CHAPTER 32 Cranioplasty
Diagnostic Evaluation
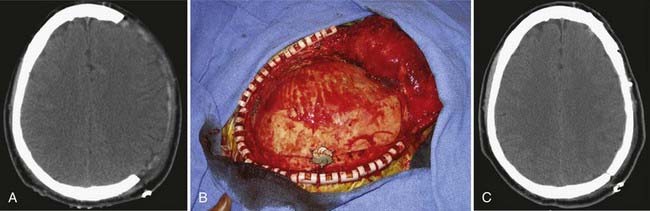
One of the most common indications for delayed cranioplasty is after hemicraniectomy for refractory intracranial hypertension (Fig. 32-1). Before the autologous bone flap may be replaced, the overlying scalp must be well healed and vascularized, intracranial hypertension and neurological status should be completely stabilized, and infections (both systemic and cranial) must be fully treated. Although cranioplasty is typically performed approximately 3 months after traumatic brain injury, recent reports indicate that in select, otherwise healthy patients, early cranioplasty after 5 to 8 weeks may aid recovery.1,2 Other recent literature suggests that the improvement in neurological status after early cranioplasty depends on restoration of normal CSF flow dynamics. Several reports indicate that communicating hydrocephalus occurs at a high incidence after decompressive hemicraniectomy.3 Early cranioplasty combined with implantation of a programmable shunt improved patient outcomes and reduced complications.4 On a technical note, early cranioplasty after 5 to 8 weeks may allow easier discrimination of the various tissue layers when the skin flap is reflected. However, onlay synthetic dural substitutes, if used, may not have formed an adherence to the underlying native dura and will often be inadvertently reflected with the skin flap.
Patients commonly exhibit hemispheric collapse on the side of the calvarial defect, often referred to as syndrome of the trephined.5 Miscellaneous neurological symptoms are attributed to the hemispheric collapse and include headache, dizziness, fatigue, and psychiatric changes.6 The most common explanation of this depression of the brain is an alteration of CSF dynamics with resultant neurological compromise.5,7,8 A report showed that cerebral blood flow, as measured by xenon-enhanced computed tomography (CT), increases after cranioplasty.9 However, without some degree of hemispheric collapse, replacement of the bone flap is technically challenging and requires additional CSF drainage at the time of surgery.
Infection after cranial surgery is another clinical scenario in which the native bone sometimes must be discarded, and delayed cranioplasty is preferred. Several reports have indicated that cranioplasty may be avoided after operative débridement of an infected bone flap.10,11 However, when the infection has devitalized the bone flap or is extensive within overlying scalp or brain parenchyma, discarding the bone flap is required. A standard does not exist for the duration of time required after bone flap infection before cranioplasty may be performed. Conservative estimates have specified delay of reconstructive surgery for 6 months to 1 year.12–14 Inflammatory markers, such as C-reactive protein and erythrocyte sedimentation rate, as well as serial imaging may assist in the determination of cranioplasty timing.
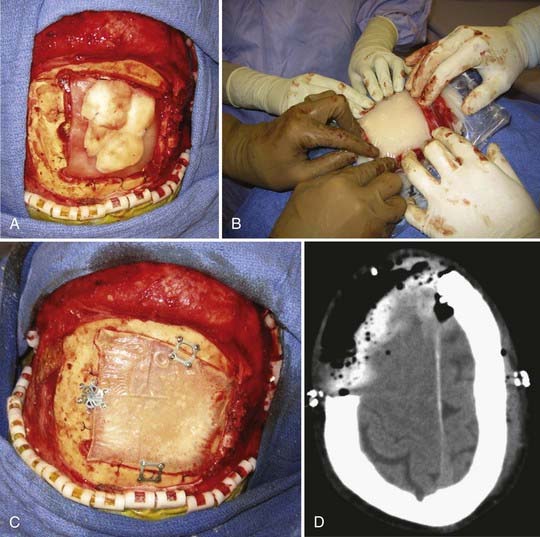
Immediate cranioplasty is indicated after resection of bone neoplasm, such as a meningioma, that has eroded through the skull (Fig. 32-2). In the adult population, use of synthetic cranioplasty materials such as methyl methacrylate is preferred for large skull defects (>10 cm) because of their ease of use for irregularly shaped defects. For smaller defects, titanium mesh alone is sufficient to achieve an excellent cosmetic result.14
Preoperative Management
In cases of traumatic brain injury or stroke, the patient’s autologous bone flap must be removed from storage before cranioplasty. These bone flaps are usually either placed into deep-freeze preservation or subcutaneously preserved in abdominal fat. Some reports indicate that the preservation in subcutaneous tissue improves the bone viability, thereby lowering cranioplasty revision rate.15 However, storage at less than −28°C has been shown to be an effective method of preservation and avoids the additional morbidity of an abdominal incision.16–19 The largest disadvantage of frozen autologous bone graft is a higher rate of reported resorption compared with other cranioplasty materials, especially in children.20,21
