Chapter 3 Cosmeceutical Formulation Considerations
VEHICLES
• Emulsions
OIL-IN-WATER EMULSIONS
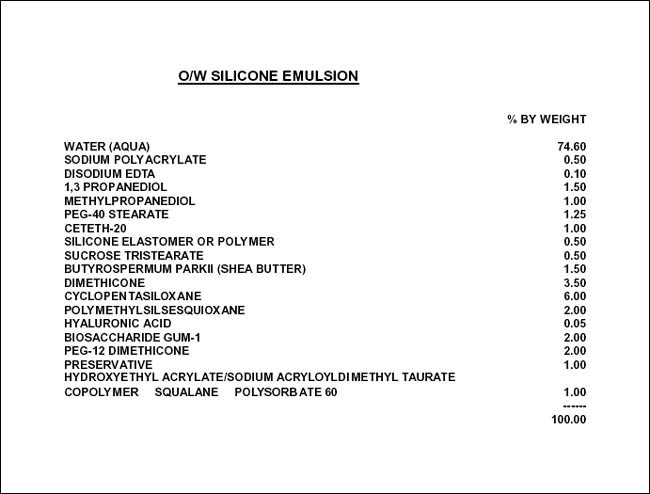
The choice of ingredients now needs to consider the effect on performance as well as aesthetics. Figure 3.1 is an O/W emulsion in which the predominant components of the oil phase are a blend of silicones. This emulsion can be modified with the addition of bioactive ingredients—antioxidants and skin-soothing ingredients. The other aspect of this formulation that is worth noting is the use of an emulsion-stabilizing system that can be added at the end. This ingredient serves a dual function—adjusting viscosity and improving emulsion stability. Care must be taken, however, because these emulsion-stabilizing systems can also affect the feel of the product.
WATER-IN-OIL AND WATER-IN-SILICONE EMULSIONS
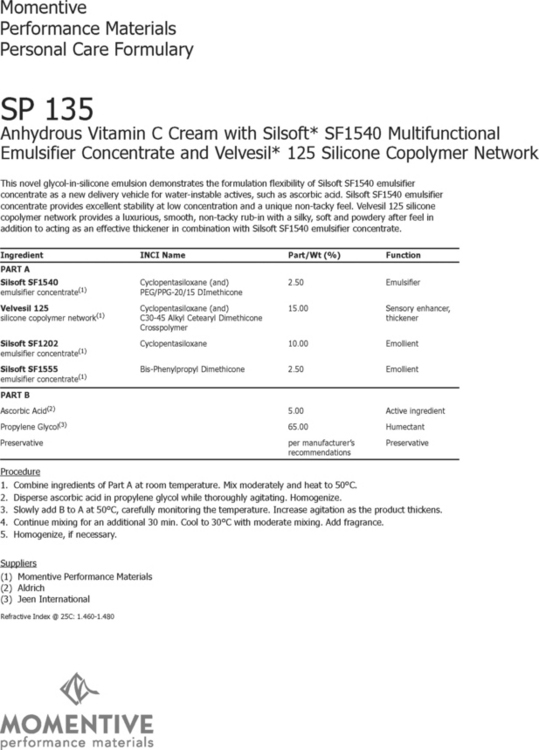
Water-in-oil (W/O) and water-in-silicone (W/S) emulsions, sometimes referred to as ‘inverse emulsions’, are formulations in which the internal or dispersed phase is a water phase, and the oil (or silicone) phase is the continuous or external phase. While still much less popular than O/W emulsions, recent advances in silicone-based emulsifier systems have caused an increase interest in these types of formulation. The formulation from Dow Corning (Fig. 3.2) demonstrates the use of these newer emulsifier systems. They have become increasingly useful when developing water-resistant sunscreen products and when looking to incorporate more lipophilic (oil-loving) functional materials into formulations that deliver skin care benefits such as barrier protection. The formulation of ‘inverse emulsions’ has been extended to where the internal phase is a hydrophilic polyol in place of water. The formulation from Momentive (Fig. 3.3) is an example of an ‘anhydrous’ emulsion which can be used as a delivery system for cosmeceutical ‘actives’ that have stability issues in water (i.e. ascorbic acid—vitamin C).
