Chapter 2 Cornea and Sclera
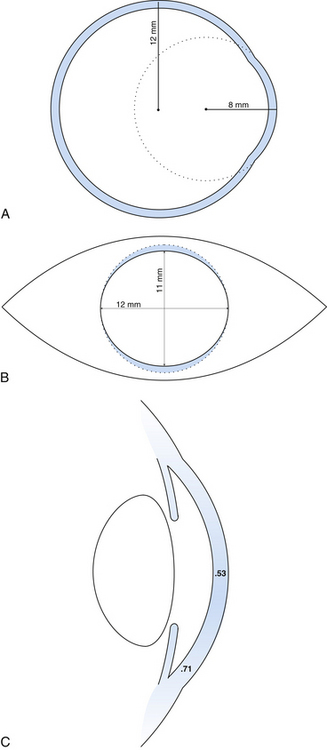
The outer connective tissue coat of the eye has the appearance of two joined spheres. The smaller, anterior transparent sphere is the cornea and has a radius of curvature of approximately 8 mm. The larger, posterior opaque sphere is the sclera, which has a radius of approximately 12 mm (Figure 2-1, A). The globe is not symmetric; its approximate diameters are 24 mm anteroposterior, 23 mm vertical, and 23.5 mm horizontal.1
Cornea
Corneal Dimensions
The transparent cornea appears from the front to be oval, as the sclera encroaches on the superior and inferior aspects. The anterior horizontal diameter is 12 mm, and the anterior vertical diameter is 11 mm.1,2 If viewed from behind, the cornea appears circular, with horizontal and vertical diameters of 11.7 mm (Figure 2-1, B).1
In profile, the cornea has an elliptic rather than a spherical shape, the curvature being steeper in the center and flatter near the periphery. The radius of curvature of the central cornea at the anterior surface is 7.8 mm and at the posterior surface is 6.5 mm.1,3 The central corneal thickness is 0.53 mm, whereas the corneal periphery is 0.71 mm thick (Figure 2-1, C).1,3–5 (All values given are approximations.)
Clinical Comment: Astigmatism
Regular astigmatism occurs when the longest radius of curvature and shortest radius of curvature lie 90 degrees apart. The usual presentation occurs when the radius of curvature of the vertical meridian differs from that of the horizontal meridian. The most common situation, called with-the-rule astigmatism (Figure 2-2, A), occurs when the steepest curvature lies in the vertical meridian. Thus the vertical meridian has the shortest radius of curvature. Against-the-rule astigmatism (Figure 2-2, B) is not as common and occurs when the horizontal meridian is the steepest; the greatest refractive power is found in the horizontal meridian. If the meridians that contain the greatest differences are not along the 180- and 90-degree axes (± 30 degrees) but lie along the 45- and 135-degree axes (± 15 degrees), the astigmatism is called oblique. Irregular astigmatism is an uncommon finding in which the meridians corresponding to the greatest differences are not 90 degrees apart.
Corneal Histologic Features
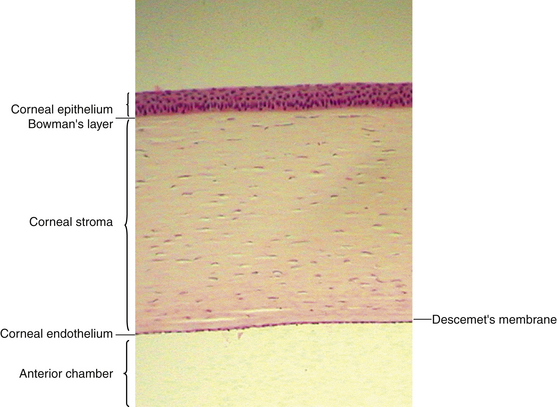
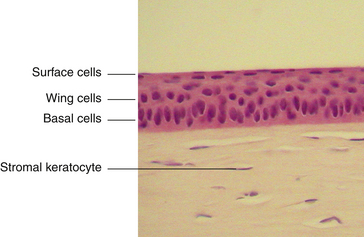
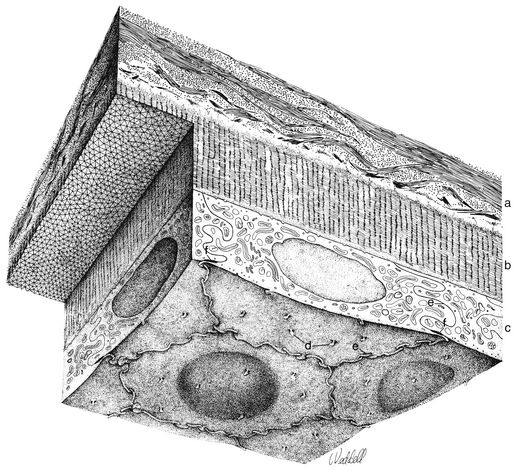
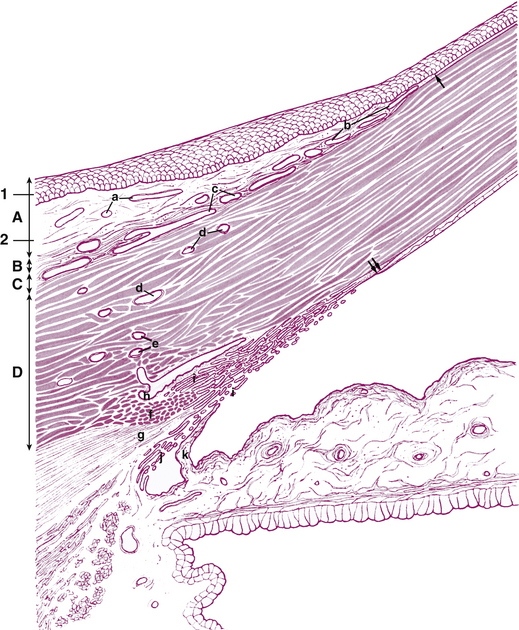
The cornea is the principal refracting component of the eye. Its transparency and avascularity provide optimal light transmittance. The anterior surface of the cornea is covered by the tear film, and the posterior surface borders the aqueous-filled anterior chamber. At its periphery, the cornea is continuous with the conjunctiva and the sclera. From anterior to posterior, the five layers that compose the cornea are epithelium, Bowman’s layer, stroma, Descemet’s membrane, and endothelium (Figure 2-3).
Epithelium
The outermost layer of stratified corneal epithelium is five to seven cells thick and measures approximately 50 μm.1,6 The epithelium thickens in the periphery and is continuous with the conjunctival epithelium at the limbus.
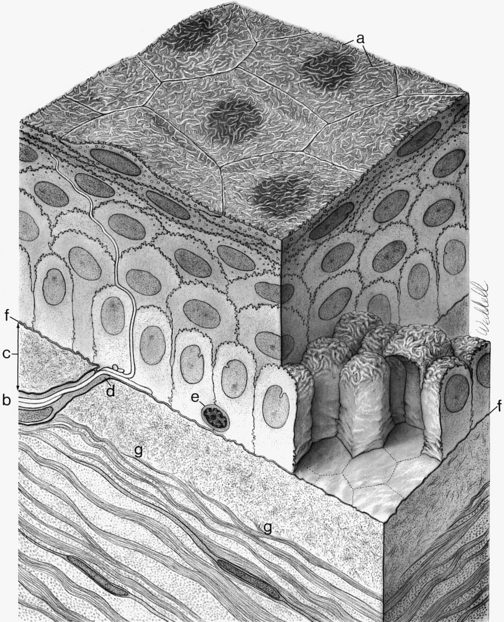
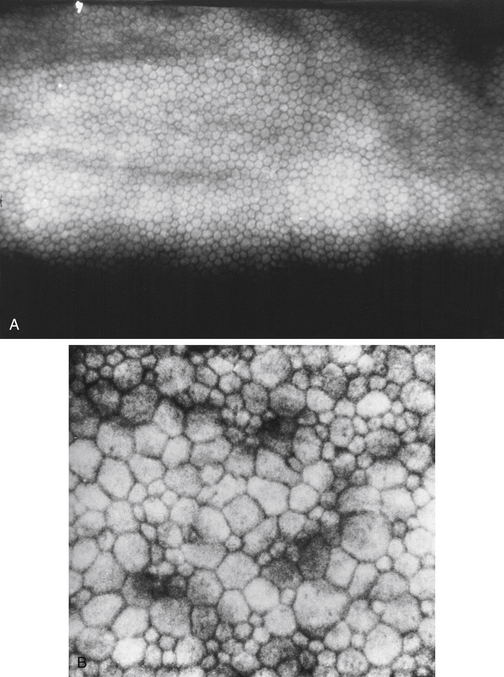
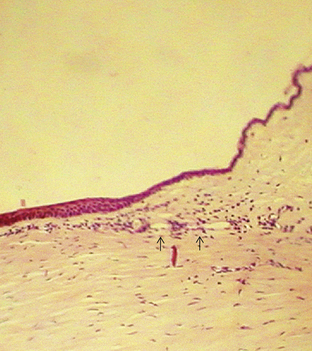
The surface layer of corneal epithelium is two cells thick and displays a very smooth anterior surface. It consists of nonkeratinized squamous cells, each of which contains a flattened nucleus and fewer cellular organelles than deeper cells. Cell size varies but a superficial cell can be 50 μm in diameter and 5 μm in height.7 The plasma membrane of the surface epithelial cells secretes a glycocalyx component that adjoins the mucin layer of the tear film.8–10 Many projections located on the apical surface of the outermost cells increase the surface area, thus enhancing the stability of the tear film. The fingerlike projections are microvilli, and the ridgelike projections are microplicae (Figure 2-4).
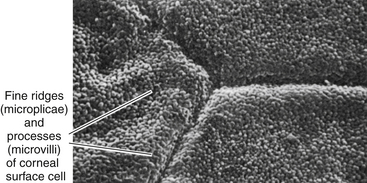
FIGURE 2-4 Scanning electron micrograph of junction of three superficial cells in cornea. (×5000.)
(From Krause WJ, Cutts JH: Concise text of histology, Baltimore, 1981, Williams & Wilkins.)
As the surface cells age, they degenerate. The cytoskeleton disassembles and the cytoplasm condenses. The cells lose their attachments and are sloughed off, being constantly replaced from the layers below. On scanning electron microscopy, the corneal surface consists of variously sized cells, ranging from small to large. The lighter cells are newer replacement cells, whereas the darker cells are those that are degenerating and will soon be sloughed.11
The middle layer of the corneal epithelium is made up of two to three layers of wing cells. These cells have winglike lateral processes, are polyhedral, and have convex anterior surfaces and concave posterior surfaces that fit over the basal cells (Figure 2-5). The diameter of a wing cell is approximately 20 μm.7 Desmosomes and gap junctions join wing cells to each other, and desmosomes join wing cells to surface and basal cells.12
The innermost basal cell layer of the corneal epithelium is a single layer of columnar cells, with diameters ranging from 8 to 10 μm (Figure 2-6).7 These cells contain oval-shaped nuclei displaced toward the apex and oriented at right angles to the surface. The rounded, apical surface of each cell lies adjacent to the wing cells, and the basal surface attaches to the underlying basement membrane (basal lamina). The basal cells secrete this basement membrane, which attaches the cells to the underlying tissue through hemidesmosomes. Anchoring fibrils pass from these junctions through Bowman’s layer into the stroma.13 Although less numerous here than in the wing cell layer, desmosomes and gap junctions join the columnar cells; interdigitations and desmosomes connect the basal cells with the adjacent layer of wing cells. The basal layer is the germinal layer where mitosis occurs.
