage of severe burns. Ann N Y Acad Sci. 1968; 150:895–904.
47. Carvajal, HF, Linares, HA, Brouhard, BH. Relationship of burn size to vascular permeability changes in rats. Surg Gynecol Obstet. 1979; 149:193–202.
48. Leape, LL. Initial changes in burns: Tissue changes in burned and unburned skin of rhesus monkeys. J Trauma. 1970; 10:488–492.
49. Zetterstrom, H, Arturson, G. Plasma oncotic pressure and plasma protein concentration in patients following thermal injury. Acta Anaesthesiol Scand. 1980; 24:288–294.
50. Demling, RH, Kramer, G, Harms, B. Role of thermal injury-induced hypoproteinemia on fluid flux and protein permeability in burned and nonburned tissue. Surgery. 1984; 95:136–144.
51. Demling, RH, Kramer, GC, Gunther, R, Nerlich, M. Effect of nonprotein colloid on postburn edema formation in soft tissues and lung. Surgery. 1984; 95:593–602.
52. Guyton, AC. Interstitial fluid pressure. I. Pressure-volume curves of interstitial space. Circ Res. 1965; 16:452–460.
53. Tanaka, H, Lund, T, Wiig, H, et al. High dose vitamin C counteracts the negative interstitial fluid hydrostatic pressure and early edema generation in thermally injured rats. Burns. 1999; 25:569–574.
54. Miserocchi, G, Negrini, D, Passi, A, De Luca, G. Development of lung edema: Interstitial fluid dynamics and molecular structure. News Physiol Sci. 2001; 16:66–71.
55. Pruitt, BA Jr, Mason, AD, Jr., Moncrief, JA. Hemodynamic changes in the early postburn patient: The influence of fluid administration and of a vasodilator (hydralazine). J Trauma. 1971; 11:36–46.
56. Hilton, JG, Marullo, DS. Effects of thermal trauma on cardiac force of contraction. Burns Incl Therm Inj. 1986; 12:167–171.
57. Warden, GD. Burn shock resuscitation. World J Surg. 1992; 16:16–23.
58. Schwartz, SI. Supportive therapy in burn care. Consensus summary on fluid resuscitation. J Trauma. 1979; 19:876–877.
59. Navar, PD, Saffle, JR, Warden, GD. Effect of inhalation injury on fluid resuscitation requirements after thermal injury. Am J Surg. 1985; 150:716–720.
60. Yowler, CJ, Fratianne, RB. Current status of burn resuscitation. Clin Plast Surg. 2000; 27:1–10.
61. Pruitt, BA. Protection from excessive resuscitation: “pushing the pendulum back”. J Trauma. 2000; 49(3):567–568.
62. Cancio, LC, Chavez, S, Alvarado-Ortega, M, et al. Predicting increased fluid requirements during the resuscitation of thermally injured patients. J Trauma. 2004; 56:404–413.
63. Engrav, LH, Colescott, PL, Kemalyan, N, et al. A biopsy of the use of the Baxter formula to resuscitate burns or do we do it like Charlie did it? J Burn Care Rehabil. 2000; 21:91–95.
64. Kaups, KL, Davis, JW, Dominic, WJ. Base deficit as an indicator of resuscitation needs in patients with burn injuries. J Burn Care Rehabil. 1998; 19:346–348.
65. Choi, J, Cooper, A, Gomez, M, et al. The 2000 Moyer Award. The relevance of base deficits after burn injuries. J Burn Care Rehabil. 2000; 21:499–505.
66. Cancio, LC, Reifenberg, L, Barillo, DJ, et al. Standard variables fail to identify patients who will not respond to fluid resuscitation following thermal injury: Brief report. Burns. 2005; 31:358–365.
67. Jeng, JC, Lee, K, Jablonski, K, Jordan, MH. Serum lactate and base deficit suggest inadequate resuscitation of patients with burn injuries: Application of a point-of-care laboratory instrument. J Burn Care Rehabil. 1997; 18:402–405.
68. Dries, DJ, Waxman, K. Adequate resuscitation of burn patients may not be measured by urine output and vital signs. Crit Care Med. 1991; 19:327–329.
69. Tuchschmidt, JA, Mecher, CE. Predictors of outcome from critical illness. Shock and cardiopulmonary resuscitation. Crit Care Clin. 1994; 10:179–195.
70. Fiddian-Green, RG, Haglund, U, Gutierrez, G, Shoemaker, WC. Goals for the resuscitation of shock. Crit Care Med. 1993; 21:S25–S31.
71. Velmahos, GC, Demetriades, D, Shoemaker, WC, et al. Endpoints of resuscitation of critically injured patients: Normal or supranormal? A prospective randomized trial. Ann Surg. 2000; 232:409–418.
72. Schiller, WR, Bay, RC, Garren, RL, et al. Hyperdynamic resuscitation improves survival in patients with life-threatening burns. J Burn Care Rehabil. 1997; 18:10–16.
73. Barton, RG, Saffle, JR, Morris, SE, et al. Resuscitation of thermally injured patients with oxygen transport criteria as goals of therapy. J Burn Care Rehabil. 1997; 18:1–9.
74. Holm, C, Tegeler, J, Mayr, M, et al. Effect of crystalloid resuscitation and inhalation injury on extravascular lung water: Clinical implications. Chest. 2002; 121:1956–1962.
