CHAPTER 15 Control of hypothalamic–pituitary–ovarian function
Anatomy of the Hypothalamic–Pituitary Axis
The adenohypophysis consists of:
Neural connections
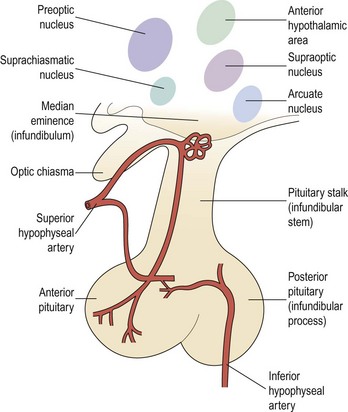
There are numerous and extensive neural pathways connecting the hypothalamus with the rest of the brain. The majority of afferent hypothalamic nerve fibres run in the lateral hypothalamic areas, whilst efferent pathways are more medially placed. One important efferent connection is the supraopticohypophyseal nerve tract carrying fibres from the supraoptic and paraventricular nuclei to the infundibular process of the pituitary, whilst other fibres carry hypothalamic-releasing or -inhibiting factors from the medial and basal parts of the hypothalamus to the anterior pituitary (Figure 15.2).
Gonadotrophin-releasing hormone (GnRH)-secreting neurones appear in the medial olfactory placode and enter the brain with the nervus terminalis, a cranial nerve that projects from the nose to the septal preoptic nuclei in the brain. By migration during embryogenesis, the cells, between 1000 and 3000 GnRH-producing neurones, predominantly settle in the arcuate nucleus of the hypothalamus (Schwanzel-Fukunda et al 1989). Failure of this migration has been shown to result in Kallmann’s syndrome; a disorder associated with an absence of GnRH secretion and a defect of smell-anosmia (a failure of both olfactory axonal and GnRH neuronal migration from the olfactory placode). A 5–7-fold increased frequency of this condition is found in males, indicating that an X-linked transmission is the most common, although autosomal-dominant and autosomal-recessive modes of transmission have also been established (Waldstreicher et al 1996).
The mutations responsible for this syndrome result in the failure to produce a protein, homologous to members of the fibronectin family, responsible for cell adhesion and protease inhibition that is necessary for migration of these neurones (Bick et al 1992). GnRH neurones have cilia — as do olfactory epithelial cells in the nose — and the olfactory origin and structural similarity of these cells suggest an evolution from reproduction controlled by pheromones.
Hypothalamic Regulation of Pituitary Secretion
Considerable efforts have been made in the past 25 years to identify, characterize and synthesize the substances thought to be produced in the neural elements of the infundibulum. Several substances which can either stimulate or suppress the rate of release of one or more hormones from the pituitary gland have been found in the infundibular complex. These can be classified into hypophysiotrophic, neurohypophyseal and pituitary peptide hormones and are listed in Table 15.1. Other substances will probably be added to this list in the future.
| Hypothalamic hormones | |
| Gonadotrophin-releasing hormone | GnRH |
| Thyrotrophin-releasing hormone | TRH |
| Corticotrophin-releasing factor | CRF |
| Growth-hormone-releasing hormone | GHRH |
| Somatostatin | |
| Prolactin-inhibiting factor | PIF |
| Growth hormone secretagone receptor | Ghrelin |
| Posterior pituitary products | |
| Vasopressin | |
| Oxytocin | |
| Neurophysin I and II | |
| Anterior pituitary peptide hormones | |
| Adrenocorticotrophic hormone | ACTH |
| Prolactin | PRL |
| Luteinizing hormone | LH |
| Follicle-stimulating hormone | FSH |
| Growth hormone | GH |
| Thyroid-stimulating hormone | TSH |
GnRH
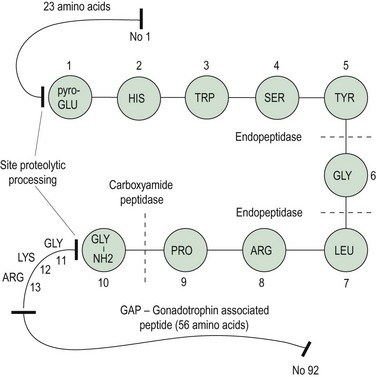
In 1971, Schally et al isolated pure preparations of porcine LH-releasing hormone (LHRH) from hypothalamic extracts. Subsequently, its structure was discovered and synthesis was achieved (Matsuo et al 1971a, b). The finding of FSH-releasing activity of this LHRH led to the hypothesis of a single hypothalamic-releasing hormone, GnRH, controlling secretion of both LH and FSH from the pituitary gland, with the suggestion that sex steroids might play a role in modulating the proportions of LH and FSH released. The amino acid sequence of GnRH is shown in Figure 15.3; it is a decapeptide secreted with a large pre- and postprecursor molecule.
GnRH neurone system
The GnRH neurone system has been mapped in detail using primarily immunocytochemical methods. The GnRH neurones are not grouped into specific nuclei but form a loose network in several anatomical divisions. However, GnRH neurone bodies are found principally in two areas: the preoptic anterior hypothalamic area and the tuberal hypothalamus, particularly the arcuate nucleus and periventricular nucleus. Axons from GnRH neurones project to many sites in the brain; the most distinct tract is from the medial basal hypothalamus to the median eminence, where extensive plexuses of boutons are found on the primary portal vessels. GnRH, then, has ready and direct access to the anterior pituitary gonadotroph cells via the portal capillary plexus (see Figure 15.2). There are also numerous projections of GnRH-secreting neurones to the limbic system and the circumventricular organ, other than the median eminence. The role of these connections is currently unknown, but they may connect with other cells or GnRH may bind to different receptors (type II) which may modulate sexual behaviour or sexual arousal.
Regulation of gonadotrophin secretion by GnRH
The GnRH gene sequence was first isolated by Seeburg and Adelman in 1984. The GnRH decapeptide is derived from the post-translational processing of a larger precursor molecule that has been termed ‘pre–pro-GnRH’. This appears to be a tripartite structure with a preceding 23-amino acid sequence joined to the decapeptide GnRH, which is then attached via a 3-amino acid sequence, glycine–lycine–arginine (GLY–LYS–ARG), to a 56-amino acid terminal peptide, which is termed the ‘gonadotrophin-associated peptide’ (GAP). The post-GnRH-decapeptide GLY–LYS–ARG 3-amino acid section is an important site for proteolytic processing. GAP itself is thought to have some prolactin-inhibiting properties (Figure 15.3). GnRH genes are encoded from a single gene located on the shorter arm of chromosome 8.
Using radioimmunoassays, GnRH has been demonstrated in the hypophyseal portal blood from a number of animal species (Carmel et al 1976). Electrical stimulation of the preoptic area of the brain in female rats on the day of pro-oestrus increases the GnRH concentration in portal blood, and the stimulus induces a marked release of LH from the anterior pituitary. In contrast, administration of antibodies against GnRH prevents this electrically stimulated LH release. These data provide evidence favouring a cause-and-effect relationship between GnRH release by the hypothalamus and LH release by the anterior pituitary.
In the female, the magnitude of gonadotrophin release, particularly LH, in individuals varies with the stage of the menstrual cycle, being greatest in the preovulatory phase, less marked in the luteal phase and least in the follicular phase of any individual cycle (Yen et al 1972, Shaw et al 1974) (Figure 15.4).
Mechanism of action of GnRH on pituitary cells
There are three principal positive actions of GnRH on gonadotrophin secretion:
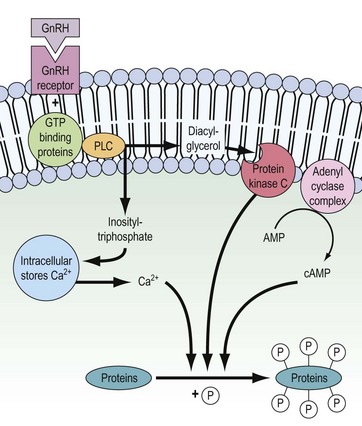
The binding of GnRH to its receptor induces a complex series of intracellular responses, which result in hormone secretion and biosynthesis of the α and β subunits of LH and FSH. In addition, dimerization of α and β subunits and the glycosylation processes are induced. The mechanism of action of GnRH is depicted in Figure 15.5. Within seconds of GnRH binding to and activating GnRH receptors on the pituitary gonadotrophs, intracellular free Ca2+ concentrations increase. This Ca2+ is initially mobilized from intracellular stores (e.g. endoplasmic reticulum) but also, to maintain sustained LH release, extracellular Ca2+ enters the gonadotroph through receptor-regulated voltage-dependent Ca2+ channels.
The initial mobilization of intracellular Ca2+ is induced by inositol triphosphate, released as a consequence of receptor activation of the membrane-bound phospholipase-C enzyme. Diacylglycerol is also released by the action of phospholipase-C and in turn activates the phosphorylating enzyme protein kinase C. The adenyl cyclase complex is also stimulated and cyclic adenosine monophosphate (cAMP) is generated. Ca2+, protein kinase C and cAMP then interact to stimulate release of stored LH and FSH and subsequent biosynthesis (for review, see Clayton 1989).
GnRH agonist analogues
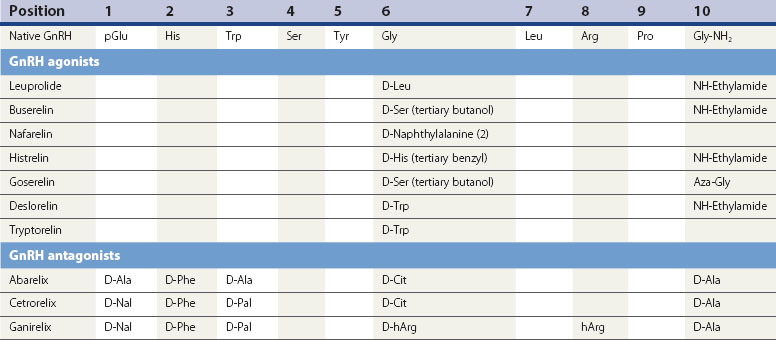
The current generation of GnRH analogues in clinical use are predominantly agonists. GnRH is degraded by peptidases in the pituitary and hypothalamus which cleave the native decapeptide at the Gly6-Leu7 bond and at position 10. Substitution at position 6 with another amino acid renders the analogue less susceptible to enzymatic breakdown, resulting in a half-life 2.5–6 times longer than the native GnRH, whilst still allowing the analogue to bind to the pituitary gonadotroph GnRH receptor site. Several agonistic analogues are available for clinical use (see Table 15.2). Following an initial few days of increased secretion of LH and FSH after the first administration of a GnRH agonist, downregulation of the GnRH receptor is achieved with subsequently suppressed release of LH and FSH, and hence a reduced stimulus to the ovary resulting in failure of follicle growth and reduced ovarian steroidogenesis.
GnRH antagonist analogues
GnRH antagonists are characterized by multiple modification of the amino acid sequence of native GnRH; predominantly the pyroglutamic and glycine termini (position 1 and 10) and deletion and substitution of hydrophobic D amino acids at positions 2 and 3 (see Table 15.2).
The wide-ranging group of clinical conditions in which GnRH, GnRH agonists or GnRH antagonists have found application are listed in Box 15.1. These are discussed in greater depth in Chapters 22 and 32–34.
Modulatory Role of Monoamines, Other Neurotransmitters and Second Messengers on GnRH Secretion
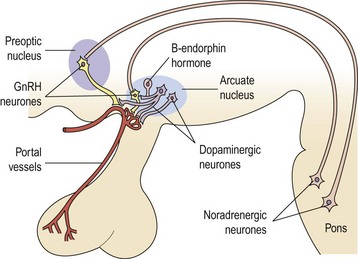
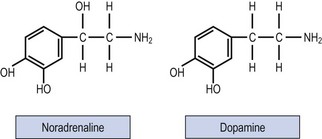
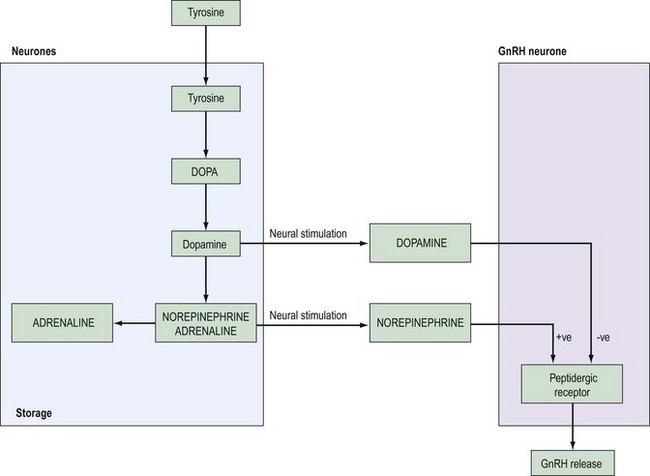
Past studies indicated that LH release and ovulation were dependent upon drug-affected neural stimuli of both cholinergic and adrenergic origin. The infundibulum contains large stores of noradrenaline (norepinephrine), a lesser quantity of dopamine and a small amount of adrenaline (epinephrine) (Figure 15.6).
Dopamine
The addition of dopamine to pituitaries coincubated with hypothalamic fragments increases the release of LH, while the addition of phentolamine, an α-receptor blocker, prevents dopamine-induced LH release. These early in-vitro experiments suggested that the hormonal background was capable of modifying the response to dopamine, since it seemed ineffective in ovariectomized animals or during oestrus or dioestrus day 1 of the oestrous cycle. Dopamine was more effective at pro-oestrus or in oestrogen- and progesterone-primed rats (McCann et al 1974, Ojeda and McCann 1978).
In humans, the inhibitory role of dopamine and its agonists on LH, as well as that of prolactin release, has been demonstrated (LeBlanc et al 1976, Lachelin et al 1977). Elevated levels of prolactin can also stimulate dopamine turnover in the hypothalamus, and it is postulated that the stimulated dopamine secretion alters GnRH secretion and hence reduces FSH and LH release in situations of hyperprolactinaemia. Hence, dopamine in the human may principally have an inhibitory effect on GnRH secretion.
Noradrenaline
Most experimental evidence supports a stimulatory role for noradrenaline in the control of gonadotrophin release. Turnover of hypothalamic noradrenaline is increased during the preovulatory surge of gonadotrophins at pro-oestrus, and noradrenaline synthesis in the anterior hypothalamus is enhanced in ovariectomized rats. These effects on GnRH secretion appear to be mediated by α receptors, since phenoxybenzamine, an α blocker, suppresses the postcastration rise in gonadotrophins in male rats, and phentolamine blocks the pulsatile release of LH in ovariectomized monkeys (for review, see McCann and Ojeda 1976).
Most of the cell bodies that synthesize noradrenaline are located in the mesencephalon and lower brainstem, and these cells also synthesize serotonin. The axons from these cells ascend into the medial forebrain bundle to terminate in various brain structures including the hypothalamus. The probable mode of action of catecholamines is to influence the frequency of GnRH release (see Figure 15.7).
Serotonin
High concentrations of serotonin are found in the median eminence, with most of the serotonin-containing neurones originating from the raphe nucleus in the midbrain–pons area. Present evidence shows that serotonin plays a predominantly inhibitory role in gonadotrophin release (see McCann and Ojeda 1976).
Neuropeptide Y
Gonadal steroids regulate the secretion and gene expression of neuropeptide Y in hypothalamic neurones (Sahu et al 1992). Neuropeptide Y stimulates pulsatile release of GnRH and potentiates gonadotrophin response to GnRH (Pau et al 1995). In the absence of oestrogens, neuropeptide Y inhibits gonadotrophin secretion, and increased amounts of neuropeptide Y are found in cases of under-nutrition and the cerebrospinal fluid of women with anexoria and bulimia nervosa. These findings explain a link between under-nutrition and suppressed LH and FSH secretion in those conditions.
Endogenous opioids
A single injection of morphine, administered to oophorectomized monkeys, brings about immediate cessation of GnRH pulse generation (Yen et al 1985). Hypothalamic opioidergic neurones are found in the arcuate nucleus of the medial basal hypothalamus, in close contact with GnRH neurones. The administration of an opioid antagonist, naloxone, produces an increased frequency and amplitude of GnRH and LH secretion. These changes are most marked in the luteal phase of the cycle, and it is thought that the negative feedback of oestrogen may partly be effected through opioid-induced inhibition of GnRH secretion (Ropert et al 1981), as is the negative feedback of progesterone on GnRH secretion.
Changes in opioid tone seem to mediate the hypogonadotrophic state seen in hyperprolactinaemia, exercise and other causes of hypothalamic amenorrhoea. Treatment of patients with hypothalamic amenorrhoea (who have suppressed GnRH pulsatile secretion) with naltrexone — an opioid receptor blocker — allows return of pulsatile LH and FSH secretion and return of ovulation (Wildt et al 1993).
Corticotrophin-releasing hormone
Experimental studies indicate that corticotrophin-releasing hormone inhibits hypothalamic GnRH secretion both directly and by augmenting endogenous opioid secretion. Corticotrophin-releasing hormone infusions inhibit gonadotrophin release in primates (Xiao et al 1989), and suppress the electrophysiological activity of the GnRH pulse generator (Knobil 1989). