Chapter 13 Cosmeceutical Metals
INTRODUCTION
Are certain topically applied metal ions simply innocuous treatments or do they provide real technical benefit? Their use goes back to the earliest recorded medical text (∼1500 BC), the Ebers papyrus of ancient Egypt. For example, calamine (a natural material containing zinc oxide) was described for treating many skin and eye ailments; green copper-based minerals (likely malachite) were used for burn wounds and itching. Many of these applications have withstood the ensuing 3500 years of history, providing a first clue of real technical merit. For example, zinc is still the first choice to soothe a crying baby’s bottom.
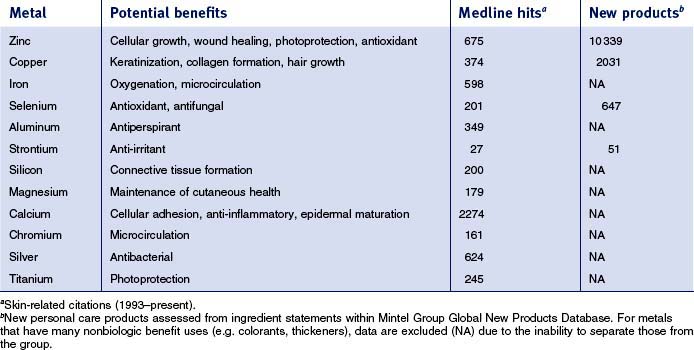
This review will focus specifically on five metals—zinc, copper, selenium, aluminum, and strontium—which are currently used in cosmeceuticals. Each metal will be covered sequentially, reviewing commonly used materials followed by clinical and scientific data supporting their use. There are many other metals that have found usage in cosmeceuticals (see Table 13.1). Searching the skin-related literature (Medline, 1993–present) demonstrates that there is substantial scientific activity exploring the technical basis for utilization of some of these metals. Also summarized in Table 13.1 for some of the metals is the introduction of new personal care products containing them; substantial commercial activity is evident.
ZINC IN COSMECEUTICAL PRODUCTS
• Materials
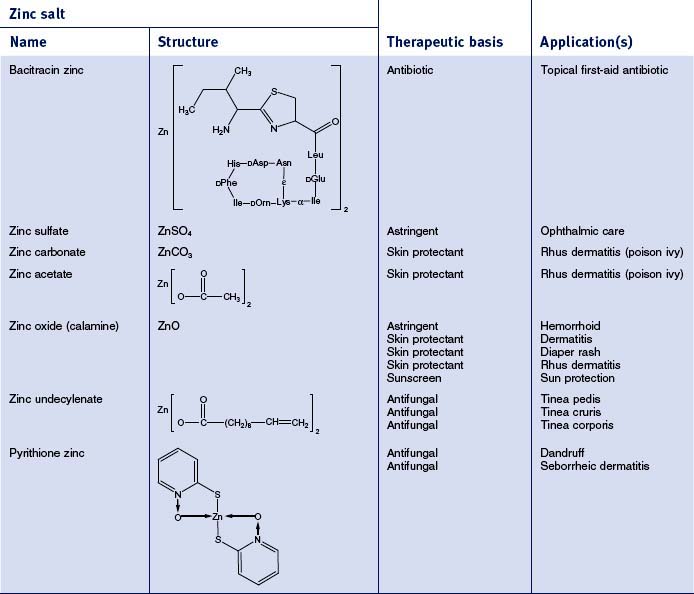
There are 55 different zinc-containing materials listed in the International Cosmetic Ingredient (INCI) Dictionary and Handbook (a tabulation of all materials used in cosmetic and personal care products). Of those, seven have been approved by the US Food and Drug Administration (FDA) for over-the-counter (OTC) usage as safe and effective for a range of benefits, including skin protection, antimicrobial activity, and astringency (Table 13.2). The skin protective benefits of these zinc materials find applications treating various inflammatory dermatitis conditions such as poison ivy and diaper rash. The wide range of zinc materials approved by the FDA provides a strong indication of the general utility of zinc as an effective treatment.
• Basis for use of zinc materials
CLINICAL PERSPECTIVE
Zinc has also recently been shown to improve skin elasticity, reducing the signs of aging skin.
SCIENTIFIC FOUNDATION
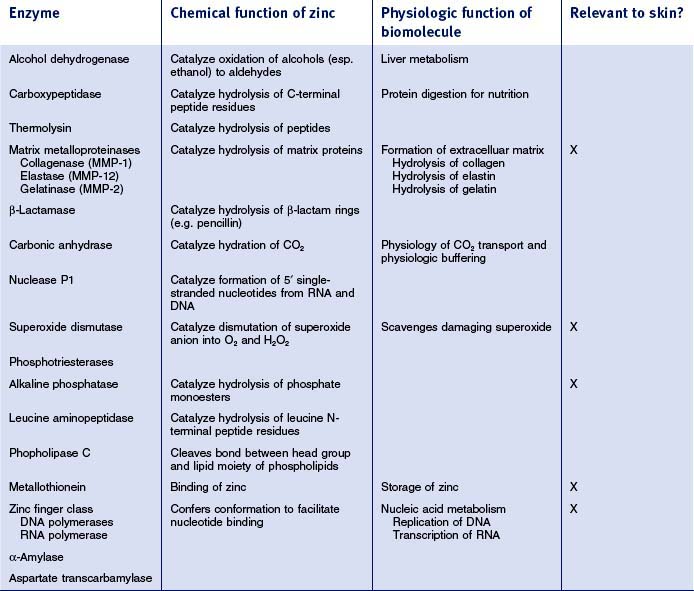
An average human contains 2.5 g of zinc and requires 15 mg/day to remain healthy (this is exceeded only by iron for trace elements). The vast majority of the zinc is present in metalloenzymes and proteins. This field was opened in 1940 with the discovery that carbonic anhydrase, a ubiquitous enzyme required for maintaining physiologic pH, was zinc-containing and that the zinc was required for catalytic activity. Since that time, over 300 enzymes requiring zinc for activity have been structurally characterized. Even more impressive are the thousands of zinc-containing proteins that require zinc for conferring a three-dimensional structure that allows them to regulate replication of DNA and transcription of RNA. These proteins form the class called ‘zinc fingers’ and regulate the fundamental biologic process of transcribing genetic information to functional proteins. At least 3% of all proteins encoded for by the human genome have zinc fingers; this has led Berg to coin the phrase ‘galvanization of biology’ to acknowledge the importance of this metal in human physiology.
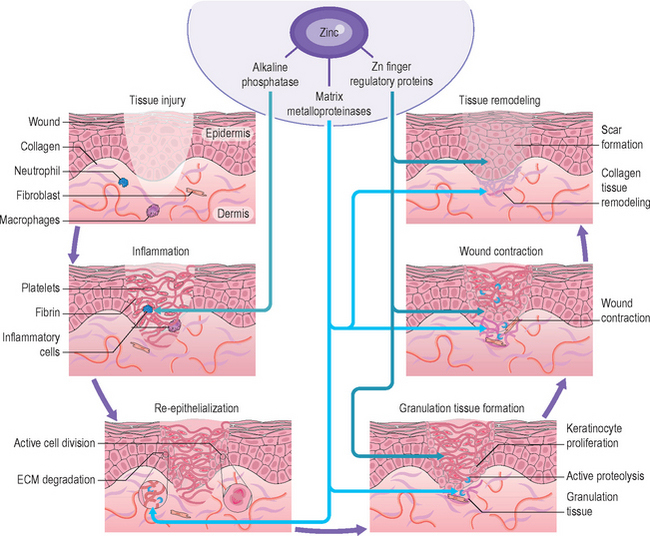
While it is beyond the scope here to review many of the zinc-containing biomolecules (see Table 13.3 for an overview of important examples), a few will be highlighted that have specific relevance to cutaneous biology and supporting the clinical observations reviewed above. Matrix metalloproteinases (MMPs) are zinc-dependent proteases capable of degrading many molecules important in wound healing, including signaling factors as well as the structural proteins of the extracellular matrix (including collagen and elastin). Wound healing requires intensive cell division and protein synthesis, thus the zinc finger proteins DNA and RNA polymerases are critical throughout this process. The anti-inflammatory role observed clinically for zinc, relevant both in wound healing as well as in other dermatitis conditions, may lie partially in the importance of alkaline phosphatase (AP). AP requires multiple zinc ions and is involved in adenosine monophosphate metabolism, which has a role in restraining an inflammatory response. The breadth of the impacts of these zinc biomolecules on the wound healing process is represented schematically in Figure 13.1.
The observed antioxidant activity of zinc may have its roots in multiple effects:
 Zinc is a component of both superoxide dismutase and metallothionein, both of which have strong antioxidant activity.