75. Oda, J, Yamashita, K, Inoue, T, et al. Resuscitation fluid volume and abdominal compartment syndrome in patients with major burns. Burns. 2006; 32:151–154.
76. Britt, RC, Gannon, T, Collins, JN, et al. Secondary abdominal compartment syndrome: Risk factors and outcomes. Am Surg. 2005; 71:982–985.
77. Moylan, JA, Mason, AD, Jr., Rogers, PW, Walker, HL. Postburn shock: A critical ealuation of resuscitation. J Trauma. 1973; 13:354–358.
78. Monafo, WW, Halverson, JD, Schechtman, K. The role of concentrated sodium solutions in the resuscitation of patients with severe burns. Surgery. 1984; 95:129–135.
79. Oda, J, Ueyama, M, Yamashita, K, et al. Hypertonic lactated saline resuscitation reduces the risk of abdominal compartment syndrome in severely burned patients. J Trauma. 2006; 60:64–71.
80. Caldwell, FT, Bowser, BH. Critical evaluation of hypertonic and hypotonic solutions to resuscitate severely burned children: A prospective study. Ann Surg. 1979; 189:546–552.
81. Bowser-Wallace, BH, Caldwell, FT, Jr. A prospective analysis of hypertonic lactated saline v. Ringer’s lactate-colloid for the resuscitation of severely burned children. Burns Incl Therm Inj. 1986; 12:402–409.
82. Bowser-Wallace, BH, Cone, JB, Caldwell, FT, Jr. Hypertonic lactated saline resuscitation of severely burned patients over 60 years of age. J Trauma. 1985; 25:22–26.
83. Cooper, DJ. Hypertonic saline resuscitation for head injured patients. Crit Care Resuscitation. 1999; 1:161.
84. Thomas, SJ, Kramer, GC, Herndon, DN. Burns: Military options and tactical solutions. J Trauma. 2003; 54:S207–S218.
85. Vassar, MJ, Perry, CA, Gannaway, WL, Holcroft, JW. 7. 5% sodium chloride/dextran for resuscitation of trauma patients undergoing helicopter transport. Arch Surg. 1991; 126:1065–1072.
86. Huang, PP, Stucky, FS, Dimick, AR, et al. Hypertonic sodium resuscitation is associated with renal failure and death. Ann Surg. 1995; 221:543–554.
87. Griswold, JA, Anglin, BL, Love, RT, Jr., Scott-Conner, C. Hypertonic saline resuscitation: Efficacy in a community-based burn unit. South Med J. 1991; 84:692–696.
88. Roberts, I, Alderson, P, Bunn, F, et al. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2004.
89. O’Mara, MS, Slater, H, Goldfarb, IW, Caushaj, PF. A prospective, randomized evaluation of intra-abdominal pressures with crystalloid and colloid resuscitation in burn patients. J Trauma. 2005; 58:1011–1018.
90. Goodwin, CW, Dorethy, J, Lam, V, Pruitt, BA, Jr. Randomized trial of efficacy of crystalloid and colloid resuscitation on hemodynamic response and lung water following thermal injury. Ann Surg. 1983; 197:520–531.
91. Greenhalgh, DG, Housinger, TA, Kagan, RJ, et al. Maintenance of serum albumin levels in pediatric burn patients: A prospective, randomized trial. J Trauma. 1995; 39:67–73.
92. Du, GB, Slater, H, Goldfarb, IW. Influences of different resuscitation regimens on acute early weight gain in extensively burned patients. Burns. 1991; 17:147–150.
93. Kravitz, M, Warden, GD, Sullivan, JJ, Saffle, JR. A randomized trial of plasma exchange in the treatment of burn shock. J Burn Care Rehabil. 1989; 10:17–26.
94. Guha, SC, Kinsky, MP, Button, B, et al. Burn resuscitation: Crystalloid versus colloid versus hypertonic saline hyperoncotic colloid in sheep. Crit Care Med. 1996; 24:1849–1857.
95. Waters, LM, Christensen, MA, Sato, RM. Hetastarch: An alternative colloid in burn shock management. J Burn Care Rehabil. 1989; 10:11–16.
96. Wiedermann, CJ. Hydroxyethyl starch—Can the safety problems be ignored? Wien Klin Wochenschr. 2004; 116:583–594.
97. Tanaka, H, Wada, T, Simazaki, S, et al. Effects of cimetidine on fluid requirement during resuscitation of third-degree burns. J Burn Care Rehabil. 1991; 12:425–429.
98. Holliman, CJ, Meuleman, TR, Larsen, KR, et al. The effect of ketanserin, a specific serotonin antagonist, on burn shock hemodynamic parameters in a porcine burn model. J Trauma. 1983; 23:867–871.
99. Bjork, J, Arturson, G. Effect of cimetidine, hydrocortisone superoxide dismutase and catalase on the development of oedema after thermal injury. Burns Incl Therm Inj. 1983; 9:249–256.
100. Tanaka, H, Matsuda, T, Miyagantani, Y, et al. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: A randomized, prospective study. Arch Surg. 2000; 135:326–331.
101. Sullivan, SR, Ahmadi, AJ, Singh, CN, et al. Elevated orbital pressure: Another untoward effect of massive resuscitation after burn injury. J Trauma. 2006; 60:72–76.
102. Singh, CN, Klein, MB, Sullivan, SR, et al. Orbital compartment syndrome in burn patients. Ophthalmic plastic and reconstructive surgery. 2008; 24:102–106.
103. Smith, DJ, Jr., Bendick, PJ, Madison, SA. Evaluation of vascular compromise in the injured extremity: A photoplethysmographic technique. J Hand Surg (Am). 1984; 9:314–319.
104. Saffle, JR, Zeluff, GR, Warden, GD. Intramuscular pressure in the burned arm: Measurement and response to escharotomy. Am J Surg. 1980; 140:825–831.
105. Salisbury, RE, Taylor, JW, Levine, NS. Evaluation of digital escharotomy in burned hands. Plast Reconstr Surg. 1976; 58:440–443.
106. Krieger, Y, Rosenberg, L, Lapid, O, et al. Escharotomy using an enzymatic debridement agent for treating experimental burn-induced compartment syndrome in an animal model. J Trauma. 2005; 58:1259–1264.
107. Fusco, MA, Martin, RS, Chang, MC. Estimation of intra-abdominal pressure by bladder pressure measurement: Validity and methodology. J Trauma. 2001; 50:297–302.
108. Hong, JJ, Cohn, SM, Perez, JM, et al. Prospective study of the incidence and outcome of intra-abdominal hypertension and the abdominal compartment syndrome. Br J Surg. 2002; 89:591–596.
109. Latenser, BA, Kowal-Vern, A, Kimball, D, et al. A pilot study comparing percutaneous decompression with decompressive laparotomy for acute abdominal compartment syndrome in thermal injury. J Burn Care Rehabil. 2002; 23:190–195.
110. Hobson, KG, Young, KM, Ciraulo, A, et al. Release of abdominal compartment syndrome improves survival in patients with burn injury. J Trauma. 2002; 53:1129–1133.
111. Miller, PR, Meredith, JW, Johnson, JC, Chang, MC. Prospective evaluation of vacuum-assisted fascial closure after open abdomen: Planned ventral hernia rate is substantially reduced. Ann Surg. 2004; 239:608–614.
112. Jensen, AR, Hughes, WB, Grewal, H. Secondary abdominal compartment syndrome in children with burns and trauma: A potentially lethal complication. J Burn Care Res. 2006; 27:242–246.
113. Barrow, RE, Jeschke, MG, Herndon, DN. Early fluid resuscitation improves outcomes in severely burned children. Resuscitation. 2000; 45:91–96.
114. Colic, M, Ristic, L, Jovanovic, M. Emergency treatment and early fluid resuscitation following electrical injuries. Acta Chir Plast. 1996; 38:137–141.
115. Abassi, ZA, Hoffman, A, Better, OS. Acute renal failure complicating muscle crush injury. Semin Nephrol. 1998; 18:558–565.
116. Holm, C, Horbrand, F, von Donnersmarck, GH, Muhlbauer, W. Acute renal failure in severely burned patients. Burns. 1999; 25:171–178.
117. Tremblay, R, Ethier, J, Querin, S, et al. Veno-venous continuous renal replacement therapy for burned patients with acute renal failure. Burns. 2000; 26:638–643.
118. Beal, AL, Deuser, WE, Beilman, GJ. A role for epinephrine in post-traumatic hypokalemia. Shock. 2007; 27:358–363.
119. Dolecek, R. Endocrine changes after burn trauma—A review. Keio J Med. 1989; 38:262–276.
120. Mozingo, D, Mason, A. Hypophosphatemia. In: Herndon D, ed. Total Burn Care. Philadelphia: WB Saunders; 2002:309–315.
121. Burke, JF, Bondoc, CC, Quinby, WC. Primary burn excision and immediate grafting: A method shortening illness. J Trauma. 1974; 14:389–395.
122. Herndon, DN, Barrow, RE, Rutan, RL, et al. A comparison of conservative versus early excision. Therapies in severely burned patients. Ann Surg. 1989; 209:547–552.
123. Ong, YS, Samuel, M, Song, C. Meta-analysis of early excision of burns. Burns. 2006; 32:145–150.
124. Desai, MH, Herndon, DN, Broemeling, L, et al. Early burn wound excision significantly reduces blood loss. Ann Surg. 1990; 211:753–759.
125. Saffle, J. Role of surgical management. In: Webb A, Shapiro M, Singer M, Suter P, eds. Oxford Textbook of Critical Care. Oxford: Oxford Medical Publishers; 1999:774–776.
126. Muller, M, Herndon, D. Operative wound management. In: Herndon D, ed. Total Burn Care. Philadelphia: WB Saunders; 2002:170–182.
127. Supp, DM, Boyce, ST. Engineered skin substitutes: Practices and potentials. Clin Dermatol. 2005; 23:403–412.
128. Mozingo, DW, McManus, AT, Kim, SH, Pruitt, BA, Jr. Incidence of bacteremia after burn wound manipulation in the early postburn period. J Trauma. 1997; 42:1006–1010.
129. Budny, PG, Regan, PJ, Roberts, AH. The estimation of blood loss during burns surgery. Burns. 1993; 19:134–137.
130. Warden, GD, Saffle, JR, Kravitz, M. A two-stage technique for excision and grafting of burn wounds. J Trauma. 1982; 22:98–103.
