Corneal Epithelial Adhesion Disorders
Pathophysiology
Normal Anatomy of the Epithelial Adhesion Complex
There are two major adherence mechanisms for corneal epithelial cells to adhere to basement membrane. One mechanism is through direct molecular interaction of receptors with ligands located in the extracellular matrix. Three major families of such molecular interactions have been identified. These include the N-CAM family, the cadherin family, and the integrins, which are a family of integral membrane proteins interacting with an extracellular matrix ligand at cell–matrix interfaces.1
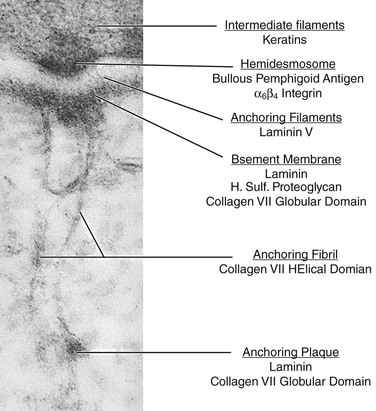
The second mechanism of cell–matrix adhesion is through adhesive junctions, called hemidesmosomes (Fig. 26.1).2 The hemidesmosomes are located on the basal membranes of the epithelial cells. On the external side of the cell membrane at the hemidesmosome, an electron-dense line parallels the membrane and, from it, anchoring filaments extend through the lamina lucida to the lamina densa of the basement membrane (Fig. 26.1). Opposite the lamina densa, anchoring fibrils insert from the stromal side. These fibrils form an intertwining network in the anterior stroma. Distal from their insertions in the basement membrane, anchoring fibrils insert into anchoring plaques, which appear structurally as small segments of basement membrane (Fig. 26.1). Collectively, all these structurally linked components, including intermediate filaments, hemidesmosomes, anchoring filaments, anchoring fibrils and anchoring plaques, are termed the adhesion complex.3
Epithelial Cell Adhesions in Recurrent Corneal Erosions
As basal cells of the corneal epithelium begin to migrate to cover a wound, they lose their hemidesmosomes.4 Re-establishment of the tight adhesion of the corneal epithelium is associated with re-formation of hemidesmosomes and components of the adhesion complex.5 An interim adhesion junction, termed ‘focal adhesion,’ is constructed along the cell–matrix interface of epithelial cells during migration, as evident by a dramatic increase in protein synthesis during migration to cover a wound.6
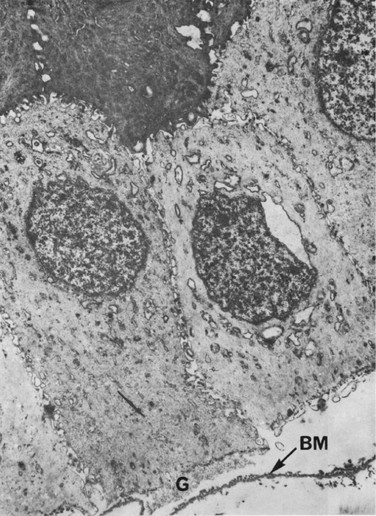
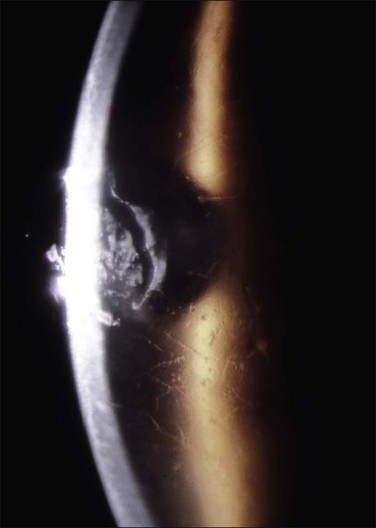
Ultrastructural studies of the cornea in RCE have demonstrated defective junctional complexes following epithelial trauma, resulting in delayed adhesion of epithelial cells to underlying structures (Fig. 26.2).5,7 These adhesion complex defects are characterized primarily by the focal absence of the basement membrane and of the hemidesmosomes. The basement membrane may appear multilayered and folded between epithelial cells. In addition, some of the basal corneal epithelial cells appear pale and swollen.7,8 Areas of healthy epithelium contain intraepithelial pseudocysts, with collections of cellular and amorphous debris.7 This cellular debris is probably the result of entrapment of the epithelium by aberrant basement membrane. The abnormal adhesion complexes between the basal epithelial cells and the basement membrane, may lead to focal areas of elevation of the epithelium and the accumulation of underlying cellular debris (Fig. 26.2). This leads to formation of abnormal basement membrane, with further focal detachments of the basal epithelium and accumulation of cellular debris, leading to a vicious cycle of aberrant epithelial adhesion and recurrent erosions.
