CHAPTER 21 Corneal surgery
Corneal and anterior segment trauma
Amit Patel, Massimo Busin and Carlo Enrico Traverso
Corneal laceration
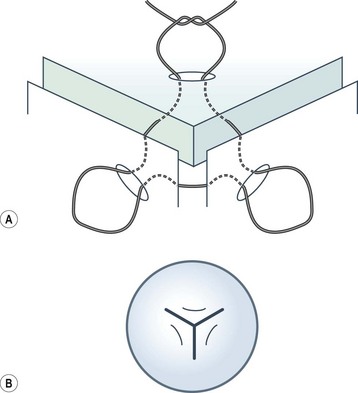
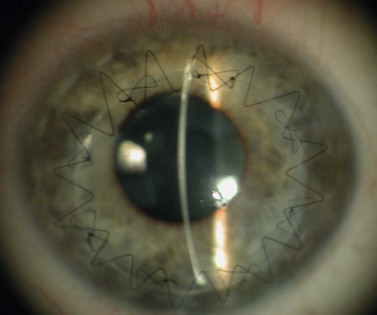
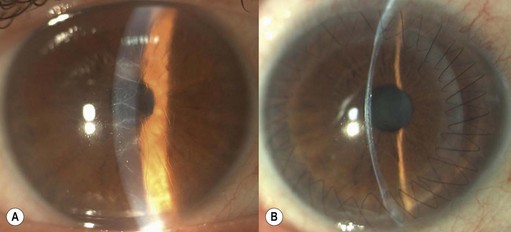
Viscoelastic may be injected through the wound or via a paracentesis to reform the anterior chamber if required. 10.0 Nylon is preferably used to close the corneal laceration. The placement of the primary suture depends on the configuration of the wound. When linear, it is placed at the center of the wound with subsequent sutures bisecting the remaining portion. If angulated, the primary suture is placed at the apex of the laceration. Stellate ruptures are difficult to repair and may require triangular, ‘X’ or purse-string sutures (Fig. 21.1). Attempts should be made to place sutures outside the visual axis if possible.
Particular care should be taken when placing sutures in bevelled lacerations. The entry and exit points of the sutures should be appropriately placed in order to avoid overriding of the wound edges or dehiscence secondary to ‘cheese-wiring’ of the tissue (Fig. 21.2).
Hyphema
Most traumatic hyphemas may be treated medically; however, surgical intervention should be considered in cases with prolonged and severely raised IOP, which may lead to optic nerve damage and corneal staining1. The corneal staining may develop rapidly, especially in younger individuals, but can take several months or years to clear.
Removal of the blood clot may be carried out with a mechanical vitrector or irrigation and aspiration2. Two paracenteses are made and an irrigation cannula or anterior chamber maintainer placed in one and a vitrector in the other. The aspiration and cutting functions of the vitrector are effective in removing the clot whilst minimizing traction on the iris and iridocorneal angle. Irrigation and aspiration may also be used without the cutter; however, the clot may be too thick to be evacuated and may result in unnecessary traction.
Postoperative care
Post-traumatic septic endophthalmitis carries a poor prognosis and, given the higher risk as well as the fact that it may be difficult to distinguish from the effects of trauma itself (i.e. pain, redness, inflammation, and reduced vision)3, prophylactic antibiotics must be prescribed in all cases.
Conjunctival flap surgery
Amit Patel, Carlo Enrico Traverso and Massimo Busin
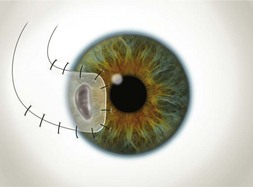
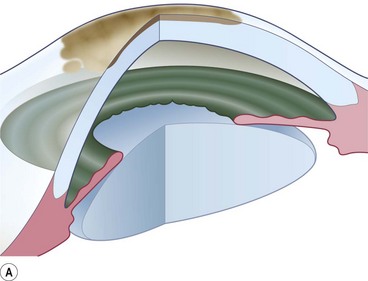
Of the numerous techniques to cover the cornea with conjunctiva, the total conjunctival flap popularized by Gundersen4 and the partial conjunctival flap are the most effective. The aim of conjunctival flap surgery in ocular surface disease and ulceration (both infectious and non-infectious) is to prevent stromal melting and possible perforation, thus stabilizing the eye and eventually allowing later surgery aimed at rehabilitating vision. However, for conditions that do not allow later corneal surgery (i.e. extreme dry eyes, high grade stem cell deficiency, etc.) a conjunctival flap may be compatible with vision up to 20/400 (see Fig. 21.4C).
Indications
Conjunctival flaps may be utilized in a variety of corneal diseases that are not responsive to medical management, induced ptosis, or tarsorrhaphy. Chronic ocular surface disease and non-healing corneal epithelial defects are excellent candidates for flap surgery5. It has also been used to stabilize the eye in chronic and non-response viral, bacterial, and fungal corneal infections6–8. In the presence of a descemetocele or perforation, a corneal patch must be carried out prior to considering a conjunctival flap.
Along with providing corneal support and fibrovascular tissue to fill epithelial defects, the associated blood supply aids to bring immunoglobulins, anticollagenases, and systemic antibiotics to the lesion9. This advantage is not afforded when applying non-living tissue such as amniotic membrane. Other indications include neurotrophic ulcers, marginal ulcers, exposure keratopathy, and bullous keratopathy with low visual potential.
Surgical technique
Total conjunctival flap
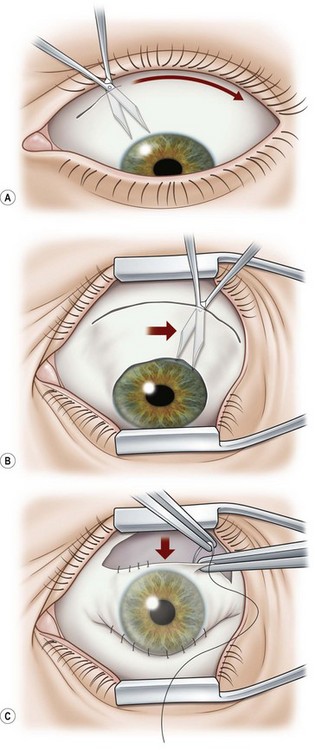
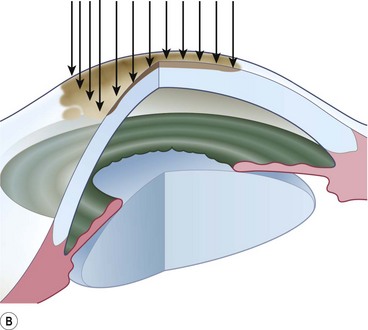
A 6.0 silk or polyglactin 910 traction suture may be placed through the cornea near the limbus to allow downward rotation of the globe during flap dissection. The superior conjunctiva is dissected from the underlying Tenon’s tissue by injecting 1–2 ml of local anesthesia (e.g. 1–2% lidocaine) or balanced salt solution with a 27 gauge needle. Epinephrine in a dilution of 1 : 100 000 may be added to achieve temporary vasoconstriction. The injection site should be situated away from the conjunctiva intended for flap use so as to prevent creating a ‘buttonhole’. A small horizontal incision is made with Wescott scissors at the fornix through conjunctiva, but not into Tenon’s fascia. Then the blunt and sharp dissection is performed with Wescott scissors and non-toothed forceps, separating conjunctiva from Tenon’s, ideally in the plane created by the local anesthesia. Dissecting the conjunctiva with the scissors placed underneath (Fig. 21.3A and B), as opposed to under direct visualization, ensures that the correct plane is maintained and minimizes the risk of buttonhole formation. The dissection is carried out in a meticulous fashion to the limbus and care is taken to avoid buttonholes, although this may be difficult in eyes that have had chronic inflammation, scarring, surgery, or trauma.
Once most of the conjunctiva is dissected down to the limbus, the horizontal incision can be extended several millimeters toward the nasal and temporal canthi (Fig. 21.3B). The nasal and temporal portions of the flap then need to be undermined. Once the entire flap has been created, the conjunctival dissection is continued forward until it comes free at the limbus. A 360° conjunctival peritomy is then performed. A small amount of undermining of the inferior limbal conjunctiva often makes the peritomy and subsequent suturing of the conjunctival edge easier. The limbus is then debrided of any remaining epithelium.
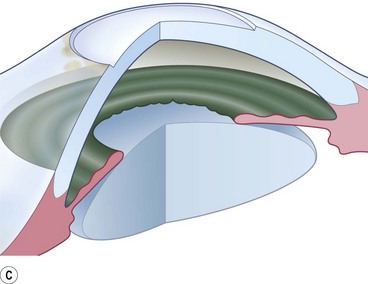
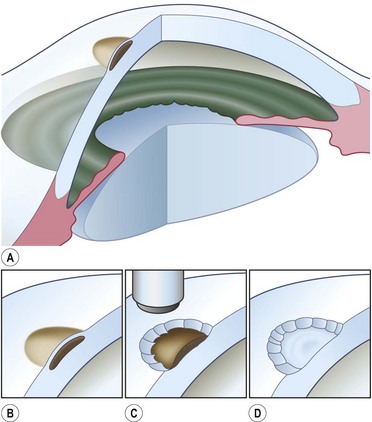
Buttonholes of the flap are the most common intraoperative complication and can lead to flap retraction. These should be closed with interrupted or purse string sutures and, if located in an area of flap that covers the cornea, the sutures should be anchored to the underlying cornea. Fig. 21.4 illustrates the pre- and postoperative appearance of a total conjunctival flap. Failure to separate Tenon’s fascia from the overlying conjunctiva leads to fibrovascular proliferation and poor transparency (Fig. 21.5).
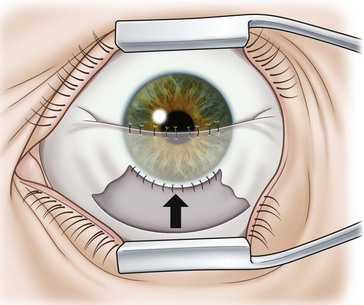
Partial conjunctival flaps
Localized limbal and paralimbal lesions in an otherwise healthy cornea may occasionally be treated with a partial conjunctival flap. The corneal epithelial debridement is carried out in the area expected to be covered by the conjunctival flap and the conjunctiva is ballooned with local anesthesia as described above. The width of the conjunctival flap should be approximately 1.5 times the width of the lesion to be covered in order to allow for proper suture placement and account for late flap retraction. In order to secure the conjunctival edge onto the cornea and prevent dehiscence and retraction, it is important to create a ledge of cornea into which to suture the conjunctiva. A groove is made into healthy cornea at the edge of the intended extension of the flap with a diamond or metal blade and a small triangular strip of cornea is removed to create a ledge (Fig 21.6).
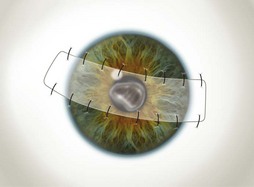
The partial flaps may be fashioned in various ways. A simple advancement flap is created much in the same manner as a total flap; however, the amount of conjunctival dissection is smaller and the edge of the flap is sutured on to the cornea with 10-0 nylon sutures. An advancement flap is prone to retraction with time and a relaxing incision through the conjunctiva and Tenon’s tissue, away from the limbus, may reduce the likelihood and allow the bulbar conjunctiva distal to the incision to retract. A pedicle (Fig. 21.7) or bridge flap (Fig. 21.8) may be considered as alternatives. A bridge flap may be created with a narrow band of conjunctiva that runs across the corneal lesion allowing a greater view of the anterior chamber. Edges of the flap overlying the cornea are sutured with 10-0 nylon and those overlying episclera with 8-0 polyglactin 910 (e.g. Vicryl). Non-absorbable suture knots must be buried.
Pterygium surgery
Amit Patel, Carlo Enrico Traverso and Massimo Busin
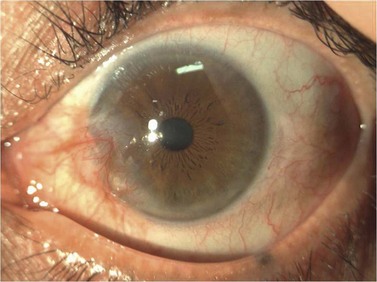
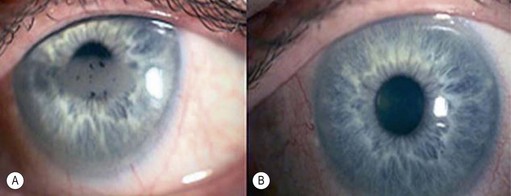
A pterygium (from the Greek pterygion meaning ‘wing’) is a degenerative wing-like fleshy mass of bulbar conjunctival and subconjunctival tissue that arises at the limbus and encroaches onto the corneal surface (Fig. 21.9). It consists of an elevated leading edge (head) and a broad triangular fibrovascular mass (body). Although it most commonly originates from the nasal limbus, temporal or simultaneous nasal and temporal pterygia may occasionally exist. Histopathologically, it is characterized by fibrovascular proliferation and ‘elastotic degeneration’, i.e. positive to elastic tissue stain, but is not sensitive to elastase. In addition, there is alteration of the basal corneal epithelial layer, destruction of Bowman’s layer, and scarring of the superficial corneal stroma.
Although the precise pathogenesis of pterygia formation remains speculative, it is largely thought to be related to ultraviolet light exposure10,11. This is supported by its formation in the interpalpebral fissure as well as the higher prevalence among people living closer to the equator and in those with outdoor occupations. Ocular surface irritation from dust, sand, etc. has also been implied as a causative factor.
Surgical technique
Primary pterygium
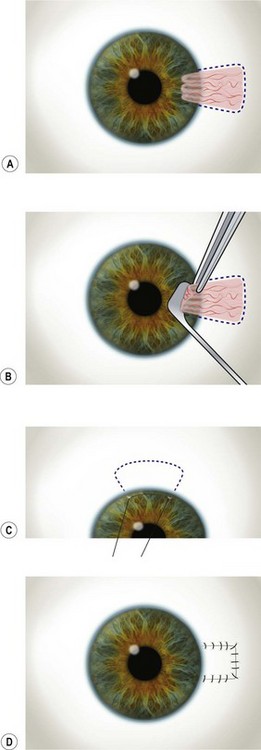
The outline of the pterygium is marked with a gentian violet marker, and anesthetic or balanced salt solution is injected subconjunctivally to balloon the pterygium (Fig. 21.10A). This allows easier dissection and protects the underlying rectus muscle from accidental damage.
The head of the pterygium is lifted with fine toothed forceps and gentle dissection using a rounded sharp blade (e.g. Beaver No. 57) or Tooke’s knife is employed to roll the tissue towards the limbus (Fig. 21.10B). A superficial keratotomy may be made to outline the leading edge prior to the dissection and a superficial plane of dissection is maintained up to the limbus. Attempts must be made to maintain a level and smooth plane of dissection. On reaching the limbus, the bulbar portion (body) of the pterygium is then excised carefully down to the sclera using Wescott scissors. Particular attention should be paid in dissecting and removing all the underlying Tenon’s tissue. To minimize the risk of recurrence, this dissection should lead to complete removal of all the hypertrophic fibrotic tissue underlying the pterygium and must be carried out up to the recti perimysium, into which it usually blends. The gentian violet marks allow visualization of the margins following the subconjunctival injection and avoid inadvertent excision of the plica semilunaris and caruncle. Wet field cautery may be applied sparingly to achieve hemostasis. Remnant tissue at the limbus and sclera is scraped using a rounded blade or large diamond burr.
The rolled edges of the remaining conjunctiva are unraveled with forceps, and the conjunctival defect is then measured with calipers. The globe is rotated upward with the limbal traction suture and the inferior bulbar conjunctiva away from the pterygium excision is exposed. In general, it is advisable to spare the superior conjunctiva, in case filtration surgery is needed later in life. A limbal edge is preferred and a gentian violet marker is used to mark the outline of the remaining three sides of conjunctival graft to be created (Fig. 21.10C). The conjunctiva is dissected from the underlying Tenon’s tissue by injecting 1–2 ml of local anesthesia (e.g. 1%–2% lidocaine) with a 27-gauge needle. The injection site should be situated away from the conjunctival graft intended for use so as to prevent creating a buttonhole. A small incision is made just peripheral to the superior corner of the marked area. Blunt and sharp dissection is performed with Wescott scissors and non-toothed forceps to ensure that the conjunctiva is separated from Tenon’s, ideally in the plane created by the local anesthesia. The dissection is carried out in a meticulous fashion to the limbus and care is taken to avoid buttonholes. Once the conjunctiva is dissected free from the underlying Tenon’s, the edges of the graft are cut slightly larger than the outline marks. This allows for the natural tissue shrinkage and also incorporates the gentian violet marks, which are useful in aiding graft orientation.
Once the edges of the graft are approximated to the edges of the bulbar conjunctiva, absorbable sutures (e.g. polyglactin) are used to secure the graft. The first sutures are positioned through the two limbal corners of the graft, into sclera, and then into conjunctiva to place the limbal edge of the graft on gentle stretch. If the limbal edge is on good stretch, no additional sutures are required. The next two sutures secure the posterior corners of the graft to the bulbar conjunctiva. Additional sutures are placed to close the wound edges and horizontal mattress sutures may be used to anchor the edges to the underlying sclera (Fig. 21.10D). It is important to suture the graft to conjunctival edges, and not just Tenon’s, as these two tissues may appear similar. A wet swab may be used to distinguish between the two, as Tenon’s tends to stick to the swab whereas conjunctival tissue does not. The conjunctival defect in the area from which the graft is harvested may be sutured, but is usually allowed to re-epithelialize spontaneously. Suture knots are not generally buried and any buttonholes are sutured and anchored to the underlying sclera.
Excimer laser phototherapeutic keratectomy
Angelo Macrì and Carlo Enrico Traverso
Excimer laser phototherapeutic keratectomy (PTK) has been performed to treat anterior corneal pathology for the last 20 years. FDA approved PTK in 199513. Since then indications and techniques have continuously evolved.
