Chapter 2 Cosmeceuticals: Function and the Skin Barrier
THE STRATUM CORNEUM STRUCTURE AND FUNCTION
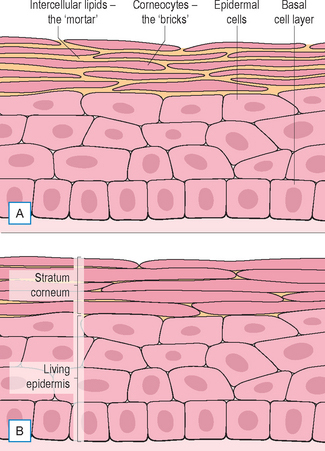
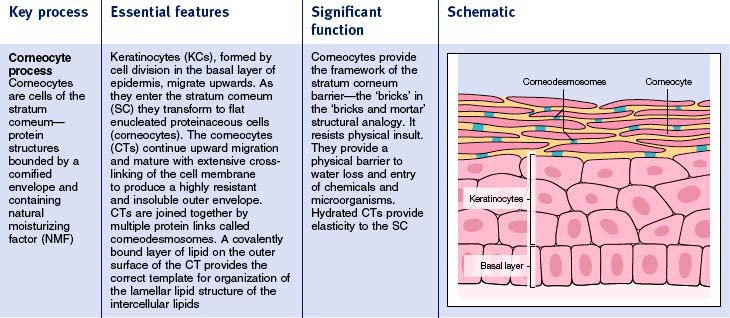
The stratum corneum is an essentially impermeable structure with layers of pancake-like cells (corneocytes) embedded in a matrix of lamella lipids (Fig. 2.1). This complex structure is continuously renewed by transformation of cells (keratinocytes) migrating up from the living epidermis. There are four key processes of stratum corneum function (Fig. 2.2). The main features are summarized in Table 2.1. When all four processes are functioning optimally the stratum corneum is an excellent moisture barrier (without which all mammals would quickly dehydrate and perish) and a very effective barrier to micro-organisms and chemicals.
THE STRATUM CORNEUM BARRIER AND THE ENVIRONMENT
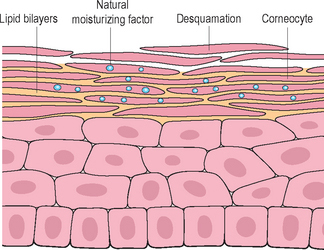
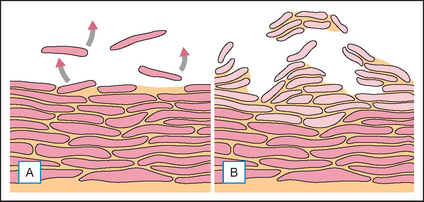
Although the stratum corneum is an excellent and resilient barrier, the superficial layers are readily disturbed by low humidity, wind, sun, detergents, solvents, and other chemicals. The result is dry skin. What we experience as dry skin is not simply skin that lacks water but dysfunctional skin where aberrant desquamation leads to an accumulation of corneocytes at the skin surface (Fig. 2.3). The skin feels rough; looks dull because light is scattered by the uneven surface; looks pale because the pinky glow from the microcirculation is obscured; may show visible scaling, and is susceptible to irritation. All this is a consequence of dehydration at the skin surface.
• The stratum corneum and moisturization
Simple moisturizers are not normally classed as cosmeceuticals but in practice they have much greater benefit for skin than many cosmetic ingredients described as cosmeceutical actives. Moisturization is not the most dramatic or exciting effect of skincare products but it is a benefit delivered with great certainty and to a maximum extent. An effective moisturizer contains a good humectant, such as glycerol, to hold water in the stratum corneum, and lipid emollients that seal in moisture and prevent the washout of humectant when the skin is next in contact with water. Moisturizers reverse the negative effects of skin dryness leaving skin soft and smooth with a natural looking healthy glow. Moisturizers also restore the elasticity of the stratum corneum, making skin feel firmer and more vibrant. Skin looks better, looks healthier, and looks rejuvenated. All this from using a good moisturizer!
• The stratum corneum and cosmeceuticals
Moisturizers exert their effects in the upper regions of the stratum corneum whereas cosmeceuticals are supposed to penetrate the stratum corneum barrier to exert their effects more deeply although still at a level where they are properly regarded as cosmetic rather than drugs. To penetrate the barrier and reach a skin target in sufficient concentration to be effective is not easy. Strategies to enhance penetration are discussed later but there is an increasing recognition that there are benefits for overall skin condition by maintaining barrier integrity. Moisturizers help skin restore and maintain barrier function by activating skin processes that are switched off in dry skin, but is it possible to directly enhance barrier integrity?
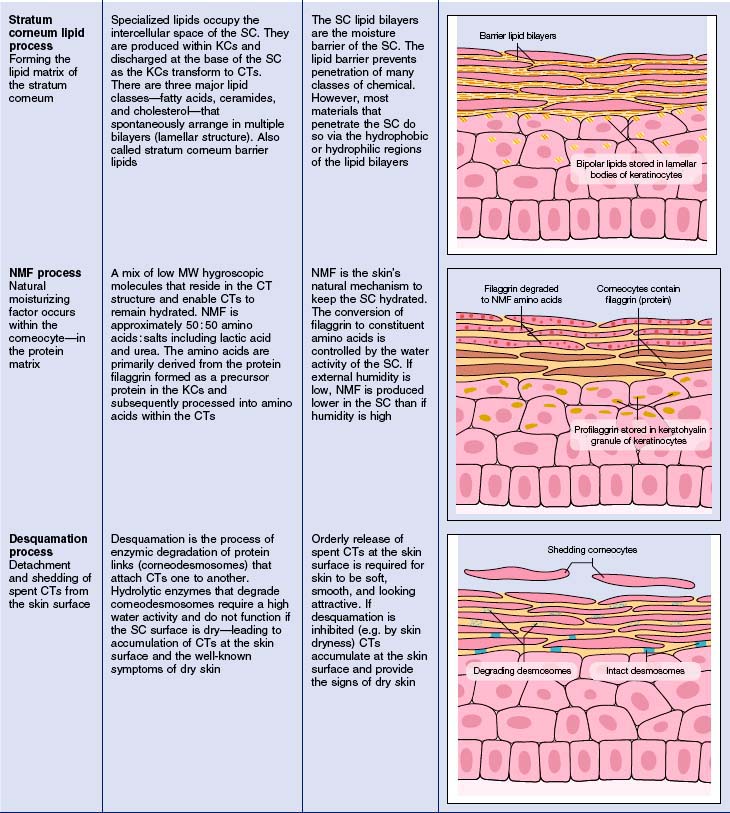
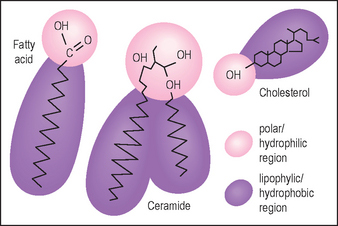
• The stratum corneum intercellular lipids and ceramides
Lotions containing the three classes of stratum corneum lipids (Fig. 2.4) are claimed to restore normal barrier function, but most are no more effective than products containing the same amount of any emollient lipids. To restore the barrier requires the three lipid types, the correct subspecies of ceramide (there are nine subclasses), the right molar ratio (1 : 1 : 1) delivered into the skin, and an effective amount (well above the low levels present in some cosmetic products). Partial mixtures, cholesterol and fatty acids without ceramides, may disrupt stratum corneum lipid structures rather than improve them.
NATURAL REJUVENATION OF SKIN
Skin has remarkable ability to repair and compensate for environment insults. The healing of wounds is an example. Many of the skin rejuvenation techniques used in cosmetic dermatology, such as chemical peels, ablative laser therapies, and dermabrasion, involve controlled injury of ‘bad skin’ with the expectation that wound healing processes will replace the damaged skin with new skin that looks and functions better. The biologic mechanisms involved are simple in concept although complex in detail. Injury to skin kills some cells and damages others. Many chemical messengers (cytokines and other mediators) are released and activate/recruit wound-healing cells (immune, inflammatory, hematopoietic) to the damaged area. Scavenger cells remove the debris and tissue remodeling cells rebuild the structure.
THE BARRIER IS A CHALLENGE FOR COSMECEUTICALS
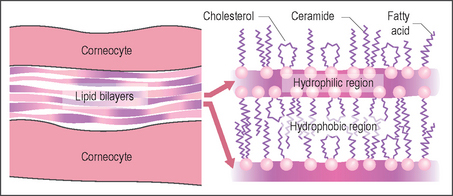
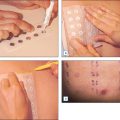
A route through the corneocytes at their points of intercellular connections (the desmosomes) is the most direct way across the stratum corneum barrier but the corneocyte outer membrane, the cornified envelope, is so extensively cross-linked and insoluble that very few substances are able to penetrate. The main route of penetration of the stratum corneum is a tortuous path through the multiple bilayer lipid matrix between the corneocytes (Fig. 2.5). The lipid bilayers create a formidable barrier to water, microorganisms, and many chemical types.
There has been extensive research to find methods to enhance delivery of actives through the stratum corneum. The main methods are summarized in Table 2.2. None of these methods is uniformly effective. Chemical penetration enhancers function by temporarily disrupting the lipid bilayer structures of the stratum corneum to allow easier passage of active molecules. The more effective chemical enhancers tend to cause irritation and some are toxic—these are not used in cosmeceuticals. SCOPE is a high-throughput screening system to identify Synergistic Combinations of Penetration Enhancers.
| Penetration enhancer | Effectiveness comment |
| Chemicals | Variable effect; often irritating |
| Liposomes | Overclaimed (see text); mostly ineffective |
| Occlusive patches | Effective; practical limitations |
| Iontophoresis | Effective; requires charged molecules |
| Sonophoresis | Limited evidence; safety to consider |
| Thermal poration | Limited evidence; safety limitations |
| Massage | Limited effect; transfollicular |
| Microneedle arrays | Effective; invasive |
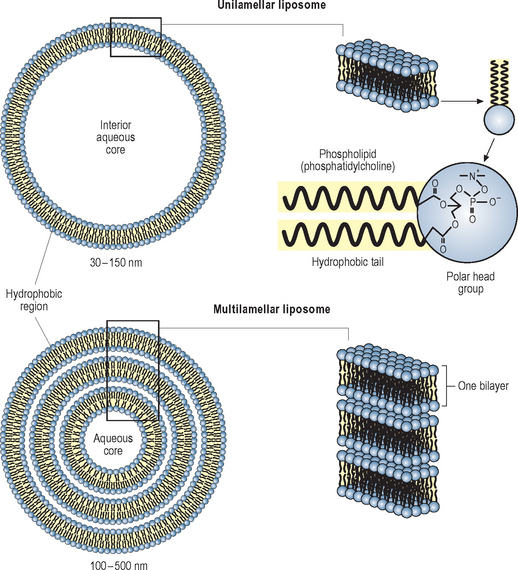
Liposomes are widely used in cosmeceuticals. They are spherical vesicles bounded by a bilayer membrane formed from phospholipid or other polar lipids. This may be a single bilayer (unilamellar) or many layers (multilamellar). The multilamellar membranes are similar in structure to the stratum corneum lipid bilayers but generally less complex (Fig. 2.6). They are claimed to enhance penetration by carrying their hydrophilic contents through the lipid barrier layers as intact vesicles but their effectiveness as penetration enhancers is controversial. It seems likely that most effects of liposomes are due to disruption of the skin lipid bilayers and not to penetration of intact vesicles.
While the focus of this chapter is the stratum corneum barrier, there is increasing interest in transfollicular delivery. Because hair follicles occupy less than 0.1% of the skin surface they have been discounted as a significant route for delivery. This may be true for cosmetically uninteresting sites like the forearm but for sites like the forehead the transfollicular route may be important. Transfollicular penetration depends on the state of the hair follicle which can be open or closed (closed pores are opened by massage).
NANOPARTICLES
Nanoparticles are microscopic particles with one dimension less than 100 nm (Table 2.3). Nanoparticles are not new but the possibility to use submicron size as a strategy to achieve hitherto difficult technical goals has spawned nanotechnology as a new field in medicine and cosmetology. It is no panacea. There are concerns that small particles entering the body may be a hazard to health. Nanoparticles are being promoted for cosmetic applications but optimism that they will overcome the skin barrier to penetration may turn out to be premature. Submicron liposomes penetrate human skin deeper and more rapidly than solid particles the same size that barely penetrate, indicating that permeability is determined by chemistry more than size. Nanoparticles greater than 5 nm do not penetrate the lipid layers of the stratum corneum but can enter the openings of the hair follicle.
| Description | Size nm (10−9 m) | Size microns (10−6 m) |
| Angstrom unit | 0.1 | 0.00001 |
| Length of C–C bond | 0.154 | 0.000154 |
| Stratum corneum hydrophilic pathways | 0.4 | 0.0004 |
| Carbon nanotube diameter | 3 | 0.003 |
| Cell membrane | 6–10 | 0.006–0.01 |
| Stratum corneum lipid bilayer | 13 | 0.013 |
| TiO2 in sunscreens | 10–100 | 0.01–0.1 |
| Rhino(cold) virus | 20–30 | 0.02 |
| Stratum corneum intercorneocyte space | 20–75 | 0.025–0.075 |
| ZnO in sunscreens | 30–200 | 0.03–0.2 |
| Flu virus | 80–120 | 0.1 |
| Upper limit nanoparticles | 100 | 0.1 |
| Staphylococcus | 700 | 0.7 |
| Escherichia coli | 2000 | 2 |
| Human sperm head | 5000 | 5 |
| Red blood cell | 8000 | 8 |
| Stratum corneum thickness | 10–40 000 | 10–40 |
| Human hair diameter | 80 000 | 80 |
| Head of pin | 2 000 000 | 2000 |
FUTURE PROSPECTS
Although we can expect incremental progress as cosmetic scientists seek ways to increase penetration of cosmeceuticals, it looks likely that the skin barrier will maintain the upper hand. Our safety and well-being are secure!! Perhaps there is a need for a new strategy for skin care. Instead of seeking to propel putative actives to the deeper layers of skin to fix damage, maybe we should concentrate on depositing ingredients in the superficial regions of skin to prevent damage in the first place by protecting from the environmental factors that drive poor skin condition and premature skin aging.
Barry BW. Breaching the skin’s barrier to drugs. Nature Biotechnology. 2004;22:165–167.
Chan CK 2005 Percutaneous penetration enhancers: an update. Proceedings of the 9th Biennial International Conference of Perspectives in Percutaneous Penetration, La Grand Motte, France, April 13, 2004
Chandrasekhar NS, Rani S. Current status and future prospects in transdermal drug delivery. Available http://www.centerwatch.com/pharminfo.net, 2006.
Coderch L, De Pera M, Fonollosa J, De La Maza A, Parra J. Efficacy of stratum corneum lipid supplementation on human skin. Contact Dermatitis. 2002;47:139–146.
Current Stratum Corneum Research. Optimizing barrier function through fundamental skin care. Dermatological Therapy. 2004;17:1-68. [A full issue of the journal (9 papers) dedicated to the biology of the stratum corneum barrier and the impact of cleansing and moisturizing products]
Elias PM, Tsai J, Menon GK, Holleran WM, Feingold KR. The potential of metabolic interventions to enhance transdermal drug delivery. Journal of Investigative Dermatology Symposium Proceedings. 2002;7:79–85.
Forster T, editor. Cosmetic lipids and the skin barrier. New York: Marcel Dekker, 2002.
Gooris GS, Bouwstra JA. Infrared spectroscopic study of stratum corneum model membranes prepared from human ceramides, cholesterol and fatty acids. Biophysical Journal. 2007;92:2785–2795.
Kandlikar M, Ramachandran G, Maynard A, Murdock B. Health risk assessment for nanoparticles: a case for using expert judgment. Journal of Nanoparticle Research. 2007;9:137–156.
Kanikkannan N, Kandimalla K, Lamba SS, Singh M. Structure–activity relationship of chemical penetration enhancers in transdermal drug delivery. Current Medicinal Chemistry. 2000;7:593–608.
Karande P, Jain A, Ergun K, et al. Design principles of chemical penetration enhancers for transdermal drug delivery. Proceedings of the National Academy of Sciences. 2005;102:4688–4693.
Karande P, Jain A, Mitragotri S. Discovery of transdermal penetration enhancers by high-throughput screening. Nature Biotechnology. 2004;22:192–197.
Lademann J, Richter H, Teichmann A, et al. Nanoparticles: an efficient carrier for drug delivery into the hair follicles. European Journal of Pharmaceuticals and Biopharmaceutics. 2007;66:159–164.
Leyden JJ, Rawlings AV, editors. Skin moisturization. New York: Marcel Dekker, 2002.
McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles for gene and drug delivery. Annual Review of Biomedical Engineering. 2000;2:289–313.
Morgan CJ, Renwick AG, Friedmann PS. The role of stratum corneum and dermal microvascular perfusion in penetration and tissue levels of water-soluble drugs investigated by microdialysis. British Journal of Dermatology. 2003;148:434-443.
Tamarkin D. Using iontophoresis to enhance cosmetics delivery. Cosmetics and Toiletries. 2004;119:63–74.
Teichmann A, Ossadnik M, Richter H, Sterry W, Lademann J. Semiquantitative determination of the penetration of a fluorescent hydrogel formulation into the hair follicle with and without follicular closure by microparticles by means of differential stripping. Skin Pharmacology and Physiology. 2006;19:101–105.
Wiechers JW, Kelly CL, Blease TG, Dederen JC. Formulating for efficacy. International Journal of Cosmetic Science. 2004;26:173–182.