Chapter 29 Cosmeceutical Antiaging Myths
The most numerous dermatologic myths relate to cosmeceuticals and their ability to improve the appearance of facial aging. Notice that the word ‘appearance’ is always used when referring to the effects of cosmeceuticals on wrinkling. This is due to the fact that improving appearance insures that claims made regarding the cosmeceutical active are perceived as cosmetic in nature and not pharmaceutical. Claims regarding improving appearance deal with how an active alters looks and not function. Yet, there are some claims and myths that seem to continue due to their consumer and marketing appeal. These cosmeceutical antiaging myths represent the most common patient questions encountered by the practicing dermatologist.
EXPENSIVE MOISTURIZERS ARE MORE EFFECTIVE
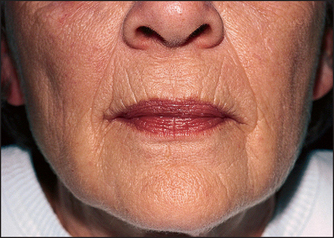
For many consumers, price equates with quality. This may be true for some commercial purchases, but is not necessarily true for moisturizers. The most expensive part of any facial moisturizer is the fragrance, the bottle, and the packaging. None of these contributes to the efficacy of the moisturizer, only the aesthetic appeal. A quality moisturizer should cost less than $30 a bottle and products priced above this amount are selling more than skin efficacy. A quality moisturizer should contain an occlusive agent, a humectant, and some form of silicone. Ideally, it should also contain a sunscreen to provide the added benefit of sun protection. No moisturizer in any price range will improve the wrinkling on the lower face demonstrated in Figure 29.1.
MOISTURIZERS REMOVE WRINKLES
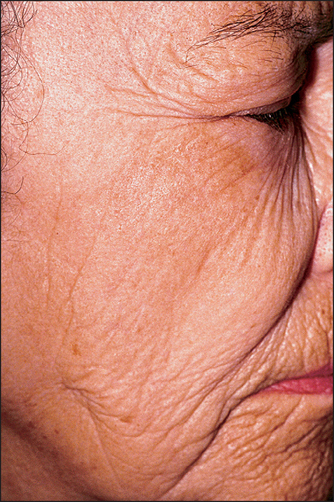
Moisturizers do not remove wrinkles, they simply minimize their appearance. The role of moisturizers in reducing wrinkles is primarily through increased skin hydration. The moisturizer contains an occlusive agent, such as petrolatum, mineral oil, or dimethicone, which prevents transepidermal water loss from the skin surface and increases the water content of the skin. This increased water content decreases or even eliminates wrinkles that are due to stratum corneum barrier defects. Barrier repair will prevent the wrinkles from recurring and the moisturizer creates an environment to initiate barrier repair. Thus, moisturizers do not remove wrinkles (Fig. 29.2), they simply can provide an environment to reverse dehydration due to stratum corneum barrier defects.
BLEACHING CREAMS CAN IMPROVE BROWN SPOTS QUICKLY
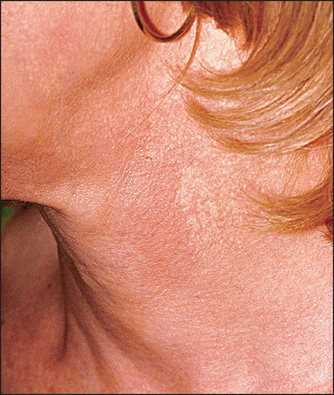
Bleaching creams are most effective when used on the face due to enhanced penetration through thin skin. Neck, chest, and forearm hyperpigmentation responds even more slowly to treatment due to the decreased penetration of the active (Fig. 29.3).
GLYCOLIC ACID PEELS MUST HURT TO BE EFFECTIVE
There is a misnomer that the ‘no pain, no gain’ philosophy applies to both muscles and the skin. While muscles must be exercised to the point of exhaustion to promote increased muscle mass, this is not the case with the skin. Any procedure that induces pain is injuring the skin. Sometimes dermatologists injure the skin for what are considered to be medically beneficial reasons, but injury is still occurring. For example, glycolic acid peels are intended to exfoliate the outer layer of skin, removing unwanted melanin pigment and producing smoother skin with better color (Fig. 29.4). However, pain may indicate that the skin has been injured and the resulting inflammation may actually worsen skin color through postinflammatory hyperpigmentation. Peels that are intended simply to remove the stratum corneum should not hurt. Thus, glycolic acid peels do not have to hurt to be effective.