131. MacLennan, N, Heimbach, DM, Cullen, BF. Anesthesia for major thermal injury. Anesthesiology. 1998; 89:749–770.
132. McGuinness, SK, Wasiak, J, Cleland, H, et al. A systematic review of ketamine as an analgesic agent in adult burn injuries. Pain Med. 2011; 12:1551–1558.
133. Jaehde, U, Sorgel, F. Clinical pharmacokinetics in patients with burns. Clin Pharmacokinet. 1995; 29:15–28.
134. Weinbren, MJ. Pharmacokinetics of antibiotics in burns patients. J Antimicrob Chemother. 2001; 47:720.
135. Rovers, J, Knighton, J, Neligan, P, Peters, W. Patient-controlled analgesia in burn patients: A critical review of the literature and case report. Hosp Pharm. 1994; 29(106):108–111.
136. Faucher, L. Modern pain management in burn care. Problems in general surgery. Burns. 2003; 20:80–87.
137. Herndon, DN, Traber, DL, Traber, LD. The effect of resuscitation on inhalation injury. Surgery. 1986; 100:248–251.
138. Thompson, JT, Molnar, JA, Hines, MH, et al. Successful management of adult smoke inhalation with extracorporeal membrane oxygenation. J Burn Care Rehabil. 2005; 26:62–66.
139. Musgrave, MA, Fingland, R, Gomez, M, et al. The use of inhaled nitric oxide as adjuvant therapy in patients with burn injuries and respiratory failure. J Burn Care Rehabil. 2000; 21:551–557.
140. Carman, B, Cahill, T, Warden, G, McCall, J. A prospective, randomized comparison of the volume diffusive respirator vs conventional ventilation for ventilation of burned children. 2001 ABA paper. J Burn Care Rehabil. 2002; 23:444–448.
141. Cartotto, R, Ellis, S, Smith, T. Use of high-frequency oscillatory ventilation in burn patients. Crit Care Med. 2005; 33:S175–S181.
142. Cortiella, J, Mlcak, R, Herndon, D. High frequency percussive ventilation in pediatric patients with inhalation injury. J Burn Care Rehabil. 1999; 20:232–235.
143. Sheridan, RL, Kacmarek, RM, McEttrick, MM, et al. Permissive hypercapnia as a ventilatory strategy in burned children: Effect on barotrauma, pneumonia, and mortality. J Trauma. 1995; 39:854–859.
144. Saffle, JR, Sullivan, JJ, Tuohig, GM, Larson, CM. Multiple organ failure in patients with thermal injury. Crit Care Med. 1993; 21:1673–1683.
145. Fitzwater, J, Purdue, GF, Hunt, JL, O’Keefe, GE. The risk factors and time course of sepsis and organ dysfunction after burn trauma. J Trauma. 2003; 54:959–966.
146. Hollingsed, TC, Saffle, JR, Barton, RG, et al. Etiology and consequences of respiratory failure in thermally injured patients. Am J Surg. 1993; 166:592–596.
147. Baxter, AD, Allan, J, Bedard, J, et al. Adherence to simple and effective measures reduces the incidence of ventilator-associated pneumonia. Can J Anaesth. 2005; 52:535–541.
148. Desai, MH, Mlcak, R, Richardson, J, et al. Reduction in mortality in pediatric patients with inhalation injury with aerosolized heparin/N-acetylcystine (correction of acetylcystine) therapy. J Burn Care Rehabil. 1998; 19:210–212.
149. Levine, BA, Petroff, PA, Slade, CL, Pruitt, BA, Jr. Prospective trials of dexamethasone and aerosolized gentamicin in the treatment of inhalation injury in the burned patient. J Trauma. 1978; 18:188–193.
150. Saffle, JR, Morris, SE, Edelman, L. Early tracheostomy does not improve outcome in burn patients. J Burn Care Rehabil. 2002; 23:431–438.
151. Arbabi, S, Ahrns, KS, Wahl, WL, et al. Beta-blocker use is associated with improved outcomes in adult burn patients. J Trauma. 2004; 56:265–269.
152. Marik, PE, Zaloga, GP. Adrenal insufficiency in the critically ill: A new look at an old problem. Chest. 2002; 122:1784–1796.
153. Reiff, DA, Harkins, CL, McGwin, G, Jr., et al. Risk factors associated with adrenal insufficiency in severely injured burn patients. J Burn Care Res. 2007; 28:854–858.
154. Marik, PE, Zaloga, GP. Adrenal insufficiency during septic shock. Crit Care Med. 2003; 31:141–145.
155. Cooper, MS, Stewart, PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003; 348:727–734.
156. Burchard, K. A review of the adrenal cortex and severe inflammation: Quest of the “eucorticoid” state. J Trauma. 2001; 51:800–814.
157. Barquist, E, Kirton, O. Adrenal insufficiency in the surgical intensive care unit patient. J Trauma. 1997; 42:27–31.
158. Sheridan, RL, Ryan, CM, Tompkins, RG. Acute adrenal insufficiency in the burn intensive care unit. Burns. 1993; 19:63–66.
159. Pruitt, BA, Jr., McManus, AT, Kim, SH, Goodwin, CW. Burn wound infections: Current status. World J Surg. 1998; 22:135–145.
160. Becker, WK, Cioffi, WG, Jr., McManus, AT, et al. Fungal burn wound infection. A 10-year experience. Arch Surg. 1991; 126:44–48.