Infection of the autologous bone flap is also a common complication, and sterile technique and care must be taken during the collection and storage preservation of the bone flap at the time of hemicraniectomy. Use of ethylene oxide gas to sterilize autologous bone graft before storage at room temperature has been shown to be an effective alternative to freezing the bone flap.22 Cultures of the bone flap obtained at this time must be reviewed before cranioplasty, as bacterial contamination of the bone flap often occurs in an indolent fashion.23 Bacterial contamination of an autologous bone flap has been a contraindication for reinsertion during cranioplasty. Although it is not the standard of practice, some reports suggest insertion of infected autograft into the subcutaneous fat to allow the patient’s immune system to clear the infection before cranioplasty is attempted.24
Cranioplasty Material Options
There is a large selection of possible materials for repair of skull defects, which may be categorized into autografts, allografts, xenografts, and bone substitutes. The success and durability of the operation require careful selection of a material tailored to the clinical scenario. The ideal material is malleable, sterilizable, nonmagnetic, radiolucent, lightweight, and able to be easily secured to existing skull (Table 32-1). Although a multitude of different animal bones, metals, and even coconut shell have been used in cranioplasty, this discussion focuses on those materials used in modern neurosurgery.14
| MATERIAL | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Autologous bone | Viable, potential for growth, low rate of plate fracture or migration | Bone resorption, infection, possible poor cosmesis |
| Methyl methacrylate | Ease of use, excellent cosmesis, low cost, strength and durability | Infection, plate fracture, no growth potential, exothermic reaction, inflammatory reaction |
| Calcium phosphate bone cement | Osteoconductive, osteoinductive, useful for difficult to reach defects, no inflammatory reaction | Brittle, fragile, difficult to contour, cannot bear stress |
| Titanium mesh | No inflammatory reaction, low infection rate, osteointegrative | High cost, poor malleability, possible poor cosmesis, loosens over time; image artifact on magnetic resonance images and computed tomographic scans, rendering resolution of adjacent tissue difficult |
Methyl methacrylate is polymerized ester of acrylic acid that exists in powdered form and is mixed with liquid monomer, benzoyl peroxide. In an exothermic reaction, methyl methacrylate slowly cools from a paste-like substance into a translucent material with strength comparable to that of native bone.25 During this cooling phase, methyl methacrylate may be shaped to fit any skull defect. Methyl methacrylate may be used for technically challenging areas of the skull, and reconstruction and growth from the native bone edge adjacent to the prosthesis will secure it to the skull. Disadvantages of methyl methacrylate include postoperative infection, at a rate of approximately 5% to 10%, and plate breakdown or fracture.26,27 A methyl methacrylate prosthesis is at higher risk of infection compared with autologous bone flap because it is not viable, and a fibrous layer will grow around the plate, to which bacteria may adhere. The most common organisms are Staphylococcus aureus and Propionibacterium acnes.28 Deep wound infection may be latent and not become clinically apparent for several years. Liquid methyl methacrylate may be absorbed by tissues and has been reported to cause acute hypotension and hypersensitivity.29,30 Different types of methyl methacrylate are commercially available. We are most familiar with Aneuroplastic, a product of Codman and Shurtleff (Raynham, MA). Surgical Simplex is a product of Stryker Howmedica Osteonics (Mahwah, NJ) and is more commonly used in orthopedic procedures. It is a composite material of polymethyl methacrylate and barium sulfate, creating a radiopaque bone cement.
Another option of synthetic prostheses is calcium phosphate bone cement, which like methyl methacrylate exists as a powder and forms a malleable substance when it is mixed with liquid sodium phosphate. When it is fully cured, the calcium phosphate prosthesis approximates the mineral phase of bone and will be integrated into the native skull and remodeled over time to fit the defect. The most commonly used calcium phosphate material is hydroxyapatite, shown to be ideally suited for small craniofacial defects.31,32 When it is used directly against exposed dura, titanium mesh is recommended as an underlay to prevent small fractures in the hydroxyapatite plate from dural pulsations.33 In contrast to to methyl methacrylate, which does not allow further expansion of a growing skull, hydroxyapatite bone cement is often used for skull defects in the pediatric population. Norian calcium phosphate bone cement (Synthes, West Chester, PA) is used by our institutions. Certain types of calcium phosphate prostheses, including hydroxyapatite, have the additional advantage of being osteoconductive, so it serves as scaffolding for growth of new bone.
Titanium mesh, either alone or in combination with methyl methacrylate, is another useful material for cranioplasty. Titanium is non-ferromagnetic and noncorrosive, and it does not elicit an inflammatory reaction. Several series have reported a low incidence of infection while still achieving excellent cosmetic results.34,35 Most commonly, titanium exists as a metallic alloy with other metals to improve its strength and malleability. Titanium is also employed to preform prostheses on the basis of three-dimensional computed tomographic reconstructions of the skull base defect.
Computer-designed implants from computed tomographic reconstructions are expensive but effective for complex skull defects.36 Anatomic models may be formed by polymerization of ultraviolet light–sensitive liquid resin with use of a laser, based on computed tomographic data. These stereolithographic models are then used to manufacture customized titanium plates,37 hydroxyapatite implants, or methyl methacrylate prostheses.37 Costs for these prefabricated prostheses may be as high as $4000; however, the precision has been reported to be 0.25 mm for implants as large as 18 cm.38
New biocompatible materials and composite implants have recently been used for cranioplasty with excellent results. Porous polyethylene implants are composed of high-density polyethylene microspheres that create interconnected pores, allowing ingrowth of native bone. This unique implant structure rapidly incorporates fibrovascular tissue from the patient and decreases the infection rate of the implant. Porous polyethylene implants may be shaped to cover a large variety of skull defects and secured with titanium screws to native bone. A distinct advantage of this material over titanium is that it does not produce artifact on postoperative computed tomographic scans and magnetic resonance images. In a study of 611 cranioplasty procedures using porous polyethylene, all patients achieved excellent cosmetic results with no postoperative infections.39
Further efforts to decrease cranioplasty implant infection have focused on antibiotic elution from hydroxyapatite cement materials. Hydroxyapatite cement is able to be impregnated with a variety of antibiotics intraoperatively. Tobramycin, a broad-spectrum aminoglycoside with activity against S. aureus, gram-negative bacteria, and gentamicin-resistant pseudomonal species, has shown promise in cranioplasty materials. Studies have demonstrated a predictable concentration and sustained release of tobramycin from hydroxyapatite cement for approximately 10 days.40 There is currently a paucity of data about the use of these implants for cranioplasty procedures. Joint arthroplasty procedures have more extensively used antibiotic-eluting materials, with some mixed success in decreasing infection.41,42 There remains some concern about increasing bacterial resistance from widespread use of these materials.
Because each cranioplasty material has its own advantages and disadvantages, studies have examined hydroxyapatite–polymethyl methacrylate composites. Hydroxyapatite has good osteoconductivity but is fragile and cracks easily. Conversely, methyl methacrylate is easier to shape and is stronger, but it has relatively poor osteoconductivity. A composite of both materials using two-thirds hydroxyapatite and one-third methylmethacrylate showed almost the same osteoconductivity as hydroxyapatite alone at the surface of the implant, but it did not penetrate inside the composite.43 Various formulas of composites show excellent promise in cranioplasty because of the different properties of each substance.
Operative Technique
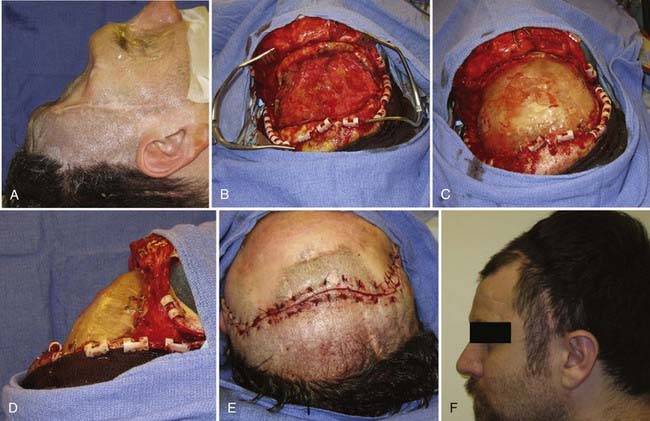
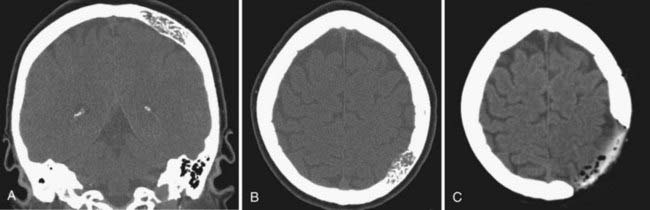
![]() In formation of the methyl methacrylate prosthesis, shaping of the plate to achieve an excellent cosmetic result requires careful planning. Large saline-soaked cotton balls are placed into the skull defect above the dura and molded until they form the appropriate contour (Fig. 32-3). With use of a container hooked to a vacuum system to remove fumes, the powdered methyl methacrylate and benzoyl peroxide are mixed slowly, with care taken to stir slowly so that air bubbles do not form. When the mixture reaches a thick syrup consistency, it is placed into a sterile plastic sleeve and quickly smoothed into a thin sheet. The entire plastic sleeve is placed over the skull defect, pulled taut, and pressed firmly onto the edge of the native skull. Methyl methacrylate will undergo the exothermic reaction; however, the saline-soaked cotton balls protect the underlying cortex. When the edges of the plate become more transparent, the methyl methacrylate prosthesis should be removed from the plastic sleeve and soaked briefly in cold saline. Excess acrylic may be shaved off with a high-speed drill (Fig. 32-4; Video 32-1).
In formation of the methyl methacrylate prosthesis, shaping of the plate to achieve an excellent cosmetic result requires careful planning. Large saline-soaked cotton balls are placed into the skull defect above the dura and molded until they form the appropriate contour (Fig. 32-3). With use of a container hooked to a vacuum system to remove fumes, the powdered methyl methacrylate and benzoyl peroxide are mixed slowly, with care taken to stir slowly so that air bubbles do not form. When the mixture reaches a thick syrup consistency, it is placed into a sterile plastic sleeve and quickly smoothed into a thin sheet. The entire plastic sleeve is placed over the skull defect, pulled taut, and pressed firmly onto the edge of the native skull. Methyl methacrylate will undergo the exothermic reaction; however, the saline-soaked cotton balls protect the underlying cortex. When the edges of the plate become more transparent, the methyl methacrylate prosthesis should be removed from the plastic sleeve and soaked briefly in cold saline. Excess acrylic may be shaved off with a high-speed drill (Fig. 32-4; Video 32-1).
Common Pitfalls and Complications
Complications related to cranioplasty may be categorized as plate failure or bone resorption, infection, and subdural fluid collection or hydrocephalus. When the plate is secured to the native bone, the titanium plates and screws should not be under tension. In addition, placement of titanium mesh under the acrylic plate can increase the strength of the construct. Autologous bone resorption may occur if the flap has become irreversibly devitalized or does not come in contact with vascular native bone edge. This occurs when the soft tissue and scar tissue have not been adequately removed from the bone edge before replacement of the bone flap. Some reports have indicated that resorption of autologous bone occurs more frequently in children and is associated with the size of the skull defect.20 Risk factors for bone flap infection include previous infection in the skull defect, communication between the operative site and cranial-facial sinuses, devascularized scalp, and persistent subdural or subgaleal fluid collection.14 In most cases, cranioplasty improves CSF flow dynamics and restores normal intracranial pressure relationships within the skull.3 However, in cases of traumatic brain injury or subarachnoid hemorrhage, the loss of brain parenchyma and obstruction of CSF pathways may not become apparent until after cranioplasty. Unilateral or bilateral subdural fluid collections may form if there has been intrinsic loss of brain parenchyma so that the brain does not fully reexpand to fill the skull. Attempts to decrease formation of these collections with subdural drains or central dural tacking suture are not always successful. Similarly, the ventricles may increase in size after cranioplasty, either from brain atrophy or from manifesting true hydrocephalus. Typically, ventricular-peritoneal shunts are placed in a separate procedure to address these complications after the patient’s neurological status and clinical examination findings have stabilized after cranioplasty.
, Itokawa H, Hiraide T, Moriya M, et al. A 12 month in vivo study on the response of bone to a hydroxyapatite-polymethylmethacrylate cranioplasty composite. Biomaterials. 2007;28:4922.
, Lee C, Antonyshyn OM, Forrest CR. Cranioplasty: indications, technique, and early results of autogenous split skull cranial vault reconstruction. J Craniomaxillofac Surg. 1995;23:133.
, Liu JK, Gottfried ON, Cole CD, et al. Porous polyethylene implant for cranioplasty and skull base reconstruction. Neurosurg Focus. 2004;16:ECP1.
, Marchac D, Greensmith A. Long-term experience with methylmethacrylate cranioplasty in craniofacial surgery. J Plast Reconstr Aesthet Surg. 2008;61:744.
, Rengachary SS, Benzel EC. Calvarial and Dural Reconstruction. Park Ridge, Ill: AANS; 1998.
, Rish BL, Dillon JD, Meirowsky AM, et al. Cranioplasty: a review of 1030 cases of penetrating head injury. Neurosurgery. 1979;4:381.
1 Liang W, Xiaofeng Y, Weiguo L, et al. Cranioplasty of large cranial defect at an early stage after decompressive craniectomy performed for severe head trauma. J Craniofac Surg. 2007;18:526.
2 Rish BL, Dillon JD, Meirowsky AM, et al. Cranioplasty: a review of 1030 cases of penetrating head injury. Neurosurgery. 1979;4:381.
3 Waziri A, Fusco D, Mayer SA, et al. Postoperative hydrocephalus in patients undergoing decompressive hemicraniectomy for ischemic or hemorrhagic stroke. Neurosurgery. 2007;61:489.
4 Carvi y Nievas MN, Höllerhage HG. Early combined cranioplasty and programmable shunt in patients with skull bone defects and CSF-circulation disorders. Neurol Res. 2006;28:139.
5 Dujovny M, Agner C, Aviles A. Syndrome of the trephined: theory and facts. Crit Rev Neurosurg. 1999;9:271.
6 Grant FC, Norcross NC. Repair of cranial defects by cranioplasty. Ann Surg. 1939;110:488.
7 Akins PT, Guppy KH. Sinking skin flaps, paradoxical herniation, and external brain tamponade: a review of decompressive craniectomy management. Neurocrit Care. 2008;9:269.
8 Dujovny M, Fernandez P, Alperin N, et al. Post-cranioplasty cerebrospinal fluid hydrodynamic changes: magnetic resonance imaging quantitative analysis. Neurol Res. 1997;19:311.
9 Isago T, Nozaki M, Kikuchi Y, et al. Sinking skin flap syndrome: a case of improved cerebral blood flow after cranioplasty. Ann Plast Surg. 2004;53:288.
10 Auguste KI, McDermott MW. Salvage of infected craniotomy bone flaps with the wash-in, wash-out indwelling antibiotic irrigation system. Technical note and case series of 12 patients. J Neurosurg. 2006;105:640.
11 Bruce JN, Bruce SS. Preservation of bone flaps in patients with postcraniotomy infections. J Neurosurg. 2003;98:1203.
12 Lee C, Antonyshyn OM, Forrest CR. Cranioplasty: indications, technique, and early results of autogenous split skull cranial vault reconstruction. J Craniomaxillofac Surg. 1995;23:133.
13 Manson PN, Crawley WA, Hoopes JE. Frontal cranioplasty: risk factors and choice of cranial vault reconstructive material. Plast Reconstr Surg. 1986;77:888.
14 Rengachary SS, Benzel EC. Calvarial and Dural Reconstruction. Park Ridge, Ill: AANS; 1998.
15 Movassaghi K, Ver Halen J, Ganchi P, et al. Cranioplasty with subcutaneously preserved autologous bone grafts. Plast Reconstr Surg. 2006;117:202.
16 Grossman N, Shemesh-Jan HS, Merkin V, et al. Deep-freeze preservation of cranial bones for future cranioplasty: nine years of experience in Soroka University Medical Center. Cell Tissue Bank. 2007;8:243.
17 Iwama T, Yamada J, Imai S, et al. The use of frozen autogenous bone flaps in delayed cranioplasty revisited. Neurosurgery. 2003;52:591.
18 Prolo DJ, Burres KP, McLaughlin WT, et al. Autogenous skull cranioplasty: fresh and preserved (frozen), with consideration of the cellular response. Neurosurgery. 1979;4:18.
19 Zingale A, Albanese V. Cryopreservation of autogeneous bone flap in cranial surgical practice: what is the future? A grade B and evidence level 4 meta-analytic study. J Neurosurg Sci. 2003;47:137.