TOPICAL FORMULATIONS OF VITAMINS AND SUPPLEMENTS ARE SIMILAR TO PILLS IN EFFECTIVENESS FOR SKIN IMPROVEMENT
RETINOL IN OVER-THE-COUNTER PREPARATIONS WORKS LIKE PRESCRIPTION TRETINOIN
Retinoids are a complex family of cosmeceuticals covered in Chapter 6. Retinol is the retinoid vitamin form that is necessary for vision. If the retinol is properly stabilized, it is possible that the skin can enzymatically convert small amounts of retinol to tretinoin. While this is theoretically possible, it has never been quanititated.
A SUNSCREEN WITH AN SPF ABOVE 15 DOES NOT PROVIDE ADDITIONAL PHOTOPROTECTION
Sunscreens above SPF 15 do actually provide additional photoprotection, but the increase in the amount of photoprotection provided is small. The percent UVB photoprotection provided by a given SPF rating is summarized in Table 29.1.
Table 29.1 UVB photoprotection provided by a given SPF rating
| SPF rating | % UVB radiation blocked |
| 4 | 75 |
| 8 | 88 |
| 15 | 93 |
| 30 | 97 |
| 45 | 98 |
Notice that an SPF 4 sunscreen blocks 75% of the UVB radiation, but an SPF 15 sunscreen blocks 93%. Although this is a substantial improvement in photoprotection, the percent increase in UVB protection decreases as the SPF increases such that an SPF 30 product only has 4% more photoprotection than an SPF 15 product. Thus, dermatologists typically recommend SPF 15 sunscreens to combine product function with aesthetic characteristics, since higher SPF products tend to be sticky due to increased concentration of the sunscreen actives. Higher SPF products may provide value in patients with unique medical UVB photosensitive disorders.
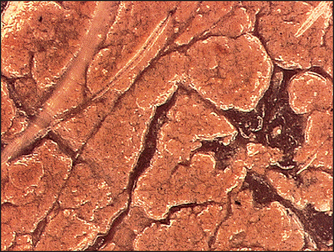
It is important to remember that the single most common reason sunscreens fail is due to incomplete film formation over the skin surface. This may be due to uneven application or migration of the sunscreen over the skin surface. Figure 29.5 is a 400 × video microscope of the appearance of a sunscreen containing facial foundation on the skin surface. Notice how the film has begun to separate 2 hours after application. This means that the sunscreen will not deliver the SPF labeled amount of sun protection after 2 hours. Frequent reapplication of sunscreen products is essential to achieving adequate sun protection.
NANOPARTICLES INCREASE ANTIAGING COSMECEUTICAL EFFICACY
At present, nanoparticles are being investigated for use in sunscreens containing zinc oxide and titanium dioxide. However, nanodispersed organic sunscreen formulations containing benzophenone or octyl methoxycinnamate have also been created. All of the nanoparticle sunscreens have the same health concerns of unwanted penetration, since sunscreen actives are intended to stay on the skin surface. The possible dangers arise over concern as to what happens once the nanoparticle titanium dioxide or zinc oxide has penetrated the skin. Both titanium dioxide and zinc oxide are chemically inert. They are theorized to remain in the body indefinitely, either forming a reservoir within the dermis or spreading throughout the body via the circulation. It appears that the sunscreen particles are capable of absorbing and reflecting UV radiation within the skin, causing the generation of oxygen radicals within the dermis and initiating the inflammatory cascade. No one knows if this nanoparticle sunscreen reservoir might enhance the photoprotective abilities of the skin or prematurely age skin due to chronic low-grade inflammation characterized by unusually high levels of interleukins 8 and 12. Thus, the safety of nanoparticles and their use in cosmeceuticals remains controversial, as no enhancement of efficacy has been demonstrated to date.