161. Matsumura, H, Meyer, NA, Mann, R, Heimbach, DM. Melting graft-wound syndrome. J Burn Care Rehabil. 1998; 19:292–295.
162. Steer, JA, Papini, RP, Wilson, AP, et al. Quantitative microbiology in the management of burn patients. I. Correlation between quantitative and qualitative burn wound biopsy culture and surface alginate swab culture. Burns. 1996; 22:173–176.
163. Jarvis, WR. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis. 1995; 20:1526–1530.
164. Sasaki, TM, Panke, TW, Dorethy, JF, et al. The relationship of central venous and pulmonary artery catheter position to acute right-sided endocarditis in severe thermal injury. J Trauma. 1979; 19:740–743.
165. Kealey, GP, Chang, P, Heinle, J, et al. Prospective comparison of two management strategies of central venous catheters in burn patients. J Trauma. 1995; 38:344–349.
166. Fearonce, G, Faraklas, I, Saffle, JR, Cochran, A. Peripherally inserted central venous catheters and central venous catheters in burn patients: A comparative review. J Burn Care Res. 2010; 31:31–35.
167. O’Grady, NP, Alexander, M, Dellinger, EP, et al. Guidelines for the prevention of intravascular catheter-related infections. The Hospital Infection Control Practices Advisory Committee, Center for Disease Control and Prevention, U. S. Pediatrics. 2002; 110:e51.
168. Greenhalgh, DG, Saffle, JR, Holmes, JHT, et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007; 28:776–790.
169. Keen, A, Knoblock, L, Edelman, L, Saffle, J. Effective limitation of blood culture use in the burn unit. J Burn Care Rehabil. 2002; 23:183–189.
170. Uzzan, B, Cohen, R, Nicolas, P, et al. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: A systematic review and meta-analysis. Crit Care Med. 2006; 34:1996–2003.
171. Neely, AN, Fowler, LA, Kagan, RJ, Warden, GD. Procalcitonin in pediatric burn patients: An early indicator of sepsis? J Burn Care Rehabil. 2004; 25:76–80.
172. Sheridan, RL, Weber, JM, Pasternack, MS, Tompkins, RG. Antibiotic prophylaxis for group A streptococcal burn wound infection is not necessary. J Trauma. 2001; 51:352–355.
173. Boss, WK, Brand, DA, Acampora, D, et al. Effectiveness of prophylactic antibiotics in the outpatient treatment of burns. J Trauma. 1985; 25:224–227.
174. Mackie, DP, van Hertum, WA, Schumburg, T, et al. Prevention of infection in burns: Preliminary experience with selective decontamination of the digestive tract in patients with extensive injuries. J Trauma. 1992; 32:570–575.
175. Barret, JP, Jeschke, MG, Herndon, DN. Selective decontamination of the digestive tract in severely burned pediatric patients. Burns. 2001; 27:439–445.
176. Cuthbertson, D. The disturbance of metabolism produced by bony and nonbony injury with notes of certain abnormal conditions of bone. Biochem J. 1930; 24:1244–1263.
177. Hart, DW, Wolf, SE, Chinkes, DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000; 232:455–465.
178. Kudsk, K, Brown, R. Nutritional support. In: Mattox K, Feliciano D, Moore E, eds. Trauma. New York: McGraw-Hill; 2000:1369–1405.
179. Bessey, P, Jiang, Z, Johnson, D, et al. Posttraumatic skeletal muscle proteolysis: The role of the hormonal environment. World J Surg. 1989; 13:465–470.
180. Long, C, Kinney, J, Geiger, C. Nonsuppressibility of gluconeogenesis by glucose in septic patients. Metabolism. 1976; 25:193.
181. Long, C, Schaffel, N, Geiger, C, et al. Metabolic response to injury and illness: Estimation of energy and protein needs from indirect calorimetry and nitrogen balance. J Parenter Enteral Nutr. 1979; 3:452–456.
182. Wilmore, D, Long, J, Mason, A, et al. Catecholamines: Mediators of the hypermetabolic response to thermal injury. Ann Surg. 1974; 180:653–658.
183. Newsome, T, Mason, A, Pruitt, B. Weight loss following thermal injury. Ann Surg. 1973; 178:215–217.
184. Wilmore, D. Nutrition and metabolism following thermal injury. Clin Plast Surg. 1974; 1:603–619.
185. Royall, D, Fairholm, L, Peters, WJ, et al. Continuous measurement of energy expenditure in ventilated burn patients: An analysis. Crit Care Med. 1994; 22:399–406.
186. Rutan, T, Herndon, D, VanOsten, T, Abston, S. Metabolic rate alterations in early excision and grafting versus conservative treatment. J Trauma. 1986; 26:140–146.
187. Mittendorfer, B, Hildreth, MA, Desai, MH, Herndon, DN. The 1995 Clinical Research Award. Younger pediatric patients with burns are at risk for continuing postdischarge weight loss. J Burn Care Rehabil. 1995; 16:589–595.
188. Heyland, DK, Dhaliwal, R, Drover, JW, et al. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. J Parenter Enteral Nutr. 2003; 27:355–373.
189. Magnotti, L, Deitch, E. Burns, bacterial translocation, gut barrier function, and failure. J Burn Care Rehabil. 2005; 26:383–391.
190. Andel, H, Rab, M, Andel, D, et al. Impact of duodenal feeding on the oxygen balance of the splanchnic region during different phases of severe burn injury. Burns. 2002; 28:60–64.
