Chapter 92 Craniovertebral Junction Deformities
Clinical and Radiographic Anatomy of the Craniovertebral Junction
The normal relationships among the occiput, atlas, and axis have been studied and are well described. The use of CT with sagittal reconstruction and multiaxial MRI has greatly enhanced the understanding and definition of abnormal anatomic relationships at the CVJ. Various measurements have been described to delineate normal from abnormal pathologies. In other words, there are different indices for trauma, degenerative disease, cranial settling, and basilar impression (Table 92-1).
Trauma
Injury to the CVJ can manifest as ligamentous injury or fracture of the occiput, atlas, or axis. These injuries are as follows: occipital condyle fractures, atlanto-occipital dislocation (AOD), atlas fractures or C1 burst fractures, and C2 fractures of the odontoid or pars interarticularis. Radiographic criteria have been established to help assess clinical stability. Although many of these criteria are used traditionally, they are by no means standardized criteria for each type of injury.1 Determining instability of these fractures is of primary importance in determining management.
C0 (Occipital Condyle) Fractures
Traditionally, occipital condyle fractures are categorized by the system of Anderson and Montesano.2 This grades occipital condyle fractures according to occipital fracture (type 1), large condyle fracture (type 2), and avulsion condyle fracture (type 3). The inference from this study is that small condyle fractures represent disruption of the alar ligament. The disruption of the alar ligament has been demonstrated to increase the mobility of the C0-1 joint.3
Tuli et al. defined fractures according to evidence of ligamentous instability.4 Type 1 represents large bony fractures, condensing Anderson and Montesano types 1 and 2 into one group. Type 2 is both small bony fractures of the condyle. The fractures are further subdivided into 2a (stable) and 2b (unstable). Instability is characterized by MRI evidence of alar ligament disruption or CT/radiographic criteria. However, use of MRI to assess disruption of the alar ligament remains controversial.5
To simplify the issue, Maserati et al. focused on the C0-1 joint.6 Determination of instability is made using the elongation of the distance of the C0-1 joint described by Pang.7 This method is also used to determine AOD and will be more completely described in the next section.
Unstable condyle fractures are a form of AOD and need to be treated as such.6 However, once instability has been determined, treatment is also not standardized. Fractures without apparent ligamentous disruption can be treated conservatively with a cervical collar or halo vest. Immobilization may be performed if the fracture fragment is large enough and aligned enough to allow bony fusion.1 If the bony fragment appears small or there is an apparent alar ligament disruption, it may be necessary to perform an occipital cervical fusion because purely ligamentous injury is unlikely to heal by immobilization.6
C0-1 Fractures or Atlanto-Occipital Dislocation
In the diagnosis of AOD,vigilant clinical suspicion is most important. The deformity may reduce spontaneously because of recoil of the elastic ligamentous structures. Suspicion should be raised based on the mechanism of injury (e.g., high-velocity crash) or findings on neurologic examination (severe neurologic injury, brainstem or C1-2 level deficits), lateral cervical spine radiograph (obvious separation of the condyle-C1 joint or C1-2 prevertebral swelling), or head CT (subarachnoid hemorrhage around the brainstem or upper cervical spinal cord, or epidural/subdural blood at C1-2).7–9
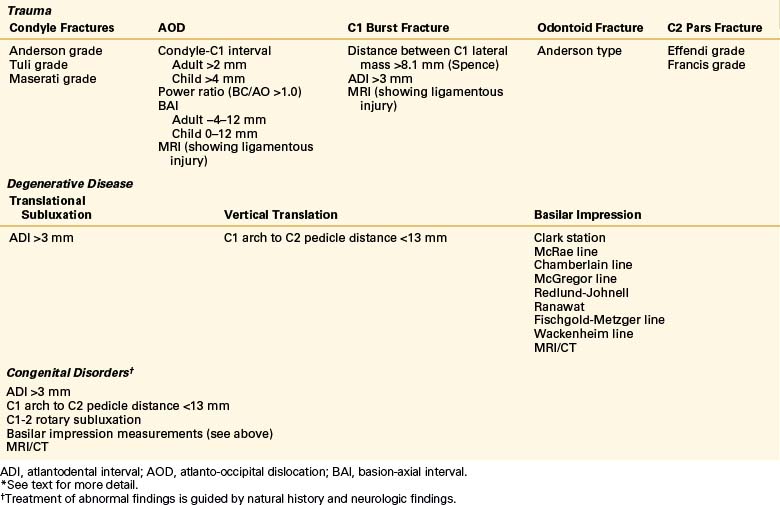
AOD is determined by measurements made from normal plain radiographs. These techniques are the Powers ratio,10 basion-axial interval (Harris),11 and the Wholey dens-basion interval (Fig. 92-1). These measurements essentially infer dislocation based on measurement of structures remote from the occipital condyle–atlas joint,10–12 which can lead to false-negative examinations and lack of interobserver reliability. It has been found that the diagnostic sensitivities for the common tests range from 25% to 50%, with false-negative rates of 50% to 75%. However, the diagnostic sensitivity of the nonstandard indicators (perimedullary blood, tectorial membrane damage, C1-2 extra-axial blood) is 63% to 75%.7
An increase in the measurement of the joint distance between the occipital condyle and C1 can be used to determine AOD. This is called the condylar distance. Thin-slice axial CT scanning allowed Pang et al. to calculate that the distance should be less than 4 mm in pediatric patients (Fig. 92-2). This test has been shown in the pediatric population to have a diagnostic sensitivity of 100%.7 Dziurzynski et al. showed that in adult patients a condylar distance greater than 2 mm was diagnostic of AOD. This has a sensitivity of 92% and specificity of 95%.13
If the patient survives the initial injury, he or she should be immediately immobilized. The use of a halo vest to immobilize the patient has been shown to be a safe and effective treatment method to prevent delayed neurologic deterioration while the patient is stabilized and prepared for definitive treatment.14 Depending on the severity of injury, operative fixation can be performed on an elective basis.14 The instability of AOD is primarily a ligamentous injury, and therefore internal fixation and fusion is recommended for definitive treatment. If reduction of the AOD is necessary, it should be done with gentle manual manipulation under fluoroscopic guidance. If the patient has a neurologic examination to follow, the reduction can be performed with the patient under mild sedation. In the anesthetized patient, somatosensory evoked responses may provide some help in determining if reduction is affecting the patient neurologically.