The timing of the erosion corresponds with an abrupt opening of the lids, which is why these episodes occur at night time or while awakening from sleep in the morning. During sleep and lid closure, there is no air between the lids and the tear film, and the surface tension of the tears creates sealing of the lid margins. Abrupt opening the lids creates a shearing force, which is greater than the force of adherence of the epithelium to its basement membrane, and this may result in epithelial avulsion.9
Meibomian Gland Disease and Inflammatory Mediators
A higher incidence of severe meibomian gland disease (MGD) and acne rosacea was noted in non-traumatic RCE.10 These patients had inspissation of the meibomian glands, reduced tear film break-up time, conjunctival injection, and facial manifestations of acne rosacea, including facial erythema, flushes, papules, and pustules. The location of the corneal erosions was at the inferior cornea, which was explained by a longer contact with the tear film, containing its inflammatory mediators, typical of MGD.10
MGD is associated with higher levels of inflammatory cytokines and matrix-degrading enzymes in the tear film, which may potentially disturb the normal healing process, by interfering with the formation of normal hemidesmosomes and adhesion complexes. Increased bacterial lipase has been demonstrated in patients with MGD, which is responsible for the production of free fatty acids, which can interfere with the assembly of the adhesion complexes.11,12 In addition, inflammatory cytokines and matrix-degrading enzymes, such as interleukin-1 and MMP-9 were found to be elevated in the tears of patients with MGD,13 further contributing to the damaged healing patterns of the corneal epithelium in RCE.
Elevated levels of MMP-2 and MMP-9 have been observed in the tear fluid of patients with RCE. These matrix-degrading enzymes were found to be up-regulated in human epithelia affected by recurrent erosion. These enzymes are concentrated in basal epithelial cells where they may play an important role in degradation of the epithelial anchoring system and result in recurrent epithelial slippage and erosion.14
Etiology
Recurrent corneal erosions are the clinical end result of multiple disorders of the corneal epithelium and basement membrane. Although most of the patients with unilateral RCE will present after a history of acute trauma to the cornea with sloughing or erosion of the corneal epithelium (Fig. 26.3), careful consideration must be given to a wide spectrum of causes, specifically in patients with a bilateral disease, having no prior injury to the cornea.
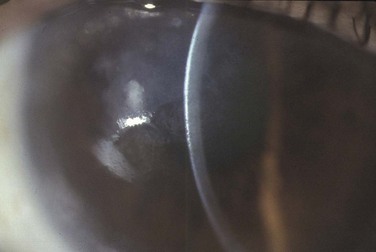
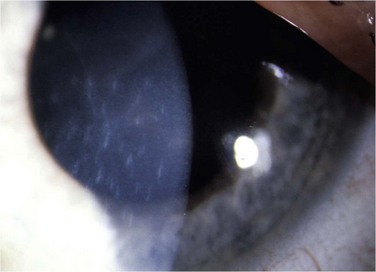
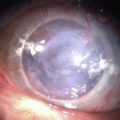
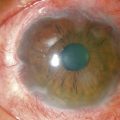
The etiology of RCE may be classified into primary and secondary disorders (Box 26.1).9 Primary disorders include genetic disorders, chiefly the corneal dystrophies that involve the epithelium, basement membrane or anterior stroma. Primary disorders are usually bilateral, symmetrical, and may occur in multiple locations in the cornea. The most common of these etiologies is the map-dot finger print dystrophy (Figs 26.4, 26.5). RCE is a common manifestation in lattice dystrophy, which involves the anterior stroma (Fig. 26.6).15
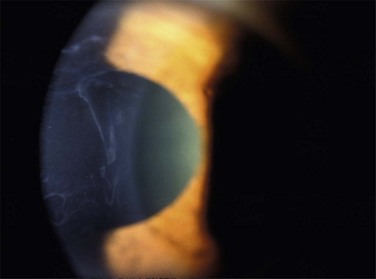
Figure 26.4 Map-like changes in epithelial basement membrane dystrophy. (Courtesy Peter Laibson, MD.)
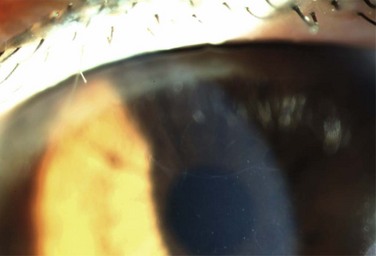
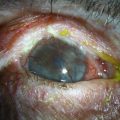
The secondary basement membrane disorders leading to RCE are more common. They are usually acquired disorders, appear in one eye, and are often limited to a single location in the cornea. Of these, minor trauma to the corneal epithelium is the most common cause for RCE. Trauma to the epithelium can be caused by a fingernail, plant material, sharp domestic objects, or the edge of a piece of paper (Fig. 26.7).16 Salzmann’s nodular degeneration is another common acquired disorder that may be associated with recurrent erosions (Fig. 26.8).
Rarely, cases may occur spontaneously without any obvious predisposing factor.
Clinical Manifestations
Two main forms of erosion have been identified: microform and macroform. The microform erosions are small epithelial breaks, while the macroform erosion is larger and surrounded with a loosely adherent epithelium. Typically, the microform erosions are less severe, occur more frequently, sometimes every night or morning, occur spontaneously and are associated with epithelial basement membrane dystrophy (EBMD). The macroform erosions are associated with a traumatic etiology, and persist for several days.17
Although little is known about the epidemiology of RCE, it is thought to be a relatively common problem in specialized cornea services. The incidence of RCE was reported to be 1 : 150 cases following trauma.9
Careful slit lamp examination is needed to find the subtle signs of this syndrome, since the epithelium in many cases had already healed. The delicate signs of basement membrane dystrophy (Fig. 26.9), the sites of a previous erosion, or clusters of small epithelial microcysts, may be seen with either a broad slit beam or with retroillumination (Fig. 26.10). Examination of the cornea after pupil dilation, against the red reflex, may disclose subtle changes in the corneal epithelium. Gentle pressure applied to the cornea through the eyelid may demonstrate wrinkling of loosely adherent epithelium. In many cases, no signs are found, and then the patient should be instructed to return immediately after the next episode of pain, without allowing time for the epithelium to heal and cover the erosion. This will facilitate proper diagnosis and correct location of the lesion for the purpose of treatment.
During the acute attack or during the first few days following the attack, the affected corneal epithelium can present as a loosely adherent and elevated epithelium, or as epithelial microcysts, or as corneal epithelial defects.17,18 Stromal infiltrates and opacities may also develop at the site of the erosions.19 The location of most of the corneal erosions is in the lower half of the cornea.16 The midline below the horizontal meridian is usually the last area to re-epithelialize, and the closure lines at this area are predisposed to frequent breakdown. In addition, this is the area of maximal exposure, since it opposes the line of lids closure.
A possible complication of RCE is infectious keratitis, occurring as a result of prolonged bandage contact lens use and topical steroids.9
When no obvious signs of RCE are evident in the slit lamp examination, the presence of impaired epithelial adhesion is detected by use of a dry cellulose sponge, which is gently rubbed over the area of suspected epithelial erosions. If the intact epithelial sheet is moveable by the sponge, then the lack of adequate epithelial–stromal adhesion must be suspected.20
The duration of symptoms may vary, and the frequency and number of attacks are also extremely variable. The frequency of recurrence may range from a minor recurrence every morning to a major recurrence every several months. Recurrences typically last from 1 to 4 hours in the microform condition and 1 to 21 days in the macroform erosions.17 A more recent study in a large cohort of RCE patients demonstrated that after 4 years of follow-up, 59% of all patients were still symptomatic, and most of them complained of symptoms occurring upon waking in the mornings.21 The median frequency of the attacks was every 60 days; however, 24% of symptomatic patients suffered with an attack at least every week, and 51% of the symptomatic patients suffered an attack at least every month.