The basal cells are joined to keratin filaments in the basement membrane by hemidesmosomes. Opposite the plaque, fine anchoring collagen fibrils form a complex branching and anastomosing network that runs from the basement membrane through Bowman’s layer and penetrates 1.5 to 2 μm into the stroma.13–18 The linkage between the hemidesmosome and the anchoring network is likely composed of basement membrane components.5 The anchoring fibrils attach to anchoring laminin-containing plaques of extracellular matrix within the stroma.16,19
Epithelial Replacement
Maintenance of the smooth corneal surface depends on replacement of the surface cells that are continually being shed into the tear film. This renewal of the stratified epithelium involves cell division, migration, differentiation, and senescence. Cell proliferation occurs in the basal layer. Basal cells move up to become wing cells, and wing cells move up to become surface cells. Only the cells in contact with the basement membrane have the ability to divide; the cells that are displaced into the wing cell layers lose this ability.20 Stem cells located in a 0.5- to 1-mm-wide band around the corneal periphery are the source for renewal of the corneal basal cell layer. A slow migration of basal cells occurs from the periphery toward the center of the cornea.21,22 Turnover time for the entire corneal epithelium is approximately 7 days, which is more rapid than for other epithelial tissues.23,24 Repair to corneal epithelial tissue proceeds quickly; minor abrasions heal within hours, and larger ones often heal overnight. If the basement membrane is damaged, however, complete healing with replacement basement membrane and hemidesmosomes can take months.14,15
Despite cells constantly being sloughed, the barrier function is maintained as the cell below moves into position to replace the one that has been shed. Tight junctions are present exclusively between the squamous cells that occupy the superficial position. The protein components necessary to form these junctions are not present in the basal cells but are increasingly present as the cells move up to the surface where the zonula occludens junctions become complete.25
The basal cell layer is continually losing and reestablishing the hemidesmosome junctions as cells divide and move up into the wing cell layers. The plaque sites remain present in the stroma for reattachment.15
Clinical Comment: Recurrent Corneal Erosion
RECURRENT CORNEAL EROSION is a condition in which the corneal epithelium sloughs off either continually or periodically. This condition may occur because of either poor attachment between the epithelium and its basement membrane or poor attachment between the basement membrane and the underlying tissue. Recurrent corneal erosion can occur after incomplete healing of an abrasion in which the hemidesmosomes are malformed, or it may be caused by an epithelial basement membrane dystrophy stemming from defective nutrition or metabolism.5
Age-related changes also can play a role in recurrent corneal erosion. Epithelium continues to secrete basement membrane throughout life; in the corneal epithelium, the thickness of the basement membrane doubles by 60 years of age. In addition, areas of reduplication of the membrane can occur with aging.26 As the basement membrane thickens or as reduplication occurs, the thickness of the membrane can exceed the length of the anchoring fibrils, allowing sloughing of epithelial layers.
Corneal erosions are very painful because the dense network of sensory nerve endings in the epithelium is disrupted. A number of treatments may be used. Acute cases may be patched and antibiotic ointment applied to allow healing of the surface without the shearing effect of opening and closing the eyelids. Bandage soft contact lenses or collagen shields often are applied in chronic situations to alleviate pain.26–29 For cases in which the suspected cause is a faulty basement membrane, treatment might include corneal puncture in which multiple perforations are made through the epithelial layers to induce new basement membrane formation and adhesion30–32 (Figure 2-7). If reduplication is the cause of corneal erosion, the doubled membrane can be removed.32
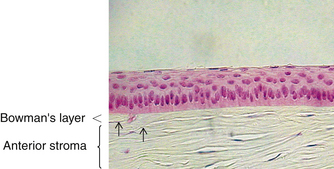
Bowman’s Layer
The second layer of the cornea is approximately 8 to 14 μm thick.1,6,33 Bowman’s layer is a dense, fibrous sheet of interwoven collagen fibrils randomly arranged in a mucoprotein ground substance. The fibrils have a diameter of 20 to 25 nm, run in various directions, and are not ordered into bundles. Bowman’s layer sometimes is referred to as a “membrane,” but it is more correctly a transition layer to the stroma rather than a true membrane. It differs from the stroma in that it is acellular and contains collagen fibrils of a smaller diameter. Bowman’s layer might provide biomechanical rigidity and shape to the cornea. The pattern of the anterior surface is irregular and reflects the contour of the bases of the basal cells of the epithelium. Posteriorly, as the layer transitions into stroma, the fibrils gradually adopt a more orderly arrangement and begin to merge into bundles that intermingle with those of the stroma (Figure 2-8). The posterior surface is not clearly defined.33
Bowman’s layer is produced prenatally by the epithelium and is not believed to regenerate. Therefore, if injured, the layer usually is replaced by epithelial cells or stromal scar tissue. However, Bowman’s layer is very resistant to damage by shearing, penetration, or infection. Speculation continues regarding the function of Bowman’s layer and whether it is necessary to maintain corneal function. No long-term effects have been documented in patients with Bowman’s layer removed by photorefractive keratoplasty, a procedure performed since the late 1980s.34
Corneal nerves passing through Bowman’s layer typically lose their Schwann cell covering and pass into the epithelium as naked nerves (see Figure 2-5). The layer tapers and ends at the corneal periphery and does not have a counterpart in either the conjunctiva or the sclera.
Stroma or Substantia Propria
The middle layer of the cornea is approximately 500 μm thick, or about 90% of the total corneal thickness7 (see Figure 2-3). The stroma (substantia propria) is composed of collagen fibrils, keratocytes, and extracellular ground substance.
The collagen fibrils have a uniform 25- to 35-nm diameter and run parallel to one another, forming flat bundles called lamellae.33 The 200 to 300 lamellae are distributed throughout the stroma and lie parallel to the corneal surface. Each contains uniformly straight collagen fibrils arranged with regular spacing, sometimes described as a “latticework.” The fibrils are also oriented parallel to the corneal surface. Adjacent lamellae lie at angles to one another, but all fibrils within a lamella run in the same direction (Figure 2-9). Each lamella extends across the entire cornea, and each fibril runs from limbus to limbus. Interweaving occurs between the lamellae.35,36
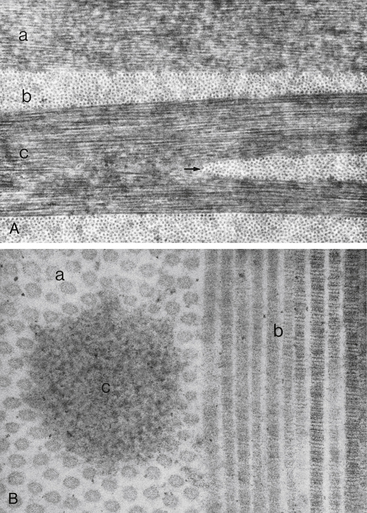
FIGURE 2-9 Corneal stroma.
(From Hogan MJ, Alvarado JA, Weddell JE: Histology of the human eye, Philadelphia, 1971, Saunders.)
The arrangement of the lamellae varies slightly within the stroma. In the anterior one third of the stroma, the lamellae are thin (0.5 to 30 μm wide and 0.2 to 1.2 μm thick), and they branch and interweave more than in the deeper layers.33,37 In the posterior two thirds of the stroma, the arrangement is more regular, and the lamellae become larger (100 to 200 μm wide and 1 to 2.5 μm thick).33 Anterior cornea has a higher incidence of cross-linking and is more rigid, helping to maintain corneal curvature.38
In the innermost layer, adjacent to Descemet’s membrane, the fibrils interlace to form a thin collagenous sheet that contributes to the binding between stroma and Descemet’s membrane.33
Keratocytes (corneal fibroblasts) are flattened cells that lie between and occasionally within the lamellae39 (Figure 2-10). The cells are not distributed randomly; a corkscrew pattern is recognizable from anterior to posterior, with the density higher in anterior stroma.40 Keratocytes have extensive branching processes joined by gap junctions along the lateral extensions, as well as the anteroposterior branches.41,42 These are active cells that maintain the stroma by synthesizing collagen and extracellular matrix components. Other cells may be found between lamellae, including white blood cells, lymphocytes, macrophages, and polymorphonuclear leukocytes, which can increase in number in pathologic conditions.
FIGURE 2-10 Summary diagram of corneal stroma.
(From Hogan MJ, Alvarado JA, Weddell JE: Histology of the human eye, Philadelphia, 1971, Saunders.)
Ground substance fills the areas between fibrils, lamellae, and cells. It contains proteoglycans (PG), macromolecules consisting of a core protein with one or more attached glycosaminoglycan (GAG) side chains. The corneal proteoglycans were once classified by their side chains: keratan sulphate (KS) and chondroitin/dermatan sulphate (CS/DS).43 They are now named for their core proteins, decorin is a CS/DS proteoglycan, and lumican, keratocan, and mimican are KS proteoglycans. Decorin is more abundant in anterior stroma; lumican, keratocan, and mimican are more abundant in posterior stroma.44 Lumican controls collagen fibril diameter keeping it within a very limited range.45 PGs have a significant role in maintaining corneal tensile strength and the GAGs contribute to the relatively high stromal hydration.44 Glycosaminoglycans are hydrophilic, negatively charged carbohydrate molecules located at specific sites around each collagen fibril. They attract and bind with water, maintaining the precise spatial relationship between individual fibrils.45
The very regular arrangement of the stromal components, as well as the small diameter of the fibrils, contributes to stromal transparency.46 The index of refraction of the fibrils is 1.411 and that of the extracellular matrix is 1.365. Studies have shown that the distance between areas of different refractive indices can affect transparency. If the change in the index of refraction occurs across a distance that is less than one half the wavelength of visible light (400 to 700 nm), destructive interference occurs, and light scattering is reduced significantly.40,47,48 In the stroma the very specific spacing between the fibrils allows destructive interference of rays reflecting from adjacent fibrils. Although the components of the epithelium, Bowman’s layer, and Descemet’s membrane are arranged irregularly, the scattering particles are separated by such small distances that light scattering is minimal in these layers.37 The cornea scatters less than 1% of the light that enters it.6,49
Clinical Comment: Keratoconus
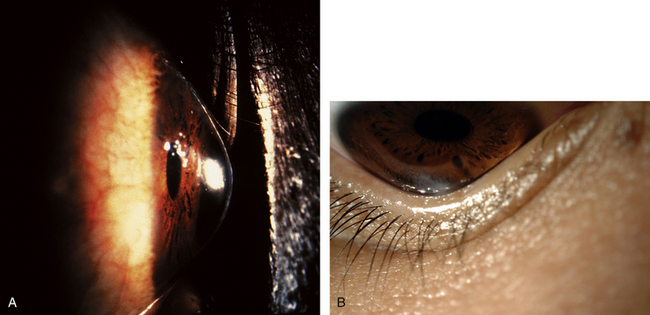
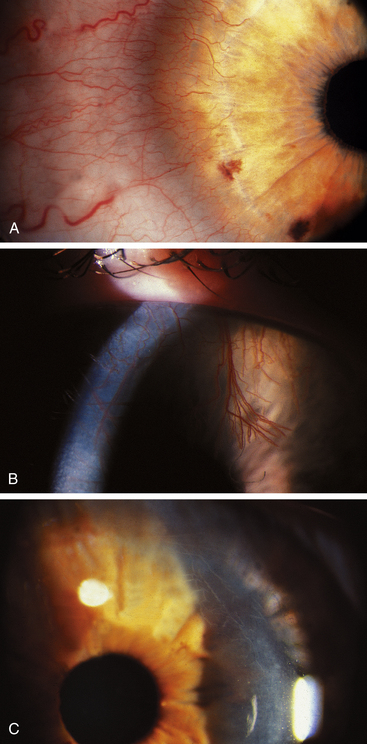
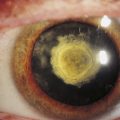
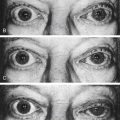
KERATOCONUS is a corneal dystrophy that first presents with focal disruptions of basement membrane and Bowman’s layer. Metabolic and nutritional disturbances are among the possible causes. Normal corneal shape and strength are maintained by the arrangement and density of the collagen fibrils that lie parallel to each other and the surface. Two mechanisms likely play some role in keratoconus: tissue loss caused by enzymatic degradation and the loss of adhesive forces between collagen fibrils that can cause slippage and displacement of lamellae.50 The process usually begins in central cornea; the stroma eventually degenerates and thins, and the affected area projects outward in a cone shape because of the force exerted on the weakened area by intraocular pressure (Figure 2-11, A). The cone shape is most evident in downgaze when the lower lid conforms to the cone shape; this is known as Munson’s sign (Figure 2-11, B). Folds occur in the posterior stroma and Descemet’s membrane.51,52
Spectacles may be used for a time for correction of refractive error, but with increasing irregular astigmatism, rigid gas-permeable contact lenses usually are necessary to achieve best corrected vision.53 When contact lenses no longer correct vision, penetrating keratoplasty may be performed to replace the defective cornea with a donor cornea.