Zinc is a component of both superoxide dismutase and metallothionein, both of which have strong antioxidant activity.
 Zinc may also displace more harmful metal ions (such as copper and iron) which cause oxygen-based free radical formation due to their oxidation–reduction activity.
Zinc may also displace more harmful metal ions (such as copper and iron) which cause oxygen-based free radical formation due to their oxidation–reduction activity.
 Zinc does not have this capacity to generate radicals since it does not undergo redox activity.
Zinc does not have this capacity to generate radicals since it does not undergo redox activity.
COPPER IN COSMECEUTICAL PRODUCTS
• Materials
The number of copper compounds commonly used in personal care products is far less than for zinc. There are 19 copper materials listed within the INCI, none of which is accepted by the FDA as safe and effective for OTC topical drugs. As with zinc, the normal form is ionic Cu2+, with various counterions that can impact solubility. The nature of the counterion and the resultant impact on bioavailability is not well known and requires substantial expertise in product pharmacology to achieve the desired benefit. Copper also has a very strong affinity for other product matrix ingredients that must be carefully monitored to minimize negative effects either to the matrix or to the copper itself. As mentioned above, copper also has the potential for oxidation–reduction activity, which can enhance reactive oxygen species formation.
• Basis for use of copper materials
SCIENTIFIC FOUNDATION
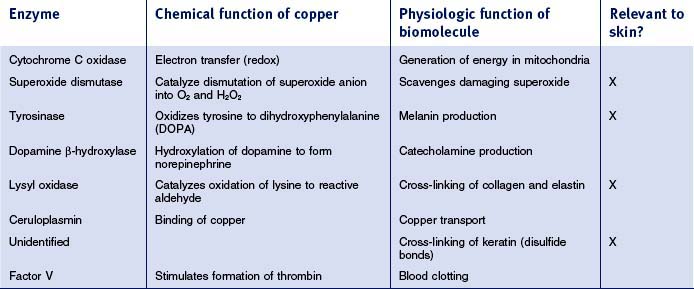
Copper is also ubiquitous in all cells, but the overall amount in the body, 0.1 g, is far lower than that of zinc. As with other metals, copper is primarily bound to enzymes; over 100 have been structurally characterized and major ones are summarized in Table 13.4. Melanins are responsible for skin pigmentation, providing a natural photoprotective effect. Synthesis of melanin requires the copper-based enzyme tyrosinase. The role that copper plays in damage repair is probably based on several copper-containing enzymes: lysyl oxidase cross-links molecules of tropocollagen to form collagen and an as yet unidentified enzyme is involved in cross-linking proteins by disulfide bond formation (this one is probably specifically important in the kinky hair formed as a symptom of Menkes syndrome). Antioxidant activity is most likely also beneficial for damage repair and can be traced to the copper enzymes superoxide dismutase (which has both copper and zinc ions) and ceruloplasmin.
SELENIUM IN COSMECEUTICAL PRODUCTS
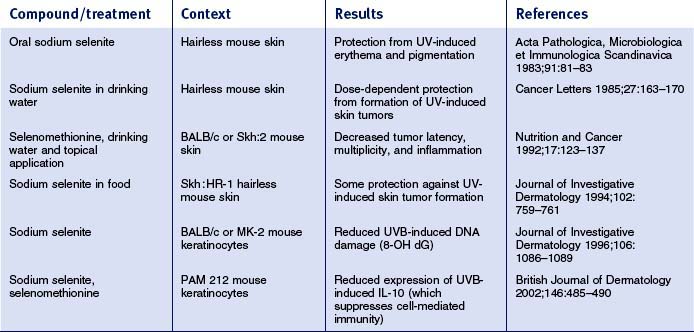
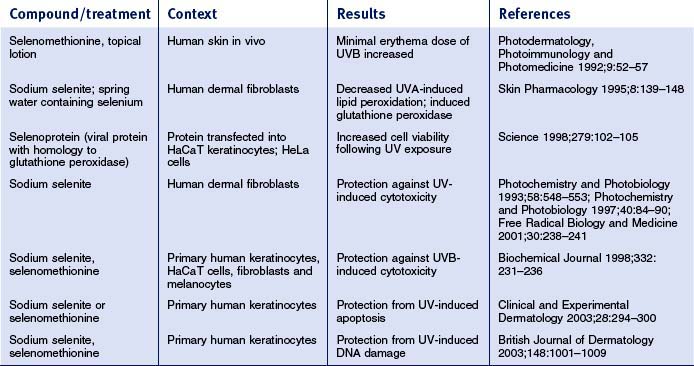
Overall, relatively little is known about how selenium affects skin physiology. The tables below review available published literature related to selenium effects on skin and skin-derived cells. The studies have been separated into work done in mouse skin (Table 13.5) and in human skin/skin-derived cells (Table 13.6). In mouse skin, selenium compounds can protect against UV-induced skin tumors, erythema, pigmentation, DNA damage, and changes in cell-mediated immunity. In human skin/skin-derived cells, selenium compounds have been shown to alter the UVB minimal erythema dose (MED), induce glutathione peroxidase, and decrease UV-induced lipid peroxidation, DNA damage and apoptosis. Some have speculated that all of these effects can be traced to the direct and indirect antioxidant effects of selenium. However, studies in human mammary cells have shown that selenium compounds (e.g. methylselinic acid; MSA) can elicit gene expression profiles that involve alterations in expression of genes involved in cell cycle regulation and apoptosis. In prostate cancer cells, MSA was also shown to modulate the expression of a number of cell cycle regulatory genes, as well as genes involved in inhibition of cell invasion, DNA repair, and stimulation of transforming growth factor-beta signaling. Moreover, work in human lung cancer cell lines has shown that MSA can inhibit cell growth, arrest cell cycle progression at G1, and induce apoptosis. Interestingly, dietary supplementation with selenized yeast is capable of reducing the overall human cancer morbidity rate by nearly 50%. These and other data prompted the National Cancer Institute to launch the Selenium and Vitamin E Chemoprevention Trial (SELECT) in 2001, a 12-year trial to assess the effect of selenium and vitamin E, individually and in combination, on the incidence of prostate cancer. Thus, there is an emerging appreciation that selenium can influence not only redox status, but also cell cycle control, apoptosis, and differentiation in a variety of epithelia, which has implications for the skin.
While the potential of selenium-based pharmacologic agents for the treatment of skin disease has not been adequately explored, published data from studies in skin and other epithelia provide an intriguing basis for further study. Little is known about the penetration and metabolism of topically administered selenocompounds, or their possible effects on gene expression in the skin. However, if selenium compounds could be adequately delivered to and metabolized by the skin, their pleiotropic effects could prove useful in the treatment of skin conditions that are characterized by altered cell proliferation, differentiation, and inflammation. Synthetic selenocompounds could also be considered as possible topical selenium supplements.
Ågren MS. Influence of two vehicles for zinc oxide on zinc absorption through intact skin and wounds. Acta Dermato-venereologica. 1991;71:153–156.
Albergoni V. Physiological properties of copper and zinc. In: Rainsford K, Milanino R, editors. Copper and zinc in inflammatory and degenerative diseases. New York: Kluwer; 1998:7–17.
Alonis M, Pinnell S, Self WT. Bioavailability of selenium from the selenotrisulphide derivative of lipoic acid. Photodermatology, Photoimmunology and Photomedicine. 2006;22:315–323.
Baumann L, Weinkle S. Improving elasticity: the science of aging skin. Cosmetic Dermatology. 2007;20:168–172.
Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271:1081–1085.
Clark LC, Combs GFJr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. Journal of the American Medical Association. 1996;276:1957–1963.
Danks D. Copper deficiency and the skin. In: Goldsmith L, editor. Physiology, biochemistry, and molecular biology of the skin. 2nd edn. New York: Oxford University Press; 1991:1351–1361.
Dong Y, Ganther HE, Stewart C, Ip C. Identification of molecular targets associated with selenium-induced growth inhibition in human breast cells using cDNA microarrays. Cancer Research. 2002;62:708–714.
Dong Y, Zhang H, Hawthorn L, Ganther HE, Ip C. Delineation of the molecular basis for selenium-induced growth arrest in human prostate cancer cells by oligonucleotide array. Cancer Research. 2003;63:52–59.
Frydrych A, Arct J, Kasiura K. Zinc: a critical important element in cosmetology. Journal of Applied Cosmetology. 2004;22:1–13.
Hostýnek J, Maibach H. Copper and the skin. New York: Informa Healthcare, 2006.
Idson B. Trace minerals in cosmetics, Part 1. Drug and Cosmetic Industry. 1990;146:18–20.
Idson B. Trace minerals in cosmetics, Part 2: Physiology and potential uses. Drug and Cosmetic Industry. 1990;146:37-38. 88
Ip C, Hayes C, Budnick RM, Ganther HE. Chemical form of selenium, critical metabolites, and cancer prevention. Cancer Research. 1991;51:595–600.
Ip C, Dong Y, Ganther HE. New concepts in selenium chemoprevention. Cancer Metastasis Reviews. 2002;21:281–289.
Klein EA. Selenium: epidemiology and basic science. Journal of Urology. 2004;171(2 Pt 2):S50-53. discussion S53
Kohrl J, Brigelius-Flohe R, Bock A, et al. Selenium in biology: facts and medical perspectives. Biological Chemistry. 2000;381:849–864.
Laden K, Felger C. Antiperspirants and deodorants. New York: Marcel Dekker, 1988.
Lansdown ABG, Mirastschijski U, Stubbs N, et al. Zinc in wound healing: theoretical, experimental and clinical aspects. Wound Repair and Regeneration. 2007;15:2–16.
Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. Journal of the American Academy of Dermatology. 2007;56:116–124.
May SW. Selenium-based pharmacological agents: an update. Expert Opinion on Investigational Drugs. 2002;11:1261–1269.
Mullin CH, Frings G, Abel J, et al. Specific induction of metallothionein in hairless mouse skin by zinc and dexamethasone. Journal of Investigative Dermatology. 1987;89:164–166.
Neldner K. The biochemistry and physiology of zinc metabolism. In: Goldsmith L, editor. Physiology, biochemistry, and molecular biology of the skin. 2nd edn. New York: Oxford University Press; 1991:1328-1350.
Neumann P, Coffindaffer T, Cothran PE, et al. Clinical investigation comparing 1% selenium sulfide and 2% ketoconazole shampoos for dandruff control. Cosmetic Dermatology. 1996;9:20–26.
Nitzan Y, Cohen A. Zinc in skin pathology and care. Journal of Dermatological Treatment. 2006;17:205–210.
Parat MO, Richard MJ, Meplan C, Favier A, Beani JC. Impairment of cultured cell proliferation and metallothionein expression by metal chelator NNN’N’-tetrakis-(2-pyridylmethyl)ethylene diamine. Biological Trace Element Research. 1999;70:51–68.
Pirot F, Millet J, Kalia YN, et al. In vitro study of percutaneous absorption, cutaneous bioavailability and bioequivalence of zinc and copper from five topical formulations. Skin Pharmacology. 1996;9:259–269.
Rittenhouse T. The management of lower-extremity ulcers with zinc-saline wet dressings versus normal saline wet dressings. Advances in Therapy. 1996;13:88–94.
Rostan EF, DeBuys HV, Madey DL, Pinnell SR. Evidence supporting zinc as an important antioxidant for skin. International Journal of Dermatology. 2002;41:606–611.
Sheretz E, Goldsmith L. Nutritional influences on the skin. In: Goldsmith L, editor. Physiology, biochemistry, and molecular biology of the skin. 2nd edn. New York: Oxford University Press; 1991:1315–1328.
Siméon A, Wegrowski Y, Bontemps Y, Maquart FX. Expression of glycosaminoglycans and small proteoglycans in wounds: modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. Journal of Investigative Dermatology. 2000;115:962–968.
Skaare AB, Rolla G, Barkvoll P. The influence of triclosan, zinc or propylene glycol on oral mucosa exposed to sodium lauryl sulphate. European Journal of Oral Sciences. 1997;105:527–533.
Swede H, Dong Y, Reid M, et al. Cell cycle arrest biomarkers in human lung cancer cells after treatment with selenium in culture. Cancer Epidemiology, Biomarkers and Prevention. 2003;12(11 Pt 1):1248–1252.
Tasaki M, Hanada K, Hashimoto I. Analyses of serum copper and zinc levels and copper/zinc ratios in skin diseases. Journal of Dermatology. 1993;20:21–24.
Warren R, Schwartz J, Sanders L, et al. Attenuation of surfactant-induced interleukin 1α expression by zinc pyrithione. Exogenous Dermatology. 2003;2:23–27.
Zhai H, Hannon W, Hahn GS, et al. Strontium nitrate suppresses chemically induced sensory irritation in humans. Contact Dermatitis. 2000;42:98–100.