191. Saito, H, Trocki, O, Alexander, J, et al. The effect of route of nutrient administration on the nutritional state, catabolic hormone secretion and gut mucosal integrity after burn injury. J Parenter Enteral Nutr. 1987; 11:1–7.
192. Herndon, D, Stein, M, Rutan, T, et al. Failure of TPN supplementation to improve liver function, immunity, and mortality in thermally injured patients. J Trauma. 1987; 27:195–204.
193. Herndon, D, Barrow, R, Stein, M, et al. Increased mortality with intravenous supplemented feeding in severely burned patients. J Burn Care Rehabil. 1989; 10:309–313.
194. Barret, JP, Jeschke, MG, Herndon, DN. Fatty infiltration of the liver in severely burned pediatric patients: Autopsy findings and clinical implications. J Trauma. 2001; 51:736–739.
195. Mochizuki, H, Trocki, O, Dominioni, L, et al. Mechanism of prevention of postburn hypermetabolism and catabolism by early enteral feeding. Ann Surg. 1984; 200:297–310.
196. Peck, MD, Kessler, M, Cairns, BA, et al. Early enteral nutrition does not decrease hypermetabolism associated with burn injury. J Trauma. 2004; 57:1143–1149.
197. Moore, E, Jones, T. Benefits of immediate jejunostomy feeding after major abdominal trauma—A prospective, randomized study. J Trauma. 1988; 26:874–881.
198. Gottschlich, M, Jenkins, M, Mayes, T, et al. The 2002 Clinical Research Award: An evaluation of the safety of early vs delayed enteral support and effects on clinical nutritional, and endocrine outcomes after severe burns. J Burn Care Rehabil. 2002; 23:401–415.
199. Jenkins, M, Gottschlich, M, Mayes, T, et al. Enteral feeding during operative procedures. J Burn Care Rehabil. 1994; 15:199–205.
200. Booth, CM, Heyland, DK, Paterson, WG. Gastrointestinal promotility drugs in the critical care setting: A systematic review of the evidence. Crit Care Med. 2002; 30:1429–1435.
201. Curreri, P, Richmond, D, Marvin, J, Baxter, C. Dietary requirements of patients with major burns. J Am Diet Assoc. 1974; 65:415–417.
202. Saffle, J, Medina, E, Raymond, J, et al. Use of indirect calorimetry in the nutritional management of burn patients. J Trauma. 1985; 25:32–39.
203. Dickerson, RN, Gervasio, JM, Riley, ML, et al. Accuracy of predictive methods to estimate resting energy expenditure of thermally-injured patients. J Parenter Enteral Nutr. 2002; 26:17–29.
204. Harris, J, Benedict, F. A Biometric Study of Basal Metabolism in Man. Washington, DC: Carnegie Institute of Washington; 1919.
205. Peck, M. Practice guidelines for burn care: Nutritional support. J Burn Care Rehabil. 2001; 22:59S–66S.
206. Adam, S, Batson, S. A study of problems associated with the delivery of enteral feed in critically ill patients in five ICUs in the UK. Intensive Care Med. 1997; 23:261–266.
207. De Jonghe, B, Appere-De-Vechi, C, Fournier, M, et al. A prospective survey of nutritional support practices in intensive care unit patients: What is prescribed? What is delivered? Crit Care Med. 2001; 29:8–12.
208. Saffle, J, Hildreth, M. Metabolic support of the burned patient. In: Herndon D, ed. Total Burn Care. 2nd ed. New York: WB Saunders; 2002:271–287.
209. Solano, T, Totaro, R. Insulin therapy in critically ill patients. Curr Opin Clin Nutr Metab Care. 2004; 7:199–205.
210. van den Berghe, G, Wouters, P, Weekers, F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001; 345:1359–1367.
211. Wu, X, Thomas, SJ, Herndon, DN, et al. Insulin decreases hepatic acute phase protein levels in severely burned children. Surgery. 2004; 135:196–202.
212. Gore, DC, Wolf, SE, Sanford, A, et al. Influence of metformin on glucose intolerance and muscle catabolism following severe burn injury. Ann Surg. 2005; 241:334–342.
213. Demling, R, Signe, P. Metabolic management of patients with severe burns. World J Surg. 2000; 24:673–680.
214. Garrel, D, Razi, M, Lariviere, R. Improved clinical status and length of care with low-fat nutrition support in burn patients. J Parenter Enteral Nutr. 1995; 19:482–491.
215. Mochizuki, H, Trocki, O, Dominioni, L, et al. Optimal lipid content for enteral diets following thermal injury. J Parenter Enteral Nutr. 1984; 8:638–646.
216. Battistella, FD, Widergren, JT, Anderson, JT, et al. A prospective, randomized trial of intravenous fat emulsion administration in trauma victims requiring total parenteral nutrition. J Trauma. 1997; 43:52–58.
217. Huschak, G, Zur Nieden, K, Hoell, T, et al. Olive oil based nutrition in multiple trauma patients: A pilot study. Intensive Care Med. 2005; 31:1202–1208.
218. Alexander, J, Saito, H, Trocki, O, Ogle, C. The importance of lipid type in the diet after burn injury. Ann Surg. 1986; 204:1–8.