C1 Fractures
Fractures of the atlas (C1) can manifest in multiple ways: isolated ventral or dorsal arch, burst, and lateral mass fractures. Isolated arch fractures are a controversial diagnosis because it is unlikely that a ring can have a fracture in one place without fracturing in another, although they have been described.15 An axially directed force that translates into C1 through the wedge-shaped occipital condyles causes burst fractures of the atlas. These fractures were first described by Geoffrey Jefferson in 1920.16 These fractures are detected with an open-mouth odontoid radiograph demonstrating spread of the lateral masses of C1 beyond the lateral borders of the C2 lateral masses. Assessment of the integrity of the transverse ligament is critical in determination of the treatment of C1 burst fractures. Initial assessment of the competence of the ligament was made by a cadaveric study performed by Spence et al.17 in 1970. Spence showed that the transverse ligament typically failed if the spread between lateral masses was 6.9 mm or more. When corrected for the magnification of the radiographs, this distance should be increased to 8.1 mm.18 This allows for indirect determination of rupture of the ligament based on plain radiographs. Again, the advent and widespread use of CT and MRI have allowed for direct visualization of ligament integrity. Dickman et al. used MRI to evaluate the transverse ligament and found an abnormal atlantodental interval of 3 mm or more implies the incompetence of the transverse ligament.19 A ruptured transverse ligament was found in cadaver studies to produce hypermobility at C1-2, increasing flexion-extension (42%), lateral bending (24%), and axial rotation (5%).20–22
There is not enough evidence to provide standardized treatment guidelines, but there are recommendations for this treatment of C1 fractures.23 Isolated ventral or dorsal ring fractures may be treated with cervical immobilization (collar or halo) for 8 to 12 weeks with good results. C1 burst fractures without ligamentous injury can be treated with collar or halo immobilization for 12 weeks. C1 burst fractures with rupture of the transverse ligament may be treated with halo immobilization for 12 weeks or with internal fixation of C1 to C2 with fusion.
C2 Fractures
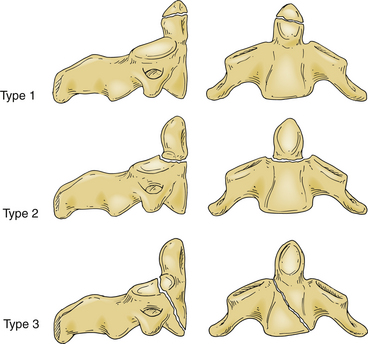
C2 fractures can be broadly divided into odontoid, C2 body, and pedicle/pars fractures. Odontoid fractures are classified by the system of Anderson and D’Alonzo (Fig. 92-3).24 Type 1 fractures are rare and are at the distal tip of the odontoid process. Type 2 fractures occur at the base of the odontoid where it meets the body of the axis. Type 3 fractures occur through the body of the axis. The management options for odontoid fractures depend on the type of fracture, the degree of subluxation of the cranial fragment, and the status of the transverse ligament. Type 1 and type 3 fractures are often managed by external immobilization alone, collar or halo. Type 2 fractures can be managed by immobilization or operative intervention depending on patient factors and the degree of subluxation. An increased rate of nonunion has been associated with patient age younger than 60 years and/or subluxation greater than 4 to 6 mm.25–27 Nonunion rates can be as high as 28%. Type 2a fractures, characterized by comminution of the C2 body, are associated with lower healing rates without surgery.28,29 C2 pars and pedicle fractures may require surgical intervention, depending on the degree of angulation and distraction between the fragments (see subsequent discussion).27,29
Os odontoideum is defined as an ossicle of cortical bone in the position of the odontoid process often attached to the C2 body by a cartilaginous segment (Fig. 92-4). The cause of this remains unclear. There is some evidence to suggest that this is a consequence of old trauma, often at an early age.30 It is unlikely that this is a failure of fusion during development, because the normal somite pattern of development of the axis does not normally have a site of fusion where the axis meets the body.31 However, os odontoideum is associated with congenital disorders, such as Down and Morquio syndromes, and spondyloepiphyseal dysplasia. Patients who have neurologic compromise are offered surgical decompression and fusion. Patients with gross instability or narrow canal diameter are also offered surgery. The treatment of incidentally found os odontoideum is controversial. Most authors recommend close follow-up, with surgery reserved for the development of symptoms or radiographic evidence of instability or progressive deformity.31
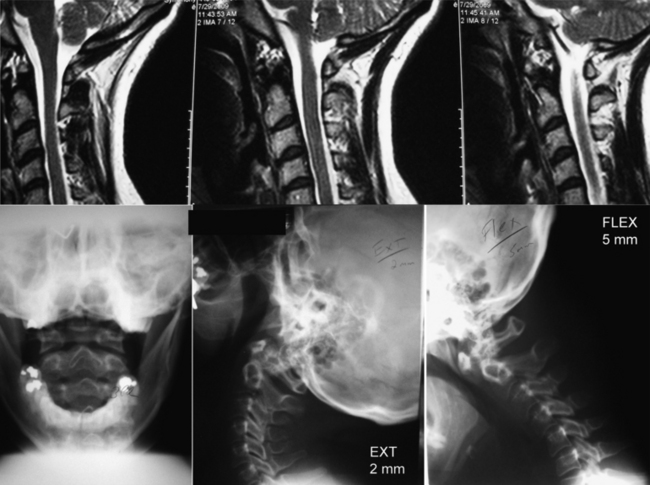
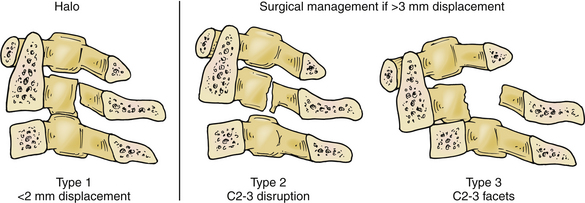
Fractures of the C2 pars interarticularis are called hangman’s factures because of the similarity to those seen in judicial hangings.32 These fractures are also called C2 traumatic spondylolisthesis fractures. These fractures have been classified into three types by Effendi et al. (Fig. 92-5).33,34 Type 1 fractures are displaced less than 2 mm and minimally angulated, and the C2-3 disc space remains intact. Type 2 fractures have a displaced and angulated body of the axis and a disrupted C2-3 disc space. Type 3 fractures are like type 2 fractures with locked C2 and C3 facets, and the body of the axis is ventrally displaced.