Currently clinical trials are being conducted to evaluate a procedure called collagen cross-linking. In this treatment, after removing the epithelium, the stroma is saturated with topical riboflavin; it is then exposed to ultraviolet radiation that interacts with the riboflavin creating chemical bonds between and within the collagen fibrils. The corneal collagen stiffens, decreasing the progression of keratoconus.54 Initial results are promising.55,56
Descemet’s Membrane
Descemet’s membrane is considered the basement membrane of the endothelium. It is produced continually and therefore thickens throughout life, such that it has doubled by age 40 years.26 In children it is 5 μm thick and will increase to approximately 15 μm over a lifetime (Figure 2-12).
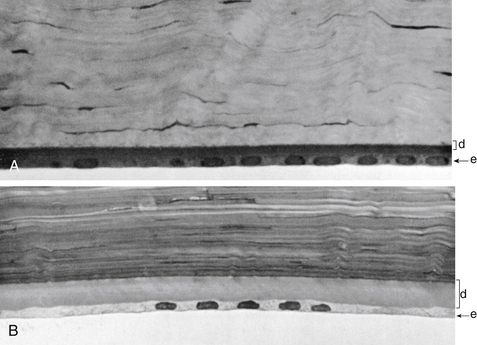
FIGURE 2-12 Thickness of Descemet’s membrane changes with increasing age.
(From Hogan MJ, Alvarado JA, Weddell JE: Histology of the human eye, Philadelphia, 1971, Saunders, p 94.)
Descemet’s membrane consists of two laminae. The anterior lamina, approximately 3 μm thick, exhibits a banded appearance and is a latticework of collagen fibrils secreted during embryonic development. The posterior lamina is nonbanded and homogeneous; it is the portion secreted by the endothelium throughout life.57
The method of attachment between Descemet’s membrane and the neighboring layers is poorly understood. Attachment sites between the stroma and Descemet’s membrane are relatively weak; the membrane can be detached easily from the posterior stroma.19 The anchoring fibrils characteristic of the connective tissue component of the hemidesmosome are not seen in Descemet’s58 and so the adhesions between Descemet’s membrane and the endothelium are not the typical hemidesmosomes.59
Endothelium
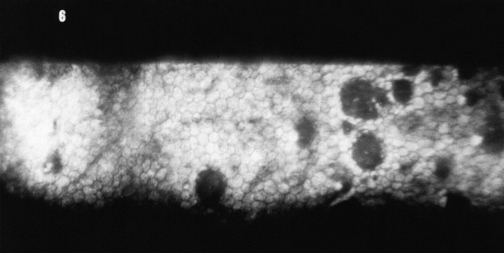
The innermost layer of the cornea, the endothelium, lies adjacent to the anterior chamber and is composed of a single layer of flattened cells. It normally is 5 μm thick.6 The basal part of each cell rests on Descemet’s membrane, and the apical surface, from which microvilli extend, lines the anterior chamber (Figure 2-13). Endothelial cells are polyhedral: five-sided and seven-sided cells can be found in normal cornea, but 70% to 80% are hexagonal. The hexagon is considered the most efficacious shape to provide area coverage without gaps.5,60 The very regular arrangement of these cells is described as the endothelial mosaic (Figure 2-14).
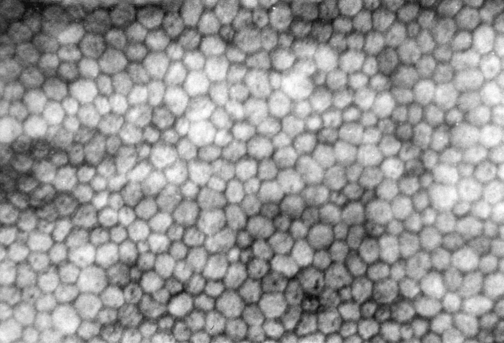
FIGURE 2-14 View with specular reflection through biomicroscope showing the endothelial mosaic.
(Courtesy Patrick Caroline, C.O.T., Pacific University College of Optometry, Forest Grove, Ore.)
Although Descemet’s membrane is considered a basement membrane, the nature of the junctions joining it to the endothelium are undefined.61 Extensive interdigitations join the lateral walls of the cells, and gap junctions provide intercellular communication.12 Tight junctional complexes joining the endothelial cells are located near the cell apex; these are a series of macula occludens rather than zonula occludens.62,63
The barrier formed by these adhesions is slightly leaky; in experiments, large molecules have penetrated the intercellular spaces.64 This incomplete barrier allows the entrance of nutrients, including glucose and amino acids, from the aqueous humor. Excess water that accompanies these nutrients must be moved out of the cornea if proper hydration is to be maintained. Metabolic pump mechanisms are active throughout the cells of the endothelium and function continually to move ions across the cell membranes; lateral infoldings increase the surface area providing space necessary for the number of ionic pumps needed. With changes in solute concentration, water flows down the concentration gradient, thus maintaining a balance of fluid movement across the endothelium. The endothelial cell is rich in cellular organelles; mitochondria reflect high metabolic activity and are more numerous in these cells than in any other cells of the eye, except the retinal photoreceptor cells.6
Endothelial cells do not divide and replicate. Studies now suggest that endothelial cells in the adult possess proliferative capacity but are in an arrested phase in the cell cycle. The cell-to-cell contact may be one factor that maintains this layer in the nonproliferative state.59,61,65 The lack of proliferation may be necessary for the layer to maintain its barrier and pump functions.59 Even in children, cells migrate and spread out to cover a defect, with resultant cell thinning. The cell density (cells per unit area) of the endothelium decreases normally with aging because of cell disintegration; density ranges from 3000 to 4000 cells/mm2 in children to 1000 to 2000 cells/mm2 at age 80 years.5,62,66,67 The minimum cell density necessary for adequate function is in the range of 400 to 700 cells/mm2.68 Disruptions to the endothelial mosaic can include endothelial cell loss or an increase in the variability of cell shape (pleomorphism) or size (polymegathism) (Figure 2-15). The active pump function can be detrimentally affected by polymegathism or morphologic changes, although the endothelial barrier function is not compromised by a moderate loss of cells.69 An excessive loss of cells can disrupt the intercellular junctions and allow excess aqueous to flow into the stroma, and the endothelial pumps may be unable to compensate for this loss of barrier function.
Clinical Comment: Hassall-Henle Bodies and Guttata
The endothelium can produce mounds of basement membrane material, which are seen as periodic thickenings in Descemet’s membrane that bulge into the anterior chamber. Those located near the corneal periphery are called Hassall-Henle bodies. These bodies are a common finding, and their incidence increases with age. Such deposits of basement membrane in the central cornea are called corneal guttata and are indicative of endothelial dysfunction. The endothelium that covers these mounds is thinned and altered, and the endothelial barrier may be compromised. Both Hassall-Henle bodies and guttata are visible as dark areas when viewed with specular reflection with the biomicroscope. These may be interpreted as holes in the endothelium, but the endothelium is merely displaced posteriorly from the plane of reflection (Figure 2-16).
Clinical Comment: Effects of Contact Lenses
Clinical studies indicate that epithelial thinning, stromal thinning, and a decreased number of keratocytes are associated with long-term extended wear of contact lenses.70,71 Numerous studies show that contact lens wear can induce changes in the regularity of the endothelial mosaic.72–76 Pleomorphism and polymegathism have been documented after only six years of either rigid gas-permeable or soft contact lens wear, although cell density remained normal.77,78 Endothelial stress resulting from contact lens wear, disease, surgery, or age can lead to endothelial remodeling, including change in size and shape or both.
Corneal Function
The cornea has two primary functions: to refract light and to transmit light. Factors that affect the amount of corneal refraction include (1) the curvature of the anterior corneal surface, (2) the change in refractive index from air to cornea (actually the tear film), (3) corneal thickness, (4) the curvature of the posterior corneal surface, and (5) the change in refractive index from cornea to aqueous humor. The total refractive power of the eye focused at infinity is between 60 and 65 diopters (D), with 43 to 48 D attributable to the cornea.5
In the transmission of light through the cornea, it is important that minimal scattering and distortion occur. Scattering of incident light is minimized by the smooth optical surface formed by the corneal epithelium and its tear film covering. The regular arrangement of the surface epithelial cells provides a relatively smooth surface, and the tear film fills in slight irregularities between cells producing negligible scatter of incident light. The absence of blood vessels and the maintenance of the correct spatial arrangement of components account for minimal scattering and distortion as light rays pass through the tissue. The cornea scatters less than 1% of the visible incident light6,49 and the majority of that scatter as determined by examination with the confocal microscope occurs due to the epithelium and endothelium.48 The epithelial and endothelial cell cytoplasm contain large amounts of water-soluble proteins, which enable the cytoplasm to appear homogenous and help to diminish light scattering. These proteins are now called corneal crystallins, and they share many of the attributes of the long recognized lens crystallins, important in maintaining the transparency of the lens.77
Because the stroma makes up 90% of the cornea, the regularity of spacing between the collagen fibrils is important in maintaining corneal transparency. The negatively charged molecules located around each collagen fibril maintain this precise arrangement by their bonds with water molecules, and corneal transparency is optimal when the stroma is 75% to 80% water.4,78
Corneal Hydration
This relative corneal deturgescence (78% water content) requires precise control of stromal extracellular water content and is dependent upon: (1) the barrier functions of the epithelium and endothelium, (2) the anionic characteristics of molecules within the stromal matrix that account for the tendency of the stroma to imbibe water, and (3) water and ion transport through the epithelial and endothelial cell membranes (including ion channels, ion cotransporters, and energy-utilizing ion pumps). Fluid is continually entering the cornea through the leaky barrier formed by the junctions joining the endothelial cells. Ion transporters in both the epithelium and the endothelium help to maintain the concentration gradient change that can facilitate water movement from the stroma into the tear film through the epithelium and into the anterior chamber through the endothelium. Net transport of solute into the anterior chamber exceeds that into the tears and corneal deturgescence is primarily reliant on endothelium and minimally on epithelium.79
The movement of water out of the cornea from the stroma through the endothelium and into the aqueous or through the epithelium into tears is mediated by ion flow and osmotic gradients. As ions are exchanged and the concentration is altered, water passage follows, moving down its concentration gradient. Cl− extrusion and Na+ absorption are the major driving forces for water transport across the epithelium and endothelium.80 However, an additional avenue of water movement occurs through water transport channels called aquaporins, identified in human corneal epithelial and endothelial cell membranes.
Aquaporins
Aquaporins (AQPs) are small integral membrane proteins residing in the plasma membrane; some are water-selective and others also transport glycerol.81 They form bidirectional osmotic water transport channels across the plasma membrane. The most constricted portion of the channel might allow only single file water molecule flow.82 AQP1, AQP2, AQP4, AQP5, and AQP8 are water selective and AQP3, AQP7, and AQP9 transport glycerol and perhaps other small solutes.81 AQP1 is found in corneal epithelium, endothelium, and keratocytes; AQP3 is found in cornea and conjunctival epithelium; and AQP5 is found in corneal epithelium.81 AQP5 is a significant pathway for water movement into the hypertonic tear film and water moves from the stroma into the aqueous through AQP1.81 Aquaporins function not only as channels but have some role in cellular processes, particularly in cell migration.81
Epithelium
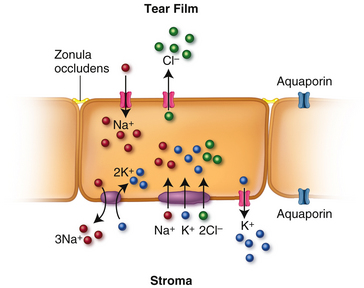
The cations, Na+ and K+, and the anion Cl− move across the epithelial cell membrane by various mechanisms (Figure 2-17). Channels in the apical membrane allow Na+ to move into the epithelium from the tear film; Na+ is extruded from the cell via Na+/K+ ATPase pumps in the basolateral membrane. Na+, K+, and Cl− are transported from stroma into the cell via the Na+/K+/2Cl− cotransporter in the basolateral membrane.83 Cl− moves out of the cell through channels in the apical surface. K+ movement out of the cell down its concentration gradient through channels in the basal membrane is opposed by the cotransporter and the Na+/K+ ATPase pump resulting in intracellular accumulation of K+. The net K+ transport creates the electrical force for Cl− diffusion across the apical side of the cell.83 This Cl− movement in turn has been identified as the driving force for osmotic water transport out of the epithelial cell into the tear film.82
Endothelium
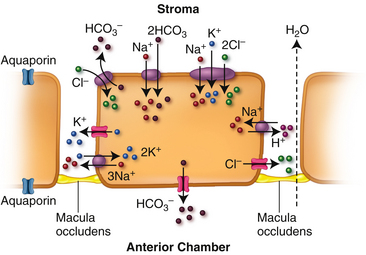
The endothelial layer allows a slow leak of fluids and solutes from the aqueous into the cornea due to the “leaky” occluding type of tight junction that joins them. Discontinuity and focal disruptions have been observed between the adhesion molecules forming these tight junctions.84 The rate of leakage into the cornea is dependent upon the swelling pressure of the stroma and must be balanced by water exit through the endothelium to maintain homeostasis and prevent edema.85
Water movement through the endothelium into the anterior chamber follows the concentration gradient as ions accumulate in the anterior chamber. In addition there are aquaporins throughout the endothelial cell membrane.86 Several types of processes transport ions across the membrane. The Na+/K+ ATPase pump located in the basolateral membrane of the endothelial cell is the most thoroughly understood; other methods of ion movement include channels, cotransporters, and exchangers. Figure 2-18 depicts some of the established methods, however there is still uncertainty about the control of many of these processes. The Na+/K+/2Cl− cotransporter and the Na+/2HCO3− cotransporter (although there is some question whether these two ions are directly coupled87) move ions into the cell from the stroma. Ion channels transport K+ and Cl− out of the cell into the intracellular space; however, the gating mechanism governing these channels is uncertain.85 Cl− and HCO3− also exit the apical membrane via ion channels, some of which are permeable to both ions.87 H+ and HCO3−, (both generated intracellularly as by-products of metabolism,) are moved out of the endothelial cell via Cl−/HCO3− and Na+/H+ exchangers. Water movement out of the endothelium and into the anterior chamber is believed to be driven by the anions Cl− and HCO3−.86
Clinical Comment: Fuchs’ Dystrophy
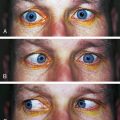
FUCHS’ DYSTROPHY (see Figure 2-16) is a bilateral, noninflammatory loss of endothelial function. It is inherited and progressive and may be caused by mutation of the gene that codes for collagen VII. The changes start in central cornea and gradually extend to the periphery. Guttata first form and endothelial cells lose Na+K+ ATPase pumps, although the barrier function remains. As guttata increase in number, some will fuse and the disruption of the endothelial mosaic, visible with specular reflection, is described as having the appearance of beaten metal. Stromal edema occurs with the reduction of ion movement. If the edema moves into the epithelium, it can cause a painful microcystic epithelial edema and recurrent corneal erosion can follow.85 Fuchs’ dystrophia was given as the reason for 15% of penetrating keratoplasty procedures performed in the United States in 2000.88
Corneal Metabolism
The metabolically active cornea depends on a stable supply of oxygen and glucose. Oxygen is derived primarily from atmospheric oxygen dissolved in the tear film, with small amounts obtained from the aqueous humor and limbal capillaries. In closed eye conditions, approximately two thirds of the oxygen is supplied by palpebral capillaries with the rest from the aqueous.89 Most nutrients, including glucose, amino acids, and vitamins, readily enter the cornea from the aqueous humor through the leaky endothelium; a lesser amount is obtained from limbal capillaries.90,91
Glucose is metabolized by aerobic glycolysis via the tricarboxylic acid cycle (TCA or Krebs cycle), anaerobic glycolysis, and the hexose monophosphate shunt. About 85% of the glucose utilized by the cornea is metabolized anaerobically.92,93 Because the basal cells of the corneal epithelium are in a constant state of replication, they have significant stores of glycogen, and 35% of the glucose processed within the epithelium is via the hexose monophosphate shunt.79 The hexose monophosphate shunt provides NADP, a reducing agent (part of the defense against free radicals) and nucleotides for the synthesis of cellular components necessary for the constant replacement of epithelial cells.
The endothelium requires significant stores of energy to maintain its metabolic function; each cell contains a large number of mitochondria and each cell is estimated to have 1.5 × 106 Na+/K+ ATPase pumps.49 In certain diseases, in which there is an increase in the permeability of the endothelial layer, the body can increase the number of pumps per cell, thus expanding pump function and compensating for the increased membrane permeability.94
Hydrogen ions (also a by-product of glycolysis) can also build up, causing a decrease in intracellular pH. Acidification can prompt a change in K+ channels, resulting in a rapid and massive loss of intracellular K+, which causes cell shrinkage and apoptosis.95 If acidification involves keratocytes, cellular damage can cause a dysfunction in collagen production, resulting in scar formation.
Clinical Comment: Overnight Corneal Swelling
During sleep the cornea swells because of the limited oxygen available to the endothelium.96 The cornea is thickest upon awakening but returns to baseline within the first two hours of waking.97 With stromal hydration increase, there is a decrease in swelling pressure and for a short time, the endothelial pumps exceed the water leak, decreasing the edema and reattaining normal hydration.87
Clinical Comment: Corneal Edema
CORNEAL EDEMA is manifested by a change in corneal thickness; the swelling is directed posteriorly and the anterior surface curvature remains the same (due to the fixed nature of Bowman’s layer).98 The more closely packed lamellae in the anterior cornea may make the anterior stroma more resistant to edema than the posterior stroma, with the larger spaces between lamellae in the posterior stroma allowing more fluid collection.40 The reduction in the curvature of the posterior surface can cause buckling of Descemet’s membrane and the appearance of vertical folds (striae). Corneal diameter remains the same. An increase in corneal hydration is positively and linearly correlated with corneal thickness. Normally the cornea scatters 1% of incident light, but with fluid retention light scattering increases.
Corneal edema caused by the loss of endothelial function is generally of a magnitude greater than that caused by the loss of the epithelial barrier and causes generalized stromal edema.85 Fluid accumulates in the stromal matrix around the collagen fibrils. Moderate stromal edema is usually symptom-free. Mild to moderate corneal edema can temporarily be cleared with instillation of a hypertonic solution of glycerin.
Corneal Repair: Wound Healing
Corneal injury initiates a cascade of mechanisms designed to repair damaged tissue. These processes are directed by various biomolecules such as integrins, cytokines, and growth factors Corneal integrins are integral membrane glycoproteins that have multiple roles in maintaining corneal function. Some facilitate interactions between cells and extracellular matrix; some have a role in matrix assembly; some impact cell adhesion and the formation of intercellular junctions; and others sense change in the extracellular environment and communicate to the cell nucleus by an alteration in the cytoskeleton.99 Cytokines are signaling molecules that facilitate cellular communication between cells and with surrounding tissues. Cellular proliferation and differentiation are mediated by growth factors.100–108
Epithelium
Because of the high rate of cell turnover, mitosis is constantly occurring in the basal layer of corneal epithelium. With corneal injury mitosis stops, and growth factors and cytokines are released from damaged epithelial and stromal cells. These molecules play key roles in initiating and continuing the processes necessary for corneal repair.99,109 Hemidesmosomes in the basal layer are dissembled along the leading edge of the wound.110 Changes in the cytoskeleton occur allowing for a rapid change in cell shape as those cells at the wound edges develop membrane extensions (filopodia) enabling the cell to migrate and cover the wound.99,111,112 Cell migration requires precise control of the hemidesmosomes, the cytoskeleton structure, and cell-to-matrix adhesion, which preserves the structural integrity of the epithelial sheet.109
Adhesion molecules allow the leading edge of the epithelial sheet to adhere to the basement membrane, in the absence of hemidesmosomes, and also to “pull cells forward” as the sheet moves to cover the injury. Growth factors stimulate the production of matrix components that enhance this cell-to-substrate adhesion; fibronectin is likely a key factor in the substrate that establishes adhesion during cell migration.113 Proliferation is suppressed until migration occurs, but then proliferation is enhanced in the region behind the advancing front.109
Once the defect is covered by a single layer of cells, cell-to-cell junctions are constructed between neighboring cells. Mitosis resumes and glycogen utilization and protein synthesis increases.80 Cell proliferation continues until normal cell density is reached and the stratified nature of the tissue is reestablished; apoptosis prevents epithelial hyperplasia.114 Biochemical bonds hold the basal cell to its substrate before hemidesmosomes are formed.14,15 A small lesion in the epithelium can heal in 24 to 48 hours with hemidesmosomes reformed, but if the basement membrane is damaged, normal adhesion may take months to complete.17,110 Basal cells are replenished by proliferation in the limbus. Epithelial healing generally is scar-free.
Stroma
When corneal injury extends into the stroma, keratocytes increase in number and some are stimulated to become myofibroblasts. These cells cause the wound bed to contract, allowing for more rapid wound coverage by the epithelium.99 The characteristics of the newly formed connective tissue components of the stroma differ slightly from those of the original tissue. The diameter of regenerated corneal stromal collagen is larger than the original fibrils, comparable to that found in the sclera, and the alignment and organization of the replacement fibrils are not as precise. These factors increase the probability that a scar will result.115 The tensile strength of the collagen fibrils in repaired cornea is diminished and may take months to approach the typical strength.116 Once healing is complete, the myofibroblasts undergo apoptosis or revert back to keratocytes.99
Descemet’s
Descemet’s membrane is a strong, resistant membrane but, if damaged, can be secreted and re-formed by stromal keratocytes and the endothelium.
Endothelium
Very little mitosis occurs in the endothelium; with cell loss, the neighboring cells generally enlarge and flatten to cover the area of loss; a decrease in endothelial cell density results. The cells remodel into the hexagonal shape, and pump and barrier functions are reestablished. In certain conditions, the number of ion pumps in an endothelial cell can increase dramatically to compensate for the loss of pumps that occur when cells are lost.94 There may be endothelial cells with proliferative ability that are inhibited and quiescent because of some as yet unknown factor.59,117
Absorption of Ultraviolet Radiation (UVR)
The cornea transmits light with wavelengths between 310 and 2500 nm.49,118 Wave lengths below 300 nm are absorbed by the epithelium and Bowman’s layer and do not penetrate deeper; those between 300 to 320 nm are absorbed by corneal stroma.119–121 The ability of the cornea to absorb the shorter wavelengths of ultraviolet radiation is protective to deeper structures (lens and retina), but the cornea is vulnerable to damage from this constant exposure.119 UVR induces oxidative stress by generating reactive oxygen species (ROS). These free radicals are highly reactive because of an unpaired electron and can damage cellular structures. The corneal epithelium has some protection against the damage caused by UVR absorption. Its cells have high concentrations of ascorbate (vitamin C) and glutathione. Ascorbate can absorb UVR, and is also a cellular antioxidant that can reduce free radicals and neutralize their activity.119 Glutathione is both a reducing agent and a free radical scavenger.77 Crystallins, present in cellular cytoplasm, also absorb UVR and are free radical scavengers.77 The epithelial cell also has a cellular repair system to minimize or reverse UVR damage to DNA.77
Clinical Comment: UVR Overexposure or Photokeratitis
Because the epithelium and Bowman’s layer are the primary sites for UVR absorbance, acute overexposure to UVR can result in a painful photokeratitis. This can occur with exposure to sunlamps, tanning beds, a welder’s arc, or the highly reflective rays from snow. Cellular defense mechanisms are overcome causing disruption of the epithelial tight junctions, inducing edema. Hyperactivation of the K+ channels in cell membranes results in a massive loss of intracellular K+, which causes cell shrinkage and apoptosis.95 Chronic exposure can result in keratopathies affecting the epithelium and anterior stroma or can cause endothelial pleomorphism.122
Corneal Innervation
The cornea is densely innervated with sensory fibers. Seventy to 80 large nerves, branches of the long and short ciliary nerves, enter the peripheral stroma. Approximately 2 to 3 mm after they pass into the cornea, the nerves lose their myelin sheath, but the covering from the Schwann cell remains.123,124 Considerable branching occurs, and three nerve networks are formed. One network is located in midstroma, and a subepithelial network is located in the region of Bowman’s layer and anterior stroma. Branches from this second network enter the epithelium, where the final nerve network is located (see Figure 12-2).