219. Soeters, PB, van de Poll, MC, van Gemert, WG, Dejong, CH. Amino acid adequacy in pathophysiological states. J Nutr. 2004; 134:1575S–1582S.
220. Prelack, K, Cunningham, J, Sheridan, R, Tompkins, R. Energy and protein provisions for thermally injured children revisited: An outcome-based approach for determining requirements. J Burn Care Rehabil. 1997; 18:117–181.
221. Matsuda, T, Kagan, R, Hanumadass, M, Jonasson, O. The importance of burn wound size in determining the optimal calorie:nitrogen ratio. Surgery. 1983; 94:562–568.
222. Alexander, JW, MacMillan, BG, Stinnett, JD, et al. Beneficial effects of aggressive protein feeding in severely burned children. Ann Surg. 1980; 192:505–517.
223. Waymack, J, Herndon, D. Nutritional support of the burned patient. World J Surg. 1992; 16:80–86.
224. Yu, YM, Ryan, CM, Castillo, L, et al. Arginine and ornithine kinetics in severely burned patients: Increased rate of arginine disposal. Am J Physiol Endocrinol Metab. 2001; 280:E509–517.
225. Novak, F, Heyland, DK, Avenell, A, et al. Glutamine supplementation in serious illness: A systematic review of the evidence. Crit Care Med. 2002; 30:2022–2029.
226. Souba, W. Glutamine: A key substrate for the splanchnic bed. Ann Rev Nutr. 1991; 11:285–289.
227. De-Souza, DA, Greene, LJ. Intestinal permeability and systemic infections in critically ill patients: Effect of glutamine. Crit Care Med. 2005; 33:1125–1135.
228. Wischmeyer, PE. Can glutamine turn off the motor that drives systemic inflammation? Crit Care Med. 2005; 33:1175–1178.
229. Wischmeyer, PE, Lynch, J, Liedel, J, et al. Glutamine administration reduces gram-negative bacteremia in severely burned patients: A prospective, randomized, double-blind trial versus isonitrogenous control. Crit Care Med. 2001; 29:2075–2080.
230. Zhou, YP, Jiang, ZM, Sun, YH, et al. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: A randomized, double-blind, controlled clinical trial. J Parenter Enteral Nutr. 2003; 27:241–245.
231. Barbul, A, Larzarrow, S, Efron, O. Arginine enhances wound healing and lymphocyte immune response in humans. Surgery. 1990; 108:331–335.
232. Kirk, S, Barbul, A. Role of arginine in trauma, sepsis, and immunity. J Parenter Enteral Nutr. 1990; 14:226S–229S.
233. Gottschlich, M, Jenkins, M, Warden, G, et al. Differential effects of three enteral dietary regimens on selected outcome variables in burn patients. J Parenter Enteral Nutr. 1990; 14:225–236.
234. Heys, S, Walker, L, Smith, I, Eremin, O. Enteral nutritional supplementation with key nutrients in patients with critical illness and cancer: A meta-analysis of randomized controlled trials. Ann Surg. 1999; 229:467–477.
235. Heyland, DK, Samis, A. Does immunonutrition in patients with sepsis do more harm than good? Intensive Care Med. 2003; 29:669–671.
236. Saffle, J, Wiebke, G, Jennings, K, et al. Randomized trial of immune-enhancing enteral nutrition in burn patients. J Trauma. 1997; 42:793–802.
237. Gamliel, Z, DeBiasse, M, Demling, R. Essential microminerals and their response to burn injury. J Burn Care Rehabil. 1996; 17:264–272.
238. Gottschlich, M, Warden, G. Vitamin supplementation in the patient with burns. J Burn Care Rehabil. 1990; 11:275–279.
239. Shippee, R, Wilson, S, King, N. Trace mineral supplementation of burn patients: A national survey. J Am Diet Assoc. 1987; 87:300–303.
240. Gump, F, Kinney, J. Energy balance and weight loss in burned patients. Arch Surg. 1971; 103:442–448.
241. Streat, S, Beddoe, A, Hill, G. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma. 1987; 27:262–266.
242. Hart, DW, Wolf, SE, Herndon, DN, et al. Energy expenditure and caloric balance after burn: Increased feeding leads to fat rather than lean mass accretion. Ann Surg. 2002; 235:152–161.
243. Zdolsek, HJ, Lindahl, OA, Angquist, KA, Sjoberg, F. Non-invasive assessment of intercompartmental fluid shifts in burn victims. Burns. 1998; 24:233–240.
244. LeBlanc, A, Gogia, P, Schneider, V, et al. Calf muscle area and strength changes after five weeks of horizontal bed rest. Am J Sports Med. 1988; 16:624–629.
245. Rettmer, R, Williamson, J, Labbe, R, Heimbach, D. Laboratory monitoring of nutrition status in burn patients. Clin Chem. 1992; 38:334–337.
246. Manelli, J, Abdetii, C, Botti, G, Golstein, M. A reference standard for plasma proteins is required for nutritional assessment of adult burn patients. Burns. 1998; 24:337–345.
247. Williamson, J. Actual burn nutrition care practices: A national survey (Part II). J Burn Care Rehabil. 1989; 10:185–194.
248. Pereira, CT, Herndon, DN. The pharmacologic modulation of the hypermetabolic response to burns. Adv Surg. 2005; 39:245–261.