Decisions of treatment of C2 pars fractures are primarily guided by degree of subluxation of C2 on C3. A type 1 fracture without significant ligamentous injury can be treated with immobilization. A halo ring can be used to achieve reduction by extension and capital flexion, reversing the mechanism of fracture. When significant ligamentous injury exists, care must be taken with the use of traction to avoid iatrogenic separation of C2 and C3. In type 2 or 3 fractures, if there is displacement greater than 3 mm, operative intervention may be indicated for reduction and fixation.25,34,35
C2 transverse process fractures do not cause instability, but potential injury to the vertebral artery is an area of concern. It is unclear whether aggressive imaging or treatment of these injuries affects patient outcomes, and decisions should be individualized depending on patient symptoms and anatomy.36
Degenerative Disease
Abnormalities of bone metabolism, degeneration of synovial joints, or abnormal stresses placed on the CVJ can result in basilar impression. The principles of diagnosis and treatment remain the same, regardless of the cause.
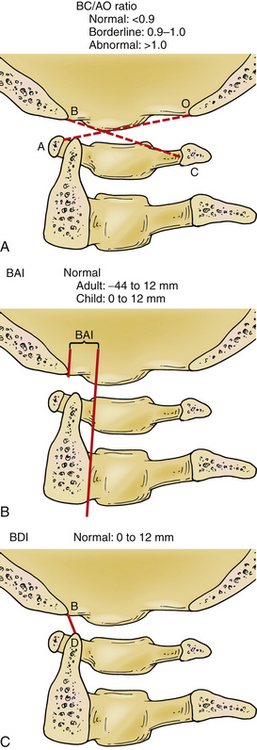
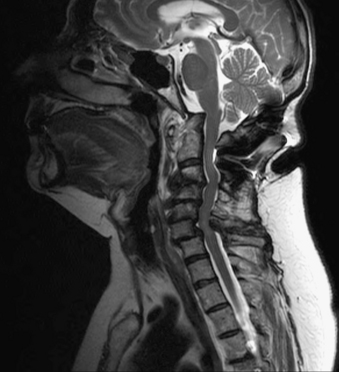
Rheumatoid arthritis (RA) is the most common degenerative disorder of the CVJ. RA is characterized by destruction of synovial joints. The disease is estimated to affect 0.8% of the Caucasian adult population in the United States, about 2.2 million people. The cervical spine is the second most commonly involved region of the body.37 The degenerative changes seen in the cervical spine are progressive in nature. Translational subluxation of C1-2 occurs first, followed by vertical subluxation of C1 on C2.38,39 Compression of the spinal cord and brainstem occurs as the lateral mass joints are eroded by inflammatory synovitis and the odontoid ascends through the atlas and the foramen magnum (Fig. 92-6). Oda et al. found a predictable progression of transverse subluxation to reducible vertical subluxation to irreducible vertical atlantoaxial subluxation.38 Fujiwara et al. redemonstrated this progression and also noted an association between the severity of RA and the progression of subluxation. Patients with less severe RA develop transverse subluxation, those with RA of moderate severity develop a combination of transverse and vertical subluxation, and those with more severe RA develop vertical subluxation.39 Basilar impression is the ascension of the odontoid process into the posterior cranial fossa and is defined by an abnormal position of the dens with respect to the foramen magnum. As the dens ascends into the posterior fossa, variable symptoms, which include but are not limited to myelopathy and lower cranial nerve deficits, develop. Although motor weakness and sensory changes due to myelopathy are the most common signs, the earliest sign of spinal cord dysfunction is posterior column function.40
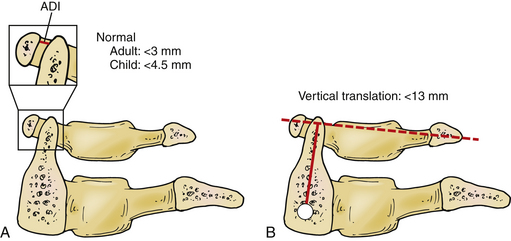
Determination of transverse C1-2 instability is performed using the anterior dental interval (ADI) (Fig. 92-7A). An ADI greater than 3 mm is considered to be abnormal.41 Vertical subluxation is measured using the Ranawat method. The vertical distance between the center of the pedicles on the axis to a line connecting the ventral and dorsal arches of the atlas is measured (Fig. 92-7B). If this distance is less than 13 mm in men and 15 mm in women, vertical subluxation is diagnosed.38
Many indices are used to screen for basilar impression from plain radiographs. These indices use bony anatomic landmarks and are the Clark station, McRae line,42 Chamberlain line,43 McGregor line,44 Redlund-Johnell criterion,45 Ranawat criterion,46 Fischgold-Metzger line,47 and Wackenheim line.48 Riew et al. evaluated the sensitivity and specificity of these standard screening measurements.49 The most sensitive measurement (the test with the fewest false-negative results) is the Wackenheim line, at 88%, and the Clark station, at 83%. The Redlund-Johnell criterion is the most specific measurement (fewest false-positive results) at 76%. The Redlund-Johnell measurement has the highest positive predictive value (PPV) of 68%. The Wackenheim line has a positive predictive value of 48%. The Fischgold-Metzger line has a negative predictive value of 100%. The McRae line has the lowest negative predictive value of 75%. The study also found that identification of bony landmarks is difficult and precludes accurate application of these measurement techniques in many cases. Riew et al. recommend a combination of tests to screen for basilar invagination: the Clark station, the Redlund-Johnell criterion, and the Ranawat criterion (Fig. 92-8).
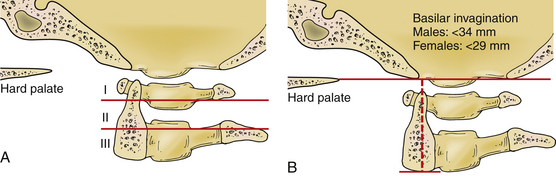
FIGURE 92-8 Measurements of basilar impression. A, The Clark station is determined by dividing the C2 body into three equal parts. If the ventral arch of C1 travels into the region of station II or station II itself, basilar invagination is suspected. B, The Redlund-Johnell criterion is measured by drawing a line from the tip of the hard palate to the rim of the occiput. A second line is drawn from the midpoint of the caudal margin of the body of C2. Basilar invagination is diagnosed if the measurement is less than 34 mm in men and 29 mm in women. The Ranawat classification is described in Figure 92-7B. The combination of these measurements should be used to screen for basilar invagination, and if suspected, an MRI should be performed to evaluate for evidence of neurologic compression.
The goals of treatments of basilar impression are to decompress the brainstem and spinal cord and reestablish support for the head. Decompression of the neural elements can be achieved either directly or indirectly. Indirect decompression is performed via closed reduction through the use of traction and manual manipulation. Long-standing lesions are unlikely to be reducible. However, a trial of craniocervical traction is warranted. Use of a halo ring provides multiple points of skull fixation and allows for fixation to the thoracic vest once the deformity is reduced. Traction is started with a weight of approximately 5 pounds (2.3 kg) and is increased as necessary. Attempted reduction is generally limited for 5 to 7 days because the likelihood of further benefit is limited after this period, and complications related to immobilization increase.37 Special beds have been designed to help prevent complications of immobilization.
The ventral rheumatoid pannus often resolves once the C1-2 junction is fused, which can indirectly decompress the brainstem and spinal cord.50,51 However, occasionally a ventral transoral approach needs to be used to obtain adequate decompression.52–56 Intraoperative image guidance may be used to help with the decompression in a region where anatomic landmarks have been distorted.57–59
Congenital Disorders
CVJ abnormalities are common in a number of congenital disorders. These disorders can be broadly grouped as connective tissue disorders (Down syndrome); Klippel-Feil syndrome; osteochondrodysplasias (e.g., achondroplasia); mucopolysaccharidoses (e.g., Morquio and Lesch-Nyhan syndromes); and skeletal dysplasias (i.e., osteogenesis imperfecta); as well as other disorders of development: Goldenhar syndrome, Conradi syndrome, and Klippel-Feil triad.60–64 The incidence of atlantoaxial subluxation is about 20% in Down syndrome65 and 50% in Morquio syndrome.66 The anatomic abnormalities produced by these disorders and the natural history of these can help guide treatment decisions. The following will review the more common disorders of Down syndrome, Morquio syndrome, and achondroplasia to help delineate some concepts. Description and management of other mentioned syndromes can be found in the listed references.37,67–70
CVJ abnormalities in these patients have variable causes. C1-2 subluxation is likely related to ligamentous laxity that is a common consequence of connective tissue disorders, such as Morquio syndrome and Lesch-Nyhan syndrome, and it is also a component of Down syndrome. Aberrant ossification of the dens occurs; this could be due to ligamentous laxity and disturbances in blood supply during development because of inordinate mobility.71,72 Patients with skeletal dysplasias, such as osteogenesis imperfecta, have abnormal collagen deposition, resulting in brittle bones that easily develop multiple microfractures. Accumulation of these microfractures leads to ascension of the dens and medial skull base, causing basilar invagination.72
Treatment of these lesions is guided by symptoms and the syndrome involved. For example, many children with Down syndrome have asymptomatic increased ADIs and atlanto-occipital hypermobility.73,74 The usual measurements of CVJ instability may not apply to patients with Down syndrome. Large cohort studies have not demonstrated increased rates of neurologic injury in children with Down syndrome and abnormal ADIs as compared with their peers without abnormal ADIs. These studies also do not demonstrate a protective effect of restricted activity.74,75 Reduction and stabilization of the CVJ is probably not indicated unless the patient has clear signs of brainstem or upper cervical spinal cord compression.73,76
Morquio syndrome and other skeletal dysplasias are often found to have an os odontoideum and ligamentous laxity.77 The cartilaginous os odontoideum deforms with flexion and extension. Radiographically, C1-2 instability manifests as changes in the ADI and is a late finding in affected children. By the time this is seen, myelopathy is nearly always present. These patients benefit from prophylactic fusion prior to the onset of myelopathy. The ideal age for this operation has not been determined. Ransford et al.77 suggest that the surgery be performed at 4 years of age unless myelopathic signs develop earlier. Dorsal occipitocervical fusion can result in complete ossification of the dens, which supports the role of ligamentous laxity in the formation of os odontoideum.72
Infection: Atlantoaxial Rotatory Subluxation and Fixation
Infection may lead to a rare syndrome termed atlantoaxial subluxation or atlantoaxial rotary fixation. It was originally described by Bell78 in 1830 but was named after Grisel,79 a French otolaryngologist who described this syndrome after upper respiratory infection. It is more common in children who present with torticollis and the head in a “cock-robin” position. There is no clear mechanism of pathogenesis for this entity, although it is associated with infection, trauma, head and neck surgery, RA, Down syndrome, Morquio disease, and other congenital cervical anomalies.80 Battiata et al.81 hypothesize a baseline ligamentous laxity along with an inflammatory response to an infectious process. Pang et al. hypothesize that rather than ligamentous laxity, there is increased friction of the C1-2 joints.80
The diagnosis is made by the clinical presentation along with findings of rotation of C1 on C2 seen on axial CT imaging. It is important to differentiate the presentation from muscular torticollis that has other etiologies. Pang et al. have used a dynamic CT scanning protocol to diagnose and grade the degree of pathologic “stickiness” of C1 on C2.80,82 Type I shows movement of less than 20% with movement of the head away from the affected side. Type II is less sticky, and C1 moves on C2 greater than 20%. Type III allows movement of C1 on C2 past midline but still remains abnormal compared with normal controls. MRI can be performed to evaluate for infectious etiology.
Primary treatment of atlantoaxial rotary fixation involves traction and muscle relaxants. Traction can be performed using a head halter. Once a reasonable amount of clinical reduction has been achieved, the patient can be placed in a cervical collar and treated with muscle relaxants for 2 weeks. Any infection needs to be treated completely to help prevent recurrence.54 Patients whose condition does not reduce or continues to recur may need more aggressive treatment. Closed reduction under general anesthesia can be used in selected cases. Operative C1-2 fixation and fusion can be used to permanently prevent recurrence. Surgical intervention is controversial. None of the patients in Menezes’ series of 52 patients required fusion.54
The outcomes of treatment have recently been shown by Pang et al.83 to be associated with the degree of rotary fixation seen with dynamic CT imaging. Patients with type I disease were more likely to have difficult reductions and recurrences than those with type III disease. Patients with type I disease were also more likely to require surgical correction. Delay in treatment also was associated with difficulty in reduction.
Tumors
Tumors of the CVJ produce signs and symptoms of neural element compression and mechanical instability (pain and progressive deformity). The treatment of these tumors depends on prognosis, symptoms, and anatomic configuration. The most common presentations of adults are myelopathy, radiculopathy, and occipitocervical pain.84 The presentations of children in descending frequency are occipitocervical pain, paresthesias or dysesthesias of the hands, cranial nerve palsies (most commonly diplopia), and myelopathy.85 The most commonly encountered tumors in this region are chordomas and meningiomas, but other primary tumors—osteoblastoma, eosinophilic granuloma, plasmacytoma, chondrosarcoma, and Ewing sarcoma—also occur. Metastatic tumors, including breast tumors and paragangliomas, have also been encountered.84–86
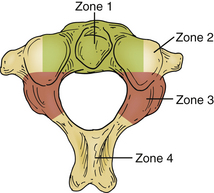
Treatment of patients with CVJ tumors involves decompression of neural structures and then determining if surgery has destabilized the CVJ to the point that fusion is necessary. The first step is to determine the direction of the surgical approach. Piper and Menezes divide the axis into four zones that help guide the surgical approach for tumor resection (Fig. 92-9).84 Zone 1 tumors are in the ventral midline involving the axis, atlas, and lower clivus and are best accessed by the transoral approach, with or without division of the palate or the mandible. Zone 2 tumors are more ventrolateral and often involve the lateral mass of C1 and C2. They are best accessed using a retropharyngeal approach. Zone 3 tumors are located dorsal to the lateral mass and may extend into the occipital condyle or dorsal fossa. These tumors are best accessed using a far lateral approach. However, this approach may not be good for later instrumention of the spine. Zone 4 tumors are in the dorsal midline and are resected through a standard midline approach.84
Ventral Odontoid Resection
Dickman et al. performed a biomechanical study of transoral odontoidectomy and concluded that resection of the ventral C1 ring, odontoid, and transverse ligament causes increased motion of C1 on C2 and acute/chronic instability.53 The conclusion from this study was that ventral odontoid resection requires subsequent dorsal fixation. Menezes87 reported that a minority of patients undergoing odontoidectomy could go without dorsal stabilization. Disagreement also exists regarding the timing of dorsal stabilization. Menezes delayed fixation for 1 week postsurgery and maintained patients in a halo to allow for wound healing and assessment of instability. Crockard and Stevens71 recommended performing the dorsal stabilization at the time of ventral resection. An increased incidence of infection has not been seen due to immediate dorsal surgery after a transoral resection.88
Lateral Condyle Resection and Lateral Mass Resection
Biomechanical studies have shown a significant increase in hypermobility with resection of greater than 50% of the condyle. Resection of the condyle affected the stability of the C1-2 junction as well.89 Based on their retrospective case series, Shin et al. recommended an occipital cervical fusion if greater than 50% of a condyle is resected or if the C1 or C2 lateral masses are resected.86
Deformity Reduction
Reduction of deformity can be performed by either closed or open methods depending on the anatomic configuration. Successful closed reduction with axial traction can sometimes obviate the need for open ventral decompression, or in some cases of trauma, such as traumatic spondylolisthesis, closed reduction can potentially obviate the need for surgical intervention. Closed reduction is achieved with the use of axial traction with a halo fixator. In some cases, manual manipulation under fluoroscopic guidance is used for reduction. The halo is applied carefully, and the force of pin application is tailored to patient age and underlying pathology. Children younger than 2 years of age or with underlying pathology affecting the skull may not be candidates for a halo ring, and the use of a custom-built Minerva device may be preferable. Children 2 to 4 years of age should have an eight-point halo fixation with an MRI-compatible device. The pins should be tightened to between 1 to 1⅓ pounds of torque.90 The maximum pin torque is 4 pounds for children who are 5 years of age. Traction should be initiated with low weight (4 pounds) in 5-year-old children, and it should not exceed 7 pounds.54 Halo application and traction are performed under general anesthesia or moderate sedation in some cases. Fluoroscopy, plain radiographs, or even CT or MRI may be used to determine if reduction has been achieved.91
Patients who have long-standing degenerative disorders or tumors are generally not candidates for preoperative reduction. These patients require direct surgical decompression via the appropriate surgical route. This can be aided with the use of computerized stereotactic navigation.58,92 After the decompression, reduction of the deformity can be achieved by direct manipulation and appropriate shaping of implants and postoperative immobilization.