Comparing patients with a traumatic etiology with patients who had RCE secondary to EBMD, patients with EBMD were significantly more likely to be symptomatic: 75% of patients with EBMD were symptomatic compared with 46% of patients with traumatic etiology.21
Management
The management of RCE is challenging, and to date, not one single therapy was found to be definitive and sufficiently effective. Over the years, multiple treatment strategies were developed, including topical conservative treatments, such as hypertonic lubricants, soft bandage contact lenses, and various aggressive surgical modalities, such as anterior stromal puncture and photorefractive keratectomy, which aim at creating a new basement membrane–epithelial interface (Box 26.2).
Medical Management
Topical Lubricants and Hyperosmotic Agents
Most patients with an acute episode of RCE can be managed initially with patching and a topical antibiotic ointment.16,17 To prevent recurrences, the most widely used therapies include topical lubrication and hypertonic saline.10,16,17,22 Topical lubrication can be administered as drops, gels or ointment. The bedtime application of an ointment will reduce the amount of friction between the tarsal conjunctiva and the corneal epithelium overnight, during the rapid eye movements (REM), and will protect the corneal epithelium from the shearing action of the eyelids upon awakening, which is the major trigger of recurrence.
Most patients will do well with these conservative treatments, which are effective in relieving the pain and promoting the initial epithelial growth.22 However, these modalities will not reduce the likelihood of recurrences.16
Therapeutic Bandage Contact Lenses
Therapeutic bandage contact lenses promote epithelial migration and regeneration of the basement membrane by protecting the corneal epithelium from the friction created by the upper tarsal conjunctiva.23 To be effective, a bandage contact lens should be worn for a few weeks to several months,16 replacing it every 2 weeks. This may enable the formation of stable adhesion complexes between the corneal epithelium and the basement membrane.
These contact lenses should be used under close supervision, since long-term continuous use of contact lenses may predispose to bacterial keratitis and neovascularization.16,24 However, the introduction of the silicon hydrogel extended-wear contact lenses, in recent years, has significantly increased the safety of long-term use of bandage contact lenses.25,26
Autologous Serum
Autologous serum has been used effectively in RCE, significantly reducing the incidence of recurrence.27,28 It is composed of substances that are essential for epithelial healing, such as vitamin A, epidermal growth factor, transforming growth factor β and fibronectin. Fibronectin promotes epithelial cell migration and participates in the adhesion process.29 The lipids in the serum may act as a substitute for the lipids produced by the meibomian glands. When appropriately prepared and used, autologous serum is safe and no adverse effects have been reported with its use.28
Managing Lid Disease
Meibomian gland disease (MGD) and chronic blepharitis are associated with RCE.10 The tear film in MGD may have increased levels of bacterial lipases, fatty acids, interleukins and MMPs, which can interfere with corneal epithelial healing. Therefore, the usual therapeutic measures employed in the treatment of MGD should be used in RCE as well. These include lid hygiene and oral tetracyclines. Oral tetracyclines were shown to be beneficial in reducing episodes of RCE by reducing the free fatty acids in the tear film of MGD, by inactivating the MMPs, and by reducing the number of colony forming units from lid cultures.10,30 Low doses of oral tetracyclines should be continued for a few months.
The corneal epithelium produces matrix-degrading enzymes, such as MMP-2 and MMP-9. These enzymes have roles in the wound healing process, and are part of the inflammatory activity in many ocular surface disorders. Increased production of these enzymes, specifically MMP-2, was demonstrated in RCE.14 Therefore, MMPs may be responsible for the degradation of anchoring molecules in the adhesion complexes of the basement membrane during epithelial healing. This is the basis for the use of inhibitors of MMP in the treatment of resistant RCE.31 Indeed, a combination of oral doxycycline and topical steroids was successfully used in recalcitrant cases of RCE.31 Both topical steroids and oral doxycycline were reported to decrease the frequency of RCE in a randomized, controlled clinical trial.30 In addition, doxycycline and corticosteroids have significant anti-inflammatory properties. Doxycycline decreases the synthesis and bioactivity of interleukin-1, produced by cultured human epithelial cells.32 The combination of steroids and doxycycline can inhibit the inflammatory cytokines and MMPs and promote rapid resolution and prevent further recurrence in RCE.31
Surgical Management
Debridement of Loose Epithelium
Epithelial debridement is probably indicated when a large area of the epithelium is loose and mobile with lid movements. Debridement of this large area of loose epithelium is necessary for pain relief, and can promote healing from the healthy adherent edges of the intact epithelium. Epithelial debridement may be performed under topical anesthesia with the slit lamp, by removing the loose epithelium with a sterile sponge. A therapeutic bandage contact lens should be placed if the epithelial defect is large. Topical antibiotics and cyclopentolate drops may be added. Epithelial debridement alone cannot reduce the recurrences of epithelial erosions.16,17,22
Anterior Stromal Puncture
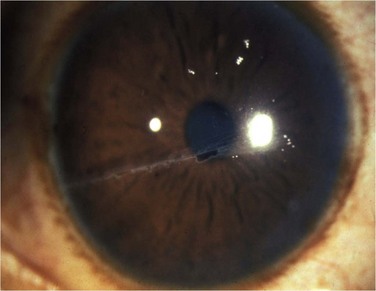
Anterior stromal puncture is a highly effective and widely used office technique, involving the use of a straight 25-gauge needle to make multiple shallow penetrations through the epithelium into anterior corneal stroma, thus, improving epithelial adhesion. It is theorized that this procedure incites reactive fibrosis and scarring, and production of extra cellular matrix proteins, that are responsible for proper adhesion of the epithelium to its substrate.33 The procedure is performed with topical anesthesia with a bent 25 gauge (0.1–0.3 mm turned end) needle attached to a 3-mL syringe. Topical nonsteroidal drops, such as ketorolac or diclofenac should be instilled prior to the procedure to reduce postoperative pain. Fluorescein can be instilled topically before the treatment to better define the affected area (Fig. 26.11). The angled tip of the needle is kept perpendicular to the surface of the cornea, and multiple superficial punctures are placed approximately 0.5 mm apart in the affected area (Fig. 26.11). Treatment is extended to 1–2 mm into the normal epithelium bordering the lesion, because the loose epithelium usually extends beyond the visible limits of the erosion. Treatment within the pupil area should be minimized if possible. Since anterior stromal puncture may result in subepithelial scars (Fig. 26.12), its use is not advisable if the erosion directly involves the visual axis, since a central scar may result in glare and a reduction in visual acuity. Immediately after the treatment, a bandage contact lens is placed and antibiotics are given for 1 week.18,22
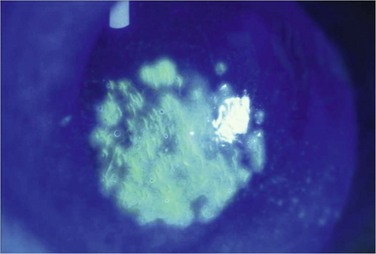
Figure 26.11 Fluorescein staining immediately following anterior stromal puncture. (Courtesy Peter Laibson, MD.)
Success rates of up to 80% have been reported in recalcitrant RCE.18 Treatment failure is associated with a too small treatment area, and erosions then develop outside of the treated area. A second, larger treatment can often resolve the erosions in patients in whom the initial procedure was unsuccessful.
Anterior stromal puncture can also be performed with a short-pulsed Nd:YAG laser.34 Energy levels ranging of 1.8–2.2 mJ can be used. The advantage of laser puncture over needle puncture is that the laser puncture is more reproducible, shallow, and translucent.
Phototherapeutic Keratectomy (PTK)
Studies have shown that partial ablation of Bowman’s layer, with the excimer laser, provides a smooth bed for migrating epithelium, and results in new hemidesmosomal adhesion complexes.35,36 Human studies have shown that the basal epithelial layer forms hemidesmosomes and new basement membrane within 2 weeks of laser photoablation.37 Thus, new hemidesmosomes, anchoring fibrils, and epithelial basement membrane are synthesized rapidly and in increasing amounts after PTK. In addition, removing the basement membrane allows the epithelium to come into direct contact with stromal elements, stimulating the synthesis of new anchoring fibrils and hemidesmosomes.38
The safety and efficacy of PTK for RCE have been well established.38,39 The advantages of PTK are removal of corneal tissue with extreme precision, without damage to the non-ablated area, and simultaneous treatment of wider areas. The aim is to remove a 6.0-mm diameter of thick anterior stromal layer. The defective epithelium is removed with a cellulose sponge and a 7.0–8.0-mm diameter flat beam is programmed to ablate a 6.0-mm diameter layer of the anterior stroma. The eye is usually padded after cycloplegic and antibiotic drops. Bandage contact lenses may be needed during the postoperative period. The success rate of PTK is between 60% and 100%.40,41 PTK results in a higher rate of success for RCE following trauma than corneal dystrophies.40,41
Diamond Burr Superficial Keratectomy
The results of diamond burr are comparable to those of PTK.42 A subtle granular subepithelial deposit is initially noticed. The haze clears in up to 3 months postoperatively. A study on cases with RCE and anterior basement membrane dystrophy showed significantly less haze after diamond burr polishing compared to PTK. Diamond burr polishing is a simple, less expensive procedure with a smaller incidence of haze and fewer recurrences compared to PTK. In addition, it can be used to treat RCE involving the visual axis.
