CHAPTER 3 Coronary Anatomy
Multidetector CT has become an important tool for noninvasive evaluation of cardiovascular structures.1 With advances in multidetector CT technology, the role of imaging has progressed from detection and quantification of the calcium score on noncontrast CT to detection of luminal stenoses, and detection and characterization of plaque composition on coronary CT angiography.2,3 Basic knowledge of the normal coronary arterial anatomy is essential for evaluation and interpretation of cardiac CT studies. Properly performed, coronary CT angiography provides high spatial resolution, three-dimensional data for detailed visualization of the coronary arteries, which are typically small, and rapidly moving, and have tortuous and complex anatomic arrangements. The anatomy of the coronary arteries and their normal variants is well described using conventional coronary angiography. The cross-sectional nature of CT has the benefit, however, of more precisely displaying the spatial relationship of coronary arterial and venous anatomy with respect to adjacent cardiac structures.
Multidetector CT is a continually evolving technique that in recent years has been mainly used to evaluate the coronary arteries. Less attention has been paid to the coronary venous system until more recently.4,5 Evaluation of the cardiac venous anatomy is important for preprocedural planning of percutaneous resynchronization therapy and treatment of resistant ventricular arrhythmias. In this chapter, we discuss the detailed normal anatomy of the coronary arteries and their normal variants and the anatomy of the cardiac veins.
CORONARY ARTERIES
The definition of the abnormal versus normal anatomy of the coronary arteries presents a complex problem. Angelini and colleagues6,7 have proposed the following terms to define these categories: normal—any morphologic feature observed in greater than 1% of an unselected population; normal variant—an alternative, relatively unusual, morphologic feature seen in greater than 1% of the population; and anomaly—a morphologic feature (e.g., number of ostia, proximal course, termination) rarely encountered (<1%) in the general population. The normal coronary arteries and their variations can be described in terms of their ostia (with respect to their number, location, size, and angle of origination), the size and length of the coronary arteries, their proximal and mid course (intramyocardial bridging), arteriolar ramifications, and terminations.
NORMAL CORONARY ARTERY ANATOMY
There are two coronary arteries, the right coronary artery (RCA) and left coronary artery (LCA), which originate from their respective sinuses of Valsalva—the RCA from the right sinus of Valsalva and the LCA from the left sinus of Valsalva (Fig. 3-1). The right sinus of Valsalva is located anteriorly and the left sinus of Valsalva posteriorly and to the left. The third sinus of Valsalva, located posteriorly and to the right, does not give rise to a coronary artery, and is referred to as “noncoronary cusp/sinus.” There may be variations in the number, shape, and location of coronary ostia or origins of the coronary arteries, most of which are of no clinical significance.
The coronary ostia are usually located in the upper third of the respective sinus of Valsalva. Ostia located near the aortic valve commissures, ostia located in the posterior (noncoronary) sinus, and ostia showing a high takeoff are considered abnormal. The high takeoff of the coronary artery may reduce diastolic coronary artery blood flow, and the patient may become symptomatic.8 High takeoff of a coronary artery may result in a long intramural course which could be potentially pathologic (Fig. 3-2).
In terms of the number of the coronary ostia, two ostia are considered normal, one in the respective sinus for the RCA and LCA; however three or four are considered as normal variants, such as a separate origin of a conal branch from the aorta (prevalence 11.6%)9 or absence of the left main (LM) coronary artery with the left anterior descending artery (LAD) and left circumflex artery (LCX) having separate origins from the aorta. An absent LM is considered a benign anomaly. A separate origin of the sinus node artery from the aorta has a prevalence of 0.2%.9 The single coronary artery and ectopic origin of the coronary arteries from the aorta and pulmonary artery are considered as anomalous and are discussed in Chapter 33.
Right Coronary Artery
On axial images, the RCA arises from the right sinus of Valsalva, inferior to the origin of the LCA (Fig. 3-3). It courses anteriorly and inferiorly under the right atrial appendage along the right atrioventricular (AV) groove, toward the acute margin of the heart, where it turns posteriorly and inferiorly toward the crux of the heart and divides into the posterior descending coronary artery (PDA) and the posterolateral ventricular branch (PLB) (Fig. 3-4; see Fig. 3-3). The RCA supplies the right atrium, right ventricle, posterior third of the interventricular septum, and sinoatrial and AV nodes.
In 50% to 64% of the population, the conus branch arises as the first branch of the RCA (Fig. 3-5A).9 The conus may arise from the ostial RCA in 22.3% or separately from the aorta in 11.6% of the population (Fig. 3-5B).9 The conus branch courses anteriorly and to the right and supplies the pulmonary outflow tract. Rarely, this branch can arise from the LCA.
The sinoatrial nodal artery is the second branch that arises from the proximal RCA in 65.4%, immediately distal to the RCA origin (Fig. 3-6A). In 16.6% of cases, the sinoatrial nodal artery arises from the LCX (Fig. 3-6B); in 9.2%, it arises from the RCA and the LCX; in 0.2%, it arises from the LCX and the pulmonary artery; and in 0.2%, it arises directly from the aorta.9 This artery courses toward the superior vena cava near the cranial aspect of the interatrial septum.
The next branches of the RCA are the marginal branches that supply the right ventricular myocardium (see Figs. 3-3 and 3-4). The acute marginal artery comes off the acute margin of the heart and courses anteriorly and to the right, anterior to the right ventricle. The acute marginal branches supply the free wall of the right ventricular myocardium. In 10% to 20% of patients, an acute marginal branch runs on the diaphragmatic surface of the heart to supply the distal posterior interventricular septum. After the RCA gives off the acute marginal branches, it continues in the right AV groove toward the diaphragmatic aspect of the heart. At the crux of the heart, the RCA makes a U-turn and branches into the PDA and PLB (see Fig. 3-4).
The PDA is of variable size and runs along the diaphragmatic surface in the posterior interventricular groove toward the inferior septum. Short septal branches arising perpendicularly from the PDA supply the posterior third of the septum and can connect with the septal branches from the LAD to form a collateral circulation. The PLB runs in the posterior left AV groove and gives off multiple branches that supply the posterior and inferior wall of the left ventricle (see Fig. 3-4). The average angle between the PDA and PLB is 53 ± 27 degrees.10 Within 1 to 2 cm of the crux, the PLB runs on the diaphragmatic surface of the left ventricle parallel to the PDA to supply the posterolateral diaphragmatic surface of the left ventricle. Here the RCA can serve as a collateral for an occluded LCX. Also close to the crux of the heart, just distal to the PDA origin, the RCA gives rise to a small AV nodal artery that supplies the AV node of the conduction system (Fig. 3-7). The AV nodal artery arises from the LCX in a left dominant system.
Left Coronary Artery
On axial images, when scrolling from the cranial to caudal direction, the LCA or LM is the first coronary artery that is seen arising from the left sinus of Valsalva (Fig. 3-8). It courses to the left, beneath the left atrial appendage and posterior to the right ventricular outflow tract, before branching into the LAD and the LCX (Fig. 3-9). The LM varies in length from 5 to 25 mm (mean 13.5 mm), and its diameter ranges from 2 to 5.5 mm (mean 4 mm) (Fig. 3-10).7,11 In 543 patients, Cademartiri and colleagues9 reported a length of less than 1 cm in 41.6%, 1 to 2 cm in 47.3%, and greater than 2 cm in 7% of patients. Pflederer and coworkers10 examined average angles of coronary bifurcations in 100 patients at four branch points: LAD/LCX, LAD/first diagonal branch, LCX/first obtuse marginal branch (OM1), and PDA/right posterolateral branch. The average LAD/LCX angle was reported as 80 ± 27 degrees. The interobserver variability of bifurcation angle measurements by contrast-enhanced 16-slice multidetector CT was significantly better (r = 0.91) than invasive angiography (r = 0.62).10 A normal variation in the anatomy is a true trifurcation of the LM, when the middle branch between the LAD and LCX is called the ramus intermedius (RI).9,12 The LM may be absent in fewer than 1% of the population; in these cases, the LAD and LCX arise separately from the aorta.7
Left Anterior Descending Artery
The LAD runs anteriorly and inferiorly in the anterior interventricular groove to the apex of the heart, and supplies the anterior and anterolateral wall of the left ventricular myocardium and the anterior two thirds of the interventricular septum (Figs. 3-11 and 3-12; see Fig. 3-10). In approximately 82% of cases, the LAD curves around the cardiac apex to supply part of the inferior wall of the left ventricle. In 7%, it may not reach the apex of the heart, and in about 11% of cases, the LAD terminates in the distal anterior interventricular groove or even more proximally.13 In such cases, the distal territory may be supplied by an unusually long diagonal branch or by RCA branches that traverse the posterior interventricular groove or the inferior surface of the heart, a normal variant.14 This is one of the potential collateral routes if either the RCA or the LAD is occluded.
The LAD gives off septal perforators and diagonal branches (see Figs. 3-11 and 3-12). The diagonal branches course along and supply the anterior and anterolateral wall of the left ventricular myocardium (see Figs. 3-11 and 3-12). They can vary in size and number, and are sequentially numbered as they arise from the LAD. In a series of 543 consecutive patients who underwent 64-detector coronary CT angiography, Cademartiri and colleagues9 reported variable numbers of diagonal branches: a single diagonal branch in 136 cases (25%), two diagonal branches in 270 cases (49.7%), and more then two branches in 130 cases (24%) (see Figs. 3-11 and 3-12). Diagonal branches were absent in seven cases (1.3%). The septal perforator branches arise at right angles from the LAD and supply the anterior two thirds of the interventricular septum. They are numbered sequentially as they arise from the LAD and are of smaller caliber then diagonal branches and vary in number (one to five) and distribution. The first septal branch is more constant in position than the first diagonal. It may branch early with both branches running parallel within the septum. Occasionally, a septal branch runs parallel to the LAD within the myocardium of the septum. The average angle between the LAD and the first diagonal has been reported as 46 ± 19 degrees.10
Left Circumflex Artery
The LCX runs posteriorly and to the left in the left AV groove (Fig. 3-13). It gives rise to obtuse marginal branches that are also numbered sequentially as they arise from the LCX (OM1, OM2, and OM3) (Fig. 3-14). Cademartiri and colleagues9 reported the prevalence of the number of the marginal branches arising from the LCX as 35.2% for one obtuse marginal branch, 46.2% for two obtuse marginal branches, and 18% for more than two obtuse marginal branches. The average angle between LCX/OM1 is 48 ± 24 degrees.10 Marginal branches of the LCX are observed in 99.4% (n = 540).9 The LCX and its branches supply the lateral and posterolateral wall of the left ventricle. Additional branches of the LCX are small atrial branches that supply the lateral and posterior regions of the left atrium.
Ramus Intermedius Artery
The RI is the most common variation of LCA anatomy, occurring when the LM trifurcates; the branch between the LAD and the LCX is the RI (Fig. 3-15). The RI can supply the myocardial territory of the diagonal branch or the obtuse marginal branch depending on whether it supplies the anterior wall or the lateral wall of the left ventricular myocardium.9,15 When large, the RI perfuses a significant portion of the myocardial territory of the diagonal branch and OM1. An RI was seen in approximately 21.9% of the cases reported by Cademartiri and colleagues.9 When the RI supplied vascularization to the anterolateral wall of the left ventricle, a decreased number of diagonal branches was observed: no diagonals in 3.4%, a single diagonal in 38.6%, two diagonals in 43.7%, and more than two diagonals in 14.3%.9 The first diagonal not only may be small, but also may arise more distally from the LAD than from the proximal third of the LAD (see Fig. 3-15A).
Coronary Artery Dominance
The artery that supplies the inferior portion of the posterior interventricular septum is considered to be the dominant artery. In 80% to 85% of cases, the RCA is dominant; when at the crux of the heart, it gives rise to the PDA and PLB (Fig. 3-16A). In a left dominant system, the LCX continues in the posterior left AV groove and gives rise to the PDA and PLB; this is seen in 7% to 8% of the population (see Fig. 3-16B). In the remaining 7% to 8%, there is a codominant system or balanced circulation in which the RCA gives rise to the PDA and terminates in the posterior interventricular groove; the LCX may also give rise to a PDA with two PDAs running parallel in the interventricular septum, or the LCX may give rise to all posterolateral branches (see Fig. 3-16C).12,15 The nondominant artery is usually smaller in size and terminates early in its respective AV groove.
NORMAL VARIANTS OF CORONARY ARTERIAL ANATOMY
Duplication of the coronary arteries is rare and can be seen with the LAD, RCA, or PDA. With LAD duplication, one branch (short LAD) terminates in the proximal aspect of the anterior interventricular sulcus (AIVS), and the second, longer branch has a variable course outside the AIVS and returns to the AIVS distally.14 The long LAD usually arises from the LAD; however, it rarely can arise from the RCA and enter the distal AIVS. Four subtypes of dual LAD have been described. In all subtypes, the short LAD terminates in the proximal AIVS. The long LAD takes one of four courses before entering the distal AIVS. In type 1, the long LAD courses to the left of the AIVS over the left ventricular myocardium (Fig. 3-17). In type 2, the long LAD courses to the right of the AIVS over the left ventricular myocardium. Type 3 is extremely rare with the proximal long LAD taking an intramyocardial course before entering the distal AIVS. Type 4 dual LAD is a distinct type in which the long LAD originates from the RCA, takes an anomalous course, and enters the anterior interventricular groove. Recognition of these variants is important for correct surgical identification of the short and long LADs for revascularization purposes or correct placement of an arteriotomy.14
Myocardial Bridge
Extramural arteries are expected to plunge into the myocardium only once, at their distal end. An exception to this is an intramural/intramyocardial course of the coronary artery, or “myocardial bridging,” which is considered an anomaly of aberrant course of the involved coronary artery. Myocardial bridging is most commonly seen in the LAD, predominantly in the mid LAD (Fig. 3-18). Konen and associates16 reported a mid LAD myocardial bridge in 27 of 47 (57%) cases and a distal LAD myocardial bridge in 7 of 47 (15%). Three patterns of myocardial bridging were observed: superficial septal in 10 of 34 (29.4%), deep septal in 14 of 34 (41.1%), and right ventricular type in 10 of 34 (29.4%), with the length of the intramuscular segment ranging from 13 to 40 mm.16 Multiple segments of myocardial bridging can be seen in a single vessel, and several vessels in a single patient can show myocardial bridging. If data are available in diastole and systole, the tunneled segment can be evaluated for potential narrowing during systole—the so-called milking effect that has been described on conventional catheter angiography.
Reported prevalence of intramuscular coronary arteries ranges from 5% to 86% at autopsy and 0.8% to 4.9% at coronary angiography.16–18 The incidence of myocardial bridging as reported in the study from Konen and associates16 is 30.5%. Intramuscular coronary arteries can cause technical problems during coronary artery bypass surgery, including inadvertent perforation of the right ventricle.16 Myocardial bridging also occurs in the diagonal branches (23.4%), obtuse marginal branches (19.1%), and RCA.18
CORONARY ARTERY SEGMENTAL ANATOMY
Nomenclatures for segmental anatomy of the coronary arteries that have been developed and modified by the American Heart Association (AHA) are used predominantly for research purposes.19,20 In 1975, Austen and coworkers19 developed a 15-segment coronary arterial model by dividing the coronary arteries into multiple segments. The proximal RCA segment commences from the ostium to the acute marginal, the mid segment curves around the acute margin of the heart, and the distal segment runs along the posterior AV groove. Segments 1 through 4 denote the RCA and its branches—1, proximal RCA; 2, mid RCA; 3, distal RCA; 4a, PDA; and 4b, PLB. Segment 5 denotes the LM.
For practical catheterization or coronary CT reports, the LAD is divided into proximal, mid, and distal segments using the origins of septal perforators or diagonals as follows: the proximal segment starts from the origin of the LAD to either the first diagonal or the first septal perforator, the mid segment commences from that point to the second diagonal, and the distal segment commences distal to that point.19 The proximal, mid, and distal LAD segments correspond to segments 6, 7, and 8, and the first and second diagonals correspond to segments 9 and 10.
Regarding the LCX, the proximal segment is from the origin to OM1, and the distal segment is distal to that point. The LCX and its branches correspond to segments 11 through 15. More recently, a 17-segment model has been suggested (Fig. 3-19). Other modifications include the Bypass Angioplasty Revascularization Investigation (BARI) classification, used for revascularization procedures.20
Segmentation of the Left Ventricle
All cardiac imaging modalities should define, orient, and display the heart using the long axis of the left ventricle and selected planes oriented at 90-degree angles relative to the long axis that provide precise localization of the coronary distribution and link the segments to known coronary arterial topography by using anatomic landmarks.21 The AHA has standardized the nomenclature of cardiac planes generated by cardiovascular magnetic resonance (CMR), single photon emission tomography (SPECT), positron emission tomography (PET), and cardiac CT. The nomenclature short-axis, vertical long-axis, and horizontal long-axis views (Fig. 3-20) is used and corresponds to the nomenclature short-axis, apical two-chamber, and apical four-chamber planes traditionally used with two-dimensional echocardiography.21
Despite the tremendous variability in the coronary artery blood supply to the ventricular myocardial segments, it is important to assign myocardial segments to specific coronary artery territories. A standard 17-segment model was established using the precise data on mass and size of the myocardium from autopsy studies.22 These segments correlate with the segments proposed and used in echocardiography. On this 17-segment model, a distribution of myocardial mass of 35%, 35%, and 30% for the basal, mid-cavity, and apical thirds of the heart, respectively, reflects the autopsy data closely. This model does not include the true apical myocardial segment.23
The myocardial segments are named and localized with reference to the long axes of the left ventricle and the 360-degree circumferential locations on the short-axis views. With regard to the circumferential location, the basal and mid-cavity slices are divided into six segments of 60 degrees each,21 and the apical slice is divided into four segments (Fig. 3-21). Segment 17, the left ventricular apex, is represented on the vertical and horizontal long-axis views (see Fig. 3-21); this leads us to the recommended 17 segments that are represented on a bull’s-eye display. The circumferential locations at the base and mid-cavity level are the anterior, anteroseptal, inferoseptal, inferior, inferolateral, and anterolateral myocardial segments. Segments 1 and 7 correspond to the anterior wall at the base and mid-cavity levels. The appropriate nomenclature for these segments is basal anterior and mid-anterior segments. The septum, delineated by the attachment of the right ventricle, is divided into anterior and inferior segments. Segments 2 and 3 are basal anteroseptal and basal inferoseptal. Segment 4 is the basal inferior, segment 5 is the basal inferolateral, and segment 6 is the basal anterolateral segment. Similar names are used for the six segments (numbers 7 through 12) at the mid-cavity level. Only four segments represent the left ventricular apical myocardium as the left ventricle tapers at the apex. Segments 13 through 16 represent the apical anterior, apical septal, apical inferior, and apical lateral myocardium. Segment 17 represents the extreme tip of the ventricle, the ventricular apex, where the left ventricular cavity is no longer seen.21
The greatest variability in the myocardial blood supply occurs at the extreme apex, or segment 17, which can be supplied by any of the three arteries, RCA, LCX, or LAD. Segments 1, 2, 7, 8, 13, 14, and 17 are assigned to the LAD distribution; segments 3, 4, 9, 10, and 15 are assigned to the RCA distribution; and segments 5, 6, 11, 12, and 16 are assigned to the LCX distribution.21 The anteroseptal and anterior segments of the basal, mid-cavity, and apical segments are assigned to the LAD; the inferoseptal and inferior segments of the basal, mid-cavity, and inferior apical segments are assigned to the RCA; and the inferolateral and lateral segments of the basal, mid-cavity, and lateral apical segments are assigned to the LCX. Knowledge of the correlation between the segments of the left ventricle and the corresponding coronary artery distribution is crucial to detect corresponding coronary artery disease. Abnormalities of wall motion are usually reported using the descriptive anatomic locations of the myocardium such as anterior, septal, lateral, or inferior wall abnormalities at the three levels, although some institutions use the 17-segment model.
CORONARY VENOUS ANATOMY
The coronary venous system is increasingly used for diagnostic and therapeutic electrophysiologic procedures, such as percutaneous transcatheter therapy for cardiac resynchronization, ablation of various supraventricular arrhythmias, and therapy of mitral regurgitation by percutaneous mitral annuloplasty. The cardiac venous anatomy is not as well known to clinicians as the coronary arterial anatomy. Comprehensive knowledge of the cardiac venous system and its relationship to the surrounding structures is essential for preprocedural guidance of catheter or lead placement in a tributary of the coronary sinus.4,5
The CS is a wide venous channel about 2 cm in length, located in the posterior part of the coronary sulcus covered by muscular fibers from the left atrium. It extends from the valve of Vieussens to its orifice in the right atrium (Fig. 3-22), which is between the opening of the inferior vena cava and the AV aperture; the orifice is guarded by a semilunar valve, the valve of the CS (thebesian valve). The diameter of the CS ranges from 7.3 to 18.9 mm, with the anteroposterior diameter reported as 11.5 mm and the superoinferior diameter reported as 12.6 mm, resulting in an oval-shaped ostium.24 The CS is larger in men than in women and in patients with ischemic cardiomyopathy compared with patients with nonischemic cardiomyopathy. The RCA is inferior to the CS at the crux of the heart.
The tributaries of the CS are the great, small, and middle cardiac (posterior interventricular) veins; the posterior vein of the left ventricle; and the oblique vein of the left atrium, all of which have a valve at their orifices except for the oblique vein of the left atrium (see Fig. 3-22). The diameter of the tributaries also varies from 1.3 to 10.5 mm.24 An acute takeoff angle from the CS, a small diameter, and a tortuous course are anatomic characteristics that make cannulation of the vein difficult. Blendea and colleagues24 reported an acute takeoff in 27% of posterior veins and 25% of lateral veins. In principle, the veins run parallel to the arteries.
The anterior interventricular vein originates in the lower or middle third of the anterior interventricular groove, runs parallel to the LAD, connects with diagonal veins, and drains the lateral and anterolateral portions of the left ventricle and receives blood from the anterior two thirds of the interventricular septum (Fig. 3-23). It continues to run vertically and upward in the anterior interventricular groove, and as it turns posteriorly at the AV groove and courses horizontally it becomes the great cardiac vein (GCV) or left coronary vein. The GCV is the main tributary of the CS and is the longest venous channel of the heart that runs in the left AV groove, to reach the back of the heart, draining into the left side of the CS (Figs. 3-24 and 3-25; see Figs. 3-22 and 3-23). It receives tributaries from the left atrium and from both ventricles, and one of the tributaries, the left marginal vein, is of considerable size and ascends along the left margin of the heart (see Fig. 3-24). At the origin of the LAD, the GCV crosses over the LAD and LCX arteries, where it forms the base of the triangle of Brocq and Mouchet, and then turns into the left AV groove, running parallel to the circumflex artery.
The distance from the GCV and the LM is variable (0 to 7 mm), and sometimes the GCV touches the LM and turns with a very sharp angle to the left AV groove, crossing under the branches of the LM coronary artery. The LCX is covered by the GCV in 60% of cases so that the underlying anatomy of the LCX is obscured or inadequately visualized, especially on volume rendered images (see Fig. 3-23). The proximal diameter of the GCV is 7.2 ± 1.4 mm, and the distal diameter is 4.9 ± 1.1 mm.25 Christiaens and coworkers4 reported an angle of greater than 90 degrees between the lateral marginal vein and GCV in 12% of cases, 60 to 90 degrees in 22%, and less than 60 degrees in 58%. The GCV becomes the CS at the entrance site of the left atrial oblique vein of Marshall. In cases where the left atrial oblique vein of Marshall is not present, the CS begins at the valve of Vieussens. The GCV and CS encircle most of the left AV conduction system and are frequently used for location of assessorial electrical pathways that are present in Wolff-Parkinson-White syndrome.
The tributaries of the CS that originate off the lateral wall are referred to as marginal veins. These marginal veins are defined further by the location of the veins as they empty into the GCV in the left anterior oblique projection—anterolateral marginal, lateral marginal, and inferolateral marginal (see Fig. 3-24). The prevalence of the lateral marginal vein is 73% to 88%, whereas the prevalence of the posterolateral marginal vein varies from 13% to 80% of cases.25–27 The diameters of posterolateral and lateral marginal veins have been documented as 3.8 ± 0.7 mm and 3.1 ± 0.8 mm.25 The lateral veins are less prevalent in patients with a history of lateral myocardial infarction than in patients without such a history (33% vs. 96%)24 and (27% vs. 71%).25
The posterior lateral ventricular vein (PLVV) drains the inferior (posterior) aspect of the left ventricle. The PLVV is often confused with the posterolateral marginal vein because of their similar location. The PLVV is usually larger in caliber than the posterolateral marginal vein, and sometimes drains into the middle cardiac vein. The PLVV runs on the diaphragmatic surface of the left ventricle to the CS, but may end in the great cardiac vein. (see Fig. 3-25).
The middle cardiac vein originates near the cardiac apex, runs in the posterior interventricular groove, and drains either directly into the right atrium or into the CS in 87% of cases just before the CS drains into the right atrium (see Figs. 3-22, 3-24, and 3-25). The middle cardiac vein receives blood from the posterior third of the septum, and runs parallel to and stays to the left of the PDA. It crosses over the PLB branch of the RCA, and when the LCX is dominant, it drains blood from the inferior wall of the left ventricle and crosses over the artery before entering the CS.
The small cardiac vein (vena cordis parva; right coronary vein) is present in only 36% of cases; it runs in the right AV groove, and opens into the right side of the CS, but may drain into the middle cardiac vein or directly into the right atrium. It receives blood from the back of the right atrium and ventricle. The most frequent anatomic variant observed (52%) is a connection of the small cardiac vein to the CS at the crux cordis (Fig. 3-26).
The oblique vein of the left atrium or oblique vein of Marshall is a small vessel that descends obliquely on the back of the left atrium and drains into the CS near its left edge. This vein was identified in 37 of 51 patients included in the study by Blendea and colleagues24 with diameters of 1.7 ± 0.5 mm and takeoff angles of 154 ± 15 degrees, which makes the vein accessible for cannulation for ablation procedures for atrial fibrillation.28
Cardiac veins that do not drain into the CS are the anterior cardiac veins, comprising three or four small vessels that collect blood from the front of the right ventricle and open into the right atrium via atrial sinuses. The sinus coronarius atrii dextri is so large that it can be confused with the RCA. The right marginal vein opens into the right atrium, and is regarded as belonging to this group. The smallest cardiac veins (thebesian veins) consist of numerous minute veins arising in the muscular wall of the atria and ventricles and draining into their respective chambers.5,29,30
Achenbach S. Computed tomography coronary angiography. J Am Coll Cardiol. 2006;48:1919-1928.
Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation. 2007;115:1296-1305.
Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1999;33:1756-1824.
1 Achenbach S. Computed tomography coronary angiography. J Am Coll Cardiol. 2006;48:1919-1928.
2 Hamon M, Morello R, Riddell JW, et al. Coronary arteries: diagnostic performance of 16- versus 64-section spiral CT compared with invasive coronary angiography—meta-analysis. Radiology. 2007;245:720-731.
3 Knollmann F, Ducke F, Krist L, et al. Quantification of atherosclerotic coronary plaque components by submillimeter computed tomography. Int J Cardiovasc Imaging. 2008;24:301-310.
4 Christiaens L, Ardilouze P, Ragot S, et al. Prospective evaluation of the anatomy of the coronary venous system using multidetector row computed tomography. Int J Cardiol. 2008;126:204-208.
5 Jongbloed MRM, Lamb HJ, Bax JJ, et al. Noninvasive visualization of the cardiac venous system using multislice computed tomography. J Am Coll Cardiol. 2005;45:749-753.
6 Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation. 2007;115:1296-1305.
7 Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002;105:2449-2454.
8 Burck H. High and funnel-like origin of the coronary arteries. Beitr Pathol Anat. 1963;128:139-156.
9 Cademartiri F, La Grutta L, Malago R, et al. Prevalence of anatomical variants and coronary anomalies in 543 consecutive patients studied with 64-slice CT coronary angiography. Eur Radiol. 2008;18:781-791.
10 Pflederer T, Ludwig J, Ropers D, et al. Measurement of coronary artery bifurcation angles by multidetector computed tomography. Invest Radiol. 2006;41:793-798.
11 Sos TA, Sniderman KW. A simple method of teaching three-dimensional coronary artery anatomy. Radiology. 1980;134:605-606.
12 Patel S. Normal and anomalous anatomy of the coronary arteries. Semin Roentgenol. 2008;43:100-112.
13 Paulin S. Coronary angiography: a technical, anatomic and clinical study. Acta Radiol (Diagn) (Stockh). 1964;233(Suppl):1.
14 Spindola-Franco H, Grose R, Solomon N. Dual left anterior descending coronary artery: angiographic description of important variants and surgical implications. Am Heart J. 1983;105:445-455.
15 Kini S, Bis KG, Weaver L. Normal and variant coronary arterial and venous anatomy on high-resolution CT angiography. AJR Am J Roentgenol. 2007;188:1665-1674.
16 Konen E, Goitein O, Sternik L, et al. The prevalence and anatomical patterns of intramuscular coronary arteries: a coronary computed tomography angiographic study. J Am Coll Cardiol. 2007;49:587-593.
17 Mohlenkamp S, Hort W, Ge J, et al. Update on myocardial bridging. Circulation. 2002;106:2616-2622.
18 De Rosa R, Sacco M, Tedeschi C, et al. Prevalence of coronary artery intramyocardial course in a large population of clinical patients detected by multislice computed tomography coronary angiography. Acta Radiol. 2008;49:895-901.
19 Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5-40.
20 Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1999;33:1756-1824.
21 Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539-542.
22 Edwards WD, Tajik AJ, Seward JB. Standardized nomenclature and anatomic basis for regional tomographic analysis of the heart. Mayo Clin Proc. 1981;56:479-497.
23 Henry WL, DeMaria A, Gramiak R, et al. Report of the American Society of Echocardiography Committee on Nomenclature and Standards in Two-dimensional Echocardiography. Circulation. 1980;62:212-217.
24 Blendea D, Shah RV, Auricchio A, et al. Variability of coronary venous anatomy in patients undergoing cardiac resynchronization therapy: a high-speed rotational venography study. Heart Rhythm. 2007;4:1155-1162.
25 Van de Veire NR, Schuijf JD, De Sutter J, et al. Non-invasive visualization of the cardiac venous system in coronary artery disease patients using 64-slice computed tomography. J Am Coll Cardiol. 2006;48:1832-1838.
26 Mao S, Shinbane JS, Girsky MJ, et al. Coronary venous imaging with electron beam computed tomographic angiography: three-dimensional mapping and relationship with coronary arteries. Am Heart J. 2005;150:315-322.
27 Meisel E, Pfeiffer D, Engelmann L, et al. Investigation of coronary venous anatomy by retrograde venography in patients with malignant ventricular tachycardia. Circulation. 2001;104:442-447.
28 de Oliveira IM, Scanavacca MI, Correia AT, et al. Anatomic relations of the Marshall vein: importance for catheterization of the coronary sinus in ablation procedures. Europace. 2007;9:915-919.
29 Gerber TC, Sheedy PF, Bell MR, et al. Evaluation of the coronary venous system using electron beam computed tomography. Int J Cardiovasc Imaging. 2001;17:65-75.
30 Singh JP, Houser S, Heist EK, et al. The coronary venous anatomy: a segmental approach to aid cardiac resynchronization therapy. J Am Coll Cardiol. 2005;46:68-74.

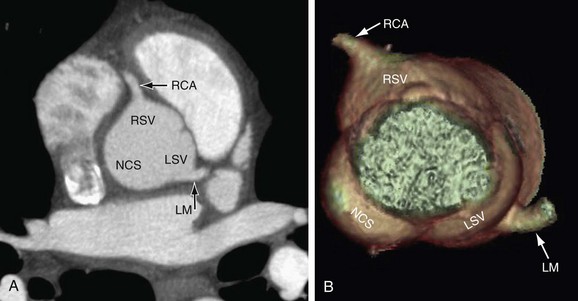
 FIGURE 3-1
FIGURE 3-1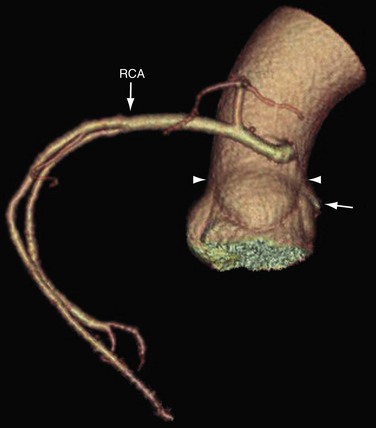
 FIGURE 3-2
FIGURE 3-2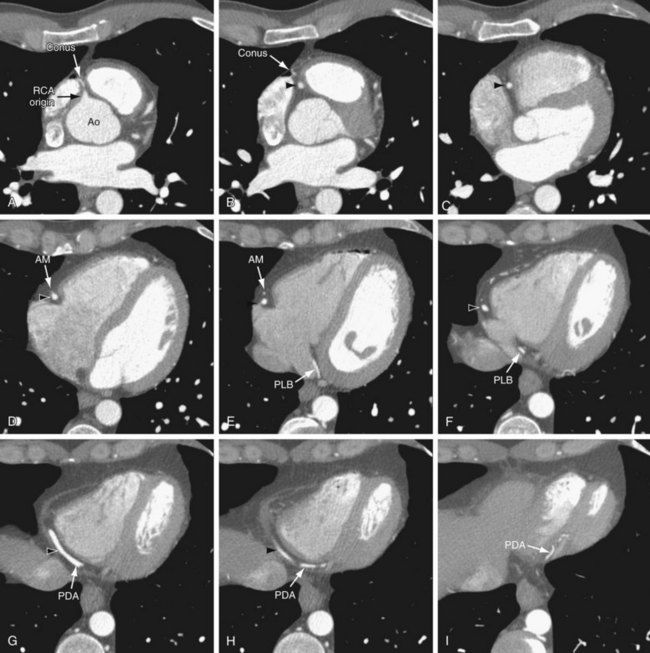
 FIGURE 3-3
FIGURE 3-3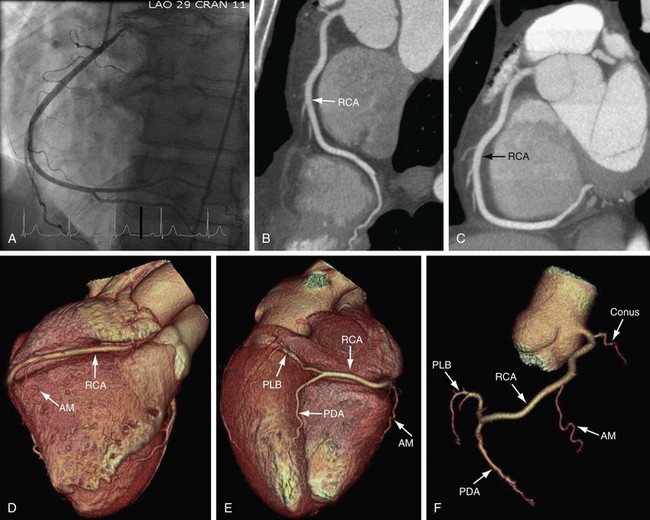
 FIGURE 3-4
FIGURE 3-4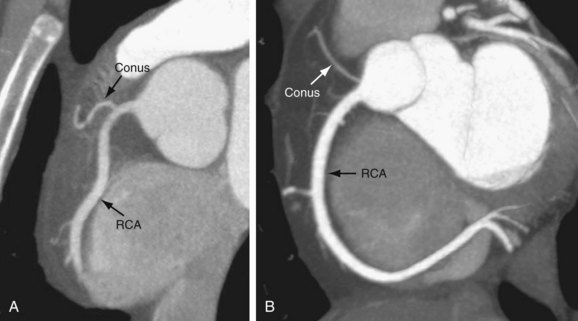
 FIGURE 3-5
FIGURE 3-5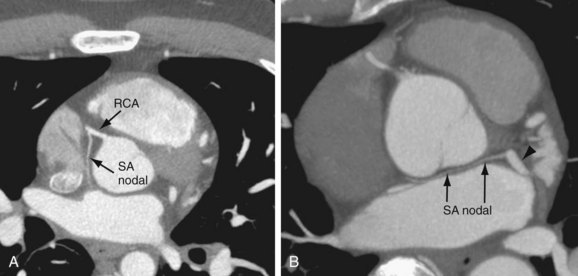
 FIGURE 3-6
FIGURE 3-6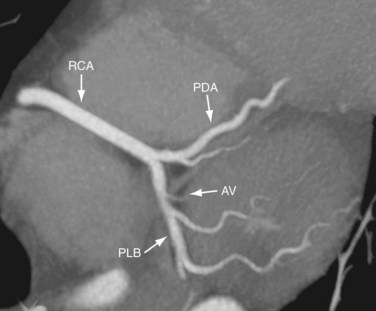
 FIGURE 3-7
FIGURE 3-7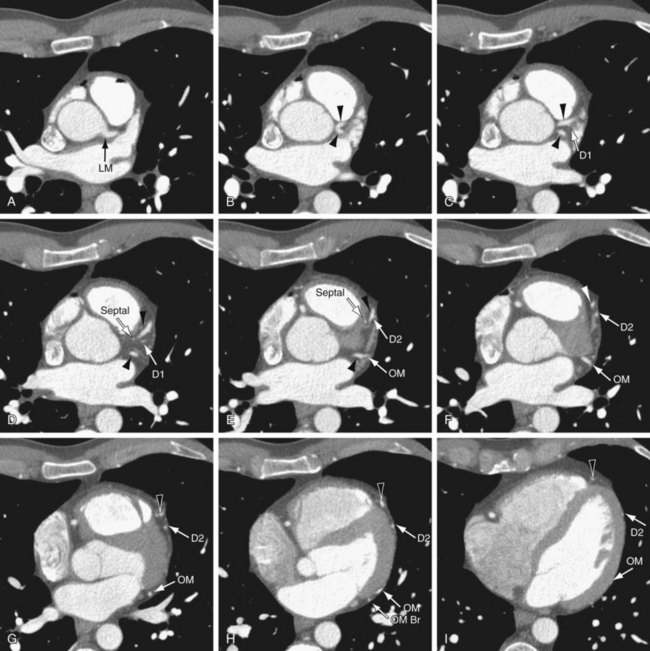
 FIGURE 3-8
FIGURE 3-8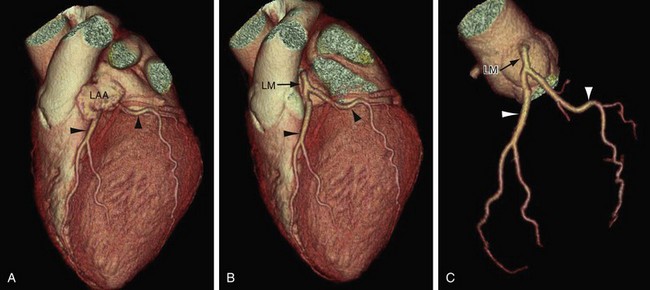
 FIGURE 3-9
FIGURE 3-9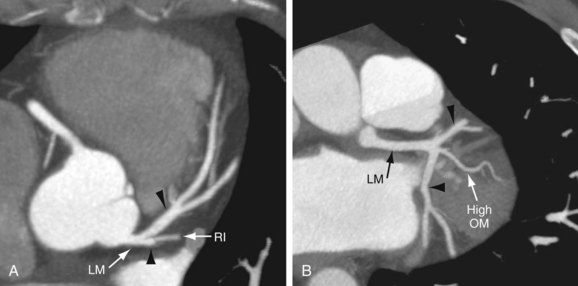
 FIGURE 3-10
FIGURE 3-10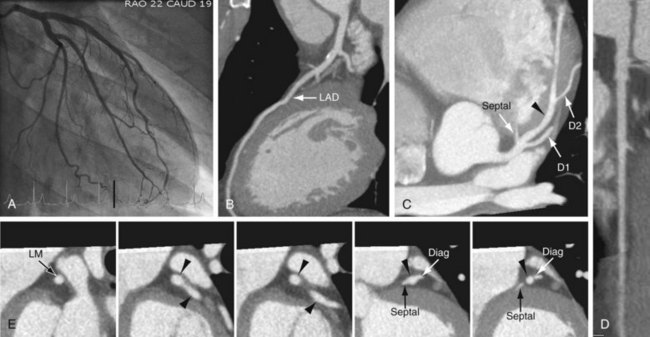
 FIGURE 3-11
FIGURE 3-11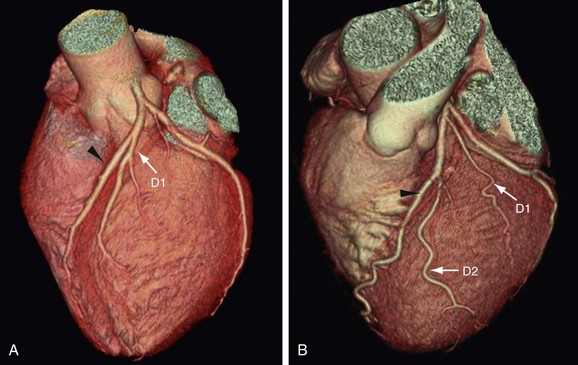
 FIGURE 3-12
FIGURE 3-12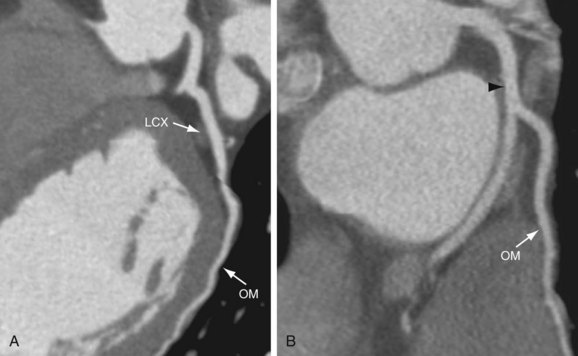
 FIGURE 3-13
FIGURE 3-13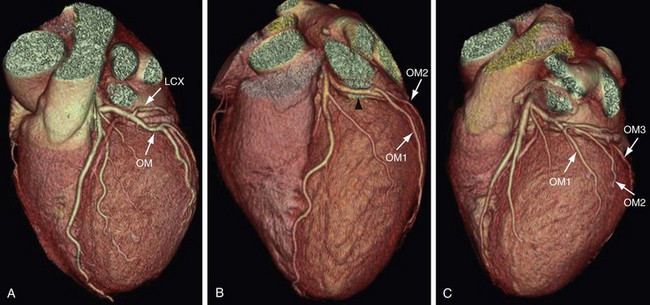
 FIGURE 3-14
FIGURE 3-14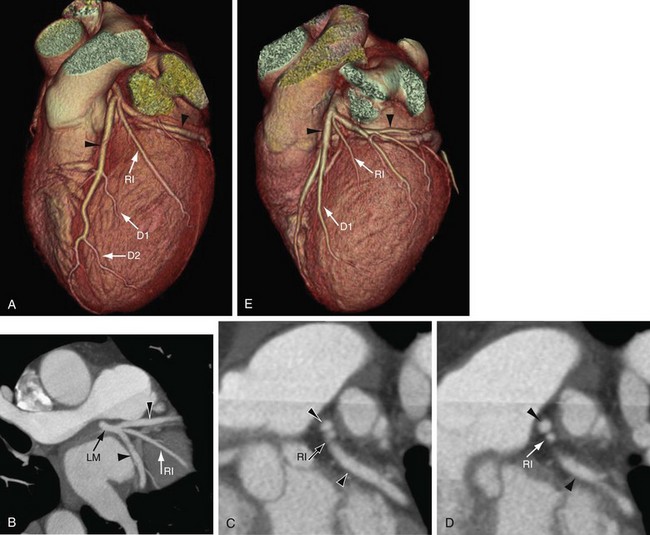
 FIGURE 3-15
FIGURE 3-15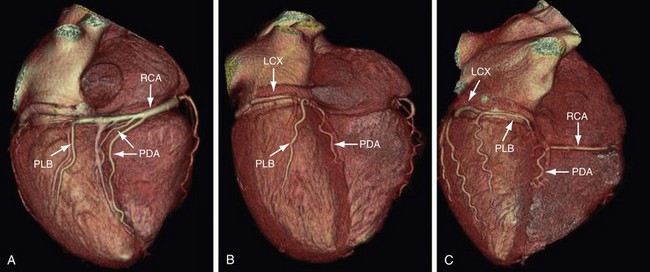
 FIGURE 3-16
FIGURE 3-16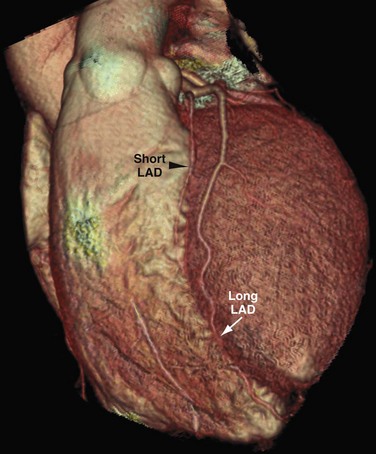
 FIGURE 3-17
FIGURE 3-17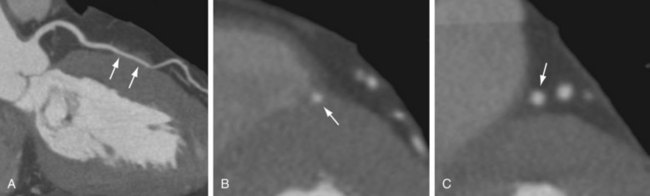
 FIGURE 3-18
FIGURE 3-18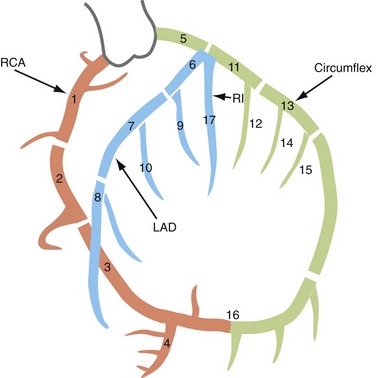
 FIGURE 3-19
FIGURE 3-19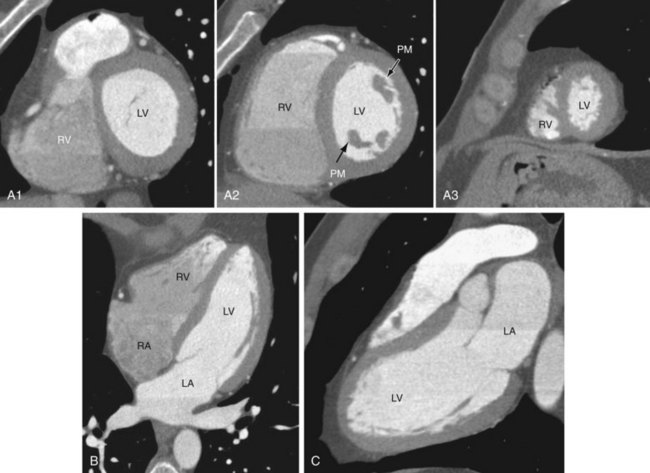
 FIGURE 3-20
FIGURE 3-20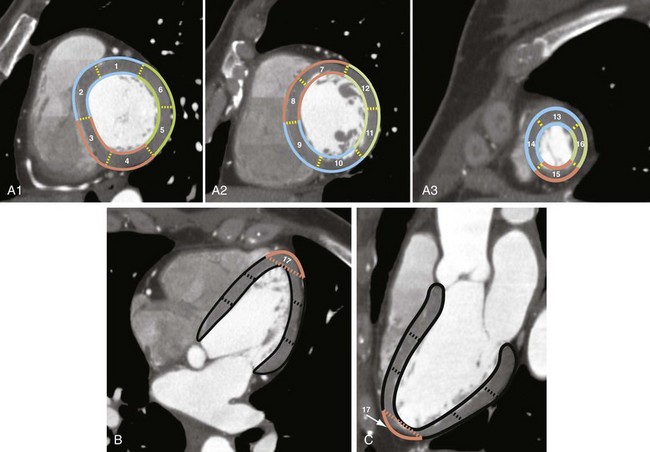
 FIGURE 3-21
FIGURE 3-21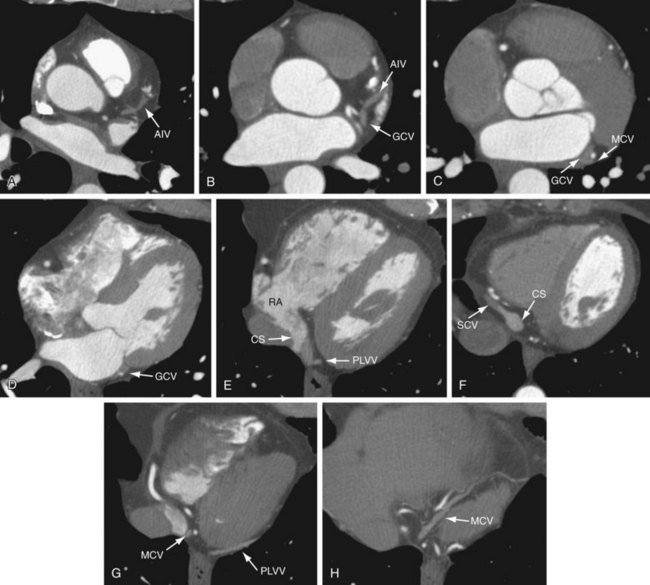
 FIGURE 3-22
FIGURE 3-22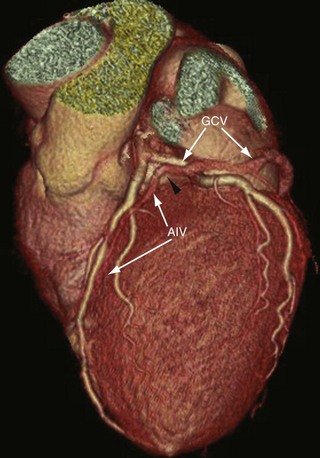
 FIGURE 3-23
FIGURE 3-23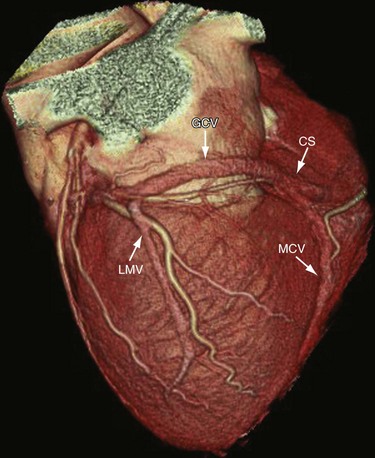
 FIGURE 3-24
FIGURE 3-24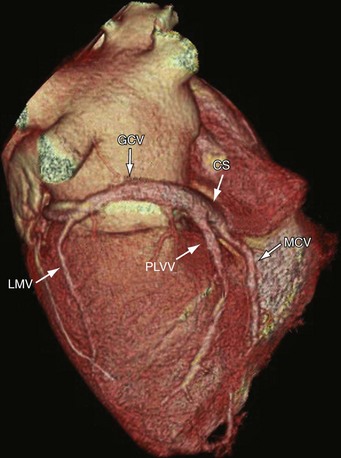
 FIGURE 3-25
FIGURE 3-25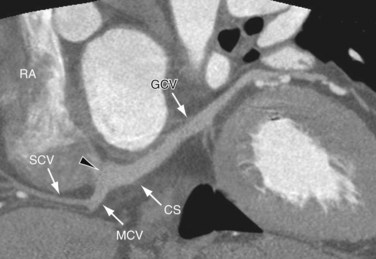
 FIGURE 3-26
FIGURE 3-26