249. Herndon, DN, Hart, DW, Wolf, SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001; 345:1223–1229.
250. Wolf, SE, Edelman, LS, Kemalyan, N, et al. Effects of oxandrolone on outcome measures in the severely burned: A multicenter prospective randomized double-blind trial. J Burn Care Res. 2006; 27:131–141.
251. Demling, RH, DeSanti, L. Oxandrolone induced lean mass gain during recovery from severe burns is maintained after discontinuation of the anabolic steroid. Burns. 2003; 29:793–797.
252. Glenn, F, Becker, CG. Acute acalculous cholecystitis. An increasing entity. Ann Surg. 1982; 195:131–136.
253. Arnoldo, BD, Hunt, JL, Purdue, GF. Acute cholecystitis in burn patients. J Burn Care Res. 2006; 27:170–173.
254. Sheridan, RL, Ryan, CM, Lee, MJ, et al. Percutaneous cholecystostomy in the critically ill burn patient. J Trauma. 1995; 38:248–251.
255. Ryan, CM, Sheridan, RL, Schoenfeld, DA, et al. Postburn pancreatitis. Ann Surg. 1995; 222:163–170.
256. Maury, E, Tankovic, J, Ebel, A, Offenstadt, G. An observational study of upper gastrointestinal bleeding in intensive care units: Is Helicobacter pylori the culprit? Crit Care Med. 2005; 33:1513–1518.
257. Robert, R, Gissot, V, Pierrot, M, et al. Helicobacter pylori infection is not associated with an increased hemorrhagic risk in patients in the intensive care unit. Crit Care. 2006; 10:R77.
258. Czaja, A, McAlhany, J, Pruitt, B. Acute gastroduodenal disease after thermal injury. N Engl J Med. 1984; 18:925–929.
259. Chung, D, Robie, D, Hernandez, A, et al. Surgical management of complications of burn injury. In: Herndon D, ed. Total Burn Care. Philadelphia: WB Saunders; 2002:442–451.
260. Kadesky, K, Purdue, GF, Hunt, JL. Acute pseudo-obstruction in critically ill patients with burns. J Burn Care Rehabil. 1995; 16:132–135.
261. Marvin, R, McKinley, B, McQuiggan, M, et al. Nonocclusive bowel necrosis occurring in critically-ill trauma patients receiving enteral nutrition manifests no reliable clinical signs for early detection. Am J Surg. 2000; 179:7–12.
262. Kowal-Vern, A, McGill, V, Gamelli, R. Ischemic necrotic bowel disease in thermal injury. Arch Surg. 1997; 132:440–443.
263. Eisenberg, P. Causes of diarrhea in tube-fed patients: A comprehensive approach to diagnosis and management. Nutr Clin Pract. 1993; 8:119–123.
264. Grube, B, Heimbach, C, Marvin, J. Clostridium difficile diarrhea in critically ill burn patients. Arch Surg. 1987; 122:655–661.
265. Scaife, CL, Saffle, JR, Morris, SE. Intestinal obstruction secondary to enteral feedings in burn trauma patients. J Trauma. 1999; 47:859–863.
266. Wolf, S, Jeschke, M, Rose, J, et al. Enteral feeding intolerance: An indicator of sepsis-associated mortality in burned children. Arch Surg. 1997; 132:1310–1313.
267. Shankar, R, Amin, C, Gamelli, R. Hematologic, hematopoietic, and acute phase response. In: Herndon D, ed. Total Burn Care. Philadelphia: WB Saunders; 2002:331–346.
268. Wallner, SF, Vautrin, R. The anemia of thermal injury: Mechanism of inhibition of erythropoiesis. Proc Soc Exp Biol Med. 1986; 181:144–150.
269. Still, JM, Jr., Belcher, K, Law, EJ, et al. A double-blinded prospective evaluation of recombinant human erythropoietin in acutely burned patients. J Trauma. 1995; 38:233–236.
270. Palmieri, TL, Greenhalgh, DG. Blood transfusion in burns: What do we do? J Burn Care Rehabil. 2004; 25:71–75.
271. Palmieri, TL, Caruso, DM, Foster, KN, et al. Effect of blood transfusion on outcome after major burn injury: A multicenter study. Crit Care Med. 2006; 34:1602–1607.
272. Valade, N, Decailliot, F, Rebufat, Y, et al. Thrombocytosis after trauma: Incidence, aetiology, and clinical significance. Br J Anaesth. 2005; 94:18–23.
273. Housinger, TA, Brinkerhoff, C, Warden, GD. The relationship between platelet count, sepsis, and survival in pediatric burn patients. Arch Surg. 1993; 128:65–66.
274. Rue, LW, 3rd., Cioffi, WG, Jr., Rush, R, et al. Thromboembolic complications in thermally injured patients. World J Surg. 1992; 16:1151–1154.
275. Wibbenmeyer, LA, Hoballah, JJ, Amelon, MJ, et al. The prevalence of venous thromboembolism of the lower extremity among thermally injured patients determined by duplex sonography. J Trauma. 2003; 55:1162–1167.
276. Klein, GL, Herndon, DN, Goodman, WG, et al. Histomorphometric and biochemical characterization of bone following acute severe burns in children. Bone. 1995; 17:455–460.
277. Celis, MM, Suman, OE, Huang, TT, et al. Effect of a supervised exercise and physiotherapy program on surgical interventions in children with thermal injury. J Burn Care Rehabil. 2003; 24:57–61.