References
1. Hynes, RO. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554.
2. Gipson, IK. Adhesive mechanisms of the corneal epithelium. Acta Ophthalmol Suppl. 1992;202:13–17.
3. Gipson, IK, Spurr-Michaud, SJ, Tisdale, AS. Anchoring fibrils form a complex network in human and rabbit cornea. Invest Ophthalmol Vis Sci. 1987;28:212–220.
4. Buck, RC. Hemidesmosomes of normal and regenerating mouse corneal epithelium. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;41(1-2):1–16.
5. Khodadoust, AA, Silverstein, AM, Kenyon, KR, et al. Adhesion of regenerating corneal epithelium. The role of basement membrane. Am J Ophthalmol. 1968;65:339–348.
6. Zieske, JD, Bukusoglu, G, Gipson, IK. Enhancement of vinculin synthesis by migrating stratified squamous epithelium. J Cell Biol. 1989;109:571–576.
7. Rodrigues, MM, Fine, BS, Laibson, PR, et al. Disorders of the corneal epithelium: a clinicopathologic study of dot, geographic, and fingerprint patterns. Arch Ophthalmol. 1974;92:475–482.
8. Tripathi, RC, Bron, AJ. Ultrastructural study of non-traumatic recurrent corneal erosion. Br J Ophthalmol. 1972;56:73–85.
9. Ramamurthi, S, Rahman, MQ, Dutton, GN, et al. Pathogenesis, clinical features and management of recurrent corneal erosions. Eye. 2006;20:635–644.
10. Hope-Ross, MW, Chell, PB, Kervick, GN, et al. Recurrent corneal erosion: clinical features. Eye. 1994;8(Pt 4):373–377.
11. Dougherty, JM, McCulley, JP. Bacterial lipases and chronic blepharitis. Invest Ophthalmol Vis Sci. 1986;27:486–491.
12. Dougherty, JM, McCulley, JP. Analysis of the free fatty acid component of meibomian secretions in chronic blepharitis. Invest Ophthalmol Vis Sci. 1986;27:52–56.
13. Afonso, AA, Sobrin, L, Monroy, DC, et al. Tear fluid gelatinase B activity correlates with IL-1alpha concentration and fluorescein clearance in ocular rosacea. Invest Ophthalmol Vis Sci. 1999;40:2506–2512.
14. Garrana, RM, Zieske, JD, Assouline, M, et al. Matrix metalloproteinases in epithelia from human recurrent corneal erosion. Invest Ophthalmol Vis Sci. 1999;40:1266–1270.
15. Zechner, EM, Croxatto, JO, Malbran, ES. Superficial involvement in lattice corneal dystrophy. Ophthalmologica. 1986;193:193–199.
16. Hykin, PG, Foss, AE, Pavesio, C, et al. The natural history and management of recurrent corneal erosion: a prospective randomised trial. Eye. 1994;8(Pt1):35–40.
17. Brown, N, Bron, A. Recurrent erosion of the cornea. Br J Ophthalmol. 1976;60:84–96.
18. Rubinfeld, RS, Laibson, PR, Cohen, EJ, et al. Anterior stromal puncture for recurrent erosion: further experience and new instrumentation. Ophthalmic Surg. 1990;21:318–326.
19. Ionides, AC, Tuft, SJ, Ferguson, VM, et al. Corneal infiltration after recurrent corneal epithelial erosion. Br J Ophthalmol. 1997;81:537–540.
20. Kenyon, KR, Paz, H, Greiner, JV, et al. Corneal epithelial adhesion abnormalities associated with LASIK. Ophthalmology. 2004;111:11–17.