As the sensory nerves pass through Bowman’s layer, the Schwann cell covering is lost, and the fibers terminate as free nerve endings between the tightly packed epithelial cells.6,124 With surface cell turnover, the nerve endings retract and shift position. As they reinsert between the new surface cells, the nerve ending pattern changes slightly. No nerve endings are located in Descemet’s membrane or the endothelium. Any abrasion of the cornea, even a superficial one, is quite painful because of the density of this sensory innervation. The density of sensory nerve endings in the epithelium is approximately 400 times that of the epidermis of the skin, with approximately 7000 nociceptors per square millimeter in the cornea.44
Stimulation of the cornea, even just touch, is recognized as pain because of the density of nociceptors. The cornea also recognizes changes in temperature. Contact lens wear over time and aging cause a decrease in sensitivity. The corneal sensory nerves have a neurotrophic effect (i.e., they influence corneal metabolism and aid in tissue maintenance).125 Individuals with corneal anesthesia and a loss of nerve endings may have increased epithelial permeability, reduced mitosis, decreased cell adhesion, and impaired wound healing.49,126,127
In addition to the rich sensory innervation, the cornea contains sympathetic nerve fibers that may have some regulatory effect on Cl− channels.49 Acetylcholine is found in significant quantities in the corneal epithelium where it functions, not as a neurotransmitter, but as a cellular signaling molecule helping to maintain transparency and cellular homeostasis. It is believed to have a role in pain recognition, cellular proliferation, ion transport, and wound healing.121,128
When corneal nerves are damaged in central cornea, the normal nerve pattern is present by week 4, but reinnervation of peripheral branches can take longer than 60 days, resulting in a less dense nerve network than is found in the normal cornea. Repair of the subepithelial plexus can occur by two methods: new nerve fibers can arise from already existing but damaged superficial nerves or new fibers might sprout from deeper stromal nerves that have not been damaged.
Clinical Comment: Neurotrophic Keratitis
NEUROTROPHIC KERATITIS is a rare degenerative disease caused by the loss of corneal sensory innervation. It confirms the role of nerve endings in maintaining corneal function. Causes include viral herpetic infection, chemical burn, corneal injury or surgery, or intracranial involvement that compromises the trigeminal innervation to the cornea. The clinical presentation can include punctate keratopathy, epithelial thinning, increased epithelial permeability, neovascularization, persistent epithelial defect, and corneal ulceration that can lead to perforation.126 Treatment can be challenging.
Corneal Blood Supply
The cornea is avascular and obtains its nourishment by diffusion from the aqueous humor and from the conjunctival and episcleral capillary networks located in the limbus. Absence of blood vessels is an important factor in corneal transparency and although it is surrounded by conjunctival capillary loops a balance between angiogenic and antiangiogenic factors maintains its avascular state.129,130 The healthy limbus also forms a physical barrier to blood vessels, preventing encroachment of conjunctival tissue into the cornea. The compact composition of the stroma impedes vessel growth.129–131 Corneal avascularity helps to establish “immune privilege” that gives some protection against immune rejection of grafts.96 The cornea is normally devoid of antigen processing but under certain conditions, such as inflammatory disease, or with mechanical irritation (such as contact lens wear) immunologically active macrophages, Langerhans cells, can migrate from the limbal periphery.132,133
Clinical Comment: Corneal Neovascularization
New vessels sprout from the perilimbal capillaries; first enzymes degrade the basement membrane of the capillary, then the endothelial cells migrate, and finally endothelial cells proliferate to form new vessels that enter the cornea (Figure 2-19, A and B). Careful monitoring of patients with neovascularization and elimination of the causative factor may prevent extensive neovascularization. When the oxygen supply to the cornea resumes, the vessels will no longer carry blood, but the structures will remain and atrophy. These are known as ghost vessels and appear as fine white lines on biomicroscopy (see Figure 2-19, C).
Corneal infections and inflammations also may induce neovascularization in the body’s attempt to increase blood supply and some diseases can release vascular endothelial growth factor (VEGF), a protein that stimulates the multiplication of vascular endothelial cells.127
Clinical Comment: Keratoplasty
Full thickness penetrating keratoplasty (PK) has been the traditional method for replacing diseased and compromised corneas. PR has significant complications such as irregular cornea and irregular astigmatism (sutures run the entire circumference of the corneal donor button, and are often left in place for years), infection, wound rupture, and sometimes graft rejection or failure. It can take months to years to heal and reestablish tensile strength.134
New surgical methods, which may replace some penetrating keratoplasty procedures, involve replacement of only the posterior cornea in those patients in whom corneal decompensation is due to endothelial dysfunction. One of these procedures is Descemet’s stripping automated endothelial keratoplasty (DSAEK). In DSAEK, Descemet’s membrane and the endothelium are removed and replaced with donor membrane and endothelium. This method offers a more stable postoperative refraction, reduced corneal surface irregularity, decreased infection risk and wound rupture, and a quicker visual recovery.134 It requires a skilled and experienced surgeon.
Clinical Comment: Corneal Reshaping
Greatly improved procedures have enhanced the methods for corneal restructuring and in these procedures corneal stroma is removed. The amount of stroma to be removed is determined by the target refractive correction desired. In photorefractive keratoplasty (PRK), the epithelium is removed first, usually by mechanical means; then Bowman’s layer and anterior stroma are ablated by laser. Bowman’s layer does not regenerate, and the basement membrane of the epithelium must be laid down on the remaining stromal surface. In laser-assisted in situ keratomileusis (LASIK), an incision is made to produce a flap consisting of epithelium and Bowman’s layer. This flap is folded back, and stroma is removed by laser. The flap is laid back down, and the edges seal as the epithelium heals. In both procedures anterior stroma is removed. Some endothelial cell loss is reported but has not been found to be clinically significant.135–139 Speculation continues about long-term effects resulting from loss of Bowman’s layer, although none has yet been determined. The role of Bowman’s layer in UVR absorption may be one of the considerations when deciding between PRK and LASIK.
The reduction of corneal thickness may have other clinical effects, considering that removal of anterior stroma eliminates an area having significant rigidity and stability.40 Studies have shown a correlation between corneal thickness and the measurement of intraocular pressure (IOP), and between corneal thickness and the incidence of glaucoma.140,141 The clinician must be aware of the increased risk of inaccurate IOP readings, as well as any implications for glaucoma risk, in patients who have had removal of stromal tissue.142,143 Pachometry (measurement of corneal thickness) may be a relevant addition to the diagnosis of glaucoma, especially in those who have had refractive surgery.
Sclera
The sclera forms the posterior five-sixths of the connective tissue coat of the globe. The sclera maintains the shape of the globe, offering resistance to internal and external forces, and provides an attachment for the extraocular muscle insertions. The thickness of the sclera varies from 1 mm at the posterior pole to 0.3 mm just behind the rectus muscle insertions.151
Scleral Histologic Features
Sclera
The sclera is a thick, dense connective tissue layer that is continuous with the corneal stroma at the limbus. The diameter of the collagen fibrils in this tissue varies from 25 to 230 nm. These fibrils are arranged in irregular bundles that branch and interlace.33,144 The fibril size, orientation, and arrangement are influenced by proteoglycans in the extracellular matrix.145 Bundle widths and thicknesses vary, with the external bundles narrower and thinner than the deeper bundles. The orientation of these scleral lamellae is very irregular compared with corneal lamellae organization. The lamella in the outer regions of the sclera run approximately parallel to the surface, with interweaving between them, whereas in the inner regions the lamellae run in all directions.33 This random arrangement and the amount of interweaving contributes to the strength and flexibility of the eye. Generally, the fibrils parallel the limbus anteriorly; the pattern becomes meridional near the rectus muscle insertions and circular around the optic nerve exit. The collagen of the extraocular muscle tendon at its insertion merges and interweaves with the fibrils of the sclera.146
Elastic fibers have a low incidence in the sclera between and sometimes within bundles.33,145,147,148 Fibroblasts are also present, although they are less numerous than in the cornea. The stromal ground substance is similar to the corneal ground substance but contains fewer GAGs.78 The innermost aspect of the sclera merges with the choroidal tissue in the suprachoroid layer.
Physiology of Scleral Changes in Myopia
Early childhood growth of the eye requires coordinated changes in refractive components and in eye size for the eye to become emmetropic. When these factors are not coordinated, refractive error develops. A myopic eye generally is larger than emmetropic or hyperopic eyes, and changes in scleral tissue may be a factor when emmetropization does not occur. Most myopia develops between ages 8 to 14 and is caused by the elongation of the vitreal chamber.145 The sclera is a dynamic tissue; the connective tissue components can change in response to changes in the visual environment.145 Animal studies have shown that poor image quality on the retina can elicit a signal to scleral tissue components to strengthen or weaken in an attempt to move the retina to the best location for a clear image.
Scleral remodeling causes the axial lengthening that occurs in myopia; the scleral tissue is weakened and thins. In progressive myopia existing collagen is degraded, the production of new collagen is reduced, and matrix proteoglycans are lost.149,150 Studies attribute these alterations during myopia development to changes in the extracellular matrix but an additional piece of the puzzle may be the role played by scleral fibroblasts; if stimulated to become myofibroblasts, they can provide biochemical signals leading to changes in collagen production and degradation of tissue.149,150
Clinical Comment: Scleral Ectasia
The progression of myopia caused by axial elongation in a highly myopic eye often causes scleral thinning, particularly at the posterior pole where the collagen fibril diameter and the bundle size are reduced.145 As the sclera thins, the tissue can bulge outward causing scleral ectasia.
Scleral Spur
The scleral spur is a region of circularly oriented collagen bundles that extends from the inner aspect of the sclera. In its entirety, the scleral spur is actually a ring, although on cross section it appears wedge shaped, resembling a spur (Figure 2-20). At the spur’s posterior edge, its fibers blend with the more obliquely arranged scleral fibers. The posterior scleral spur is the origin of the longitudinal ciliary muscle fibers and most of the trabecular meshwork sheets attach to its anterior aspect, such that the collagen of the spur is continuous with that of the trabeculae.
Scleral Opacity
The opacity of the sclera depends on a number of factors, including the number of GAGs, the amount of water, and the size and distribution of the collagen fibrils. The sclera contains one-fourth the number of GAGs that are present in the cornea, and as a probable consequence, the sclera is relatively dehydrated (68%) compared with the cornea.151 The greater variation in fibril size and the irregular spacing between scleral components induce light scattering, which renders the sclera opaque.33,145
Scleral Foramina And Canals
The sclera contains a number of foramina and canals. The anterior scleral foramen is the area occupied by the cornea. The optic nerve passes through the posterior scleral foramen, which is bridged by a network of scleral tissue called the lamina cribrosa. It is similar to a sieve, with interwoven collagen fibrils forming canals through which the optic nerve bundles pass.146,152 The lamina cribrosa is the weakest area of the outer connective tissue tunic.1
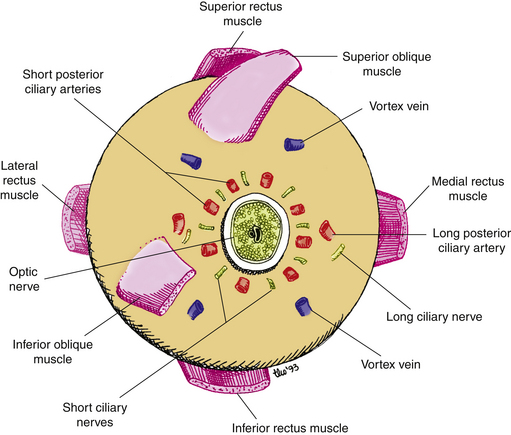
The canals that pass through the sclera carry nerves and vessels, and are possible routes by which disease can exit or enter the eye. The canals are designated by their location. The posterior apertures are located around the posterior scleral foramen and are the passages for the posterior ciliary arteries and nerves. The middle apertures lie approximately 4 mm posterior to the equator and carry the vortex veins (Figure 2-21). The anterior apertures are near the limbus at the muscle insertions and are the passages for the anterior ciliary vessels, which are branches from the muscular arteries.
Scleral Blood Supply
Because it is relatively inactive metabolically, the sclera has a minimal blood supply. A number of vessels pass through the sclera to other tissues, but the sclera is considered avascular because it contains no capillary beds. Nourishment is furnished by small branches from the episcleral and choroidal vessels, and branches of the long posterior ciliary arteries.151
Limbus
The limbus, located at the corneoscleral junction, is a band approximately 1.5 to 2 mm wide that encircles the periphery of the cornea. The radius of curvature abruptly changes at this junction of cornea and sclera, creating a narrow furrow, the external scleral sulcus. Internally at this juncture, there is a larger furrow, the internal scleral sulcus, which has a scooped-out appearance and contains the trabecular meshwork and the canal of Schlemm, the major route for drainage of the aqueous humor. These structures are discussed in Chapter 6.