DELIVERY SYSTEMS
• Polymeric entrapment systems
Frequently referred to as ‘microsponge’ entrapment systems, these polymeric systems can be visualized as a sponge with a very high internal volume that allows for entrapping up to 50% of hydrophilic (water-loving) or lipophilic (oil-loving) materials. The primary release mechanism is diffusion, in which the entrapped material slowly diffuses out from the polymer. This allows for a time release of materials so that the benefits can be extended over a longer period of time, rather than getting a ‘bolus’ effect where the entire ‘active’ is released all at once. Entrapment systems can also extend the stability and protect ‘functional’ materials that have a tendency to degrade, such as retinol and benzoyl peroxide. One of the polymer systems used is allyl methacrylate copolymers. Because of their microsponge nature they can also provide oil-absorbing benefits when applied to the skin.
• Liposomes and other nano delivery systems
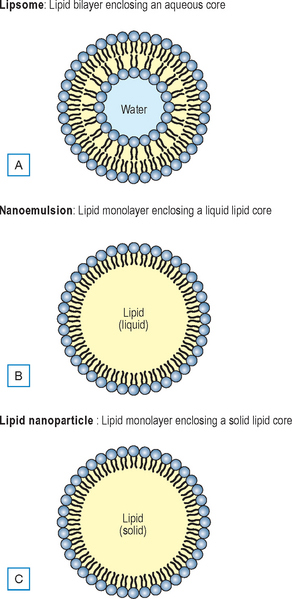
We are all familiar with liposomes. They are microscopic spherical vesicles that are formed when phospholipids are hydrated. Typically, liposomes are 200–800 nm in size. They are designed to entrap and deliver into the skin both hydrophilic and lipophilic ingredients. The core of a liposome contains water-soluble material and the ‘wall’ that makes up the liposome contains oil-soluble ingredients. Nano particles are liposomal structures in which the lipophilic material is entrapped in the core and the hydrophilic material is entrapped in the ‘walls’ (Fig. 3.4). The primary issue with liposomes, much less than with nano particles, is that they are very sensitive vesicles that can be destroyed by a number of factors (e.g. pH, type of emulsifier used, solvents, etc.), but, because their structure is very compatible with the lipid composition of the skin and with cell membranes, they are excellent transdermal delivery systems.
ADDITIONAL FORMULATION CONSIDERATIONS WHEN DEVELOPING COSMECEUTICAL PRODUCTS
• pH
The pH of your formulation can be critical to the functionality of the product. For example, if a formulation is intended to be an exfoliating product that contains either an alpha-hydroxy acid (AHA) or an enzyme, the pH is critical to the performance and the stability. In order for an AHA formulation to be effective and safe the pH should be 3.5–4.5. Above this range will compromise performance and lower than this will compromise safety/irritation. Enzymes should also have a slightly acid to neutral pH in order to maintain their stability. The color and odor of many materials will be affected by the pH environment in which they are used.
• Stability considerations
Stability testing protocols can vary with products stored at various temperatures to determine in as short a time as possible the long-term integrity of the product. It has been suggested that if a product is stored at 45°C for a period of 90 days, and no product degradation is seen, it is likely that the product will exhibit a shelf-life of at least 2 years. In some cases the product is stored for 1 month at 50°C as a more rapid test to predict shelf-life. The problem with this storage temperature is that in many cases materials used in the formulation, including the emulsifying system, can show stability issues simply because of the temperature—50°C stability will give you a ‘yes’ and will not necessarily give you a ‘no’ result. If a product is stable for 1 month at 50°C it is a very good indication that it will have good long-term shelf stability. If the product shows stability issues after 1 month at 50°C it may be because of the temperature and not be indicative of shelf-life.
Some typical storage conditions used in product stability testing are:
 40°C/70% relative humidity: 90 days (drug stability testing conditions)
40°C/70% relative humidity: 90 days (drug stability testing conditions)
 Freeze/thaw (−10°C to 20°C): 3 cycles (24 hours at each temperature)
Freeze/thaw (−10°C to 20°C): 3 cycles (24 hours at each temperature)