20 Grant GA, Jolley M, Ellenbogen RG, et al. Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg. 2004;100:163.
21 Vignes JR, Jeelani NO, Dautheribes M, et al. Cranioplasty for repair of a large bone defect in a growing skull fracture in children. J Craniomaxillofac Surg. 2007;35:185.
22 Jho DH, Neckrysh S, Hardman J, et al. Ethylene oxide gas sterilization: a simple technique for storing explanted skull bone. Technical note. J Neurosurg. 2007;107:440.
23 Tamaki T, Eguchi T, Sakamoto M, et al. Use of diffusion-weighted magnetic resonance imaging in empyema after cranioplasty. Br J Neurosurg. 2004;18:40.
24 Yano H, Tanaka K, Matsuo T, et al. Cranioplasty with auto-purified bone flap after infection. J Craniofac Surg. 2006;17:1076.
25 Lake PA, Morin MA, Pitts FW. Radiolucent prosthesis of mesh-reinforced acrylic. Technical note. J Neurosurg. 1970;32:597.
26 Blum KS, Schneider SJ, Rosenthal AD. Methyl methacrylate cranioplasty in children: long-term results. Pediatr Neurosurg. 1997;26:33.
27 Marchac D, Greensmith A. Long-term experience with methylmethacrylate cranioplasty in craniofacial surgery. J Plast Reconstr Aesthet Surg. 2008;61:744.
28 Nisbet M, Briggs S, Ellis-Pegler R, et al. Propionibacterium acnes: an under-appreciated cause of post-neurosurgical infection. J Antimicrob Chemother. 2007;60:1097.
29 Edwards SA, Gardiner J. Hypersensitivity to benzoyl peroxide in a cemented total knee arthroplasty: cement allergy. J Arthroplasty. 2007;22:1226.
30 Newens AF, Volz RG. Severe hypotension during prosthetic hip surgery with acrylic bone cement. Anesthesiology. 1972;36:298.
31 Kubo S, Takimoto H, Kato A, et al. Endoscopic cranioplasty with calcium phosphate cement for pterional bone defect after frontotemporal craniotomy: technical note. Neurosurgery. 2002;51:1094.
32 Miyake H, Ohta T, Tanaka H. A new technique for cranioplasty with L-shaped titanium plates and combination ceramic implants composed of hydroxyapatite and tricalcium phosphate (Ceratite). Neurosurgery. 2000;46:414.
33 Miller L, Guerra AB, Bidros RS, et al. A comparison of resistance to fracture among four commercially available forms of hydroxyapatite cement. Ann Plast Surg. 2005;55:87.
34 Li G, Wen L, Zhan RY, et al. Cranioplasty for patients developing large cranial defects combined with post-traumatic hydrocephalus after head trauma. Brain Inj. 2008;22:333.
35 Marbacher S, Andres RH, Fathi AR, et al. Primary reconstruction of open depressed skull fractures with titanium mesh. J Craniofac Surg. 2008;19:490.
36 Chim H, Schantz JT. New frontiers in calvarial reconstruction: integrating computer-assisted design and tissue engineering in cranioplasty. Plast Reconstr Surg. 2005;116:1726.
37 Winder J, Cooke RS, Gray J, et al. Medical rapid prototyping and 3D CT in the manufacture of custom made cranial titanium plates. J Med Eng Technol. 1999;23:26.
38 Eufinger H, Wehmoller M. Individual prefabricated titanium implants in reconstructive craniofacial surgery: clinical and technical aspects of the first 22 cases. Plast Reconstr Surg. 1998;102:300.
39 Liu JK, Gottfried ON, Cole CD, et al. Porous polyethylene implant for cranioplasty and skull base reconstruction. Neurosurg Focus. 2004;16:ECP1.
40 Pietrzak WS, Eppley BL. Antibiotic elution from hydroxyapatite cement cranioplasty materials. J Craniofac Surg. 2005;16:228.
41 Gandhi R, Razak F, Pathy R, et al. Antibiotic bone cement and the incidence of deep infection after total knee arthroplasty. J Arthroplasty. 2008 Sep 25. [Epub ahead of print]
42 Parvizi J, Saleh KJ, Ragland PS, et al. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop. 2008;79:335.
43 Itokawa H, Hiraide T, Moriya M, et al. A 12 month in vivo study on the response of bone to a hydroxyapatite-polymethylmethacrylate cranioplasty composite. Biomaterials. 2007;28:4922.