Bono C.M., Vaccaro A.R., Fehlings M., et al. Measurement techniques for upper cervical spine injuries. Spine (Phila Pa 1976). 2007;32(5):593-600.
Menezes A.H. Craniovertebral junction database analysis: incidence, classification, presentation, and treatment algorithms. Childs Nerv Syst. 2008;24(10):1101-1108.
Menezes A.H. Craniovertebral junction neoplasms in the pediatric population. Childs Nerv Syst. 2008;24(10):1173-1186.
Pang D., Nemzek W.R., Zovickian J. Atlanto-occipital dislocation–part 2: the clinical use of (occipital) condyle-C1 interval, comparison with other diagnostic methods, and the manifestation, management, and outcome of atlanto-occipital dislocation in children. Neurosurgery. 2007;61(5):995-1015. discussion 1015
Riew K.D., Hilibrand A., Palumbo M.A., et al. Diagnosing basilar invagination in the rheumatoid patient. The reliability of radiographic criteria. J Bone Joint Surg [Am]. 2001;83:194-200.
1. Bono C.M., Vaccaro A.R., Fehlings M., et al. Measurement techniques for upper cervical spine injuries. Spine (Phila Pa 1976). 2007;32(5):593-600.
2. Anderson P.A., Montesano P.X. Morphology and treatment of occipital condyle fractures. Spine (Phila Pa 1976). 1988;13(7):731-736.
3. Panjabi M., Dvorak J., Crisco J.J.III, et al. Effects of alar ligament transection on upper cervical spine rotation. J Orthop Res. 1991;9(4):584-593.
4. Tuli S., Tator C.H., Fehlings M.G., et al. Occipital condyle fractures. Neurosurgery. 1997;41(2):368-377.
5. Roy S., Hol P.K., Laerum L.T., et al. Pitfalls of magnetic resonance imaging of alar ligament. Neuroradiology. 2004;46(5):392-398.
6. Maserati M., Stephens B., Zohny Z., et al. Occipital condyle fractures: clinical decision and surgical managment. J Neurosurg Spine. 2009;11(4):388-395.
7. Pang D., Nemzek W.R., Zovickian J. Atlanto-occipital dislocation–part 2: the clinical use of (occipital) condyle-C1 interval, comparison with other diagnostic methods, and the manifestation, management, and outcome of atlanto-occipital dislocation in children. Neurosurgery. 2007;61(5):995-1015. discussion 1015
8. Przybylski G., Clyde B., Fitz C. Craniocervical junction subarachnoid hemorrhage associated with atlanto-occipital dislocation. Spine (Phila Pa 1976). 1996;21:1761-1768.
9. Menezes A., Piper J. Anatomy and radiographic pathology of injury to the occipitoatlantal complex. Park Ridge, IL: American Association of Neurological Surgeons; 1993.
10. Powers B., Miller M., Kramer R., et al. Traumatic anterior atlanto-occipital dislocation. Neurosurgery. 1979;4:12-17.
11. Harris J., Carson G., Wagner L. Radiological diagnosis of traumatic occipitocervical dissociation: 1. Normal occipitovertebral relationships on lateral radiographs of supine subjects. AJR Am J Roentgenol. 1994;162:881-886.
12. Weisel S., Rothman R. Occipitoatlantal hypermobility. Spine (Phila Pa 1976). 1979;4:187-191.
13. Dziurzynski K., Anderson P.A., Bean D.B., et al. A blinded assessment of radiographic criteria for atlanto-occipital dislocation. Spine (Phila Pa 1976). 2005;30(12):1427-1432.
14. Heary R., Hnt C., Krieger A., et al. Acute stabilization of the cervical spine by halo vest application facilitates evaluation and treatment of multiple trauma patients. J Trauma. 1992;33:445-451.
15. Landells C.D., Van Peteghem P.K. Fractures of the atlas: classification, treatment and morbidity. Spine (Phila Pa 1976). 1988;13:450-452.
16. Jefferson G. Fracture of the atlas vertebra: report of four cases and a review of those previously recorded. Br J Surg. 1920;7:407-422.
17. Spence J., Kendall B., Crockard H., et al. Bursting atlantal fracture associated with rupture of the transverse ligament. J Bone Joint Surg [Am]. 1970;52:543-549.
18. Heller J.G., Viroslav S., Hudson T. Jefferson fractures: the role of magnification artifact in assessing transverse ligament integrity. J Spinal Disord. 1993;6:392-396.
19. Dickman C.A., Greene K.A., Sonntag V.K.H. Injuries involving the transverse atlantal ligament: classification and treatment guidelines based upon experience with 39 injuries. Neurosurgery. 1996;38(1):44-50.
20. Panjabi M., Dvorak J., Crisco J.J.III, et al. Flexion, extension, and lateral bending of the upper cervical spine in response to alar ligament transections. Spine (Phila Pa 1976). 1991;4(2):157-167.
21. Oda T., Panjabi M., Crisco J.J.III, et al. Experimental study of the atlas injuries: part II–relevance to clinical diagnosis and treatment. Spine (Phila Pa 1976). 1991;16(Suppl 10):S466-S673.
22. Oda T., Panjabi M.M., Crisco J.J.III, et al. Multidirectional instabilities of experimental burst fractures of the atlas. Spine (Phila Pa 1976). 1992;17:1285-1290.