Histologically, the anterior boundary of the limbus consists of a plane connecting the termination of Bowman’s layer and the termination of Descemet’s membrane (see Figure 2-20). The posterior boundary is a plane perpendicular to the surface of the globe and passing through the posterior edge of the scleral spur.153 These boundaries are used in this discussion, although clinically the boundaries are not as definitive.
The limbus is the transitional zone between cornea and conjunctiva, and between cornea and sclera. Some layers of the cornea continue into the limbal area and others terminate (see Figure 2-20). In the limbus, (1) the very regular squamous corneal epithelium becomes the thicker columnar conjunctival epithelium, (2) the very regular corneal stroma becomes the irregularly arranged scleral stroma, (3) the corneal endothelial sheet becomes discontinuous to wrap around the strands of the trabecular meshwork, (4) Bowman’s layer and Descemet’s membrane terminate at the anterior border, and (5) the conjunctival stroma, episclera, and Tenon’s capsule begin within the limbal area.
Limbal Histologic Features
The epithelium increases at the limbus from a layer five cells thick to a layer 10 to 15 cells thick (Figure 2-22).1,153,154 Melanocytes may be present in the basal layer, and pigmentation may be evident in the limbus and conjunctiva, especially in darker-skinned individuals. Bowman’s layer tapers and terminates.
The limbus contains the transition from the very regular corneal lamellae to the irregular and random organization of collagen bundles in the sclera. This change is gradual such that, as the transparent cornea merges into the opaque sclera, no line of demarcation can be identified. The scleral fibrils extend further anteriorly on the external than on the internal side of the limbus.2 Within the limbal stroma at the corneal periphery, a distinct group of collagen fibrils has been identified that lies circumferentially, forming an annulus. This ring structure is postulated to help maintain the correct corneal curvature.50,155
Descemet’s membrane tapers at the anterior limbal boundary, and the posterior nonbanded portion becomes interlaced with the connective tissue of the anterior sheets of the trabecular meshwork. The corneal endothelium continues into the anterior chamber angle as the endothelial covering of the sheets of the trabecular meshwork.19,62
Tenon’s capsule lies just inner to the conjunctival submucosa, and the episclera is inner to Tenon’s capsule. Both begin in the limbus but do not continue into the cornea. Tenon’s capsule, the episclera, and the conjunctival stroma fuse in the anterior limbal area.2
Limbal Blood Vessels and Lymphatics
Capillary loops from conjunctival and episcleral vessels form networks in the limbus, which surround the cornea and provide nourishment to the avascular corneal tissue. Limbal veins collect blood from the anterior conjunctival veins and drain into the radial episcleral veins, which then empty into the anterior ciliary veins.156 Lymphatic channels located in the limbal area do not enter the cornea.
Palisades of Vogt
The palisades of Vogt are radial projections of fibrovascular tissue in spokelike fashion around the corneal periphery.16 They are concentrated in the superior and inferior limbus and contain nerves, blood vessels, and lymphatics. On biomicroscopy these projections appear as thin, gray pegs approximately 0.04 mm wide and 0.36 mm long.157 The surface of the limbal area where the palisades of Vogt arise remains flat. The interpalisade region contains thickened conjunctival epithelium and is the likely site for limbal stem cells.156,158–166
A population of cells residing in the basal epithelial layer in this area has been identified as possessing the characteristics of stem cells; that is, (1) a cell with self-renewal properties (i.e., after cell division one of the daughter cells remains a stem cell); (2) the cell is undifferentiated but has differentiation ability; and (3) the cell is in an arrested state most of the time, but can be stimulated to divide.117,129 The low mitotic ability of the stem cell helps to decrease possibility of DNA damage.117
Cords of cells that run from the palisade periphery into limbal stroma are the likely anatomical location of the stem cells and are called limbal epithelial crypts.167 They serve as a reserve for corneal cell proliferation; upon cell division a cell migrates toward the center of the cornea and then differentiates into a corneal epithelial cell in the basal layer.168 This centripetal movement from the limbal area is responsible for the migration and replacement of corneal epithelial basal cells in both normal cell replacement and wound healing.15 Stem cells are a source for corneal repair in disease.
Stem cells are generally located in a “niche,” a specific environment that provides the factors necessary to maintain the cell in its “stemness.”167 The palisades of Vogt are the “niche” for limbal stem cells. The undulations of the epithelium between the palisades provides increased surface area for a large number of cells. The limbal epithelial crypts are located mainly superiorly and inferiorly and thus are protected by the eyelids. The palisades contain melanocytes safeguarding against UVR damage and contain conjunctival blood vessel loops, a source of nutrition.117,129,157 The stem cells have contact with limbal stroma with no intervening Bowman’s layer and it is speculated that the contact with stroma facilitates the distribution of growth factors and cytokines and maintains the cell in its stemness.131
Clinical Comment: Limbal Stem Cell Deficiency
IN LIMBAL STEM CELL DEFICIENCY the limbus is nonfunctioning, stem cell loss occurs, and conjunctival tissue can invade the cornea, leading to neovascularization and corneal opacity. Because a key feature of limbal stem cell deficiency is corneal neovascularization, the stem cells may have a role in maintaining the antiangiogenic state of normal cornea, and when stem cells are lost neovascularization ensues. In a novel approach to treating limbal stem cell deficiency, a sheet of epithelial cells fabricated from the patient’s own oral mucosal epithelium is transplanted into the limbal area. Initial studies are promising.169
Physiological Aging Changes in Cornea
Alterations to cellular integrins in the corneal epithelium can occur with age and result in a reduction in the adhesion molecules necessary for intercellular junction construction. This causes a breakdown in the barrier function of the corneal epithelium.170 Decreased keratocyte density can adversely affect wound healing and collagen fibril degradation produces spaces that can disrupt transparency and create opacities.170 Descemet’s membrane increases in thickness, Hassall-Henle bodies increase in the periphery, and endothelial cell density decreases with cell loss.
Clinical Comment: Clinical Aging Changes
AGING produces changes in corneal appearance, but most are not detrimental to vision. Iron deposits in epithelial cell cytoplasm, more concentrated in the basal cells,171 produce a horizontal pigmented line, the Hudson-Stähli line, often evident at the level of the lower lid margin. Degeneration of Bowman’s layer produces the limbal girdle of Vogt. This yellowish white opacity is located at the 3 and 9 o’clock positions, interpalpebrally, but does not encircle the cornea. A clear interval separating the opacity from the limbus may or may not be seen. The area may include degeneration of the anterior stroma, calcium deposits, and hypertrophy of the overlying epithelium.
Corneal arcus is the most common corneal aging change. An annular yellow-white deposit located within peripheral stroma is evident (Figure 2-23). This ring is separated from the limbus by a zone of clear cornea. The deposits are cholesterol and cholesterol esters and can result from age or elevated blood cholesterol levels. With time the arcus can extend anteriorly to Bowman’s layer or into central cornea. There is no clinical significance in elderly persons, but in those under age 40, hyperlipidemia should be suspected.
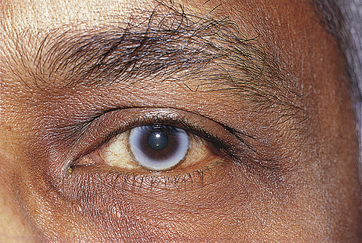
FIGURE 2-23 The white limbal band of corneal arcus.
(From Kanski JJ, Nischal JJ: Ophthalmology: clinical signs and differential diagnosis, St Louis, 1998, Mosby.)
Aging changes in the corneal endothelium include a decrease in cell density, polymegathism, and pleomorphism.66,67,172 Pigment deposits on the posterior cornea that have a vertical orientation are called Krukenberg’s spindle, and their incidence increases with age.
There is no evident change in the wavelengths transmitted by the aging cornea.173
A decrease in corneal sensitivity corresponds to a loss of corneal nerves with age.174
Fatty deposits may cause the sclera to appear yellow. Scleral collagen and elastic fibers degenerate, the concentration of certain proteoglycans is decreased, causing scleral thinning and loss of elasticity.145 The fibers of the lamina cribrosa become stiffer and less resilient with age. Changes in the laminar pores in the aged lamina cribrosa may make the nerve fibers passing through the openings more susceptible to injury, contributing to an increased susceptibility to glaucomatous damage.175–178
1. Warwick R. Eugene Wolff’s anatomy of the eye and orbit, ed 7. Philadelphia: Saunders; 1976. p 30
2. Van Buskirk E.M. The anatomy of the limbus. Eye. 1989;3:101.
3. Martola E.L., Baun J.L. A clinical study on central and peripheral corneal thickness. Arch Ophthalmol. 1968;79:28.
4. Pepose J.S., Ubels J.L. The cornea. In: Hart W.M.Jr., editor. Adler’s physiology of the eye: clinical application. ed 9. St Louis: Mosby; 1992:29.
5. Siu A., Herse P. The effect of age on human corneal thickness. Statistical implications of power analysis. Acta Ophthalmol (Copenh). 1993;71(1):51.
6. Hogan M.J., Alvarado J.A. The cornea. In: Hogan M.J., Alvarado J.A., Weddell J.E., editors. Histology of the human eye. Philadelphia: Saunders; 1971:55.
7. Guthoff R.F., Zhivov A., Stachs O. In vivo confocal microscopy, an inner vision of the cornea—a major review. Clin Exp Ophthalmol. 2009;37:100-117.
8. Gipson I.K., Yankauckas M., Spurr-Michaud S.J., et al. Characteristics of a glycoprotein in the ocular surface glycocalyx. Invest Ophthalmol Vis Sci. 1992;33:218.
9. Nichols B., Dawson C.R., Togni B. Surface features of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 1983;24:570.
10. Watanabe H., Fabricant M., Tisdale A.S., et al. Human corneal and conjunctival epithelia produce a mucin-like glycoprotein for the apical surface. Invest Ophthalmol Vis Sci. 1995;36(2):337.
11. Pfister R.R. The normal surface of corneal epithelium: a scanning electron microscopic study. Invest Ophthalmol. 1973;12:654.
12. Williams K.K., Watsky M.A. Dye spread through gap junctions in the corneal epithelium of the rabbit. Curr Eye Res. 1997;16:445.
13. Gipson I.K., Spurr-Michaud S.J., Tisdale A.S. Anchoring fibrils form a complex network in human and rabbit cornea. Invest Ophthalmol Vis Sci. 1987;28(2):212.
14. Gipson I.K., Spurr-Michaud S., Tisdale A., et al. Reassembly of the anchoring structures of the corneal epithelium during wound repair in the rabbit. Invest Ophthalmol Vis Sci. 1989;30:425.
15. Gipson I.K., Spurr-Michaud S.J., Tisdale A.S. Hemidesmosome and anchoring fibril collagen appears synchronously during development and wound healing. Dev Biol. 1988;126:253.
16. Gipson I.K. The epithelial basement membrane zone of the limbus. Eye. 1989;3:132.
17. Khodadoust A.A., Silverstein A.M., Kenyon D.R., et al. Adhesion of regenerating epithelium. The role of basement membrane. Am J Ophthalmol. 1968;65(3):339.
18. Tisdale A.S., Spurr-Michaud S.J., Rodrigues M., et al. Development of the anchoring structures of the epithelium in rabbit and human fetal corneas. Invest Ophthalmol Vis Sci. 1988;29(5):727.
19. Binder P.S., Rock M.E., Schmidt K.C., et al. High-voltage electron microscopy of normal human cornea. Invest Ophthalmol Vis Sci. 1991;32:2234.
20. Kruse F.E. Stem cells and corneal epithelial regeneration. Eye. 1994;8:170.
21. Thoft R., Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24(10):1442.
22. Tseng S.C. Concept and application of limbal stem cells. Eye. 1989;3:141.
23. Hanna C., O’Brien J.E. Cell production and migration in the epithelial layer of the cornea. Arch Ophthalmol. 1960;64:536.
24. Hanna C., Bicknell D.S., O’Brien J.E. Cell turnover in the adult human eye. Arch Ophthalmol. 1961;65:695.
25. McCartney N.D., Cantu-Crouch D. Rabbit corneal epithelial wound repair: tight junction reformation. Curr Eye Res. 1992;11:15.
26. Alvarado J., Murphy C., Juster R. Age-related changes in the basement membrane of the human corneal epithelium. Invest Ophthalmol Vis Sci. 1983;24(8):1015.
27. Bartlett J.D., Jaanus S.D. Clinical ocular pharmacology, ed 2. Boston: Butterworth; 1989. p 379
28. Susický P. Use of soft contact lenses in the treatment of recurrent corneal erosions. Cesk Oftalmol. 1990;46(5):381. (abstract)
29. Robin J.B., Keys C.L., Kaminski L.A., et al. The effect of collagen shields on rabbit corneal reepithelialization after chemical debridement. Invest Ophthalmol Vis Sci. 1990;31(7):1294.
30. Geggel H.S. Successful treatment of recurrent corneal erosion with Nd:YAG anterior stromal puncture. Am J Ophthalmol. 1990;110(4):404.
31. Katsev D.A., Kincaid M.C., Fouraker B.D., et al. Recurrent corneal erosion: pathology of corneal puncture. Cornea. 1991;10(5):418.
32. Pfister R.R. Clinical measures to promote corneal epithelial healing. Acta Ophthalmol Suppl. 1992;202:73.
33. Komai Y., Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991;32:2244.
34. Wilson S.E., Hong J.W. Bowman’s layer structure and function: critical or dispensable to corneal function? A hypothesis. Cornea. 2000;19(4):417.
35. Radner W., Zehetmayer M., Aufreiter R., et al. Interlacing and cross-angle distribution of collagen lamellae in the human cornea. Cornea. 1998;17(5):537.
36. Radner W., Mallinger R. Interlacing of collagen lamellae in the midstroma of the human cornea. Cornea. 2002;21(6):598.
37. Goldman J., Benedek G., Dohlman C., et al. Structural alterations affecting transparency in swollen human corneas. Invest Ophthalmol. 1968;7(5):501.
38. Müller L.J., Pels E., Vrensen G.F. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001;85:437-443.
39. Poole C.A., Brookes N.H., Clover G.M. Keratocyte networks visualized in the living cornea using vital dyes. J Cell Sci. 1993;106:685.
40. Müller L.J., Pels E., Vrensen G.F. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001;85:437.
41. Müller L.J., Pels L., Vrensen G.F. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995;36(13):2557.
42. Snyder M.C., Bergmanson J.P., Doughty M.J. Keratocytes: no more the quiet cells. J Am Optom Assoc. 1998;69:180.
43. Davies Y., Lewis D., Fullwood N.J., et al. Proteoglycans on normal and migrating human corneal endothelium. Exp Eye Res. 1999;68:303-311.
44. Ehlers N., Hjortdal J. The Cornea. In: Fischbarg J., editor. The biology of the eye, vol. 10. Elsevier; 2006:83-111.
45. Scott J.E., Haigh M. “Small” proteoglycan: collagen interactions: Keratin sulfate proteoglycan associates with rabbit corneal collagen fibrils at the “a” and “c” bands. Biosci Rep. 1985;5:765.
46. Maurice D.M. The structure and transparency of the cornea. J Physiol. 1957;136:263.
47. Farrell R.A., McCally R.L., Tatham P.E. Wave-length dependencies of light scattering in normal and cold swollen rabbit corneas and their structural implications. J Physiol. 1973;233:589.
48. Jester J.V. Corneal crystallins and the development of cellular transparency. Semin Cell Dev Biol. 2008;19:82-93.
49. Edelhauser H.F., Ubels J.L. The cornea and the sclera. In Kaufman P.L., Alm A., editors: Adler’s physiology of the eye: clinical application, ed 10, St Louis: Elsevier Science, 2003. p 47
50. Meek K.M., Tuft S.J., Huang Y., et al. Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46:1948-1956.
51. Bron A.J. Keratoconus. Cornea. 1988;7(3):163.
52. Chi H.H., Katzin H.M., Teng C.C. Histopathology of keratoconus. Am J Ophthalmol. 1956;42:847.
53. Astin C. Contact lens fitting after anterior segment disease. Contact Lens J. 1992;20(5):11.
http://www.nkcf.org/treatment-options/corneal-crosslinking/70-corneal-collagen-crosslinking.html. Accessed April 10, 2011.
55. Whikehart D.R., Parikh C.H., Vaughn A.V., et al. Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol Vis. 2005;11:816-824.
56. Raiskup-Wolf F., Hoyer A., Spoerl E. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34(5):796-801.
57. Johnson D.H., Bourne W.M., Campbell R.J. The ultrastructure of Descemet’s membrane: I. Changes with age in normal corneas. Arch Ophthalmol. 1942;100:1982.
58. Gipson I.K., Grill S.M., Spurr S.J., et al. Hemidesmosomes formation in vitro. J Cell Biol. 1983;9(97):849-857.
59. Joyce N.C. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003;22:359.
60. Doughty M.J. Toward a quantitative analysis of corneal endothelial cell morphology: a review of techniques and their application. Optom Vis Sci. 1989;66:626.
61. Joyce N.C., Harris D.L., Mello D.M. Mechanisms of miotic inhibition in corneal endothelium: contact inhibition and TGF-beta2. Invest Ophthalmol Vis Sci. 2002;43:2152. (abstract)
62. Waring G.O.3rd, Bourne W.M., Edelhauser H.F., et al. The corneal endothelium. Normal and pathologic structure and function. Ophthalmology. 1982;89(6):531.
63. Barry P.A., Petroll W.M., Andrews P.M., et al. The spatial organization of corneal endothelial cytoskeletal proteins and their relationship to the apical junctional complex. Invest Ophthalmol Vis Sci. 1995;36(6):1115.
64. Kaye G.I., Sibley R.C., Hoefle F.B. Recent studies on the nature and function of the corneal endothelial layer. Exp Eye Res. 1973;15:585.
65. Senoo T., Joyce N.C. Cell cycle kinetics in corneal endothelium from old and young donors. Invest Ophthalmol Vis Sci. 2000;41:660.
66. Mustonen R.K., McDonald M.B., Srivannaboon S., et al. Normal human corneal cell populations evaluated by in vivo scanning slit confocal microscopy. Cornea. 1998;17(5):485.
67. Abib F.C., Barreto J.Jr. Behavior of corneal endothelial density over a lifetime. J Cataract Refract Surg. 2001;27:1574.
68. Bourne W.M., Kaufman H.E. Specular microscopy of the human corneal endothelium in vivo. Am J Ophthalmol. 1976;81:319.
69. Bergmanson J.P. Histopathological analysis of corneal endothelial polymegathism. Cornea. 1992;11:133.
70. Efron N., Perez-Gomez I., Morgan P.B. Confocal microscopic observations of stromal keratocytes during extended contact lens wear. Clin Exp Optom. 2002;85(3):156.
71. Lui Z.G., Pflugfelder S.C. The effects of long-term contact lens wear on corneal thickness, curvature, and surface regularity. Ophthalmology. 2000;107:105.
72. Connor C.G., Zagrod M.E. Contact lens-induced corneal endothelial polymegathism: functional significance and possible mechanisms. Am J Optom Physiol Opt. 1986;63:539.
73. Holden B.A., Sweeney D.F., Vannas A., et al. Effects of long-term extended contact lens wear on the human cornea. Invest Ophthalmol Vis Sci. 1985;26:1489.
74. Holden B.A., Vannas A., Nilsson L., et al. Epithelial and endothelial effects from the extended wear of contact lenses. Curr Eye Res. 1985;4:739.
75. Matsuda M., Inaba M., Suda T., et al. Corneal endothelial changes associated with aphakic extended contact lens wear. Arch Ophthalmol. 1988;106:70.
76. MacRae S.M., Matsuda M., Shellans S., et al. The effects of hard and soft contact lenses on the corneal endothelium. Am J Ophthalmol. 1986;102:50.
77. Lassen N., Black W.J., Estey T., et al. The role of corneal crystallins in the cellular defense mechanisms against oxidative stress. Semin Cell Dev Biol. 2008;19:100-112.
78. McCulley J.P. The circulation of fluid at the limbus (flow and diffusion at the limbus). Eye. 1989;3:114.
79. Mishima S., Hedbys B.O. Physiology of the cornea. Int Ophthalmol Clin. 1968;8:527-560.
80. Levin M.H., Verkman A.S. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Invest Ophthalmol Vis Sci. 2006;47:4365-4372.
81. Verkman A.S. Aquaporins and water transport in the cornea. In: Tombran-Tink J., Barnstable C.J., editors. Ophthtlamology research: ocular transporters in ophthalmic diseases and drug delivery. Totowa NJ: Hamana Press, 2008.
82. Candia O.A. Electrolyte and fluid transport across corneal, conjunctival and lens epithelia. Exp Eye Res. 2004;78:527-535.
83. Reinach P.S., Capó-Aponte J.E., Mergler S., et al. Roles of corneal epithelial ion transport mechanisms in mediating responses to cytokines and osmotic stress. In: Tombran-Tink J., Barnstable C.J., editors. Ophthalmology research: ocular transporters in ophthalmic diseases and drug delivery. Totowa NJ: Hamana Press, 2008.
84. Mandell K.J., Berglin L., Severson E.A., et al. Expression of JAM-A in the human corneal endothelium and retinal pigment epithelium: localization and evidence for role in barrier function. Invest Ophthalmol Vis Sci. 2007;48:3928-3936.
85. Mergler S., Pleyer U. The human corneal endothelium: new insights into electrophysiology and ion channels. Prog Retin Eye Res. 2007;26:359-378.
86. Fischbarg J. The corneal endothelium. In: Fischbarg J., editor. The biology of the eye, vol 10. Elsevier; 2006:113-125.
87. Bonanno J.A. Identity and regulation of ion transport mechanism in the corneal epithelium. Prog Retin Eye Res. 2003;22:69-94.
88. McDermott M.L., Atluri H.K.S. Corneal endothelium. In: Yanoff M., Duker J.S., editors. Ophthalmology. ed 2. Mosby; 2004:422-430.
89. Fatt I., Bieber M.T., Pye S.D. Steady state distribution of oxygen and carbon dioxide in the in vivo cornea of an eye covered by a gas-permeable contact lens. Am J Optom Arch Am Acad Optom. 1969;46:3-14.
90. Hill R.M., Fatt I. How dependent is the cornea on the atmosphere? J Am Optom Assoc. 1964;5:873.
91. Smelser G.K., Ozanics V. Importance of atmospheric oxygen for maintenance of the optical properties of the human cornea. Science. 1952;115:140.
92. Baum J.P., Maurice D.M., McCarey B.E. The active and passive transport of water across the corneal endothelium. Exp Eye Res. 1984;39:335.
93. Larrea X., Büchler P. A transient diffusion model of the cornea for the assessment of oxygen diffusivity and consumption. Invest Ophthalmol Vis Sci. 2009;50:1076-1080.
94. Geroski D.H., Matsuda M., Yee R.W., et al. Pump function of the human corneal endothelium. Effects of age and cornea guttata. Ophthalmology. 1985;92:759-763.
95. Lu L. Stress-induced corneal epithelial apoptosis mediated by K+ channel activation. Prog Retin Eye Res. 2006;25:515-538.
96. Beebe D.C. Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol. 2008;19:125-133.
97. Mertz G.W. Overnight swelling of the living human cornea. J Am Optom Assoc. 1980;51:211-214.
98. Rom M.E., Keller W.B., Meyer C.J., et al. Relationship between corneal edema and topography. CLAO J. 1995;21(3):191.
99. Stepp M.A. Corneal integrins and their functions. Exp Eye Res. 2006;83:3-15.
100. Brazzell R.K., Stern M.E., Aquavella J.V., et al. Human recombinant epidermal growth factor in experimental corneal wound healing. Invest Ophthalmol Vis Sci. 1991;32(2):336.
101. Hoppenreijs V.P., Pels E., Vrensen G.F., et al. Effects of human growth factor on endothelial wound healing of human corneas. Invest Ophthalmol Vis Sci. 1946;33(6):1992.
102. Hoppenreijs V.P., Pels E., Vrensen G.F., et al. Platelet-derived growth factor: receptor expression in corneas and effects on corneal cells. Invest Ophthalmol Vis Sci. 1993;34(3):637.
103. Kim K.S., Oh J.S., Kim I.S., et al. Clinical efficacy of topical homologous fibronectin in persistent corneal epithelial disorders. Korean J Ophthalmol. 1992;6(1):12.
104. Mooradian D.L., McCarthy J.B., Skubitz A.P., et al. Characterization of FM-C/H-V, a novel synthetic peptide from fibronectin that promotes rabbit corneal epithelial cell adhesion, spreading, and motility. Invest Ophthalmol Vis Sci. 1993;34(1):153.
105. Pastor J.C., Calonge M. Epidermal growth factor and corneal wound healing. A multicenter study. Cornea. 1992;11(4):311.
106. Rieck P., Hartmann C., Jacob C., et al. Human recombinant bFGF stimulates corneal endothelial wound healing in rabbits. Curr Eye Res. 1992;11(12):1161.
107. Schultz G., Chegini N., Grant M., et al. Effects of growth factors on corneal wound healing. Acta Ophthalmol Suppl. 1992;202:60.
108. Fullwood N.J., Davies Y., Nieduszynski I.A., et al. Cell surface-associated keratan sulfate on normal and migrating corneal endothelium. Invest Ophthalmol Vis Sci. 1996;37(7):1256.
109. Zelenka P.S., Arpitha P. Coordinating cell proliferation and migration in the lens and cornea. Semin Cell Dev Biol. 2008;19:113-124.
110. Suzuki K., Tanaka T., Enoki M., et al. Coordinated reassembly of the basement membrane and junctional proteins during corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2000;41:2495-2500.
111. Crosson C.E., Klyce S.D., Beuerman R.W. Corneal epithelial wound closure. Invest Ophthalmol Vis Sci. 1986;27(4):464.
112. Tervo T., van Setten G.B., Päällysaho T., et al. Wound healing of the ocular surface. Ann Med. 1992;24:19.
113. Nishida T., Nakagawa S., Awata T., et al. Fibronectin promotes epithelial migration of cultured rabbit cornea in situ. J Cell Biol. 1983;97:1653-1657.
114. Stapleton F., Kim J.M., Kasses J., et al. Mechanisms of apoptosis in human corneal epithelial cells. Adv Exp Med Biol. 2002;506:827-834.
115. Karamichos D., Lakshman N., Petroll W.M. Regulation of corneal fibroblast morphology and collagen reorganization by extracellular matrix mechanical properties. Invest Ophthalmol Vis Sci. 2007;48:5030-5037.
116. Davison P.F., Galbary E.J. Connective tissue remodeling in corneal and scleral wounds. Invest Ophthalmol Vis Sci. 1986;27(10):1478.
117. Takács L, Tóth E, Berta A, et al: Stem cells of the adult cornea: from cytometric markers to therapeutic applications, (Cytometry Part A: The Journal of the International Society for Analytical Cytology) Cytometry A 75:54–66, 2009.
118. Boettner E.A., Wolter J.R. Transmission of the ocular media. Invest Ophthalmol Vis Sci. 1962;1:776.
119. Podskochy A. Protective role of corneal epithelium against ultraviolet radiation damage. Acta Ophthalmol Scand. 2004;82:714-717.
120. Kolozsvári L., Nógrádi A., Hopp B., et al. UV absorbance of the human cornea in the 240- to 400-nm range. Invest Ophthalmol Vis Sci. 2002;43(7):2165.
121. Ringvold A. Corneal epithelium and UV-protection of the eye. Acta Ophthalmol Scand. 1998;76:149.
122. Karai I., Matsumura S., Takise S., et al. Morphological change in the corneal endothelium due to ultraviolet radiation in welders. Br J Ophthalmol. 1984;68:544.
123. Lawrenson J.G., Ruskell G.L. The structure of corpuscular nerve endings in the limbal conjunctiva of the human eye. J Anat. 1991;177:75.
124. Müller L.J., Pels E., Vrensen G.F. Ultrastructural organization of human corneal nerves. Invest Ophthalmol Vis Sci. 1996;37(4):476.
125. Müller L.J., Marfurt C.F., Kruse F., et al. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521-542.
126. Bonini S., Rama P., Olzi D., et al. Neurotrophic keratitis: A review. Eye. 2003;(17):989-995.
127. Yu C.Q., Zhang M., Matis K.I., et al. Vascular endothelial growth factor mediates corneal nerve repair. Invest Ophthalmol Vis Sci. 2008;49:3870-3878.
128. Liu S., Li J., Tan D.T., et al. Expression and function of muscarinic receptor subtypes on human cornea and conjunctiva. Invest Ophthalmol Vis Sci. 2007;48:2987-2996.
129. Lim P., Fuchsluger T.A., Jurkunas U.V. Limbal stem cell deficiency and corneal neovascularization. Semin Ophthalmol. 2009;24:139-148.
130. Klintworth G.K., Burger P.C. Neovascularization of the cornea: current concepts of its pathogenesis. Int Ophthalmol Clin. 1983;23(1):27.
131. Secker G.A., Daniels J.T. Corneal epithelial stem cells: Deficiency and regulation. Stem Cell Rev. 2008;4:159-168.
132. Efron N., Al-Dossari M., Pritchard N. Confocal microscopy of the bulbar conjunctiva in contact lens wear. Cornea. 2010;29(1):43-52.
133. Chen W., Lin H., Dong N., et al. Cauterization of central cornea induces recruitment of major histocompatibility complex class II+ Langerhans cells from limbal basal epithelium. Cornea. 2010;29(1):73-79.
134. Chih A., Lugo M., Kowing D. Descemet stripping and automated endothelial keratoplasty: an alternative to penetrating keratoplasty. Optom Vis Sci. 2008;85:152-157.
135. Pallikaris J.G., Siganos D.S. Laser in situ keratomileusis to treat myopia: early experience. J Cataract Refract Surg. 1997;23:39.
136. Collins M.J., Carr J.D., Stulting R.D., et al. Effects of laser in situ keratomileusis (LASIK) on the corneal endothelium 3 years postoperatively. Am J Ophthalmol. 2001;131:1.
137. Jabbur N.S. Endothelial cell studies in patients after photorefractive keratectomy for hyperopia. J Refract Surg. 2003;19:142.
138. Fagerholm P. Phototherapeutic keratectomy: 12 years of experience. Acta Ophthalmol Scand. 2003;81(1):19.
139. Simaroj P., Kosalprapai K., Chuckpaiwong V. Effect of laser in situ keratomileusis on the corneal endothelium. J Refract Surg. 2003;19:S237.
140. Brandt J.D., Beiser J.A., Kass M.A., et al. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS). Ophthalmology. 2001;108(10):1779.
141. Bhan A., Browning A.C., Shah S., et al. Effect of corneal thickness on intraocular pressure measurements with the pneumotonometer, Goldmann applanation tonometer, and Tono-Pen. Invest Ophthalmol Vis Sci. 2002;43(5):1389.
142. Rashad K.M., Bahnassy A.A. Changes in intraocular pressure after laser in situ keratomileusis. J Refract Surg. 2001;17(4):420.
143. Arimoto A., Shimizu K., Shoji N., et al. Underestimation of intraocular pressure in eyes after laser in situ keratomileusis. Jpn J Ophthalmol. 2002;46(6):645.
144. Quantock A.J., Meek K.M. Axial electron density of human scleral collagen, Location of proteoglycans by x-ray diffraction. Biophys J. 1988;54:159.
145. Rada J.A., Shelton S., Norton T.T. The sclera and myopia. Exp Eye Res. 2006;82(2):185-200.
146. Thale A., Tillmann B. The collagen architecture of the sclera—SEM and immunohistochemical studies. Ann Anat. 1993;175:215.
147. Marshall G.E. Human scleral elastic system: an immunoelectron microscopic study. Br J Ophthalmol. 1995;79:57.
148. Alexander R.A., Garner A. Elastic and precursor fibres in the normal human eye. Exp Eye Res. 1983;36:305.
149. McBrien N.A., Cornell L.M., Gentle A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Invest Ophthalmol Vis Sci. 2001;42:2179. (abstract)
150. McBrien N.A., Gentle A. The role of visual information in the control of scleral matrix biology in myopia. Curr Eye Res. 2001;23(5):313.
151. Hogan M.J., Alvarado J.A. The sclera. In: Hogan M.J., Alvarado J.A., Weddell J.E., editors. Histology of the human eye. Philadelphia: Saunders; 1971:183.
152. Elkington A.R., Inman C.B., Steart P.V., et al. The structure of the lamina cribrosa of the human eye: an immunocytochemical and electron microscopical study. Eye. 1990;4:42.
153. Hogan M.J., Alvarado J.A. The limbus. In: Hogan M.J., Alvarado J.A., Weddell J.E., editors. Histology of the human eye. Philadelphia: Saunders; 1971:112.
154. Kikkawa D.O., Lucarelli M.J., Shovlin J.P., et al. Ophthalmic facial anatomy and physiology. In: Kaufman P.L., Alm A., editors. Adler’s physiology of the eye: clinical application. ed 10. St Louis: Elsevier Science; 2003:16.
155. Newton R.H., Meek K.M. Circumcorneal annulus of collagen fibrils in the human limbus. Invest Ophthalmol Vis Sci. 1998;39(7):1125.
156. Meyer P.A. The circulation of the human limbus. Eye. 1989;3:121.
157. Goldberg M.F., Bron A.J. Limbal palisades of Vogt. Trans Am Ophthalmol Soc. 1982;80:155-171.
158. Davanger M., Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560.
159. Dua H.S., Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44(5):415.
160. Kinoshita S., Adachi W., Sotozono C., et al. Characteristics of the human ocular surface epithelium. Prog Retin Eye Res. 2001;20(5):639.
161. Cotsarelis G., Cheng S.Z., Dong G.E., et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201.
162. Ebato B., Friend J., Throft R.A. Comparison of central and peripheral human corneal epithelium in tissue culture. Invest Ophthalmol Vis Sci. 1987;28:1450.
163. Lauweryns B., van den Oord J.J., De Vos R., et al. A new epithelial cell type in the human cornea. Invest Ophthalmol Vis Sci. 1983;34(6):1993.
164. Thoft R.A., Wiley L.A., Sundarraj N. The multipotential cells of the limbus. Eye. 1989;3:109.
165. Zieske J.D., Bukusoglu G., Yankauckas M.A. Characterization of a potential marker of corneal epithelial stem cells. Invest Ophthalmol Vis Sci. 1992;33(1):143.
166. Wolosin J.M., Xiong X., Schütte M., et al. Stem cells and differentiation stages in the limbo-corneal epithelium. Prog Retin Eye Res. 2000;19(2):223.
167. Shanmuganathan V.A., Foster T., Kulkarni B.B., et al. Morphological characteristics of the limbal epithelial crypt. Br J Ophthalmol. 2007;91:514-519.
168. Chang C.Y., Green C.R., McGhee C.N., et al. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem influence. Invest Ophthalmol Vis Sci. 2008;49:5279-5286.
169. Nishida K., Yamato M., Hayashida Y., et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187-1196.
170. Faragher R.G., Mulholland B., Tuft S.J., et al. Aging and the cornea. Br J Ophthalmol. 1997;81:814-817.
171. Barraquer-Somers E., Chan C.C., Green W.R. Corneal epithelial iron deposition. Ophthalmology. 1983;90:729.
172. Bourne W.M., Nelson L.R., Hodge D.O. Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci. 1997;38(3):779.
173. van den Berg T.J., Tan K.E. Light transmittance of the human cornea from 320 to 700 nm for different ages. Vision Res. 1994;34(11):1453.
174. Hollingsworth J., Perez-Gomez I., Mutalib H.A., et al. A population study of the normal cornea using an in vivo, slit-scanning confocal microscope. Optom Vis Sci. 2001;78(10):706.
175. Albon J., Karwatowski W.S., Easty D.L., et al. Age related changes in the non-collagenous components of the extracellular matrix of the human lamina cribrosa. Br J Ophthalmol. 2000;84:311.
176. Sawaguchi S., Yue B.Y., Fukuchi T., et al. Collagen fibrillar network in the optic nerve head of normal monkey eyes and monkey eyes with laser-induced glaucoma—a scanning electron microscopic study. Curr Eye Res. 1999;18(2):143.
177. Albon J., Purslow P.P., Karwatowski W.S., et al. Age related compliance of the lamina cribrosa in human eyes. Br J Ophthalmol. 2000;84:318.
178. Albon J., Karwatowski W.S., Avery N., et al. Changes in the collagenous matrix of the aging human lamina cribrosa. Br J Ophthalmol. 1995;79:368.