8 Cranial Nerve VIII
Auditory and Vestibular
Auditory Nerve
Anatomy
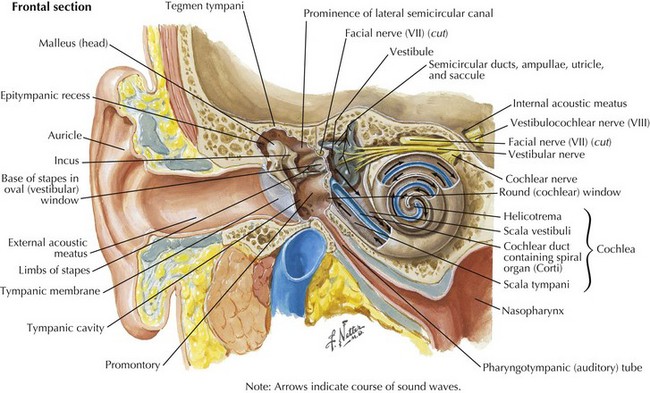
Sound waves travel through the external auditory canal and vibrate the TM, which in turn produces motion of the middle ear ossicles (incus, malleus, and stapes). The vibrations are transmitted through the oval window at the footplate of the stapes, causing a wave to travel through the endolymphatic fluid of the cochlea of the inner ear. The fluid waves vibrate the organ of Corti’s basilar membrane, stimulating inner and outer hair cells (Fig. 8-1). Hair cells, receptors of the sensorineural system, transmit action potentials to bipolar neurons, the bodies of which are in the spiral ganglion.
Afferent fibers projecting toward the CNS comprise the auditory nerve (Fig. 8-2). They travel to the dorsal and ventral cochlear nuclei located in the caudolateral pons. Most of the secondary neurons project contralaterally across the midline to the superior olivary nucleus and then travel up the lateral lemniscus into the inferior colliculus of the midbrain. Decussating fibers from the cochlear nucleus to the superior olivary nucleus are located in the trapezoid bodies and also in the base of the pons. Fibers from the inferior colliculus continue to travel rostrally to the medial geniculate body of the thalamus and then terminate in the auditory cortex located in the transverse temporal gyri of Heschl.
Clinical Presentation
Physical Examination
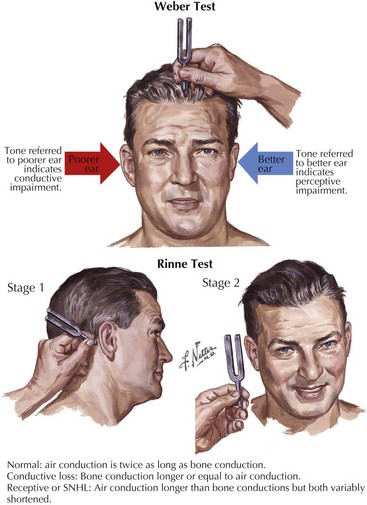
Tuning fork tests assess whether the hearing loss is conductive or sensorineural (Fig. 8-3). During the head and neck examination, a complete cranial nerve examination must also be performed to assess other potential cranial nerve abnormalities. Facial nerve weakness may be attributed to viral infections, such as herpes zoster oticus, or expanding neoplasms in the internal auditory canal or cerebello-pontine angle, such as meningiomas or facial neuromas. Auscultation of the areas around the orbit and ear may detect pulsatile tinnitus. The type of SNHL can assist in the localization of the lesion. Ototoxic drugs, excess noise exposure, and autoimmune diseases affect the hair cells within the cochlea, the primary sensory organ of hearing, and lead to hearing loss usually described as decreased sensitivity to pure tones but preserved speech discrimination. Hearing loss caused by retrocochlear lesions of the nerve fibers of CN-VIII or its central auditory projections begins as decreased speech discrimination with relatively normal pure-tone sensitivity. However, decreased speech discrimination is not exclusive to retrocochlear lesions; it is also observed with extensive hair cell damage.
Differential Diagnosis
Neoplasms
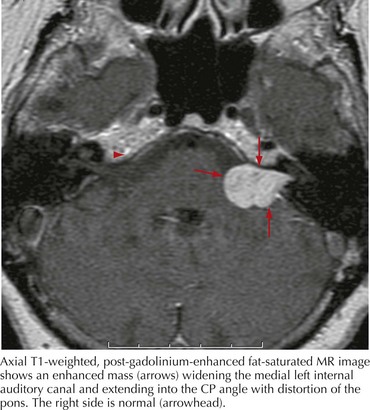
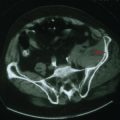
In any case of sudden, unilateral hearing loss, neoplastic lesions, although rare, should be considered in the differential until excluded by diagnostic or radiologic testing. Vestibular schwannomas (also known as acoustic neuromas) are benign tumors arising from the Schwann cells of CN-VIII and account for 6% of all intracranial tumors (Fig. 8-4). These occur on the vestibular portion of CN-VIII and involve the adjacent cochlear division by compression against the bony walls of the internal auditory canal. Less commonly, neuromas can also arise directly from the cochlear nerve.