23. No authors listed. Isolated fractures of the atlas in adults. Neurosurgery. 2002;50(3):S120-S124.
24. Anderson L., D’Alonzo R. Fractures of the odontoid process of the axis. J Bone Joint Surg [Am]. 1974;56:1663-1674.
25. Hadley M., Dickman C., Browner C., et al. Acute axis fractures: a review of 229 cases. J Neurosurg. 1989;71:642-647.
26. Nourbakhsh A., Shi R., Vannemreddy P., et al. Operative vs. nonoperative managment of acute odontoid Type II fractures: a meta-analysis. J Neurosurg Spine. 2009;11:651-658.
27. Hadley M.N., Browner C., Sonntag V.K. Axis fractures: comprehensive review of managment and treatment in 107 cases. Neurosurgery. 1985;17:281-290.
28. No author listed. Isolated fractures of the axis in adults. Neurosurgery. 2002;50(3):S125-S139.
29. Hadley M.N., Browner C.M., Liu S.S., et al. New subtype of acute odontoid fractures (type IIA). Neurosurgery. 1988;22:67-71.
30. Fielding J.W., Hensinger R.N., Hawkins R.J. Os odontoideum. J Bone Joint Surg [Am]. 1990;62:376-383.
31. Menezes A.H. Pathogenesis, dynamics, and management of os odontoideum. Neurosurg Focus. 1999;6(6):E4.
32. Schneider R., Livingston K., Cave A., et al. “Hangman’s fracture” of the cervical spine. J Neurosurg. 1965;22:141-154.
33. Francis W.R., Fielding J.W., Hawkins R.J., et al. Traumatic spondylolesthesis of the axis. J Bone Joint Surg [Br]. 1981;63:313-318.
34. Effendi B., Roy D., Cornish B., et al. Fractures of the ring of the axis: a classification based on the analysis of 131 cases. J Bone Joint Surg [Br]. 1981;63:319-327.
35. Papadopoulos S. Biomechanics of occipito-atlanto-axial trauma. Park Ridge, IL: American Association of Neurological Surgeons; 1993.
36. No authors listed. Management of vertebral artery injuries after non-penetrating cervical trauma. Neurosurgery. 2002;50(Suppl 3):S173-S178.
37. Menezes A. Congenital and acquired abnormalities of the craniovertebral junction. Philadelphia: WB Saunders, 1995;vol. 4.
38. Oda T., Fujiwara K., Yonenobu K., et al. Natural course of cervical spine lesions in rheumatoid arthritis. Spine (Phila Pa 1976). 1995;20(10):1128-1135.
39. Fujiwara K., Yonenobu K., Ochi T. Natural history of upper cervical lesions in rheumatoid arthritis. J Spinal Disord. 1997;10(4):275-281.
40. Rogers M., Crockard H., Moskovich R. Nystagmus and joint position sensation: their importance in posterior occipitocervical fusion in rheumatoid arthritis. Spine (Phila Pa 1976). 1994;19:16-20.
41. Penning L. Normal movements of the cervical spine. AJR Am J Roentgenol. 1978;130:317-326.
42. McRae D.L., Barnum A.S. Occipitalization of the atlas. Am J Roentgenol. 1953;70:23-45.
43. Chamberlain W. Basilar impression (platybasia). A bizarre developmental anomaly of the occipital bone and upper cervical spine with striking and misleading neurologic manifestations. Yale J Biol Med. 1939;11:487-496.
44. McGregor M. The significance of certain measurements of the skull in the diagnosis of basilar impression. Br J Radiol. 1948;21:171-181.
45. Redlund-Johnell I., Pattersson H. Radiographic measurements of the cranio-vertebral region. Designed for evaluation of abnormalities in rheumatoid arthritis. Acta Radiol Diagn (Stockh). 1984;25:23-28.
46. Ranawat C., O’Leary P., Pellici P. Cervical spine fusion in rheumatoid arthritis. J Bone Joint Surg [Am]. 1979;61:1003-1010.
47. Fischgold H., Metzger J. Etude radiotomographique de l’impression basilaire. Rev Rhum Ed Fr. 1952;19:261-264.
48. Wackenheim A. Roentgen diagnosis of the cranio-vertebral region. New York: Springer; 1974.
49. Riew K.D., Hilibrand A., Palumbo M.A., et al. Diagnosing basilar invagination in the rheumatoid patient. The reliability of radiographic criteria. J Bone Joint Surg [Am]. 2001;83:194-200.
50. Larsson E.M., Holtas S., Zygmunt S. Pre- and postoperative MR imaging of the craniocervical junction in rheumatoid arthritis. AJR Am J Roentgenol. 1989;152(3):561-566.
51. Milbrink J., Nyman R. Posterior stabilization of the cervical spine in rheumatoid arthritis: clinical results and magnetic resonance imaging correlation. J Spinal Disord. 1990;3(4):308-315.
52. Crockard H.A. Transoral surgery: some lessons learned. Br J Neurosurg. 1995;9(3):283-293.
53. Dickman C., Crawford N., Brantley A., et al. Biomechanical effects of transoral odontoidectomy. Neurosurgery. 1995;36:1146-1153.
54. Menezes A.H. Decision making. Childs Nerv Syst. 2008;24(10):1147-1153.
55. Menezes A.H., VanGilder J.C. Transoral-transpharyngeal approach to the anterior craniocervical junction. Ten-year experience with 72 patients. J Neurosurg. 1988;69(6):895-903.
56. Naderi S., Crawford N.R., Melton M.S., et al. Biomechanical analysis of cranial settling after transoral odontoidectomy. Neurosurg Focus. 1999;6(6):E7.
57. Pollack I.F., Welch W., Jacobs G.B., et al. Frameless stereotactic guidance. An intraoperative adjunct in the transoral approach for ventral cervicomedullary junction decompression. Spine (Phila Pa 1976). 1995;20(2):216-220.
58. Veres R., Bago A., Fedorcsak I. Early experiences with image-guided transoral surgery for the pathologies of the upper cervical spine. Spine (Phila Pa 1976). 2001;26(12):1385-1388.
59. Welch W.C., Subach B.R., Pollack I.F., et al. Frameless stereotactic guidance for surgery of the upper cervical spine. Neurosurgery. 1997;40(5):958-963. discussion 963–964
60. Baba H., Maezawa Y., Furusawa N., et al. The cervical spine in the Klippel-Feil syndrome: a report of 57 cases. Int Orthop. 1995;19:204-208.
61. Pozo J., Crockard H., Ransford A. Basilar impression in osteogenesis imperfecta: a report of three cases in one family. J Bone Joint Surg [Br]. 1984;66:233-238.
62. Shewell P., Thompson A. Atlantoaxial instability in Lesch-Nyhan syndrome. Spine (Phila Pa 1976). 1996;21:757-762.
63. Wald S., McLaurin R. Anomalies of the craniocervical junction, ed 2, Philadelphia: WB Saunders, 1989.
64. Menezes A.H., Vogel T.W. Specific entities affecting the craniocervical region: syndromes affecting the craniocervical junction. Childs Nerv Syst. 2008;24(10):1155-1163.
65. VanGilder J.C., Menezes A.H., Dolan K. Craniovertebral junction abnormalities. Mount Kisco, NY: Futura; 1987.
66. Menezes A.H. Acquired abnormalities of the craniovertebral junction. Philadelphia: Saunders; 2003.
67. Klimo P.J., Rao G., Brockmeyer D. Congenital anomalies of the cervical spine. Neurosurg Clin N Am. 2007;18(3):463-478.
68. Yamamoto T., Kurosawa K., Masuno M., et al. Congenital anomaly of cervical vertebrae is a major complication of Rubinstein-Taybi syndrome. Am J Med Genet. 2005;135(2):130-133.
69. Menezes A.H. Craniovertebral junction database analysis: incidence, classification, presentation, and treatment algorithms. Childs Nerv Syst. 2008;24(10):1101-1108.
70. Herman M.J., Pizzutillo P.D. Cervical spine disorders in children. Orthop Clin North Am. 1999;30(3):457-466.
71. Crockard H., Stevens J. Craniovertebral junction anomalies in inherited disorders: part of the syndrome or caused by the disorder? Eur J Pediatr. 1995;154:504-512.
72. Stevens J., Kendall B., Crockard H. The odontoid process in Morquio-Brailsford’s disease: the effects of occipitocervical fusion. J Bone Joint Surg [Br]. 1991;73:851-858.
73. Menezes A.H., Ryken T.C. Craniovertebral abnormalities in Down’s syndrome. Pediatr Neurosurg. 1992;18(1):24-33.
74. Cremers M., Bol E., de Roos F., et al. Risk of sports activities in children with Down’s syndrome and atlantoaxial instability. Lancet. 1993;342:511-514.
75. Morton R., Khan M., Murray-Leslie C., et al. Atlantoaxial instability in Down’s syndrome: a five-year follow-up study. Arch Dis Child. 1995;72:115-118.
76. Nader-Sepahi A., Casey A.T., Hayward R., et al. Symptomatic atlantoaxial instability in Down syndrome. J Neurosurg Pediatr. 2005;103(3):231-237.
77. Ransford A., Crockard H., Stevens J., et al. Occipito-atlanto-axial fusion in Morquio-Brailsford syndrome. J Bone Joint Surg [Br]. 1996;78:307-313.
78. Bell C. The nervous system of the human body, embracing papers delivered to the royal society on the subject of nerves. London: Longman; 1830.
79. Grisel P. Enucleatio de l’atlas et torticollis nasopharyngen. Presse Med. 1930;38:50-53.
80. Pang D., Li V. Atlantoaxial rotatory fixation: part 1–Biomechanics of normal rotation at the atlantoaxial joint in children. Neurosurgery. 2004;55(3):614-625. discussion 625–626
81. Battiata A.P., Pazos G. Grisel’s syndrome: two-hit hypothesis–a case report and literature review. Ear Nose Throat J. 2004;83(8):553-555.
82. Pang D., Li V. Atlantoaxial rotatory fixation: part 2–new diagnostic paradigm and a new classification based on motion analysis using computed tomographic imaging. Neurosurgery. 2005;57(5):941-953. discussion 941–953
83. Pang D., Li V. Atlantoaxial rotatory fixation: part 3–a prospective study of the clinical manifestation, diagnosis, management, and outcome of children with alantoaxial rotatory fixation. Neurosurgery. 2005;57(5):954-972. discussion 954–972
84. Piper J., Menezes A. Management strategies for tumors of the axis vertebra. J Neurosurg. 1996;84:543-551.
85. Menezes A.H. Craniovertebral junction neoplasms in the pediatric population. Childs Nerv Syst. 2008;24(10):1173-1186.
86. Shin H., Barrenechea I.J., Lesser J., et al. Occipitocervical fusion after resection of craniovertebral junction tumors. J Neurosurg Spine. 2006;4(2):137-144.
87. Menezes A. Surgical approaches to the craniocervical junction. New York: Raven Press; 1991.
88. Tuite G.F., Veres R., Crockard H.A., et al. Pediatric transoral surgery: indications, complications, and long-term outcome. J Neurosurg. 1996;84(4):573-583.
89. Vishteh A.G., Crawford N.R., Melton M.S., et al. Stability of the craniovertebral junction after unilateral occipital condyle resection: a biomechanical study. J Neurosurg. 1999;90(Suppl 1):91-98.
90. Garfin S.R., Bottle M.J., Waters R.L., et al. Complications in the use of halo fixation device. J Bone Joint Surg [Am]. 1986;68:320-325.
91. Darsaut T.E., Sartawi M.M., Dhaliwal P., et al. Rapid magnetic resonance imaging-guided reduction of craniovertebral junction deformities. J Neurosurg Spine. 2009;10(1):27-32.
92. Ternier J., Joshi S.M., Thompson D.N. Image-guided transoral surgery in childhood. Childs Nerv Syst. 2009;25(5):563-568.