Indications and contraindications
The three diagnostic categories better responding to PTK are:
As with any corneal surgery, caution should be taken in patients with potential corneal healing abnormalities, such as neurotrophic corneas (e.g. after herpes simplex or herpes zoster keratitis), exposure keratitis, severe keratoconjunctivitis sicca or blepharitis, collagen vascular conditions (e.g. rheumatoid arthritis) and diabetes mellitus. Additionally, patients with herpes simplex keratitis are at risk14–16.
Recurrent corneal erosions
Recurrent corneal erosion syndrome with anterior basement membrane dystrophy was treated in the past with simple epithelial debridement, therapeutic contact lenses, or anterior stromal puncture. However long-term data have proved that diamond burr superficial keratectomy at the slit lamp and, above all, PTK have significantly minor recurrences13,17,18.
Stromal lesions
Best results are obtained when the opacities are in the anterior 20% of the cornea. It is a general opinion that PTK may be tried in all patients with dense superficial opacities before undergoing a more invasive procedure, such as lamellar or penetrating keratoplasty13,19.
Corneal haze after excimer laser corneal surgery
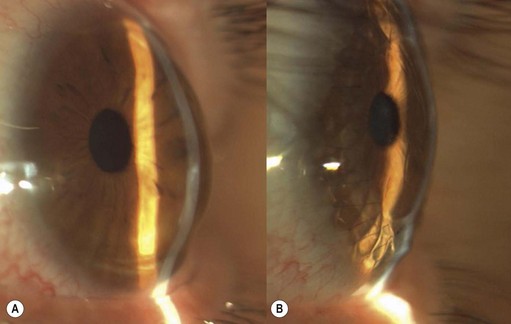
PTK can be also used to treat late haze and subepithelial scarring after PRK (Fig. 21.11). In both cases MMC is utilized because predominant effect of MMC in blocking haze formation was at the level of profound inhibition of the replication of keratocytes and myofibroblast progenitor cells in the corneal stroma that occur following the normal keratocyte apoptosis response to epithelial removal during surface ablation.
Technique
The technique varies according to size, shape, density, and numbers of the cornea lesions (Figs 21.12 and 21.13).
Elevated lesions are generally debulked with a blade before PTK.
PTK can be also performed topographically guided or ‘customized’13,21.
Commonly MMC is used at 0.02% (0.2 mg/ml) concentration, on a 6–8 mm circular sponge for 60 seconds; it is then irrigated with 30 ml of saline13,20,22. Other regimens are also suggested.
Postoperative treatment
Pain is the most important issue in the early postoperative period. Immediately after surgery, the eye is treated with either a bandage soft contact lens or a pressure patch. Ice packs are often very effective in treating the pain. Strong anti-pain medications are also widely used. Eyes with BSCLs are treated with topical antibiotic drops. The pressure patch is removed from the other eyes after 1 day and frequent antibiotic ointment is prescribed. Patients are followed every few days and the antibiotics are continued until the epithelial defect heals. There is a higher risk of postoperative problems such as poor healing, infection, and scarring after PTK than after routine keratorefractive surgery, as these corneas are usually not ‘normal’13.
Summary
PTK is a very versatile technique that is quite effective at treating a wide variety of anterior corneal lesions (Figs 21.14 and 21.15) with a low risk rate if performed properly. In particular elevated lesions and opacities in the top 10–20% of the corneal stroma generally do very well. If unsuccessful, an anterior lamellar graft can almost always be performed and thus PTK may be tried before proceeding to a corneal transplant, considering its excellent risk/benefit ratio13.
Corneal transplantation
Massimo Busin, Amit Patel, Davide Venzano and Carlo Enrico Traverso
Historical perspective
There is considerable debate regarding who first came forth with the concept of replacing diseased or scarred corneas with living tissue. Guillaume Pellier de Quengsy first suggested replacing the diseased cornea with a thin glass disc (similar to a keratoprosthesis) in 1789, and Erasmus Darwin, grandfather of the famed Charles Darwin, first suggested replacing a scarred cornea with a small piece of living donor corneal tissue. Franz Reisinger published the results of his experiments on corneal transplantation in rabbits and chickens in Germany in 182423–25. S.L. Bigger successfully replaced the scarred cornea of his pet gazelle with a healthy donor cornea from another gazelle in 183724. Richard Kissam, a New York ophthalmologist and general practitioner, was the first to operate on a human using a pig donor cornea; however, the graft became opaque shortly thereafter24. The advent of ether and chloroform inhalation anesthesia, topical cocaine anesthesia, local infiltrative anesthesia, and antiseptic surgery during this early period revolutionized corneal transplantation, as it also did for many other types of surgery.
These important companion discoveries were undoubtedly instrumental in accelerating the progress of corneal transplantation. Considerable amounts of experimental work using living corneal grafts ensued. Von Hippel was the first to improve vision with LK, consisting of a full-thickness rabbit donor cornea placed into a human recipient lamellar bed24. He was also the first to use a circular trephine. In 1906 successful total penetrating corneal graft in a human was reported by Zirm in a laborer with bilateral lye burns to the cornea26. Ironically, alkali burns like this are now known to have one of the worst transplantation prognoses. Anton Elschnig of Prague, with his extensive experimental and clinical work in the 1920s through the 1930s, is credited with refining the crude technique into an elegant, reliable procedure. Filatov, Tudor Thomas, Paton, Franceschetti, Paufique, Sourdille, Castroviejo, Arruga, and the Barraquer brothers, all eminent surgeons, based much of their knowledge and methodologies on the foundation laid by Elschnig24. Ramon Castroviejo, a Spanish ophthalmologist practicing in New York City, initiated detailed grafting methodologies, expounding the use of the square graft (Fig. 21.16), and developing and refining keratoplasty instrumentation. A. Edward Maumenee extensively studied the immunology and physiology of corneal graft rejection. Claes H. Dohlman came to the United States from Sweden and established the first cornea fellowship program in 1961, emphasizing the importance of both clinical and research training. With more recent ophthalmological advances consisting of microscopic surgery, inert sutures, antiviral and antibiotic medications, topical and systemic corticosteroids, tissue-preservation/eyebanking strategies, and progress in immunology, the success of corneal transplantation has grown rapidly over the last 30 years. Paralleling these advances, clinical indications for corneal transplantation surgery have also expanded considerably.
The last decade witnessed a strong expansion in the use of lamellar techniques.
The anterior lamellar keratoplasty performed with deep stromal separation, described by Melles (DALK) enables the surgeon to leave the recipient with healthy endothelium, with good visual results27,28.
Endothelial decompensation being the leading indication for PK procedures29–34, there has been strong interest in developing a technique able to preserve the healthy recipient stroma.
Since the original description of the posterior lamellar keratoplasty (PLK) described by Tillett and Barraquer in the 1950s and 1960s, in 1999 Melles demonstrated the first sutureless PLK35. Since then, numerous modifications in surgical technique and new instruments have led to increasing popularity of PLK.
Indications
Indications for keratoplasty have varied over the decades36–38. Box 21.1 lists the classical indications for PK; today many of these conditions may be effectively treated with LK. The Eye Bank Association of America recorded over 41 000 transplants performed in the United States in 2008, with a simultaneous increase in grafts provided for endothelial keratoplasty38. Whilst keratoconus and aphakic bullous keratopathy were major indications for corneal transplantation three decades ago, pseudophakic bullous keratopathy (PBK) rapidly emerged as a leading indication in the 1980s and 1990s38–41. This coincided with the dramatic increase in the number of cataract extractions performed. Optical indications for transplantation include corneal decompensation (e.g. bullous keratopathy and endothelial dystrophies), ectasia (e.g. keratoconus), scarring (e.g. traumatic, post-infectious or iatrogenic), and stromal dystrophies. Tectonic indications (e.g. melting, descemetoceles, perforation) are aimed primarily at preserving globe integrity and may allow optical improvement simultaneously or at a later stage. Therapeutic indications (e.g. medically unresponsive infections) provide the two-fold advantage of excisional biopsy and of elimination of the infected tissue. Causative organisms in these cases are often fungi or acanthamoeba, however, common pathogens causing microbial keratitis may also necessitate therapeutic keratoplasty.
Eye banking/donor selection
The first successful human corneal transplant by Eduard Zirm in 1905 involved the use of fresh corneal tissue from a boy whose eye was enucleated for a penetrating sclera injury. For approximately the following 30 years, all human keratoplasties were performed using fresh corneal tissue from living donors whose eyes had been enucleated due to trauma or disease not involving the anterior segment. In 1937, Filatov from the Ukraine introduced the use of whole globes from cadavers stored at 4°C in a moist chamber. Paton established in the US the first eye bank that involved procurement, processing, and distribution of donor corneas37.
Thus, cold storage and organ culture are the two main methods for preserving the donor tissue today. Cold storage (2–6°C) was introduced in 197442,43, and is commonly used in the USA. The technique is relatively simple and involves refrigeration with minimal tissue handling. No complex equipment is required. At 2–6°C, the metabolic activity of the endothelial cells is minimal and therefore deturgescent agents (e.g. dextran or chondroitin sulfate) are required to keep the cornea in a relatively dehydrated state and thus maintain corneal thickness. The original McCarey-Kaufman (M-K) medium has been modified with addition of antibiotics, energy sources, antioxidants, growth factors, and membrane stabilizing agents to improve cell survival, reduce autolysis, and maintain ultrastructural integrity. Although endothelial cell loss may be attributable to numerous factors independent of storage technique, corneas stored at 2–6°C show intercellular disruption, reduced cell adhesion as well as endothelial cell loss and death44. Modified storage media have increased the storage time for up 2 weeks, one week being most often recommended. Organ culture (31–37°C) was introduced in 197645 and is commonly used in Europe. During the storage phase in culture medium supplemented with fetal or newborn calf serum, antibiotics and antimycotics, the corneas swell considerably and can reach twice the normal thickness. Dehydrating molecules are not added as they are ingested by the corneal cells and lead to toxicity. Thus, a shorter second phase is required to reverse the swelling prior to transplantation. Dextran (4–8%), is added to the same medium to facilitate de-swelling and transportation. This phase in transport medium varies between 1 and 7 days. Overall, organ culture allows for longer periods of storage (up to 4–5 weeks). Advantages over cold storage include ease of scheduling surgery, allowing time for tissue typing/matching, and more rigorous microbiological testing.
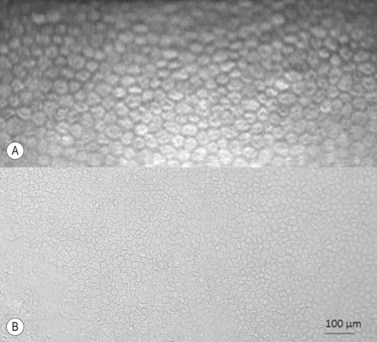
Regardless of the storage methods used, donor and tissue evaluation to assess suitability is critical. It includes obtaining a thorough medical and social history of the donor as well as serological testing, slitlamp biomicroscopy, and endothelial cell examination. The tissue is evaluated for epithelial integrity, epithelial and stromal opacities, presence of foreign bodies and infectious infiltrates, evidence of previous anterior segment surgery, degree of stromal clarity and thickness, presence of Descemet’s membrane folds and extent of endothelial guttae, snail tracks, and precipitates. Specular microscopy provides detailed microscopic analysis of individual cell morphology and an estimate of cellular density (Fig. 21.17A), however for a better and wide examination light microscopy is applied (Fig. 21.17B). The cell counts may be obtained by automated image analysis or manual counting methods.
Donor tissue has to be considered as non-sterile and effective decontamination must be in the routine. Antibiotics are less effective in cold storage than organ culture as the contaminating microbes are metabolically less active at low temperatures. For this reason, preoperative warming of the storage media is important to enhance the decontamination effect. Individuation of microbial contamination may also be better with organ culture since contamination will become more readily evident and it may therefore be considered as the storage method of choice in circumstances where corneas are suspected of being at a higher risk of contamination. However, stringent screening protocols by eye banks have minimized the risk of microbial transmission from donor to recipient and the accurate valuation of medical and social history of the donor allowed rare incidences of systemic disease like rabies, hepatitis B, Creutzfeldt–Jakob disease (CJD) and malignancies44–48. No case of seroconversion after unplanned transplantation of corneas from human immunodeficiency virus (HIV) positive patients has been reported to date49–51.
Irrespective of the storage methods, prospective studies analyzing short and long-term follow-up after PK have not found any difference in endothelial cell density or clinical outcomes52–54.
Preoperative evaluation
Examination and investigations
The status of the crystalline lens or the IOL should be assessed to ascertain the need for concurrent cataract extraction or IOL repositioning, explantation, or exchange. Preoperative biometry may not be as predictive of the final refractive status when IOL implantation is combined with PK as when it is performed in the absence of corneal disease or surgery. A combination of good clinical judgment, measurements from the other eye, reasonable choice of IOL formulas, and personalization of equations using regression analysis to estimate the surgeon’s refractive tendencies, may enhance the predictability; high refractive errors are still occurring in combined PK/IOL cases55,56.
The decision to render the eye myopic, emmetropic, or hyperopic should be influenced by the patient’s need, and the refractive status of the contralateral eye. In general, a final myopic error will lend itself better to further correction with laser or surgical treatment. The condition of the iris/iridocorneal angle and integrity of the capsular bag may be a determinant of the type of IOL to be implanted. Alternatively, the eye may be left aphakic, with view to secondary IOL implantation at a later stage. This may yield better postoperative refraction and unaided visual acuity but delays visual rehabilitation and risks damage to the graft57,58.
Penetrating keratoplasty
Preparation of recipient
This is in part an open-sky procedure and all precautions to prevent intraoperative suprachoroidal hemorrhages should be taken59.
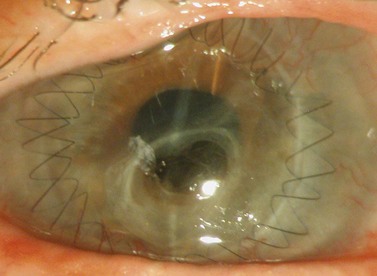
A sterile drape is applied over the eye following application of povidone-iodine into the fornices and periocular area. Various specula are available and an appropriate one must be used which offers the benefit of greatest exposure with minimal distortion and compression of the globe. Scleral support rings (e.g. Flieringa ring) should be used to maintain the structure of the globe, especially in aphakic and vitrectomized eyes or whenever lens extraction or IOL manipulation is planned, where the scleral wall may distort or collapse once the eye is open. The McNeill–Goldman speculum serves a dual purpose by combining two Flieringa rings within a speculum (Fig. 21.18). The scleral ring is secured onto the episclera with 5-0 or 6-0 polyglactin.
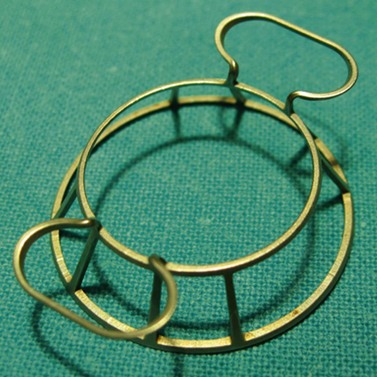
Fig. 21.18 McNeill Goldman belpharostat composed of a double scleral fixation ring and attached wire speculum.
The recipient cornea is then measured with a caliper. Sizing and centration of the host trephination depend on several factors including the size, location and extent of pathology, host corneal diameter, extent of vascularization, and risk of rejection. The area of trephination may be decentered or oversized in order to either avoid or better encompass zones of pathology, such as areas of excessive corneal thinning, overhanging glaucoma filtering blebs, foci of infection, or inferior conical apices in keratoconus. The disadvantages of large, decentered grafts include high astigmatism and problems inherently associated with the proximity of the graft–host junction to the limbus, i.e. increased risk of rejection and glaucoma. In the absence of any overriding factors, surgeons may prefer to center the graft over the pupil, i.e. a slight nasal displacement, or at the geometric center of the recipient cornea. The center of the intended trephination is marked with indentation using the tip of a cannula; gentian violet is used as a marker on the epithelium side. Radial marks to guide suture placement may be made using a gentian-violet stained 8-blade radial marker (Fig. 21.19).
Donor preparation/sizing
The donor corneoscleral buttons are commonly trephined with a disposable punch block apparatus with the endothelial side facing up. Once correct alignment is confirmed, the blade is pressed onto the corneoscleral button. Firm pressure is maintained whilst the scleral rim is moved with a pair of toothed forceps to ensure a 360° cut. The graft may occasionally remain engaged within the trephine cavity and may be gently dislodged using forceps by grasping the epithelial/stromal edge or injecting viscoelastic into the core of the trephine. The tissue is then kept submerged in storage medium or viscoelastic until the recipient cornea has been excised. This posterior punch method has been shown to result in more regular cut edges compared with an anterior approach which is performed with a suction trephine and an artificial chamber when not on a whole eye60,61. Cutting from the endothelial side results in a slightly smaller graft (by 0.2–0.3 mm) than the blade diameter as a result of the flattening of the tissue when punching from the endothelial side62–64. On the other hand, suction and downward pressure on the recipient cornea causes the tissue to protrude into the trephine, resulting in a marginally larger host cut than the trephine diameter. Thus, use of same-size trephines for the host cornea and donor cornea (posterior punch) result in a graft that is smaller than the recipient bed. For this reason, the donor is usually trephined larger in diameter than the recipient bed, compensating for the slight disparity in wound edges obtained by cutting the donor and recipient cornea by different approaches and ensuring a watertight closure62,63,65. Achieving watertight closure of the wound with even a 0.25 mm disparity is noticeably more demanding than with a 0.5 mm donor oversize; same-size or undersize grafting requires even tighter suturing and often a greater number of suture bites to maintain watertightness.
Many studies have looked into the theoretical advantages of reductions in hyperopia and myopia by either oversizing or same-/undersizing the grafts respectively66–72. Results are, however, not reliable since numerous confounding factors (i.e. tissue elasticity, trephine type, tissue healing, surgeon expertise underlying disease follow-up to name a few) do not allow accurate prediction of postoperative refraction by simply selecting a graft diameter.
Host trephination
Before trephining, a peripheral corneal paracentesis should be performed, preferably inferotemporally. This will be useful to access the AC both intraoperatively and postoperatively. The host cornea may be trephined using a variety of suction and manual trephines. A disposable vacuum trephine like the Hessburg–Barron offers the advantage of semi-quantitative gradinal circular cutting of the host. The cross hairs on the trephine are used to align the trephine with the gentian violet centration mark on the recipient cornea. Firm downward pressure with a trephine delineates the size of the intended circular cut and the trephine is lifted to check the marking. Cellulose spears may be used to dry the corneal surface in order to improve visualization of the mark. When satisfactory alignment is achieved, the vacuum is applied and the blades advanced slowly until the anterior chamber is entered with a hand-held manual trephine; centering is ensured by marking the epithelium with downward pressure, and carefully aligning the blade with the most centered mark. It is advisable to deepen the AC injecting a moderate amount of viscoelastic trough the paracentesis, to dampen the AC entrance of the blade and decrease chances of iris/lens damage. Use a 90° rotation and counter-rotation while exerting a very gentle downward pressure; as soon as the AC is entered, both rotation and downward pressure must be stopped. When the point of entry is identified curved corneal scissors (Fig. 21.20) are used to complete the trephination. The scissor tips must always be in view and held perpendicular to the corneal surface. If the posterior wound bevel is excessive, it may be trimmed with miniature Wescott or Vannas scissors. The tissue should be trimmed as a long, single, continuous strip whenever possible to avoid the formation of multiple jagged edges of stroma and Descemet’s membrane. Overly enthusiastic trimming, leading to an undermining cut of the Descemet’s membrane, may increase astigmatism and the chance of wound leakage. After excision of the host corneal button, it is important to ascertain that Descemet’s membrane from the excised button is not retained within the eye. In highly edematous corneas, Descemet’s membrane is easily stripped off and may inadvertently be left behind in the eye.
In eyes with a higher risk of suprachoroidal hemorrhage, a small portion of the recipient cornea may be left intact thereby creating a hinge which allows the cornea to be reflected back away from the surgical field, but available to flip over and close the eye should the need arise73; in some explosive cases the same procedure is performed with a surgeon finger.
Suturing
Following placement of the cardinal sutures, the graft may be closed by a variety of suturing methods including; all interrupted sutures, single or double continuous suture, antitorsion suture, double continuous suture, single continuous with interrupted sutures, deep sutures, etc. In each of these varieties, the number and orientation of suture bites may also vary. If a double continuous 10.0 nylon suture each with approximately 16 bites is adopted, the first suture is tied at the 12 o’clock position and the second placed at 6 o’clock with bites alternating with those of the first. Both knots are buried in the donor cornea. This method allows even distribution of tension, good wound closure and security in the event of one suture breaking. A combination of continuous and interrupted sutures might allow earlier visual rehabilitation by selective removal of sutures (Fig. 21.21).
Whilst the suturing technique used is highly dependent on surgeon preference, there are some instances where interrupted sutures are preferable, i.e. presence of inflammation, ulcerations, infections, or multiple previous rejections, allowing for selective removal in case of suture-related problems. Despite the numerous patterns of suturing that exist74–76, no single technique has been consistently proven to be superior77–82.
Penetrating keratoplasty with shaped wound configurations
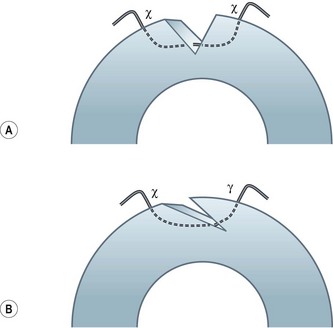
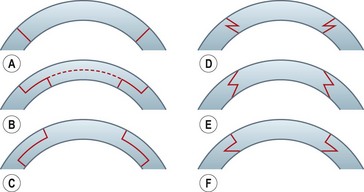
Shaped wound configurations as an alternative to perpendicular incisions for penetrating keratoplasty were described as early as the 1950s, and various modifications have been reported since83–86. Accuracy of trephination and reproducibility of the manual preparation techniques have been difficult. Furthermore, a slight mismatch in size when preparing a ‘single piece’ graft may result in high and irregular astigmatism. A ‘top-hat’ configuration described in 200387 apparently offered the advantage of more secure wound closure, and maintained the anterior graft surface at a reasonable distance from the limbus, thus reducing the theoretical risk of rejection. A microkeratome-assisted technique to perform ‘mushroom’ shaped grafts in eyes with deep central scars has also been described88. This may offer better visual and refractive outcomes compared with conventional PK, whilst preserving much of the healthy recipient endothelium. Various other types of wound configurations have since been proposed using either manual or femtosecond laser-assisted preparation, e.g. zig zag, Christmas tree, half top-hat, dovetail, etc.89–95 (Fig. 21.22). Whilst the femtosecond laser provides precise wound edges and eases graft/donor preparation, its use is greatly limited by the poor penetration through corneal opacities, and by the high cost.
Various studies have shown that shaped wound edges provide better wound stability and healing than conventional straight edge PK wounds88,92–94. Apart from providing better wound apposition, some of the shapes offer additional advantages, e.g. a top-hat configuration results in more endothelium being transplanted and would be suited to patients with endothelial decompensation who also have a central stromal opacity. The precise shape decided upon depends on the pathology and its extent.
Two different shaped techniques are described below.
Microkeratome-assisted mushroom keratoplasty
The ‘mushroom’ configuration is indicated in eyes with full-thickness central stromal opacities, for example post-traumatic and post-infectious scars, deep stromal dystrophies, and degenerations in the presence of normal endothelium. Deep anterior LK may not be feasible in these cases due to the presence of deep scarring. The ‘head’ of the mushroom offers the advantage of lower postoperative astigmatism95 whilst the ‘stem’ allows preservation of most of the recipient endothelium A microkeratome-assisted method of creating a mushroom configuration consisting of two separate lamellae can overcome the difficulties of manual preparation88 (see Fig 21.22B). The use of an anterior and posterior lamella offers the advantage of allowing: (i) the lamellae to be sized exactly as the recipient bed, thus minimizing the risk of astigmatism and (ii) optimal positioning of the lamellae, i.e. the anterior lamella can be centered on the limbus and the posterior lamella on the pupil or slightly off center to encompass the area of excised scar.
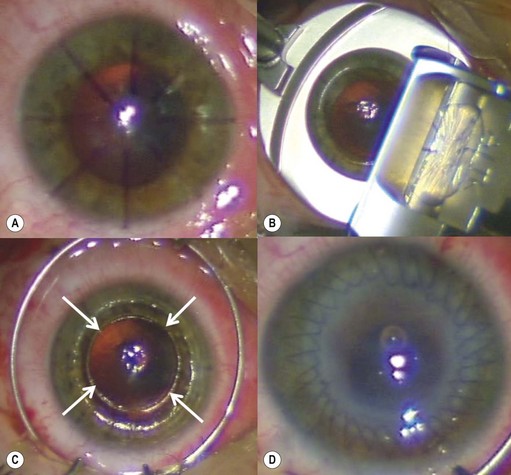
The recipient eye preparation and marking are as per PK (described above). A 9.0 mm suction trephine is used to create a partial thickness incision between 250 µm and 300 µm depth (Fig. 21.23A). Manual lamellar dissection is performed using a round crescent blade starting at the base of the incision and advancing centrally (Fig. 21.23B). A central island about 5.0–6.5 mm diameter, including the mark used for centration of the suction trephine, is spared to facilitate concentric alignment of the second trephination (Fig. 21.23C). This peripheral rim of manually dissected stroma lies off the visual axis and therefore does not affect postoperative visual acuity. On the contrary, surface irregularities induce increased scar formation over this wide area and thus speed up wound healing and allow for early suture removal.
The donor cornea is dissected with a 250 µm or 300 µm microkeratome head. The anterior lamella obtained is positioned on a trephination block and punched to 9.0 mm (Fig. 21.23D). The posterior lamella of the graft is marked with trypan blue and punched to size from the endothelial side using a 5.0–6.5 mm trephine (Fig. 21.23E). A same-sized (5.0–6.5 mm) trephination is performed in the recipient bed concentrically to the initial 9.0 mm peripheral incision (Fig. 21.23F). Oversizing the posterior graft must be avoided to prevent overlapping of the deep wound edges. Caution must be exercised to avoid damage to the intraocular structures during this central trephination, as the residual corneal layer is quite thin. It is therefore preferable to outline the incision by applying gentian violet or trypan blue and enter the anterior chamber with a 15° or diamond blade. The button is then excised with corneal scissors (Fig. 21.23G) and care must be taken to ensure clean, straight edges.
The posterior graft (endothelium and deep stroma) is simply placed on the central recipient bed (Fig. 21.23H). A small amount of viscoelastic material may be placed in the anterior chamber, especially in the presence of an intraocular lens, to avoid direct contact between the lens and the donor endothelium. No sutures are used to secure this posterior lenticule, and the 9.0 mm anterior donor lenticule is placed over it (Fig. 21.23I). The anterior lenticule is then sutured with double continuous 10.0 nylon sutures. It is essential for the superficial wound to be watertight in order to allow the intraocular pressure to push the posterior graft against the overlying anterior lamella. Figure 21.24 shows the pre- and postoperative appearance of the same surgical case illustrated in Figure 21.23. A disadvantage may be the development of interface scarring between the two lamellas that can compromise the functional recovery.
Manual top-hat penetrating keratoplasty
The ‘top-hat’ configuration is indicated in eyes that have endothelial decompensation with complete central stromal opacities and are at a high risk of rejection or inflammation, for example corneas with vascularization or limbal inflammation. This configuration offers the advantage of maximal endothelial transplantation, whilst maintaining the anterior donor cornea at a reasonable distance from the limbus87,90.
The donor button is mounted on the artificial anterior chamber, the geometric center of the cornea is marked, and a 7.0 mm Barron suction trephine is used to make a circular, deep anterior incision to 300 µm depth. A lamellar stromal dissection is performed peripherally from the base of the incision to the limbus using a bevel-up blade. The cornea is then removed from the artificial anterior chamber and placed on a suction punch with the endothelial side up; care is taken to align the mark of the geometrical center with the cross hairs of the punch and a 9.0 mm donor button is punched. As a result of the previous lamellar dissection, a superficial annular stromal lamella, 300 µm in thickness, can be removed in the area between 7.0 and 9.0 mm diameter. The donor button obtained thus consists of a central, full-thickness portion, 7.0 mm in diameter, surrounded by a peripheral lamellar wing of deep stroma and endothelium (see Fig. 21.22C).
Lamellar keratoplasty
Lamellar keratoplasty (LK) offers the advantage of retaining healthy recipient tissue and minimizing the amount of donor tissue required for transplantation. In stromal disease, the recipient endothelium may be spared, thus reducing the risk of rejection, and in many instances resulting in a faster visual rehabilitation96. Other advantages include maintenance of corneal strength, ease of donor tissue replacement e.g. in disease recurrence, and avoidance of open-sky surgery96–99. The use of LK to perform PK (mushroom keratoplasty) is discussed above.
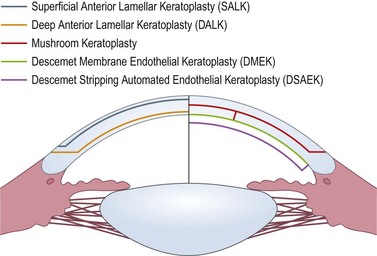
LK may be performed manually, with use of a microkeratome, or with excimer and femtosecond lasers. The improvement in surgical instrumentation and advent of lasers have led to numerous types of LK (Fig. 21.25), and various methods of performing these procedures99–104. Microkeratome-assisted preparation techniques will be discussed here.
Anterior lamellar keratoplasty (ALK)
Superficial anterior lamellar keratoplasty (SALK)
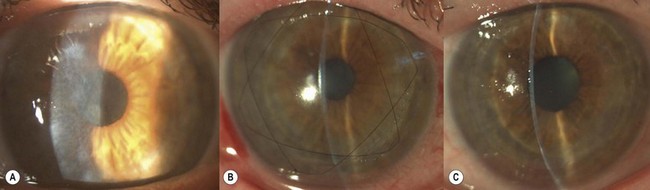
SALK is indicated in the treatment of superficial corneal opacities resulting from previous refractive surgical procedures, infections, trauma or degenerations and dystrophies (Fig. 21.26A)103,104. Tissue removal is limited to what can be encompassed within a 130–200 µm dissection; however, even in the presence of some residual opacities, sufficient visual improvement may be experienced. The procedure has a smoothing ‘soft contact lens’ like effect over minor surface irregularities, however, marked irregularities may be reproduced within the recipient bed.
A speculum with adjustable blades is positioned (Fig. 21.27). This allows maximal exposure, thereby ensuring adequate placement of the suction ring and free passage of the microkeratome head. The recipient bed is dissected using the 130 µm or 200 µm microkeratome head and a ‘0’ suction ring. This usually results in a lamella of 130–160 µm or 200–250 µm thickness with a 9.0 mm diameter. As no radial conventional suturing is performed, marking the recipient cornea before the keratectomy is not necessary. Peripheral vascularization, if present, may cause bleeding and hemostasis must be achieved prior to proceeding as interface blood may compromise long-term visual acuity. The diameter of the recipient bed is measured in both the vertical and horizontal meridians.
The graft is simply laid onto the recipient bed and adheres in a similar fashion to a LASIK flap. However, as there is no hinge to hold the graft in place, two overlay sutures are used to secure the graft position (Fig. 21.26B). To place these sutures, the needle is passed only through the peripheral recipient cornea at three equidistant points. To avoid permanent folds in the graft or lifting of the donor peripheral to the suture, the suture must not be too tight. After placing the overlay sutures, the interface should be copiously irrigated to remove any possible debris. It is also important that all portions of the sutures lie on the surface of the cornea and not in the interface. A bandage contact lens may be used to hold the graft instead of sutures. Conventional radial sutures (continuous or interrupted) are contraindicated in this procedure, as the thin graft easily distorts, inducing high degree irregular astigmatism.
The overlay sutures must be removed within 7 days of surgery. Figure 21.26C illustrates the appearance after suture removal. Delaying suture removal may cause permanent folds or prevent complete re-epithelialization of the graft as the overlay part of the sutures may act as a physical barrier against the advancement of the epithelial cells. As with any lamellar technique, interface scarring may compromise the functional recovery.
Deep anterior lamellar keratoplasty (DALK)
Microkeratome heads with slits larger than 130 µm may be used to remove mid- and deep stromal opacities (e.g. lattice dystrophy, post-trauma and infection scars); however, if the donor lamella to be sutured is cut using a head with a slit larger than 130 µm, adequate suturing is required (as per PK) and the postoperative management and rehabilitation differs from SALK105 (Fig. 21.28).
Several lamellar dissection techniques have been described previously106–112, many of which are limited by the inaccuracy of depth judgment and reproducibility. Reaching Descemet level in cases with deep stromal scarring has been challenging and the risk of perforation and uneven stromal beds has hindered the popularity of manual LK. Air, saline, and viscoelastic have been used to facilitate stromal dissection and separation of Descemet’s membrane110–115.
Manual dissection
Melles described a technique of manual dissection of the anterior lamella up to the level of Descemet membrane guided by an air bubble in the anterior chamber116,117. Aqueous is replaced with air via a small self-sealing port and the resulting air/endothelium interface acts as a convex mirror. When the specifically designed dissectors are introduced via a limbal incision, a dark band is observed immediately in front of the instrument. This band represents the residual stroma (between the dissector and air bubble) and hence the surgeon is able to advance the dissector deeper until the band disappears. At this point the dissector is positioned parallel to the surface. Once dissection is complete, air is partially removed from the anterior chamber and viscoelastic is injected into the dissected plane. This is followed by trephination of the anterior cornea. This technique relies on relative clarity of the cornea to allow view of the endothelium/air reflex for judgment of dissection depth. Residual stroma is more likely with this technique if the Descemet plane is not found at the start of dissection.
Air bubble technique
In 2002, Anwar described the ‘big-bubble’ technique, a modification of previously described air usage to achieve Descemetic separation118. Partial thickness stromal dissection between 60 and 80% depth is carried out using a calibrated trephine. A 27–30 gauge needle or a dedicated cannula mounted on an air filled syringe is then inserted bevel down, from the trephined edge and advanced 3–4 mm into the central stroma. Air is injected to form a big bubble between Descemet membrane and the overlying stroma. A paracentesis is created peripheral to the bubble to soften the eye as required. Partial anterior stroma is then removed, leaving a small layer over the bubble. A sharp pointed blade is thereafter introduced through the residual stroma and into the bubble. This is followed by careful dissection of remaining stroma. This technique is more successful in achieving a Descemetic plane of dissection compared with manual techniques and the lack of a stromal interface has been suggested to result in better visual outcomes119–121. Success rates in achieving a big bubble, however, vary and have been reported to be between 64% and 90%118,120–122. This variation even in experienced hands may be due to difficulties in obtaining a uniform plane of dissection in scarred or irregular corneas. Descemet membrane perforation may occur with both the above techniques and care must be taken to avoid these. Whilst microperforations may still allow a successful LK to be completed, the surgeon must exercise caution to avoid enlargement of the perforation during dissection of the remaining stroma and suturing of the donor tissue. Large or central perforations may necessitate conversion to PK118,122–124.
Others have described DALK without the use of viscoelastic substances or air125. DALK has been shown to produce favorable outcomes in comparison with PK125–129 and some alterations to the above techniques may help achieve better success rates130,131. These techniques require a learning curve and the meticulous separation is time consuming; should the hair bubble be only partially obtained, completion of the lamellar dissection is technically demanding.
Automated LK for keratoconus
Microkeratome-assisted LK for keratoconus and later modifications have been described in the past132.
Radial marks are placed on the recipient cornea and an anterior lamellar dissection is performed with a microkeratome (Fig. 21.29A,B). To facilitate the use of the ALTK system (Moria) in eyes with regular curvature between 38 and 46 diopters (D), the manufacturer has developed a nomogram-correlating diameter of the lamella to be obtained with keratometric readings (K readings) of the recipient cornea and the use of four different suction rings (namely –1, 0, +1, and +2). However, based on empirical in keratoconic eyes, a lamella 9 mm in diameter can be obtained in most cases by using the ‘0’ suction ring on corneas with average K readings up to 55 D and the ‘+1’ suction ring on corneas with average K readings of more than 55 D. However, if cauterization of the central cornea is performed before dissection, substantial flattening occurs, and the ‘0’ ring should be employed. In order to prevent perforation, the surgeon should aim to leave at least 100 µm of recipient stromal bed. Thus, the choice of microkeratome head is dependent on the pachymetry map obtained preoperatively. Because the microkeratome tends to cut grafts slightly thicker than the stated width of the microkeratome slit, a 250 µm head should be employed only in corneas 400 µm or thicker, while the 200 µm head may be used on corneas with a thickness as low as 350 µm. It is advisable to use the 130 µm head for corneas thinner than 300 µm.
In order to re-establish a physiologic corneal thickness, the donor tissue is dissected with a microkeratome head that has a slit at least 100 µm wider than the slit of the head used on the recipient cornea. The recipient bed is measured and the lamella obtained is punched to the same size. The recipient bed is then trephined with a 6.0–6.5 mm trephine (Fig. 21.29C). Thus the central part of the residual cornea (i.e. a lamella of deep stroma and endothelium) is practically detached from the peripheral recipient bed, similar to PK surgery, but is left in place, to attach to the posterior surface of the donor stromal graft. The cone in the recipient bed therefore ‘collapses’, and does not push against the overlying lamellar graft but simply adheres to it. This central trephination also improves the apposition between the host and donor surfaces thereby reducing the formation of folds on the interface. No particular tension is therefore needed while suturing, as the resistance from the ectactic bed is eliminated. Hence, there is no reason to undersize the donor button. With time, the 6.0–6.5 mm circular vertical incision in the recipient bed scars, binding together donor graft and host cornea at the site of trephination. This further stabilizes the postoperative structure of the cornea, thus acting as a barrier against the changes induced by suture removal.
The graft is secured with two running 10-0 nylon sutures (Fig. 21.29D). No suturing of the posterior lamella is necessary; however, the anterior chamber is reformed at the end of surgery with balanced salt solution (BSS) to ensure that the two lamellas remain attached. Figure 21.30 illustrates the pre- and postoperative appearances following automated LK.
Posterior lamellar keratoplasty (PLK)
Descemet stripping automated endothelial keratoplasty (DSAEK)
DSAEK is rapidly replacing PK as the treatment of choice in patients with endothelial failure. Whilst the vast majority of these patients would be amenable to DSAEK surgery, the presence of stromal scars or high/irregular astigmatism in post PK eyes may be relative contraindications. Indications are summarized in Box 21.2.
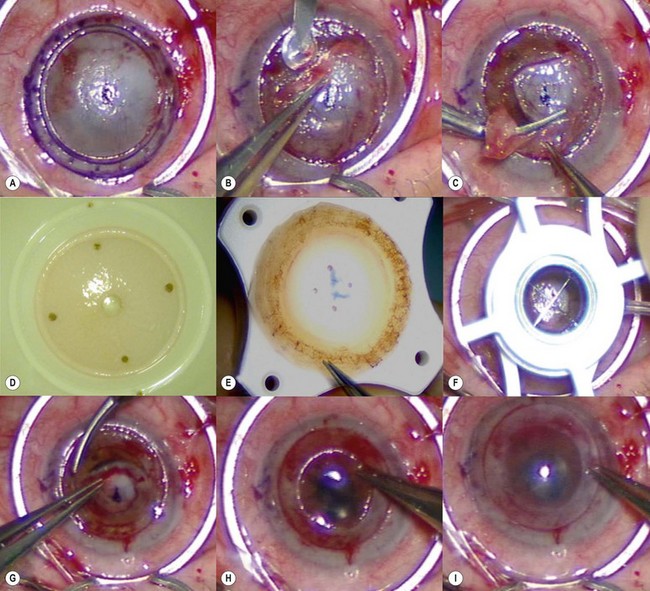
Loose and edematous epithelium is ideally removed from the recipient cornea prior to commencing surgery. This allows better intraoperative visualization, and postoperative epithelialization. A descemetorhexis is performed in the recipient eye using a 25-gauge needle mounted on a 2.5 cc empty syringe. The tip of the needle is bent approximately 45° upwards. It is then inserted into the anterior chamber at the 12 o’clock position and a small aliquot of aqueous (about 0.2–0.3 cc) is aspirated and discarded. The syringe is filled with air and reintroduced into the eye via the same incision. Air is injected into the anterior chamber and the tip of the needle is used to scour the endothelium and Descemet membrane (Fig. 21.31A). A 25-gauge cannula, Descemet stripper, or reverse Sinsky hook may be used in combination to strip the Descemet and endothelium. A temporal stab incision may be made to allow replenishment if air if required. Descemetorhexis under air fill allows better visualization of the membrane; however, it may be performed with BSS or viscoelastic material in the anterior chamber133–137. Viscoelastic use is best avoided, as retained material may hinder graft attachment. The Descemet membrane may be left in situ in the absence of central guttae, in instances where the anterior chamber visualization is poor, or where air cannot be maintained in the anterior chamber.
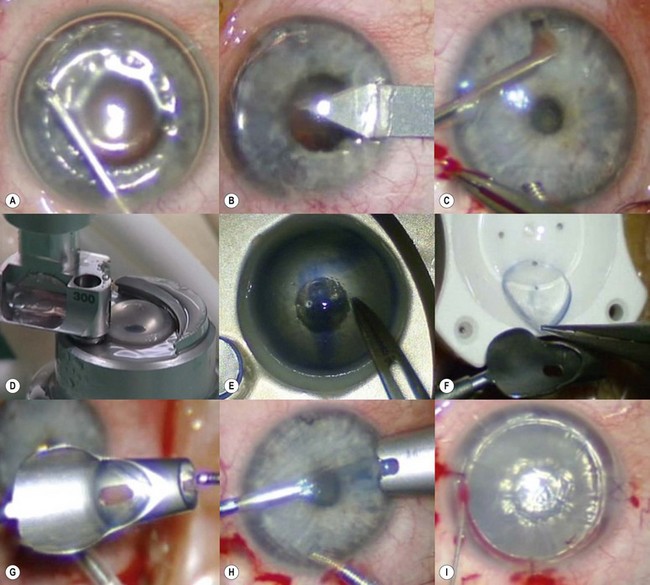
A clear-cornea tunnel, 1 mm in length and 3.2 mm in width, is prepared nasally (Fig. 21.31B) for graft insertion and a stab incision with a 15° blade is made temporally for insertion of the Busin forceps if this has not already been made for air replenishment. The 12 o’clock wound previously utilized for descemetorhexis is enlarged with the 15° blade and an anterior chamber maintainer positioned through it. The irrigation bottle connected to anterior chamber maintainer should be positioned at a height of approximately 45–50 cm. If the bottle is placed too high, it can lead to iris prolapse and malpositioning of the graft during insertion. A vertical vitreoretinal scissor is used to perform an inferior iridotomy (Fig. 21.31C) in order to prevent pupillary block and Urrets–Zavalia syndrome secondary to the complete air fill at the end of surgery.
The donor graft is mounted on an artificial chamber and the anterior stroma is removed by means of a 300–350 µm microkeratome head (Fig. 21.31D). The residual stromal bed is dried with a sponge and a 9 mm circular marker is used to outline the area of dissected stroma. This is performed to aid correct centration on the donor punch. An asymmetrical orientation mark (letter ‘F’ or similar) is made on the anterior stroma using trypan blue (Fig. 21.31E). The donor is then placed on a punch, centered using the aid of the 9 mm ring mark and punched to the desired size. Ideally the donor size should be 2 mm smaller than the vertical recipient corneal diameter to allow peripheral clearance.
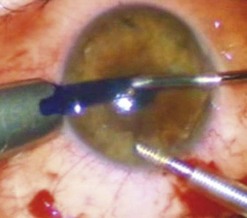
The graft is loaded onto a glide (Fig. 21.31F) and advanced through the mouth of the glide until a small area protrudes beyond the edge of the mouth (Fig. 21.31G). The glide is then turned over 180° and positioned nasally. A Busin forceps is introduced through the temporal wound and out through the nasal wound to grasp the protruding end of the graft. The graft is pulled into the anterior chamber (Fig. 21.31H) and, upon delivery, the anterior chamber maintainer is removed and the graft positioned with gentle tapping on the corneal surface. A 25-gauge cannula with a flat tip may be introduced into the interface to manipulate the graft.
Whilst studies show a lower endothelial cell loss with the use of a glide138,139, alternative methods of grafts insertion (e.g. folding and delivery with forceps, sutures, needles, or cartridges) may be employed140–142. Air is then injected under the graft when correct centration is achieved and all corneal wounds are sutured airtight with 10.0 nylon. Finally, a tight air fill is achieved via a 30-gauge needle inserted obliquely into the anterior chamber (Fig. 21.31I). Venting stab incisions may be made in the peripheral cornea to release trapped fluid from the graft–host interface to aid graft attachment137,143. These can occasionally lead to complications and may be reserved for challenging cases, e.g. post-PK eyes144. A soft bandage contact lens is placed if epithelium debridement was carried out. A patch is placed over the eye and patients are advised to remain in a supine posture for 6–8 hours.
Descemet membrane endothelial keratoplasty (DMEK)
In contrast to DSAEK, only the Descemet membrane and endothelium are transplanted in DMEK surgery. This avoids formation of a corneal interface and therefore may optimize visual outcome and speed up postoperative visual rehabilitation145. However, the grafts are considerably thinner than DSAEK grafts and pose a challenge in preparation, delivery, and attachment. Various methods of mechanical dissection of donor tissue have been described146–149. Preparation of the donor tissue is challenging and highly dependent on the surgeon’s skills. Wastage of up to 16% of donor corneas and an endothelial cell loss of >8% immediately after donor preparation have been reported146,148. Pneumatic dissection of the donor cornea results in a lower endothelial cell loss and wastage150,151 and if desired by the surgeon also allows the use of stromal support152,153.
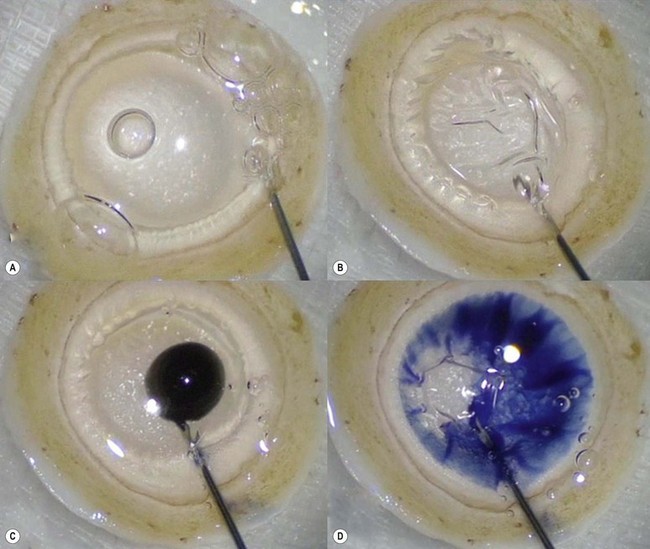
The recipient eye preparation is as per DSAEK surgery. For pneumatic dissection, the donor cornea is mounted on an artificial anterior chamber and about 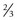 of the anterior stroma is removed by means of a microkeratome as in DSAEK surgery. This step is not essential, but allows the surgeon to convert to DSAEK in case the ensuing preparation fails. The donor cornea is removed from the artificial anterior chamber and placed on sterile gauze with the endothelium facing up. Next, a 25–30 gauge needle connected to a 10 cc syringe is inserted bevel up into the peripheral cornea at about 1 mm from the limbus and advanced in a tangential direction immediately beneath the endothelium for about 2 mm. Air is then injected until detachment of Descemet membrane is achieved and a large bubble is obtained (Fig. 21.32A). This part of the preparation can be performed either by the surgeon at the table or by a technician in the eye bank. In the latter case, the cornea with the ‘endothelial-Descemet bubble’ can be stored in tissue culture medium for up to 7 days before delivery to the surgeon. Part of the air from the bubble is then removed to achieve a partial collapse (Fig. 21.32B) so as to allow injection of trypan blue into the bubble (Fig. 21.32C). Finally the residual air is aspirated, which allows the trypan blue to stain the area of Descement dissection (Fig. 21.32D). This ensures precise punching inside the outer limit of dissection.
of the anterior stroma is removed by means of a microkeratome as in DSAEK surgery. This step is not essential, but allows the surgeon to convert to DSAEK in case the ensuing preparation fails. The donor cornea is removed from the artificial anterior chamber and placed on sterile gauze with the endothelium facing up. Next, a 25–30 gauge needle connected to a 10 cc syringe is inserted bevel up into the peripheral cornea at about 1 mm from the limbus and advanced in a tangential direction immediately beneath the endothelium for about 2 mm. Air is then injected until detachment of Descemet membrane is achieved and a large bubble is obtained (Fig. 21.32A). This part of the preparation can be performed either by the surgeon at the table or by a technician in the eye bank. In the latter case, the cornea with the ‘endothelial-Descemet bubble’ can be stored in tissue culture medium for up to 7 days before delivery to the surgeon. Part of the air from the bubble is then removed to achieve a partial collapse (Fig. 21.32B) so as to allow injection of trypan blue into the bubble (Fig. 21.32C). Finally the residual air is aspirated, which allows the trypan blue to stain the area of Descement dissection (Fig. 21.32D). This ensures precise punching inside the outer limit of dissection.
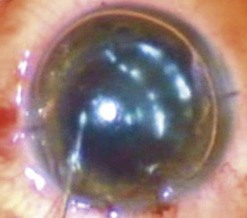
An 8–9 mm punch is used to punch the donor tissue, which consists of deep stroma and overlying detached Descemet membrane and endothelium. The Descemet/endothelium complex remains flat as it lies on the stromal bed and thus the formation of a tissue roll is prevented (Fig. 21.33). Forceps are used to transport the entire tissue onto the plate of a glide. It is then advanced through the distal part of the glide and the endothelium is selectively pulled gently from its edge until it protrudes out of the funnel. Finally, a bimanual ‘pull-through’ maneuver similar to that used for DSAEK surgery is employed to deliver the graft under continuous irrigation through an anterior chamber maintainer (Fig. 21.34). A full air fill is achieved following secure closure of all wounds with 10.0 nylon sutures (Fig. 21.35).
Various shapes (Fig. 21.36) of stromal support can be employed to aid DMEK surgery, and recently the use of a peripheral ring of stroma has been successfully described154,155. However, the sickle shape offers quick preparation and ease of handling, and insertion as well as attachment of the tissue to the level experienced in DSAEK surgery153. To achieve this, the pneumatically dissected tissue is punched slightly eccentrically to incorporate a ‘sickle’ of stroma outside the area of Descemet detachment. The frame of stromal support allows the tissue to be marked and hence reduces the risk of upside-down transplantation whilst also allowing the graft to unfold spontaneously upon insertion into the eye.
The rolled endothelium Descemet complex is introduced on the AC with an injector through a 3 mm corneal incision156.
Postoperative care
All patients are generally commenced on 2-hourly topical steroid and antibiotic combination. The frequency may be altered according to the circumstances and type of surgery. The antibiotics are withheld after 2–4 weeks and steroids are gradually tapered to a once daily administration on the absence of any adverse effects. The frequency of steroids is increased if additional procedures are undertaken, e.g. removal of sutures, cataract surgery, etc. In some circumstances, e.g. phakic patients, post-anterior lamellar surgery, steroid responders, etc. the steroids are tapered sooner. In patients with steroid induced glaucoma who still require steroid therapy, the drops are switched to preparations that are less likely to induce IOP elevation, e.g. fluoromethalone (FML), loteprednol (Lotemax). Concomitant antiglaucoma medication is continued or temporarily increased. In herpetic grafts, antiviral agents should be used as long as corticosteroids are in use. Topical antivirals should be tapered rapidly to a very low dose (paralleling the topical corticosteroid dosage) within weeks to prevent epithelial toxicity. Oral acyclovir (aciclovir) is not toxic to the corneal epithelium and its efficacy in preventing or treating herpetic relapse has been shown157. Oral antiviral agents should be prescribed prior to surgery and continued for at least 1 year postoperatively. Cycloplegics may be used for treating iritis, improving comfort and preventing synechiae. Whilst there is weak evidence to suggest that its use may lead to permanent pupillary mydriasis in keratoconic eyes, when necessary, mild, short-acting agents are recommended158,159. Topical NSAIDs should generally be avoided, due to recent reports of corneal melting and persistent epithelial defects following PK160,161.
Postoperative complications
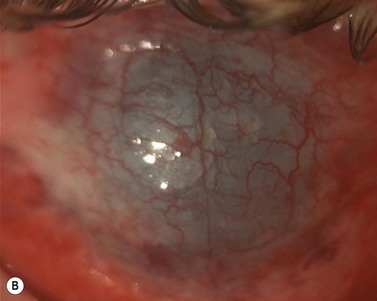
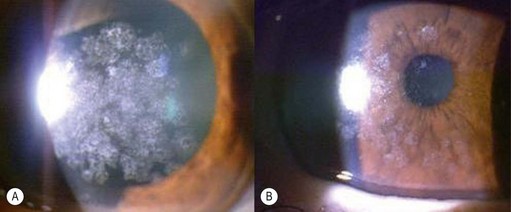
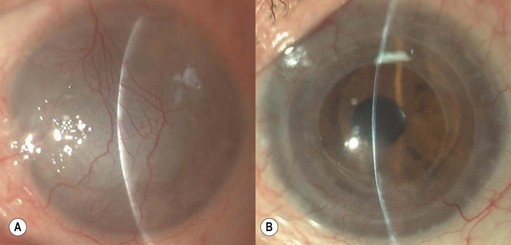
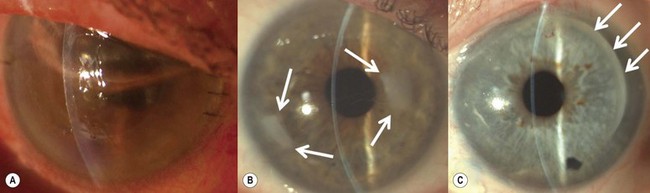
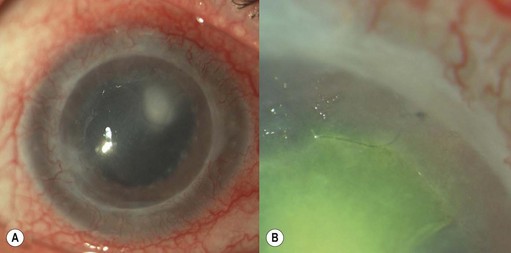
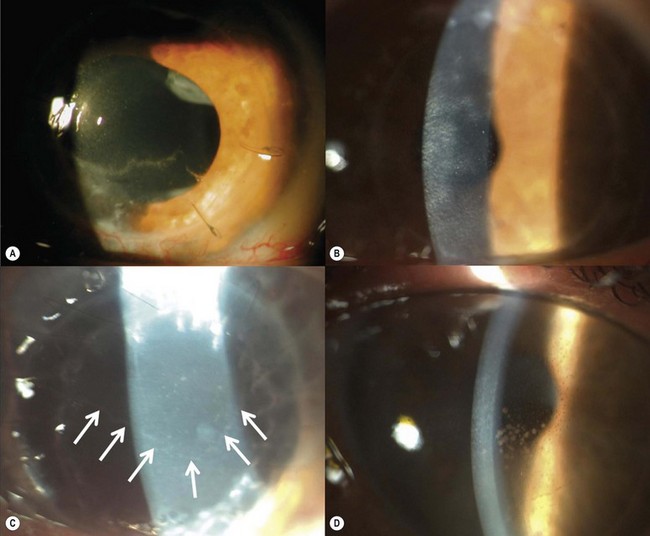
Epithelial defects are often present during the first week, but as long as they continue to diminish in size no intervention is necessary. Persistent epithelial defects are more common in eyes with pre-existing ocular surface problems such as Stevens–Johnson syndrome, ocular cicatricial pemphigoid, chemical burns, lagophthalmos, neurotrophic disease, entropion/ectropion, trichiasis, and dry eye. These conditions must be adequately controlled, preferably before corneal surgery is performed. Persistent, non-healing defects may require the application of a bandage contact lens, an increase in corneal surface lubrication (ointments), or the discontinuation or reduction of topical medications that are toxic to the epithelium. In severe, recalcitrant cases, a lateral tarsorrhaphy may need to be performed. Persistent epithelial defects may be complicated by infection, scarring, and stromal melting (Fig 21.37).
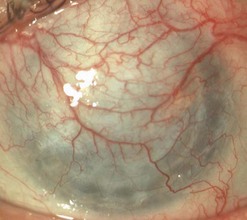
Broken or loose sutures should be immediately removed or replaced. During planned suture removal, care must be taken to ensure that sutures that are incompletely removed do not have any superficially protruding ends. Exposed suture knots and ends will likely lead to irritation, pain, epiphora, photophobia, mucus production, giant papillary conjunctivitis, and ulceration. Suture related complications include wound dehiscence, high astigmatism, vascularization along suture tracks, immune rejection, and infectious suture abscesses (Fig. 21.38). Sutures associated with an abscess or suspected infiltration must be removed and sent for microbiological investigation. Occasionally, scarring occurs along the suture track, particularly if sutures are left in place for a prolonged period of time.
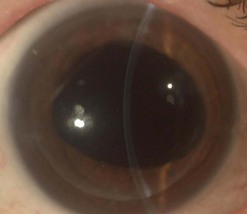
Filamentary keratitis in the early postoperative period is usually self-limiting and has been noted to occur in >20% of eyes following PK and LK162,163. These lesions do not cause any discomfort on the denervated donor cornea, but they may cause irritation and foreign body sensation on the recipient cornea. Filaments may be treated with artificial tears, N-acetylcysteine drops, debrided with fine forceps, or left untreated. Kaye dots are discrete, white specks in the peripheral donor corneal epithelium adjacent to the sutures and may represent a response to topographical elevation of the cornea in the area of wound compression by sutures164. These do not stain or cause discomfort and are seen mostly in younger patients. They usually disappear within months or with suture removal and are not associated with any secondary complications.
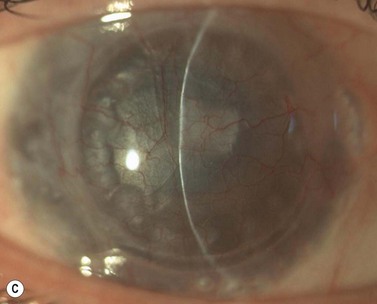
A fixed mydriasis following PK (Urrets–Zavalia syndrome) has been described159 following PK for keratoconus as well as after DALK and DSAEK165,166 (Fig. 21.39). Although the precise etiology remains unknown, iris ischemia and use of cycloplegics have been proposed as contributory factors. No treatment is indicated in asymptomatic cases, although patients with intolerable visual disturbances may require a pupilloplasty.
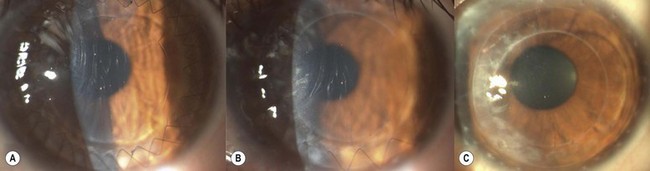
Postoperative corneal infection may occur as a sequel to suture abscess, persistent epithelial defects, ocular surface problems, suture removal, contact lens wear, prolonged corticosteroid use, or inadequate excision in cases of therapeutic grafts for infectious keratitis. Rarely, infectious keratitis in the graft may be manifested by intrastromal crystalline deposits distributed within a discrete stromal lamellar plane. This phenomenon, known as infectious crystalline keratopathy, is usually caused by low virulence organisms (e.g. Streptococcus viridans) in the setting of host immuno-compromise associated with chronic corticosteroid use167. With the exception of herpetic infections, which may occur at any period after surgery, most cases of infection occur in the first postoperative year. In lamellar surgery there may be a delay in presentation as the infection may develop in the interface and remain unnoticed (Fig. 21.40). Such infections may also be slow to respond to therapy. Scrapings for microbiological investigations must be obtained whenever possible and intensive antibiotic treatment initiated without delay. The choice and regimen of antimicrobial therapy should be dictated by the microbiological results, clinical setting, and physician preference. Unresponsive and interface infections may require a therapeutic keratoplasty. Endophthalmitis after keratoplasty occurs in less than 1% of grafts and is more common in patients with positive donor rim cultures168,169. Fibrin, hypopyon, increased white cells, and flare in the anterior chamber, as well as a reduced red reflex, may be indicators of intraocular infection. B-scan ultrasonography should be performed in all patients in whom the fundal view is obscured. Management is extrapolated from evidence for endophthalmitis following cataract surgery, and involves diagnostic vitrectomy along with intravitreal, systemic, and/or periocular antibiotics.
Primary graft failure is defined as the presence of donor corneal edema on the first postoperative day, which is unresponsive to treatment, and fails to clear for a minimum of three consecutive months. It may result from poor donor preservation, unhealthy or low donor endothelial counts, surgical trauma, or idiopathic causes170. Herpes simplex virus has been detected by polymerase chain reaction in donor corneal buttons in some cases of primary graft failure169. Although the graft with primary failure may clear and deturgesce to some degree as the normal postoperative inflammation subsides and the intraocular pressure normalizes, it will not recover full clarity as would a healthy graft. Primary graft failure is relatively rare and all failed buttons must be evaluated and reported to the supplying eye bank.
Glaucoma after keratoplasty is a common and devastating irreversible complication. Difficulty in IOP measurement, optic disc imaging/visualization, and visual field testing add to the challenge of diagnosing glaucoma in post-keratoplasty eyes. The incidence depends on the complexity of surgery, prevalence of preoperative glaucoma, previous surgery, susceptibility to corticosteroids, and indications for surgery. Eyes treated with long-tube drainage devices such as Molteno, Shocket, or Baerveldt types are somewhat more susceptible to endothelial decompensation, and when treated with subsequent PKPs tend to fail more often as well171–173.
The management of glaucoma drainage devices is discussed elsewhere in the book.
Although the avascular cornea is isolated from the immune system to a degree, the protection imparted by the blood ocular barrier is not perfect. The aqueous humor, limbal vasculature, tear film, and iridocorneal adhesions all influence rejection. Each layer of the cornea could sensitize the recipient and undergo immunologic allograft rejection. Immunologic damage to the endothelial cells, however, is most detrimental to the overall function of the donor cornea and is a major cause of graft failure174–177. Possible risk factors for corneal graft rejection include large diameter grafts, corneal vascularization (particularly deep vessels), young recipient age, regrafting (especially for prior rejection-related graft failure), glaucoma, atopy, and active ocular inflammation177–181. Rejection episodes are more likely within the first postoperative year and prompt recognition and treatment are crucial in achieving successful reversal. Although subjective reporting of redness and decreased vision have been strongly associated with rejection, patient-reported symptoms are neither sensitive nor specific for a rejection182.
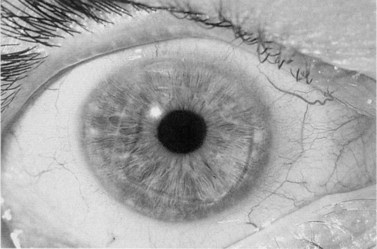
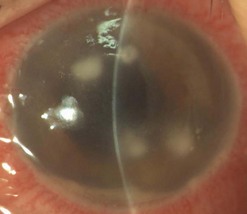
Clinical signs of graft rejection (Fig. 21.41) may be subtle and easily missed in the early stages. Mild circumcorneal injection or iritis usually signifies a rejection episode. In such cases, empirical treatment must be initiated or the frequency of existing steroid therapy increased. Keratic precipitates (KPs) signify graft rejection and may be a subtle finding in the early stages. It is important to distinguish new KPs from old ones and a photographic record (or accurate drawings) is useful. Endothelial rejection may present with corneal edema and occasionally a rejection line (Khodadoust line), which is considered to be diagnostic of endothelial rejection.
The mainstay of therapy for corneal graft rejection remains topical corticosteroids, despite a long, ongoing search for alternative treatments. Frequent topical therapy (e.g. prednisolone acetate 1%) is administered hourly in most cases. Mild cases may be treated with less frequent instillation and nocturnal therapy may be substituted with steroid ointment preparations. Periocular and oral corticosteroids may also be used in adjunct to topical treatment, especially in aggressive episodes. The periocular route of administration is particularly useful in non-compliant and poorly controlled diabetic patients. Other agents, e.g. ciclosporin, tacrolimus (FK506), and azathioprine, have been used in the treatment and prevention of graft rejection with varying degrees of success183–189. The use of these agents is generally reserved for recalcitrant and high risk cases due to the adverse systemic effects and lack of conclusive evidence regarding their efficacy.
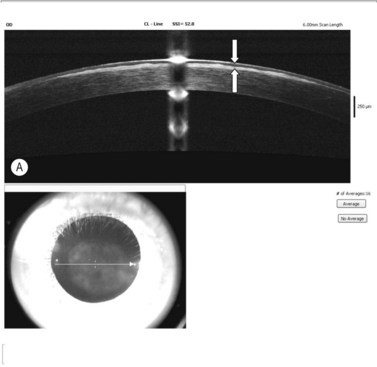
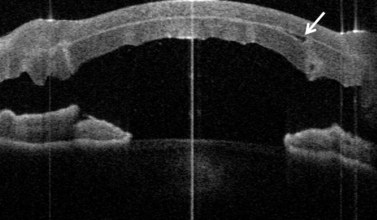
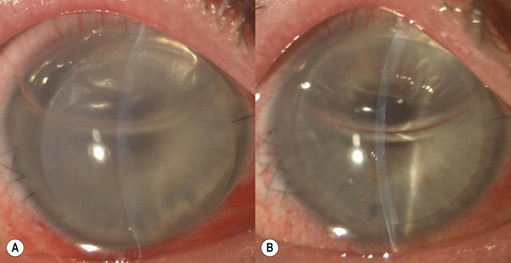
In addition to the above complications, LK may present with specific problems, the management of which depends on the type of LK surgery performed. Double anterior chamber formation may occur whenever a corneal interface may communicate with the anterior chamber, e.g. following LK performed as a patch for corneal perforations, DALK, mushroom keratoplasty, and endothelial transplantation. In DALK, this occurs secondary to Descemet membrane perforation, which is a common intraoperative complication of DALK surgery120,121,162,190. Small enclosed pockets of fluid (Fig. 21.42) may be self-limiting and may be treated conservatively with periodic observation. Re-apposition must be performed for large, persistent, and enlarging chambers by introducing air into the anterior chamber. In cases of endothelial transplantation, poor corneal clarity is a good indicator of graft detachment when it is difficult to establish the presence of a double chamber with slit-lamp examination alone (Fig. 21.43). Corneal OCT examination can be of great help in such cases. We instigate immediate re-bubbling, and have found this to be highly successful in reattaching the graft. Proper graft centration may also be attempted prior to injecting air. Patients are advised to posture supine following re-bubbling. Care must be taken to avoid overfilling the anterior chamber with air in cases where there is no peripheral iridotomy, or to ensure that air is released early.
Folds in the recipient Descemet membrane and stromal bed are common after LK. These are generally transient and tend to improve with time and following removal of sutures (Fig. 21.44). Central folds may, however, interfere with vision and induce higher order aberrations191,192. The folds may result from excessive manipulation of the grafts (in DSAEK surgery) and as a result of size mismatch in DALK. In the latter case, central recipient bed trephination (cone collapse) or oversizing the graft by 0.25–0.5 mm may overcome or reduce the likelihood of folds.
Keratoprosthesis
Marina Papadia, Alessandro Bagnis, Riccardo Scotto and Carlo Enrico Traverso
Introduction
According to a report published by the World Health Organization in 2009 there are 314 million visually impaired individuals globally and 45 million are considered blind193. Corneal disease accounted for 8 million blind, 1.5 million of those being children194.
The idea of replacing an opaque cornea with a synthetic plate dates back 200 years195 and in 1859 Heusser was possibly the first to implant a keratoprosthesis (KPro) in a human eye196.
In the first decade of the 20th century, interest in keratoprostheses declined, because good results were obtained with penetrating keratoplasty using human corneal allografts197.
Nowadays keratoplasty is a standardized technique, continuously evolving towards selective replacement of the damaged tissue (i.e. deep anterior keratoplasty or endothelial keratoplasty). Despite many advances, a subset of patients with severe corneal disease complicated with severe dry eye, corneal vascularization, total stem cell deficiency, or repeated graft failures remains unsuitable as candidates for penetrating keratoplasty198. Hence, for patients with conditions such as alkali burns, Stevens–Johnson syndrome, or recurrent graft failure, permanent keratoprosthesis remains the only viable option for visual rehabilitation and should be considered whenever the probability of maintaining a clear cornea with a keratoplasty using a human allograft is poor199.
During World War II the incidental discovery that Plexiglas fragments from airplane canopies retained in the eye were well tolerated opened a new direction for research studies on keratoprosthesis200.
Patient selection
An extended work-up to assess the presence of associated dry eye should be performed, as severe ocular surface diseases might compromise the survival of keratoprosthesis and might orient towards the use of a specific type of prosthesis. Patients with underlying autoimmune disease are most susceptible to tissue necrosis, melting, and infection201,202. Tear secretion, blink-rate, and completeness of blinking should be carefully assessed and reported. The surgeon should carefully look for the presence of symblepharon or anomalies of fornix, lids, or lid positioning preoperatively. The visual acuity should be assessed as precisely as possible, as well as the intraocular pressure (IOP), although these two measures might be particularly difficult to estimate because of the corneal anomalies. If visual acuity is not measurable, at least light projection should be tested and the absence of sensibility in the nasal projection should raise suspicion of the presence of end-stage glaucoma. The effective potential function of the retina and visual pathways should be assessed with electroretinograms (ERG) and visual evoked potentials (VEP) in order to avoid surgery if there is no reasonable chance of expecting any visual recovery.
Patients affected by severe autoimmune disease such as Stevens–Johnson syndrome (SJS) or ocular cicatricial pemphigoid (OCP) are the worst candidates, because of the potentially sight-threatening complications associated with severe dry eyes and the overall lower success rate of the surgery where these diseases are present201,203.
Keratoprosthesis surgery should be reserved for patients with bilateral corneal blindness resulting from several ocular or systemic pathologies204,205, possibly with a ‘wet’ ocular surface.
The prognosis for a ‘good outcome’ is better in eyes that have experienced little intraocular inflammation in the past201. Therefore long-standing uveitis, whether associated with rheumatoid diseases or not, or end-stage phthisis presently constitutes contraindications. Despite some evidence about the effectiveness and safety of KPro in patients with a history of herpetic keratitis, doubts about the chances of long survival of the device in such cases should be taken into account206,207.
Increasing poverty and non-white race have been associated with elevated pro-inflammatory states among adults208; black transplant recipients have decreased graft survival and increased rejection rates compared with whites209 and have a racial predisposition to diminished glucocorticoid responsiveness210.
Preoperative preparation
Boston keratoprosthesis
This procedure has been FDA approved in the USA since 1992. It is completely synthetic, consisting of medical-grade polymethyl-metacrylate (PMMA). The first prototype was developed in the 1960s. According to the WHO criteria, candidates for keratoprosthesis surgery should be monocular, or bilaterally blind205.
Assembly of the device
A 3 mm hole is trephined in the center of a donor corneal button of 8.5–9 mm diameter (Fig. 21.45).
Fig. 21.45 A 3 mm hole is trephined in the center of a donor corneal button (A) of 8.5–9 mm diameter (B).
To make the manipulation of the different parts of the device easier, the front plate of the device is secured to an adhesive surface (provided in the package) (Fig. 21.46). The fresh corneal graft with its central hole is then brought over the stem of the front plate and slid down; the back plate is then placed on top of it and slid down over the stem (Fig. 21.47).
Once the device is assembled, the patient’s cornea is trephined or the previous graft is detouched and the artificial cornea is sutured to the recipient bed using 16 interrupted 10-0 nylon sutures, burying the knots, with the same standard technique used for penetrating keratoplasty. As a rule the iris is not removed entirely at the root, since postoperative bleeding may occur and future glare problems can be increased. If the pupil is small, iridotomies will expand the pupil enough so as not to interfere with the visual axis if the positioning of the KPro should end up slightly eccentric. If the lens is in place, it should be removed in extracapsular fashion. A KPro with optics adjusted for pseudophakia can be used, but postoperative internal reflexes might worsen the visual outcome. If the vitreous surface seems intact, it is left untouched, otherwise an anterior vitrectomy is performed. A soft contact lens of 16 mm diameter and 9.8 mm base curve plano power is applied as a bandage at the end of the surgery. This surgery should not be performed without applying the contact lens (Fig. 21.48).
Osteo-odonto-keratoprosthesis (OOKP)
Strampelli in 1963 was the first to use osteo-odonto-keratoprosthesis (OOKP) in order to replace an opaque cornea that was at high risk for traditional graft211. It is composed of the patient’s own tooth root and surrounding alveolar bone to support a centrally cemented optical cylinder of PMMA which is grafted onto the cornea212 (Fig. 21.49).
The optical cylinder is cemented to the dentine. The dentine itself is coupled to the alveolar bone by the dentoalveolar ligament. The OOKP lamina is fixed by surrounding periosteum to the anterior corneal and scleral surface with sutures, but is also covered by a thick buccal mucous membrane graft204. Other biological materials are used for supporting the synthetic cylinder as cartilage213 or tibial bone214. The main theory behind all these devices is to have a inert biological skirt which can be colonized and integrated into surrounding tissues such as sclera and mucous membrane, yielding longer survival and lower extrusion rates215.
The implant of an OOKP is a multistep procedure that requires a multidisciplinary team consisting of an ophthalmologist, dental surgeon, radiologist, and psychologist. Table 21.1 summarizes the two-step focal points of the procedure.
Table 21.1 Steps for the preparation and implant of osteo-odonto-keratoprosthesis
| On the eye | Preparing the device | |
|---|---|---|
| Step A | Corneal pannus removed labial/buccal mucosa sutured to the sclera. |
A single rooted tooth with the longest and straightest root (usually a canine tooth) chosen. An approximately 2.5 mm thick slice of alveolar bone of approximately 9.5 × 14 mm cut. An optical PMMA cylinder chosen in order to have at least 1 mm rim of dentine around the central channel. The tooth-optic implant inserted in a submuscular pocket in the lower lid of the fellow eye. |
| 2–4 month period | ||
| Step B | Mucous membrane reflected inferiorly to expose the cornea. Removal of iris, cataract extraction, and anterior vitrectomy performed. Implant placed and sutured onto the corneal opening. |
Implant removed from submuscular pocket. |
Surgical technique
Preparation of the eye
The corneal pannus is removed through a keratectomy. A membrane mucous graft either from labial mucosa (Falcinelli) or from the inner side of the cheek (Falcinelli modification)216 is prepared. A 360° peritomy is performed and the corneal pannus is removed through a keratectomy. If too thin, the cornea is reinforced through a lamellar patch graft. The buccal mucosa graft is then sutured to the sclera (Fig. 21.50). After approximately 3 months the mucous membrane is usually integrated and vascularized.
AlphaCor
This type of KPro is predominantly used in Australia where it was developed, thanks to the efforts of Lions Eye Institute. Previously known as the Chirila KPro, it was first implanted in a human eye in 1998 and is FDA approved since 2003217. It is commercialized by Argus Biomedical Pty., Perth, Australia and is entirely made of poly 2-hydroxyethyl methacrylate (pHEMA), a synthetic, soft, biocompatible polymer (Fig. 21.51). The clear central optic zone is fused with a peripheral porous sponge skirt that allows biointegration into the host cornea by ingrowth of stromal fibroblasts218. The skirt is made of the same material as the clear part, but it is polymerized with a different water content (45% for the skirt and 35% for the clear optic)219.
Fig. 21.51 Picture of the AlphaCor keratoprosthesis. The opaque porous skirt and the central clear optic zone are made of the same polymer, but with different water content. http://www.hopkinsmedicine.org/wilmer/conditions/artificial_cornea.html.
The porous material remains enclosed within the cornea and allows the migration of fibroblasts. The device has a radius of 7.0 mm, a thickness of 0.5 mm, and a refractive index of 1.43219. The colonization of the skirt by the keratocytes for the integration of device is a mandatory step, as with other KPro models. The central optical zone was designed to remain clear, but various degrees of epithelialization can occur.
Patient selection is critical for reducing the risk of anatomical complications. AlphaCor should not be used in eyes with severe ocular surface diseases, extreme dry eye, history of herpes simplex disease, cicatrizing ocular surface diseases, or acute inflammation220.
This kind of device requires a two-step surgery, performed at an 8–12 week interval.
Surgical technique
Step A
On the recipient eye a peritomy is performed, followed by debridement of the corneal epithelium. 1–1.5 mm posterior to the limbus a superior 180° scleral partial thickness incision is performed and extended into the cornea to divide the cornea into an anterior and posterior lamella of similar thickness. The dissection is then continued into the inferior cornea so that a central corneal pocket of approximately 7.5 mm diameter is created. A 3–3.5 mm central disc of corneal tissue is removed from the posterior stromal bed and the device is implanted into the lamellar pocket, centered over the posterior lamellar opening220,221. The superficial flap is then replaced and sutured with interrupted 10-0 nylon sutures. A Gunderson conjunctival flap is then created to cover the ocular surface217,222.
Step B
Eight to twelve weeks later, the trephination of the center of the conjunctiva and anterior corneal lamella is performed in order to expose the optic portion of the device as a full-thickness corneal replacement; the peripheral skirt remains integrated into the corneal lamellae. Usually a 3.5 mm dermatologic trephine is used, in order to avoid the increased exposure associated with a 4 mm opening. The two-step technique is summarized in Table 21.2.
Table 21.2 Steps for the implant of AlphaCor keratoprosthesis
| On the eye | Preparing the device | |
|---|---|---|
| Step A | Starting from 1–1.5 mm from the limbus, a sclera incision continuing into the superior cornea is performed in order to create a corneal pocket. The center of the deeper lamella thephined and removed and the device positioned into the pocket. The superior sclerocorneal incision sutured and a conjunctival flap positioned over the cornea. |
No specific preparation is required for this device before implantation. |
| 2–3 month period | ||
| Step B | Trephination of the center of the conjunctival flap and anterior corneal lamella without touching the implant is performed. | |
Pintucci biointegrable keratoprosthesis (PBKP)
The first prototype dates back to 1979. The biointegrable properties of Dacron were already confirmed by the good results obtained in the use of this material in angioplasty and cardioprosthesis, so a device with a skirt that could be integrated in the surrounding tissues and which could serve as a support for a synthetic PMMA optic was developed223.
The supporting element of the PBIKP is made of a biointegrated Dacron fabric skirt that allows three-dimensional colonization by the newly formed vascularized connective tissue (Fig. 21.52).
This fabric is chemically inert and unlike osteo-odonto-keratoprosthesis is not subject to resorption. The colonized Dacron mesh should prevent the epithelial downgrowth along the shaft of the optical cylinder by contact inhibition and by leaving no empty spaces at the implant–eye interface, therefore preventing retroprosthetic membranes, leakage, and microbial contamination224.
The two-step surgical procedure of implanting this device is similar to the one used for the OOKP. Table 21.3 summarizes the two-step focal points of the procedure.
Table 21.3 Surgical steps for the implant of the Pintucci keratoprosthesis
| On the eye | Preparing the device | |
|---|---|---|
| Step A | Corneal pannus removed. Labial/buccal mucosa sutured to the sclera. |
The implant is inserted in a submuscular pocket in the lower lid of the same eye. |
| 2-month period | ||
| Step B | Cornea exposed. Removal of iris and cataract extraction. Implant placed and sutured onto the corneal opening. |
Implant removed from submuscular pocket, fixation of the optical part tested and colonization of the Dacron tissue checked. |
Supradescemetic synthetic cornea (SDSC)
It involves the implantation of a biocompatible lamellar tissue onto the Descemet membrane of a recipient cornea, and was developed in 1990 by Parel, Lacombe and Alfonso225.
Complications of keratoprostheses
Loss of contact lens
A wet ocular surface is an essential condition for the survival of the keratoprosthesis. The use of a soft contact as a bandage proved to be protective, reducing the occurrence of drying of recipient cornea, of epithelial defects, dellen formation, and corneal melt226.
Inflammation
Most of the candidates for KPro surgery have already undergone multiple, devastating surgeries or have experienced severe autoimmune diseases (such as graft-versus-host disease) and might have potentially a high degree of ocular inflammation after surgery. Intracameral injection of 0.4 mg dexamethasone at the end of Boston KPro surgery is suggested by some authors205. Patients with a history of chronic inflammation have a poorer prognosis for KPro surgery; therefore a preoperative assessment is crucial.
Retroprosthetic membrane
The formation of a retroprosthetic membrane is a relatively frequent complication due to the high degree of intraocular inflammation that occurs in these patients. Studies on Boston KPro 1 report up to 65% of patients experiencing this kind of complication227.
If the membrane is thin, it can be opened with YAG laser228. When opening the membrane with the YAG laser, attention should be given to not using too much energy, as the optical zone can be damaged. The energy should be kept lower than 2.0 mJ; if this is not enough, the power can be increased carefully until the expected result is obtained, keeping in mind that the plastic can be damaged and even crack if excessive power is applied. The opening of the membrane can elicit intraocular inflammation, therefore subTenon’s injection of 40 mg triamcinolone is recommended after the procedure.
If the membrane is thick or richly vascularized, laser is not recommended as bleeding into the vitreous can occur. Surgical membranectomy and vitrectomy can be performed by an experienced vitreoretinal surgeon229. If the procedure fails, the whole KPro can be removed, the membrane removed surgically open-sky, and a new KPro can then be fitted.
Stromal thinning or melting of the recipient tissue/extrusion
Tissue melting of the recipient bed is a severe complication that can lead to prosthesis extrusion230. Some efforts have been made to reduce the corneal melting by modifying the design of the back plate of the Boston KPro. The addition of holes in the back plate of Boston KPro allow a better nutrition and hydration from the aqueous to the endothelium, thus reducing both corneal melting and extrusion rate231. Surgical repair of such a leakage has poor chance of success.
Infectious endophthalmitis
The occurrence of endophthalmitis remains, and as with every other intraocular surgery, one of the most frightening complications. Some authors suggest the use of topical fortified vancomycin lifelong232.
Glaucoma
Glaucoma remains the greatest challenge after successful implantation of keratoprosthesis201,233–235. The assessment of IOP postoperatively remains approximate, despite the efforts made towards the development of new tools for IOP assessment (like tonopen). The digital palpation of the bulbus remains the most widely used method to evaluate IOP postoperatively, but this procedure might also be difficult to interpret because of the presence of the KPro itself and eventually of drainage devices. New imaging modalities of the optic nerve head developed to monitor nerve fiber loss, such as optical coherence tomography (OCT), GDx nerve fiber analyzer or Heidelberg retinal tomograph (HRT) progression, might be useful for the evaluation of these patients.
Future goals
Tsubota and colleagues recently tried to develop a new concept of KPro by coating the surface of poly-(vinyl alcohol) (PVA) hydrogel with a biological matrix. The results of in vitro and in vivo studies are encouraging234–236. The authors attached a collagen layer on a layer of PVA. Growth of stratified epithelia on this substrate was achieved, but the epithelia in these grafts came loose due to inflammation associated with sutures236. Recently amniotic membrane was used in order to enhance the strength of the ‘basement membrane’ where the epithelial cells can attach.
Efforts have been also made in the development of copolymers that mimic collagen type I. Engineering materials that are similar to the natural cornea might lead to the development of a material that can eventually be replaced by natural stroma through the invasion of keratocytes and remodeling of extracellular matrix237.
The use of interpenetrating polymer networks (IPN) is partly used for the production of AlphaCor. New efforts have been made in order to combine two different polymers with different characteristics in order to obtain a mesh that should end up having the beneficial properties of both polymers and compensate for some of their less desirable qualities238–242.
1 Walton W, Von Hagen S, Grigorian R, et al. Management of traumatic hyphema. Surv Ophthalmol. 2002;47(4):297-334.
2 Crouch ERJr. Traumatic hyphema. J Pediatr Ophthalmol Strabismus. 1986;23(2):95-97.
3 Reynolds DS, Flynn HWJr. Endophthalmitis after penetrating ocular trauma. Curr Opin Ophthalmol. 1997;8(3):32-38. Review
4 Gundersen T. Conjunctival flaps in the treatment of corneal disease with reference to a new technique of application. Arch Ophthalmol. 1958;60(5):880-888.
5 Lim LS, How AC, Ang LP, et al. Gundersen flaps in the management of ocular surface disease in an Asian population. Cornea. 2009;28(7):747-751.
6 Geria RC, Wainsztein RD, Brunzini M, et al. Infectious keratitis in the corneal graft: treatment with partial conjunctival flaps. Ophthalmic Surg Lasers Imaging. 2005;36(4):298-302.
7 Mauger TF, Craig E. Combined acanthamoeba and Stenotrophomonas maltophilia keratitis treated with a conjunctival flap followed by penetrating keratoplasty. Cornea. 2006;25(5):631-633.
8 Alino AM, Perry HD, Kanellopoulos AJ, et al. Conjunctival flaps. Ophthalmology. 1998;105(6):1120-1123.
9 Hankanson NE, Merideth RE. Conjunctival pedicle grafting in the treatment of corneal ulcers in the dog and cat. J Am Anim Hosp Assoc. 1987;23:641-648.
10 Coroneo MT. Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol. 1993;77(11):734-739.
11 Bradley JC, Yang W, Bradley RH, et al. The science of pterygium. Br J Ophthalmol. 2010;94(7):815-820.
12 Busin M, Halliday BL, Arffa RC, et al. Precarved lyophilized tissue for lamellar keratoplasty in recurrent pterygium. Am J Ophthalmol. 1986;102(2):222-227.
13 Rapuano CJ. Phototherapeutic keratectomy: who are the best candidates and how to treat them? Curr Opin Ophthalmol. 2010;21:280-282.
14 Netto MV, Mohan RR, Sinha S, et al. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006;82:788-797.
15 Shah RA, Wilson SE. Use of mitomycin-C for phototherapeutic keratectomy and photorefractive keratectomy surgery. Curr Opin Ophthalmol. 2010;21:269-273.
16 Netto MV, Mohan RR, Sinha S, et al. Effect of prophylactic and therapeutic mitomycin C on corneal apoptosis, cellular proliferation, haze, and long-term keratocyte density in rabbits. J Refract Surg. 2006;22:562-574.
17 Wong VW, Chi SC, Lam DS. Diamond burr polishing for recurrent corneal erosions: results from a prospective randomized controlled trial. Cornea. 2009;28:152-156.
18 Baryla J, Pan YI, Hodge WG. Long-term efficacy of phototherapeutic keratectomy on recurrent corneal erosion syndrome. Cornea. 2006;25(10):1150-1152.
19 Das S, Langenbucher A, Seitz B. Excimer laser phototherapeutic keratectomy for granular and lattice corneal distrophy: a comparative study. J Refract Surg. 2005;21(6):727-731.
20 Das S, Langenbucher A, Pogorelov P, et al. Long-term outcome of excimer laser phototherapeutic keratectomy for treatment of Salzmann’s nodular degeneration. J Cataract Refract Surg. 2005;31:1386-1391.
21 Spadea L, Bianco G, Balestrazzi E. Topographically guided excimer laser photorefractive keratectomy to treat superficial corneal opacities. Ophthalmology. 2004;111(3):458-462.
22 Elsahn AF, Rapuano CJ, Antunes VA, et al. Excimer laser phototherapeutic keratectomy for keratoconus nodules. Cornea. 2009;28:144-147.
23 Mannis MJ, Kracruner JH. Keratoplasty: A historical perspective. Surv Ophthalmol. 1981;25:333.
24 Duke-Elder S, Leigh AG. Diseases of the outer eye. In: Duke-Elder S, Leigh AG, editors. System of Ophthalmology, vol, VIII: 648. St Louis: CV Mosby, 1977.
25 Coster DJ. Doyne Lecture. Influences on the development of corneal transplantation. Eye. 1994;8:1.
26 Zirm EK. Eine erfolgreiche totale Keratoplastik [A successful total keratoplasty]. Refract Corneal Surg. 1989;5:258.
27 Melles GR, Lander F, Rietveld FJ, et al. A new surgical technique for deep stromal, anterior lamellar keratoplasty. Br J Ophthalmol. 1999;83(3):327-333.
28 Fontana L, Parente G, Tassinari G. Clinical outcomes after deep anterior lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2007;143(1):117-124.
29 Siganos CS, Tsiklis NS, Miltsakakis DG, et al. Changing indications for penetrating keratoplasty in Greece, 1982–2006: A Multicenter Study. Cornea. 2010;29(4):372-374.
30 Rahman I, Carley F, Hillarby C, et al. Penetrating keratoplasty: indications, outcomes, and complications. Eye (Lond). 2009;23(6):1288-1294. Epub 2008 Oct 24
31 Ghosheh FR, Cremona F, Ayres BD, et al. Indications for penetrating keratoplasty and associated procedures, 2001–2005. Eye Contact Lens. 2008;34(4):211-214.
32 Ghosheh FR, Cremona FA, Rapuano CJ, et al. Trends in penetrating keratoplasty in the United States 1980–2005. Int Ophthalmol. 2008;28(3):147-153. Review.
33 Busin M, Monks T, Arffa RC. Endokeratoplasty in the rabbit model: A new surgical technique for endothelial transplantation. Ophthalmology. 1996;103:167.
34 Busin M, Arffa RC, Sebastiani A. Endokeratoplasty as an alternative to penetrating keratoplasty for the surgical treatment of diseased endothelium: initial results. Ophthalmology. 2000;107(11):2077-2082.
35 Melles GR, Lander F, Beekhuis WH, et al. Posterior lamellar keratoplasty for a case of pseudophakic bullous keratopathy. Am J Ophthalmol. 1999;127:340-341.
36 Ghosheh FR, Cremona FA, Rapuano CJ, et al. Trends in penetrating keratoplasty in the United States 1980–2005. Int Ophthalmol. 2008;28(3):147-153.
37 Sharif KW, Casey TA. Changing indications for penetrating keratoplasty, 1971–1990. Eye (Lond). 1993;7(Part 4):485-488.
38 Eye Bank Association of America. 2008 Eye Banking Statistical Report. EBAA, DC, USA, 2009.
39 Waring GOIII. The 50-year epidemic pseudophakic corneal edema. Arch Ophthalmol. 1989;107:657.
40 Smith RE, McDonald HR, Nesburn AB, et al. Penetrating keratoplasty: Changing indications, 1947–1978. Arch Ophthalmol. 1980;98:1226.
41 Al-Yousuf N, Mavrikakis I, Mavrikakis E, et al. Penetrating keratoplasty: indications over a 10 year period. Br J Ophthalmol.. 2004;88(8):998-1001.
42 Mc Carey BE, Kaufman HE. Improved corneal storage. Invest Ophthalmol. 1974;13:165-173.
43 Pels E, Beele H, Claerhout I. Eye bank issues: II. Preservation techniques: warm versus cold storage. Int Ophthalmol. 2008;28(3):155-163.
44 Ferrari S, Barbaro V, Di Iorio E, et al. Advances in corneal surgery and cell therapy: challenges and perspectives for the eye banks. Expert Rev Ophthalmol. 2009;4(3):317-329.
45 Doughman DJ, Harris JE, Schmitt KM. Penetrating keratoplasty using 37°C organ cultured cornea. Trans Am Acad Ophthalmol Otolaryngol. 1976;88:778-793.
46 Duffy P, Wolf J, Collins G, et al. Possible person-to-person transmission of Creutzfeldt–Jakob disease. [Letter.]. N Engl J Med. 1974;290(12):692-693.
47 Burton RC, Wilson RW, Henson TE, et al. Human-to-human transmission of rabies virus by corneal transplant. N Engl J Med. 1979;300(11):603-604.
48 McGeorge AI, Thompson P, Elliott D, et al. Papillary adenocarcinoma of the iris transmitted by corneal transplantation. EBAA Abstracts. Cornea. 1994;13:102.
49 Simonds RJ, Holmberg SD, Hurwitz RL, et al. Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N Engl J Med. 1992;326(11):726-732.
50 Hoft RH, Pflugfelder SC, Forster RK, et al. Clinical evidence for hepatitis B transmission resulting from corneal transplantation. Cornea. 1997;16(2):132-137.
51 Schwarz A, Hoffmann F, L’age-Stehr J, et al. Human immunodeficiency virus transmission by organ donation. Outcome in cornea and kidney recipients. Transplantation. 1987;44(1):21-24.
52 Rijneveld WJ, Beekhuis WH, van Rij G, et al. Clinical comparison of grafts stored in McCarey–Kaufman medium at 4 degrees C and in corneal organ culture at 31 degrees C. Arch Ophthalmol. 1992;110(2):203-205.
53 Frueh BE, Böhnke M. Prospective, randomized clinical evaluation of Optisol vs organ culture corneal storage media. Arch Ophthalmol. 2000;118(6):757-760.
54 Rijneveld WJ, Remeijer L, van Rij G, et al. Prospective clinical evaluation of McCarey–Kaufman and organ culture cornea preservation media: 14-year follow-up. Cornea. 2008;27(9):996-1000.
55 Vajpayee RB, Sharma N, Jhanji V, et al. One donor cornea for 3 recipients: a new concept for corneal transplantation surgery. Arch Ophthalmol. 2007;125(4):552-554.
56 Soong H, Meyer RF, Sugar A. The triple procedure. Ophthalmol Clin North Am. 1990;3:697.
57 Geggel HS. Intraocular lens implantation after penetrating keratoplasty. Improved unaided visual acuity, astigmatism, and safety in patients with combined corneal disease and cataract. Ophthalmology. 1990;97(11):1460-1467.
58 Hsiao CH, Chen JJ, Chen PY, et al. Intraocular lens implantation after penetrating keratoplasty. Cornea. 2001;20(6):580-585.
59 Ling R, Kamalarajah S, Cole M, et al. Suprachoroidal haemorrhage complicating cataract surgery in the UK: a case control study of risk factors. Br J Ophthalmol. 2004;88(4):474-477.
60 Moshirfar M, Meyer JJ, Kang PC. A comparison of three methods for trephining donor corneal buttons: endothelial cell loss and microscopic ultrastructural evaluation. Curr Eye Res. 2009;34(11):939-944.
61 Radner W, Skorpik C, Loewe R, et al. Effect of trephination technique on the ultrastructure of corneal transplants: guided trephine system v posterior punch technique. Br J Ophthalmol. 1999;83(10):1172-1177.
62 Olson RJ. Variation in corneal graft size related to trephine technique. Arch Ophthalmol. 1979;97:1323.
63 Troutman RC. Astigmatic consideration in corneal graft. Ophthalmic Surg. 1979;10:21.
64 Foulks GN, Perry HD, Dohlman CH. Oversize corneal donor grafts in penetrating keratoplasty. Ophthalmology. 1979;86:490-494.
65 Jaycock PD, Jones MN, Males J, et al. Outcomes of same-sizing versus oversizing donor trephines in keratoconic patients undergoing first penetrating keratoplasty. Ophthalmology. 2008;115(2):268-275.
66 Bourne WM, Davison JA, O’Fallon WM. The effects of oversize donor buttons on postoperative intraocular pressure and corneal curvature in aphakic penetrating keratoplasty. Ophthalmology. 1982;89:242-246.
67 Perry HD, Foulks GN. Oversize donor buttons in corneal transplantation surgery for keratoconus. Ophthalmic Surg. 1987;18:751-752.
68 Wilson SE, Bourne WM. Effect of recipient–donor trephine size disparity on refractive error in keratoconus. Ophthalmology. 1989;96:299-305.
69 Goble RR, Hardman Lea SJ, Falcon MG. The use of same size host and donor trephine in penetrating keratoplasty for keratoconus. Eye. 1994;8:311-314.
70 Spadea L, Bianco G, Mastrofini MC, et al. Penetrating keratoplasty with donor and recipient corneas of the same diameter. Ophthalmic Surg Lasers. 1996;27(6):425-430.
71 Girard LJ, Esnaola N, Rao R, et al. Use of grafts smaller than the opening for keratoconic myopia and astigmatism: a prospective study. J Cataract Refract Surg. 1992;18:380-384.
72 Shimmura S, Ando M, Ishioka M, et al. Same-sized donor corneas for myopic keratoconus. Cornea. 2004;23:345-349.
73 Weiss JS. Hinge keratoplasty. Ophthalmic Surg Lasers. 1996;27(2):156-157.
74 Busin M, Arffa RC. Deep suturing technique for penetrating keratoplasty. Cornea. 2002;21(7):680-684.
75 Lin DTC, Wilson SE, Reidy JJ, et al. An adjustable running suture technique to reduce post-keratoplasty astigmatism. A preliminary report. Ophthalmology. 1990;97:934-938.
76 Filatov V, Steinert RF, Talamo JH. Post keratoplasty astigmatism with single running suture or interrupted sutures. Am J Ophthalmol. 1993;115:715-721.
77 Eliason JA, McCulley JP. A comparison between interrupted and continuous suturing techniques in keratoplasty. Cornea. 1990;9:10-16.
78 Van Meter WS, Gussler JR, Soloman KD, et al. Postkeratoplasty astigmatism control: single continuous suture adjustment versus selective interrupted suture removal. Ophthalmology. 1991;98:177-183.
79 Yue-Kong-Au, Mahjoub SB, Hart JC. Suture patterns and corneal graft rotation in the cadaver eye. Ophthalmic Surg. 1990;8:58-61.
80 Vajpayee RB, Sharma V, Sharma N, et al. Evaluation of techniques of single continuous suturing in penetrating keratoplasty. Br J Ophthalmol. 2001;85(2):134-138.
81 Karabatsas CH, Cook SD, Figueiredo FC, et al. Combined interrupted and continuous versus single continuous adjustable suturing in penetrating keratoplasty: a prospective, randomized study of induced astigmatism during the first postoperative year. Ophthalmology. 1998;105(11):1991-1998.
82 Spadea L, Cifariello F, Bianco G, et al. Long-term results of penetrating keratoplasty using a single or double running suture technique. Graefes Arch Clin Exp Ophthalmol. 2002;240(5):415-419.
83 Franceschetti A. The different techniques of corneal grafting and their indications. Am J Ophthalmol. 1955;39:61-66.
84 Stocker FW. A new technique for corneal mushroom grafts. Am J Ophthalmol. 1959;48:27-30.
85 Roberts W. The mushroom graft. Am J Ophthalmol. 1961;52:913-918.
86 Keates RH, Martinez M, Paton RT. A modified technique for mushroom corneal grafts with a new instrument. Am J Ophthalmol. 1961;52:239-241.
87 Busin M. A new lamellar wound configuration for penetrating keratoplasty surgery. Arch Ophthalmol. 2003;121:260-265.
88 Busin M, Arffa RC. Microkeratome-assisted mushroom keratoplasty with minimal endothelial replacement. Am J Ophthalmol. 2005;140(1):138-140.
89 Bahar I, Kaiserman I, McAllum P, et al. Femtosecond laser-assisted penetrating keratoplasty: stability evaluation of different wound configurations. Cornea. 2008;27:209-211.
90 Kaiserman I, Bahar I, Slomovic AR, et al. Half top-hat wound configuration for penetrating keratoplasty: 1-year results. Br J Ophthalmol. 2009;93(12):1629-1633.
91 Lee J, Winokur J, Hallak J, et al. Femtosecond dovetail penetrating keratoplasty: surgical technique and case report. Br J Ophthalmol. 2009;93(7):861-863.
92 Ignacio TS, Nguyen TB, Chuck RS, et al. Top-hat wound configuration for penetrating keratoplasty using the femtosecond laser: a laboratory model. Cornea. 2006;25:336-340.
93 Bahar I, Kaiserman I, Srinivasan S, et al. Manual top-hat wound configuration for penetrating keratoplasty. Cornea. 2008;27:521-526.
94 Price FWJr, Price MO. Femtosecond laser shaped penetrating keratoplasty: one-year results utilizing a top-hat configuration. Am J Ophthalmol. 2008;145:210-214.
95 Saelens IE, Bartels MC, Van Rij G. Manual trephination of mushroom keratoplasty in advanced keratoconus. Cornea. 2008;27(6):650-655.
96 Benson WH, Goosey CB, Prager TC, et al. Visual improvement as a function of time after lamellar keratoplasty for keratoconus. Am J Ophthalmol. 1993;116(2):207-211.
97 Melles GR, Remeijer L, Geerards AJ, et al. The future of lamellar keratoplasty. Curr Opin Ophthalmol. 1999;10:253-259. Review
98 Tan DT, Mehta JS. Future directions in lamellar corneal transplantation. Cornea. 2007;26:S21-S28. Review
99 Alio JL, Shah S, Barraquer C, et al. New techniques in lamellar keratoplasty. Curr Opin Ophthalmol. 2002;13(4):224-229. Review
100 Bilgihan K, Ozdek SC, Sari A, et al. Excimer laser-assisted anterior lamellar keratoplasty for keratoconus, corneal problems after laser in situ keratomilesis, and corneal stromal opacities. J Cataract Refract Surg. 2006;32(8):1264-1269.
101 Biser SA, Donnenfeld ED, Doshi SJ, et al. Lamellar keratectomy using an automated microkeratome. Eye Contact Lens. 2004;30(2):69-73.
102 Yoo SH, Kymionis GD, Koreishi A, et al. Femtosecond laser-assisted sutureless anterior lamellar keratoplasty. Ophthalmology. 2008;115(8):1303-1307. 1307.e1
103 Busin M. Microkeratome-assisted superficial anterior lamellar keratoplasty. Tech Ophthalmol. 2006;4:64-68.
104 Jimenez-Alfaro I, Perez-Santonja JJ, Gomez Telleria G, et al. Therapeutic lamellar keratoplasty with an automated microkeratome. J Cataract Refract Surg. 2001;27:1161-1165.
105 Vajpayee RB, Vasudendra N, Titiyal JS, et al. Automated lamellar therapeutic keratoplasty (ALTK) in the treatment of anterior to mid-stromal corneal pathologies. Acta Ophthalmol Scand. 2006;84(6):771-773.
106 Polack FM. Lamellar keratoplasty. Malbran’s ‘peeling off’ technique. Arch Ophthalmol. 1971;86:293-295.
107 Barraquer JI. Lamellar keratoplasty (special techniques). Ann Ophthalmol. 1972;4:437-469.
108 Anwar M. Dissection technique in lamellar keratoplasty. Br J Ophthalmol. 1972;56:711-713.
109 Gasset AR. Lamellar keratoplasty in the treatment of keratoconus: conectomy. Ophthalmic Surg. 1979;10:26-33.
110 Archila EA. Deep lamellar keratoplasty dissection of host tissue with intrastromal air injection. Cornea. 1984/85;3:217-218.
111 Price FW. Air lamellar keratoplasty. Refract Corneal Surg. 1989;5:240-243.
112 Chau GK, Dilly SA, Sheard CE, et al. Deep lamellar keratoplasty on air with lyophilised tissue. Br J Ophthalmol. 1992;76:646-650.
113 Manche EE, Holland GN, Maloney RK. Deep lamellar keratoplasty using viscoelastic dissection. Arch Ophthalmol. 1999;117:1561-1565.
114 Melles GRJ, Remeijer L, Geerards AJM, et al. A quick surgical technique for deep, anterior lamellar keratoplasty using visco-dissection. Cornea. 2000;19:427-432.
115 Sugita J, Kondo J. Deep lamellar keratoplasty with complete removal of pathological stroma for vision improvement. Br J Ophthalmol. 1997;81:184-188.
116 Melles GR, Lander F, Rietveld FJ, et al. A new surgical technique for deep stromal, anterior lamellar keratoplasty. Br J Ophthalmol. 1999;83:327-333.
117 Melles GR, Rietveld FJ, Beekhuis WH, et al. A technique to visualize corneal incision and lamellar dissection depth during surgery. Cornea. 1999;18(1):80-86.
118 Anwar M, Teichmann KD. Big-bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28:398-403.
119 Sarnicola V, Toro P, Gentile D, et al. Descemetic DALK and predescemetic DALK: outcomes in 236 cases of keratoconus. Cornea. 2010;29(1):53-59.
120 Noble BA, Agrawal A, Collins C, et al. Deep Anterior Lamellar Keratoplasty (DALK): visual outcome and complications for a heterogeneous group of corneal pathologies. Cornea. 2007;26(1):59-64.
121 Fontana L, Parente G, Tassinari G. Clinical outcomes after deep anterior lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2007;143(1):117-124.
122 Fogla R, Padmanabhan P. Results of deep lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2006;141(2):254-259.
123 Jhanji V, Sharma N, Vajpayee RB. Intraoperative perforation of Descemet’s membrane during ‘big bubble’ deep anterior lamellar keratoplasty. Int Ophthalmol. 2010;30(3):291-295. Epub 2009 Dec 24
124 Shimazaki J, Shimmura S, Ishioka M, et al. Randomized clinical trial of deep lamellar keratoplasty vs penetrating keratoplasty. Am J Ophthalmol. 2002;134:159-165.
125 Marchini G, Mastropasqua L, Pedrotti E, et al. Deep lamellar keratoplasty by intracorneal dissection: a prospective clinical and confocal microscopic study. Ophthalmology. 2006;113(8):1289-1300.
126 Kawashima M, Kawakita T, Den S, et al. Comparison of deep lamellar keratoplasty and penetrating keratoplasty for lattice and macular corneal dystrophies. Am J Ophthalmol. 2006;142:304-309.
127 Vajpayee RB, Tyagi J, Sharma N, et al. Deep anterior lamellar keratoplasty by big-bubble technique for treatment corneal stromal opacities. Am J Ophthalmol. 2007;143:954-957.
128 Lyons CJ, McCartney AC, Kirkness CM, et al. Granular corneal dystrophy. Visual results and pattern of recurrence after lamellar or penetrating keratoplasty. Ophthalmology. 1994;101:1812-1817.
129 Pakrou N, Fung S, Selva D, et al. Deep lamellar keratoplasty in the treatment of keratoconus. Ophthalmologica. 2006;220:164-169.
130 Hosny MH, Shalaby AM, Badr El Din N. Early bubble modification of the big bubble technique for deep anterior lamellar keratoplasty. Eur J Ophthalmol. 2010;20(2):283-289.
131 Shimazaki J. Double-bubble technique to facilitate Descemet membrane exposure in deep anterior lamellar keratoplasty. J Cataract Refract Surg. 2010;36(2):193-196.
132 Busin M, Zambianchi L, Arffa RC. Microkeratome-assisted lamellar keratoplasty for the surgical treatment of keratoconus. Ophthalmology. 2005;112(6):987-997.
133 Busin M, Bhatt PR, Scorcia V. A modified technique for Descemet membrane stripping automated endothelial keratoplasty to minimize endothelial cell loss. Arch Ophthalmol. 2008;126(8):1133-1137.
134 Melles GR, Wijdh RH, Nieuwendaal CP. A technique to excise the Descemet membrane from a recipient cornea (descemetorhexis). Cornea. 2004;23(3):286-288.
135 Price MO, Price FWJr. Descemet’s stripping with endothelial keratoplasty; comparative outcomes with microkeratome-dissected and manually dissected donor tissue. Ophthalmology. 2006;113:1936-1942.
136 Covert DJ, Koenig SB. Descemet stripping and automated endothelial keratoplasty (DSAEK) in eyes with failed penetrating keratoplasty. Cornea. 2007;26:692-696.
137 Price FWJr, Price MO. Descemet’s stripping with endothelial keratoplasty in 200 eyes: early challenges and techniques to enhance donor adherence. J Cataract Refract Surg. 2006;32:411-418.
138 Bahar I, Kaiserman I, Sansanayudh W, et al. Busin guide vs forceps for the insertion of the donor lenticule in Descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2009;147(2):220-226.
139 Mehta JS, Por YM, Poh R, et al. Comparison of donor insertion techniques for Descemet stripping automated endothelial keratoplasty. Arch Ophthalmol. 2008;126(10):1383-1388.
140 Balachandran C, Ham L, Birbal RS, et al. Simple technique for graft insertion in Descemet-stripping (automated) endothelial keratoplasty using a 30-gauge needle. J Cataract Refract Surg. 2009;35(4):625-628.
141 Kaiserman I, Bahar I, McAllum P, et al. Suture-assisted vs forceps-assisted insertion of the donor lenticula during Descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2008;145(6):986-990.
142 Kuo AN, Harvey TM, Afshari NA. Novel delivery method to reduce endothelial injury in Descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2008;145(1):91-96.
143 Price MO, Price FWJr, Trespalacios R. Endothelial keratoplasty technique for aniridic aphakic eyes. J Cataract Refract Surg. 2007;33(3):376-379.
144 Bansal R, Ramasubramanian A, Das P, et al. Intracorneal epithelial ingrowth after Descemet stripping endothelial keratoplasty and stromal puncture. Cornea. 2009;28(3):33.
145 Ham L, Balachandran C, Verschoor CA, et al. Visual rehabilitation rate after isolated Descemet membrane transplantation: Descemet membrane endothelial keratoplasty. Arch Ophthalmol. 2009;127:252-255.
146 Zhu Z, Rife L, Yiu S, et al. Technique for preparation of the corneal endothelium–Descemet membrane complex for transplantation. Cornea. 2006;25:705-708.
147 Melles GR, Wijdh RH, Nieuwendaal CP. A technique to excise the Descemet membrane from a recipient cornea (descemetorhexis). Cornea. 2004;23:286-288.
148 Ignacio TS, Nguyen TT, Sarayba MA, et al. A technique to harvest Descemet’s membrane with viable endothelial cells for selective transplantation. Am J Ophthalmol. 2005;139:325-330.
149 Lie JT, Birbal R, Ham L, et al. Donor tissue preparation for Descemet membrane endothelial keratoplasty. J Cataract Refract Surg. 2008;34:1578-1583.
150 Zarei-Ghanavati S, Khakshoor H, Zarei-Ghanavati M. Reverse big bubble: a new technique for preparing donor tissue of Descemet membrane endothelial keratoplasty. Br J Ophthalmol. 2010;94(8):1110-1111.
151 Venzano D, Pagani P, Randazzo N, et al. Descemet Membrane Air Bubble Separation (DMABS) in donor corneas: a pilot study. J Cataract Refract Surg. 2010;36(12):2022-2027.
152 Busin M, Scorcia V, Patel AK, et al. Pneumatic dissection of donor endothelial tissue and storage for Descemet membrane endothelial keratoplasty – a novel technique. Ophthalmology. 2010;117(8):1517-1520.
153 Busin M, Patel AK, Scorcia V, et al. Stromal support for Descemet membrane endothelial keratoplasty. Ophthalmology. 2010;117:2273-2277.
154 Studeny P, Farkas A, Vokrojova M, et al. Descemet’s membrane endothelial keratoplasty with a stromal rim (DMEK-S). Br J Ophthalmol. 2010;94(7):909-914.
155 McCauley MB, Price FWJr, Price MO. Descemet membrane automated endothelial keratoplasty: hybrid technique combining DSAEK stability with DMEK visual results. J Cataract Refract Surg. 2009;35:1659-1664.
156 Melles GR, Lander F, Rietveld FJ. Transplantation of Descemet’s membrane carrying viable endothelium through a small scleral incision. Cornea. 2002;21(4):415-418.
157 Herpetic Eye Disease Study (HEDS) Group. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med. 1998;339:300.
158 Geyer O, Rothkoff L, Lazar M. Atropine in keratoplasty for keratoconus. Cornea. 1991;10(5):372-373.
159 Urrets Zavalia AJr. Fixed dilated pupil, iris atrophy and secondary glaucoma. Am J Ophthalmol. 1963;56:257-265.
160 Lin JC, Rapuano CJ, Laibson PR, et al. Corneal melting associated with use of topical nonsteroidal anti-inflammatory drugs after ocular surgery. Arch Ophthalmol. 2000;118(8):1129-1132.
161 Shimazaki J, Saito H, Yang HY, et al. Persistent epithelial defect following penetrating keratoplasty: an adverse effect of diclofenac eyedrops. Cornea. 1995;14(6):623-627.
162 Feizi S, Javadi MA, Jamali H, et al. Deep anterior lamellar keratoplasty in patients with keratoconus: big-bubble technique. Cornea. 2010;29(2):177-182.
163 Rotkis WM, Chandler JW, Forstot SL. Filamentary keratitis following penetrating keratoplasty. Ophthalmology. 1982;89(8):946-949.
164 Kaye DB. Epithelial response in penetrating keratoplasty. Am J Ophthalmol. 1980;89(3):381-387.
165 Fournié P, Ponchel C, Malecaze F, et al. Fixed dilated pupil (Urrets-Zavalia syndrome) and anterior subcapsular cataract formation after Descemet stripping endothelial keratoplasty. Cornea. 2009;28(10):1184-1186.
166 Niknam S, Rajabi MT. Fixed dilated pupil (Urrets-Zavalia syndrome) after deep anterior lamellar keratoplasty. Cornea. 2009;28(10):1187-1190.
167 Sánchez Pérez A, Bueno Lozano J, Brito Suárez C, et al. Study of infectious keratitis in corneal graft. Arch Soc Esp Oftalmol. 2000;75(10):659-663.
168 Kaye S, Baddon A, Jones M, et al. A UK scheme for reporting serious adverse events and reactions associated with ocular tissue transplantation. Cell Tissue Bank. 2010;11(1):39-46.
169 Wilhelmus KR, Hassan SS. The prognostic role of donor corneoscleral rim cultures in corneal transplantation. Ophthalmology. 2007;114(3):440-445. Review
170 Cockerham GC, Bijwaard K, Sheng ZM, et al. Primary graft failure : a clinicopathologic and molecular analysis. Ophthalmology. 2000;107(11):2083-2090.
171 Hau S, Barton K. Corneal complications of glaucoma surgery. Curr Opin Ophthalmol. 2009;20(2):131-136.
172 Sarkisian SRJr. Tube shunt complications and their prevention. Curr Opin Ophthalmol. 2009;20(2):126-130. Review
173 Hollander DA, Giaconi JA, Holland GN, et al. Graft failure after penetrating keratoplasty in eyes with Ahmed valves. Am J Ophthalmol. 2010;150(2):169-178.
174 Yalniz-Akkaya Z, Burcu Nurozler A, Yildiz E, et al. Repeat penetrating keratoplasty: indications and prognosis, 1995–2005. Eur J Ophthalmol. 2009;19(3):362-368.
175 Coster DJ, Williams KA. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am J Ophthalmol. 2005;140(6):1112-1122.
176 Thompson RWJr, Price MO, Bowers PJ, et al. Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003;110(7):1396-1402.
177 Maguire MG, Stark WI, Gottsch JD, et al. Risk factors for corneal graft failure and rejection in the (CCTS). Ophthalmology. 1994;101:1536.
178 The Collaborative Corneal Transplantation Studies Research Group. The Collaborative Corneal Transplantation Studies (CCTS). Arch Ophthalmol. 1992;110:1392.
179 Küchle M, Cursiefen C, Nguyen NX, et al. Risk factors for corneal allograft rejection: intermediate results of a prospective normal-risk keratoplasty study. Graefes Arch Clin Exp Ophthalmol. 2002;240(7):580-584.
180 Sangwan VS, Ramamurthy B, Shah U, et al. Outcome of corneal transplant rejection: a 10-year study. Clin Exp Ophthalmol. 2005;33(6):623-627.
181 Williams KA, Coster DJ. The immunobiology of corneal transplantation. Transplantation. 2007;84(7):806-813.
182 Kamp MT, Fink NE, Enger C, et al. Patient-reported symptoms associated with graft reactions in high-risk patients in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Cornea. 1995;14(1):43-48.
183 Randleman JB, Stulting RD. Prevention and treatment of corneal graft rejection: current practice patterns (2004). Cornea. 2006;25(3):286-290.
184 Poon A, Constantinou M, Lamoureux E, et al. Topical cyclosporin A in the treatment of acute graft rejection: a randomized controlled trial. Clin Exp Ophthalmol. 2008;36(5):415-421.
185 Belin MW, Bouchard CS, Frantz S, et al. Topical cyclosporine in high-risk corneal transplants. Ophthalmology. 1989;96(8):1144-1150.
186 Cosar CB, Laibson PR, Cohen EJ, et al. Topical cyclosporine in pediatric keratoplasty. Eye Contact Lens. 2003;29(2):103-107.
187 Dhaliwal JS, Mason BF, Kaufman SC. Long-term use of topical tacrolimus (FK506) in high-risk penetrating keratoplasty. Cornea. 2008;27(4):488-493.
188 Joseph A, Raj D, Shanmuganathan V, et al. Tacrolimus immunosuppression in high-risk corneal grafts. Br J Ophthalmol. 2007;91(1):51-55.
189 Barraquer J. Immunosuppressive agents in penetrating keratoplasty. Am J Ophthalmol. 1985;100(1):61-64.
190 Den S, Shimmura S, Tsubota K, et al. Impact of the Descemet membrane perforation on surgical outcomes after deep lamellar keratoplasty. Am J Ophthalmol. 2007;143(5):750-754.
191 Mohamed SR, Manna A, Amissah-Arthur K, et al. Non-resolving Descemet folds 2 years following deep anterior lamellar keratoplasty: the impact on visual outcome. Cont Lens Anterior Eye. 2009;32(6):300-302.
192 Faia LJ, Baratz KH, Bourne WM. Corneal graft folds: a complication of deep lamellar endothelial keratoplasty. Arch Ophthalmol. 2006;124(4):593-595.
193 World Health Organization. Visual impairment and blindness. Available at http://who.int/mediacentre/factsheets/fs282/en/, August 20, 2009. Accessed
194 Whitcher JO, Srinivasan M, Upadhyay MP. Bull World Health Org. 2001;85:388-392.
195 Pellier de Quensgy G. Précis Ou Cours d’Operations Sur la Chirurgie Des Yeux, vol. 1. Paris: Didot & Mequignon; 1789–1790, 91.
196 Heusser I. Ein fall von Cornea artificialis. Denkschr Med Chir Gesellsch. 1860:127.
197 Von Hippel A. Cited by E. Forster. Review of keratoplastic surgery and some experiments in keratoplasty. Am J Ophthalmol. 1923;6:366-375.
198 Allan B. Artificial corneas. BMJ. 1999;318:821-822.
199 Rishi P, Maskati QB, Ray R, et al. Vitreoretinal surgery in eyes with Pintucci biointegrable keratoprosthesis. Retina. 2010;30(2):287-293.
200 Anderson OEE. Theroretical considerations for an artificial corneal implant. Br J Ophthalmol. 1951;35:628-630.
201 Yaghouti F, Nouri M, Abad JC, et al. Keratoprosthesis: preoperative prognostic categories. Cornea. 2001;20:19-23.
202 Khan B, Dudenhoefer EJ, Dohlman CH. Keratoprosthesis: an update. Curr Opin Ophthalmol. 2001;12:288-293.
203 Iaccheri B, Roque M, Fiore T, et al. Ocular cicatricial pemphigoid, keratomycosis, and intravenous immunoglobulin therapy. Cornea. 2004;23:819-822.
204 Hille K, Grabner G, Liu C, et al. Standards for modified osteo-odonto-keratoprosthesis (OOKP) surgery according to Strampelli and Falcinelli: the Rome–Vienna Protocol. Cornea. 2005;24:895-908.
205 Ament JD, Pineda R, Lawson B, et al. The Boston Keratoprosthesis International Protocol Version 1. March 31, 2009.
206 Todani A, Gupta P, Colby K. Type I Boston keratoprosthesis with cataract extraction and intraocular lens placement for visual rehabilitation of herpes zoster ophthalmicus: the ‘KPro Triple’. Br J Ophthalmol. 2009;93(1):119.
207 Pavan-Langston D, Dohlman CH. Boston keratoprosthesis treatment of herpes zoster neurotrophic keratopathy. Ophthalmology. 2008;115(2 Suppl):S21-S23.
208 Nazmi A, Victora CG. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health. 2007;7:212.
209 Pelletier SJ, Isaac RB, Raymond, et al. Ethnic disparities in outcome from posttransplant infections. Shock. 2004;22(3):197-203.
210 Federico MJ, Covar RA, Brown EE, et al. Racial differences in T-lymphocyte response to glucocorticoids. Chest. 2005;127:571-578.
211 Strampelli B. Keratoprosthesis with osteodontal tissue. Am J Ophthalmol. 1963;89:1029-1039.
212 Falcinelli G, Falsini B, Taloni M, et al. Modified osteo-odonto-keratoprosthesis for treatment of corneal blindness: long-term anatomical and functional outcomes in 181 cases. Arch Ophthalmol. 2005;123:1319-1329.
213 Casey TA. Osteo-odonto-keratoprosthesis. Proc R Soc Med. 1966;59:530-531.
214 Temprano J. Keratoprosthesis with tibial autograft. Refract Corneal Surg. 1993;9:192-193.
215 Gomaa A, Comyn O, Liu C. Keratoprostheses in clinical practice – a review. Clin Exp Ophthalmol. 2010;38:211-224.
216 Falcinelli GC, Barogi G, Caselli, et al. Personal changes and innovations in Strampelli’s osteo-odonto-keratoprosthesis. An Inst Barraquer. 1999;28(s):47-48.
217 Crawford GJ, Hicks CR, Constable IJ, et al. The chirila keratoprosthesis: Phase I human clinical trial. Ophthalmology. 2002;109:883-889.
218 Ilhan-Sarac O, Akpek EK. Current concepts and techniques in keratoprosthesis. Curr Opin Ophthalmol. 2005;16:246-250.
219 Chirilia TV. An overview of the development of artificial corneas with porous skirts and the use of pHEMA for such an application. Biomaterials. 2001;22:3311-3317.
220 Holak SA, Holak HM, Bleckmann H. AlphaCor keratoprosthesis: postoperative development of six patients. Graefes Arch Clin Exp Ophthalmol. 2009;247:535-539.
221 Ngakeng V, Hauck MJ, Price MO, et al. AlphaCor keratoprosthesis. A novel approach to minimize the risks of long-term postoperative complications. Cornea. 2008;27:905-910.
222 Hicks CR, Crawford GJ, Lou X, et al. Corneal replacement using synthetic hydrogel cornea, AlphaCor:device, preliminary outcomes and complications. Eye. 2003;17:385-392.
223 Pintucci S, Pintucci F, Caizza S, et al. New Dacron tissue colonizable keratoprosthesis: clinical experience. Br J Ophthalmol. 1995;79:825-829.
224 Hicks J, Fitton H, Chirila TV, et al. Keratoprostheses: advancing toward a true artificial cornea. Surv Ophthalmol. 1997;42:175-189.
225 Stoiber J, Fernandez V, Kaminski S, et al. Biological response to a supraDescemetic synthetic cornea in rabbits. Arch Ophthalmol. 2004;122(12):1850-1855.
226 Chew FH, Ayres BD, Hammersmith KM, et al. Boston keratoprosthesis outcomes and complications. Cornea. 2009;28:989-996.
227 Bath PE, McCord RC, Cox KC. Nd:YAG laser dissection of retroprosthetic membrane: a preliminary report. Cornea. 1983;2:225.
228 Ray S, Khan BF, Dohlman CH, et al. Management of vitreoretinal complications in eyes with permanent keratoprosthesis. Arch Ophthalmol. 2002;120:559.
229 Yaghouti F, Nouri M, Al-Merjan J, et al. Keratoprosthesis: Preoperative prognostic categories. Cornea. 2001;20:19-23.
230 Harissi-Dageher M, Khan BF, Schaumberg DA, et al. Importance of nutrition to corneal grafts when used as carrier of the Boston keratoprosthesis. Cornea. 2007;26:564-568.
231 Sayegh RR, Ang LPK, Foster CS, et al. The Boston keratoprosthesis in Stevens–Johnson syndrome. Am J Ophthalmol. 2008;145:438-444.
232 Zerbe BL, Belin MW, Ciolino JB. Boston Type 1 keratoprosthesis study group. Results from the multicenter Boston type 1 keratoprosthesis study. Ophthalmology. 2006;113:1779-1784.
233 Netland PA, Terada H, Dohlman CH. Glaucoma associated with keratoprosthesis. Ophthalmology. 1998;105:751-757.
234 Tan DTH, Tay ABG, Theng JTS, et al. Keratoprosthesis surgery for end-stage corneal blindness in Asian eyes. Ophthalmol. 2008;115:503-510.
235 Miyashita H, Shimmura S, Kobayashi H, et al. Collagen-immobilized poly(vinyl alcohol) as an artificial cornea scaffold that supports a stratified corneal epithelium. J. Biomed Mter Res. 2005;76B:56-63.
236 Shimmura S, Doillon CJ, Griffith M, et al. Collagen-poly (N-isopropylacrylamide)-based membranes for corneal stroma scaffolds. Cornea. 2003;22:S81-S88.
237 Uchino Y, Shimmura S, Miyashita H, et al. Amniotic membrane immobilized poly(vinyl alcohol) hybrid polymer as an artificial scaffold that supports a stratified and differentiated corneal epithelium. J Biomed Mater Res. 2007;81B:201-206.
238 Huang YX, Li QH. An active artificial cornea with the function of inducing new corneal tissue generation in vivo – a new approach to corneal tissue engineering. Biomed Mater. 2007;2:S121-S125.
239 Myung D, Duhamel PE, Cochran JR, et al. Development of hydrogel-based keratoprostheses: a material perspective. Biotechnol Prog. 2008;24:735-741.
240 Princz M, Scheardown H. Heparin and growth factor modified materials for artificial cornea applications. Invest Ophthalmol Vis Sci. 2006;47(5):2751.
241 Bakri A, Farooqui N, Myung N, et al. Biocompatibility of a hydrogel corneal inlay in vivo. Invest Ophthalmol Vis Sci. 47, 2006. E-abstract 3592
242 Myung D, Koh W, Ko J, et al. Characterization of poly(ethylene glycol) and poly(acryl acid) double networks for corneal implant applications. Invest Ophthalmol Vis Sci. 46, 2005. E-Abstract 5003