Vascular Etiologies
Vertebrobasilar stroke is another cause of sudden, unilateral SNHL with potentially devastating effects. Distinguishing whether hearing loss results from microvascular disease or a brainstem infarct is vital. The anterior inferior cerebellar artery supplies blood to the inferolateral portion of the pons, CN-VII, the spinal trigeminal tract, and the inferior cerebellum. A stroke from occlusion of this artery causes an infarct of the ipsilateral pons, creating a myriad of symptoms: ipsilateral hearing loss and vestibular symptoms, gait ataxia, conjugate gaze palsy, ipsilateral facial paralysis and often contralateral loss of pain and temperature sensation in the extremities (see Chapter 55).
Vestibular Nerve
Anatomy
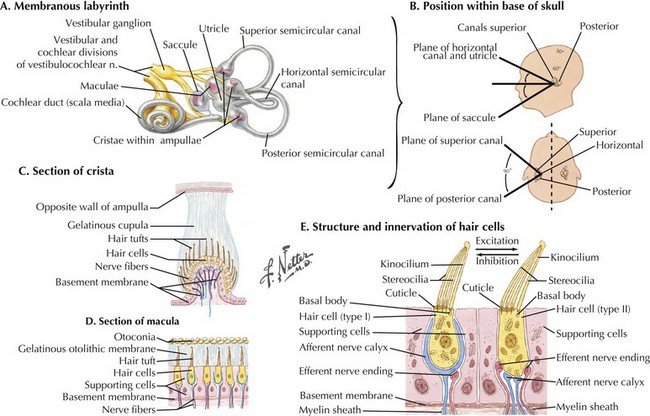
The vestibulocochlear nerve, CN-VIII, is actually composed of two nerves: the vestibular and cochlear nerves. The vestibular nerve is responsible for efferent and afferent fibers that control balance and equilibrium. The cochlear nerve, also called the auditory nerve, carries the efferent and afferent fibers for hearing. The vestibular system provides specific sensory input that influences motor function in reference to postural control (Figs. 8-1 and 8-5); the latter depends on interrelated mechanisms, including perception of position and motion in relation to gravity and orientation of the head and body in relation to the vertical axis during quiet stance. Other vestibular functions include integrating selected postural and orientation sensory cues in various environments; this aids in controlling the center of gravity when the body is static or moving and stabilizes the head during bodily movements. Because the vestibular system primarily provides sensory information about the head on the body, the CNS must rely on other sensory modalities to determine overall body position and movement.
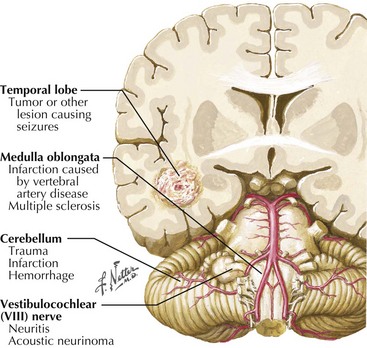
There are numerous central as well as peripheral processes that cause symptoms of vertigo (Fig. 8-6). During the patient’s initial evaluation, it is important to differentiate a CNS lesion from a peripheral localization by determining whether any associated neurologic deficits are present and their exact characteristics.
CNS Disorders
Brainstem dysfunction typically includes prominent dysmetria, diplopia, dysphagia, dysarthria, perioral numbness, or weakness. Twenty-five percent of patients with stroke risk factors who present to emergency medical settings with isolated vertigo, nystagmus, and postural instability have an infarction within the territory of the posterior inferior cerebellar artery (PICA). The acute postural instability with a PICA infarction is usually so severe that independent ambulation is not possible. Other than difficulty walking, there may be no cerebellar or central findings with a posterior inferior cerebellar artery infarction. This diagnosis is particularly important because acute postinfarction swelling or hemorrhage within the cerebellar hemisphere may cause brainstem compression and death (see Chapter 55).
Types of Vertigo and Disorders
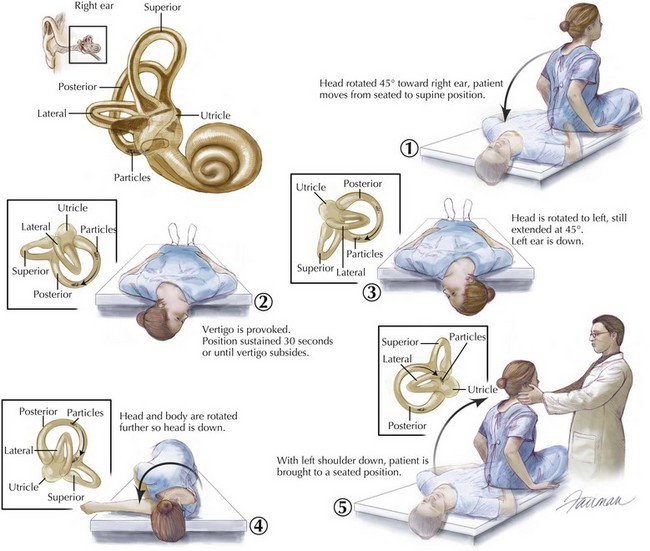
The diagnosis of BPPV in large part depends on the clinical history and bedside testing. The Dix-Hallpike maneuver, when performed and interpreted properly is diagnostic. Studies suggest that the Dix-Hallpike maneuver has a sensitivity and specificity of approximately 75%. In our experience the ability to perform the maneuver properly in anxious patients fearful of provocative maneuvers is a major limiting factor for accurate evaluation. Risk factors for BPPV include recent head trauma (which can be relatively minor); otologic surgery or disease; habitual unusual positioning such as is a daily occurrence for plumbers, mechanics, and yoga enthusiasts; or advanced age. Particle repositioning maneuvers or canalith repositioning maneuver is the main treatment for benign paroxysmal positional vertigo (Fig. 8-7). Another maneuver known as the liberatory maneuver, developed by Semont et al., relies on rapidly swinging the patient from lying initially on the involved side through 180 degrees to the opposite, uninvolved side. Unfortunately any repositioning maneuver can be limited by the inability of the patient to physically participate (musculoskeletal and orthopedic limitations, especially of the head and neck) or when induced symptoms are intolerable. In 5% of patients, symptoms may worsen with repositioning maneuvers in part due to conversion from posterior to horizontal canal involvement. Outcome studies regarding the effectiveness of the canalith repositioning maneuver provide a range of reported success, as most studies rely on subjective reporting, which is inherently unreliable because patients quickly develop adaptive behavior spontaneously. Overall, however, there is evidence to favor repositioning maneuvers, with some studies demonstrating resolution of symptoms in 90% of patients after only one treatment. Self-administered maneuvers in combination with guided treatments can often help expedite improvement in the remaining patients. Successful treatment with the repositioning maneuvers does not influence recurrence rate, which averages around 20% over a 20-month period. Persistent vertigo or frequent recurrences of BPPV is uncommon, but under such circumstances surgical occlusion of the posterior semicircular canal with bone grafts and fibrin glue is an effective treatment. Medications can provide temporary relief by controlling nausea and suppressing the vestibular responses. Meclizine and benzodiazepines are the most frequently prescribed medications but can be sedating and should be used only for a few days.
Duck SW, Prazma J, Bennett PS, et al. Interaction between hypertension and diabetes mellitus in the pathogenesis of sensorineural hearing loss. Laryngoscope. 1997;107:1596-1605.
Ho SY, Kveton JF. Acoustic neuroma: assessment and management. Otolaryngol Clin N Am. 2002;35:393-404.
Rudick RA. Multiple sclerosis and demyelinating conditions of the central nervous system. In: Goldman L, Ausiello D, editors. Cecil Textbook of Medicine. 22nd ed. Philadelphia, Pa: WB Saunders Co; 2004:2320-2327.
Schmidt RJ, Sataloff RT, Newman J, et al. The sensitivity of auditory brainstem response testing for the diagnosis of acoustic neuromas. Arch Otolaryngol Head Neck Surg. 2001;127:19-22.
Sismanis A. Pulsatile tinnitus. Otolaryngol Clin N Am. 2003;36:389-402.
Furman J, Cass S. Benign paroxysmal positional vertigo. N Engl J Med. 1999;341:1590-1596.
Furman J, Whitney S. Central causes of dizziness. Phys Ther. 2000;80:179-187.
Helminski JO, Zee DS, Janssen I, Hain TC. Effectiveness of particle repositioning maneuvers in the treatment of benign paroxysmal positional vertigo: a systematic review. Phys Ther. 2010;90:663-678.
Herdman S. Advances in the treatment of vestibular disorders. Phys Ther. 1997;77:602-618.
Horak FB, Jones-Rycewiccz C, Black FO, et al. Effects of vestibular rehabilitation on dizziness and imbalance. Otolaryngol Head Neck Surg. 1992;106:175-180.
Hotson J, Baloh R. Acute vestibular syndrome. N Engl J Med. 1998;339:680-685.
Norrving B, Magnusson M, Holtas S. Isolated acute vertigo in the elderly: vestibular or vascular disease? Acta Neurol Scand. 1995;91:43-48.
Podsialdo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142-148.
Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up and go test. Phys Ther. 2000;80:896-903.
Strupp M, Zingler VC, Arbusow V, et al. Methylprednisolone, valacyclovir, or the combination for vestibular neuritis. N Engl J Med. 2004;351:354-361.
Vassiliou A, Vlastarakos PV, Maragoudakis P, et al. Meniere’s disease: still a mystery disease with difficult differential diagnosis. Ann Indian Acad Neurol. 2011;14:12-18.
Zhou G, Gopen Q, Poe DS. Clinical and diagnostic characterization of canal dehiscence syndrome: a great otologic mimicker. Otol Neurotol. 2007;28:920-926.