21. Heyworth, P, Morlet, N, Rayner, S, et al. Natural history of recurrent erosion syndrome – a 4-year review of 117 patients. Br J Ophthalmol. 1998;82:26–28.
22. Reidy, JJ, Paulus, MP, Gona, S. Recurrent erosions of the cornea: epidemiology and treatment. Cornea. 2000;19:767–771.
23. Donnenfeld, ED, Selkin, BA, Perry, HD, et al. Controlled evaluation of a bandage contact lens and a topical nonsteroidal anti-inflammatory drug in treating traumatic corneal abrasions. Ophthalmology. 1995;102:979–984.
24. Kent, HD, Cohen, EJ, Laibson, PR, et al. Microbial keratitis and corneal ulceration associated with therapeutic soft contact lenses. CLAO J. 1990;16:49–52.
25. Blackmore, SJ. The use of contact lenses in the treatment of persistent epithelial defects. Cont Lens Anterior Eye. 2010;33:239–244.
26. Mely, R. Therapeutic and cosmetic indications of lotrafilcon a silicone hydrogel extended-wear lenses. Ophthalmologica. 2004;218(Suppl. 1):29–38.
27. Ziakas, NG, Boboridis, KG, Terzidou, C, et al. Long-term follow up of autologous serum treatment for recurrent corneal erosions. Clin Experiment Ophthalmol. 2010;38:683–687.
28. del Castillo, JM, de la Casa, JM, Sardina, RC, et al. Treatment of recurrent corneal erosions using autologous serum. Cornea. 2002;21:781–783.
29. Fujikawa, LS, Foster, CS, Harrist, TJ, et al. Fibronectin in healing rabbit corneal wounds. Lab Invest. 1981;45:120–129.
30. Hope-Ross, MW, Chell, PB, Kervick, GN, et al. Oral tetracycline in the treatment of recurrent corneal erosions. Eye. 1994;8(Pt 4):384–388.
31. Dursun, D, Kim, MC, Solomon, A, et al. Treatment of recalcitrant recurrent corneal erosions with inhibitors of matrix metalloproteinase-9, doxycycline and corticosteroids. Am J Ophthalmol. 2001;132:8–13.
32. Solomon, A, Rosenblatt, M, Li, DQ, et al. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Invest Ophthalmol Vis Sci. 2000;41:2544–2557.
33. Katsev, DA, Kincaid, MC, Fouraker, BD, et al. Recurrent corneal erosion: pathology of corneal puncture. Cornea. 1991;10:418–423.
34. Geggel, HS. Successful treatment of recurrent corneal erosion with Nd:YAG anterior stromal puncture. Am J Ophthalmol. 1990;110:404–407.
35. Aitken, DA, Beirouty, ZA, Lee, WR. Ultrastructural study of the corneal epithelium in the recurrent erosion syndrome. Br J Ophthalmol. 1995;79:282–289.
36. Marshall, J, Trokel, SL, Rothery, S, et al. Long-term healing of the central cornea after photorefractive keratectomy using an excimer laser. Ophthalmology. 1988;95:1411–1421.
37. Lohmann, CP, Gartry, DS, Muir, MK, et al. Corneal haze after excimer laser refractive surgery: objective measurements and functional implications. Eur J Ophthalmol. 1991;1:173–180.
38. O’Brart, DP, Muir, MG, Marshall, J. Phototherapeutic keratectomy for recurrent corneal erosions. Eye. 1994;8(Pt 4):378–383.
39. Cavanaugh, TB, Lind, DM, Cutarelli, PE, et al. Phototherapeutic keratectomy for recurrent erosion syndrome in anterior basement membrane dystrophy. Ophthalmology. 1999;106:971–976.
40. Dausch, D, Landesz, M, Klein, R, et al. Phototherapeutic keratectomy in recurrent corneal epithelial erosion. Refract Corneal Surg. 1993;9:419–424.
41. Fagerholm, P, Fitzsimmons, TD, Orndahl, M, et al. Phototherapeutic keratectomy: long-term results in 166 eyes. Refract Corneal Surg. 1993;9(Suppl. 2):S76–S81.
42. Soong, HK, Farjo, Q, Meyer, RF, et al. Diamond burr superficial keratectomy for recurrent corneal erosions. Br J Ophthalmol. 2002;86:296–298.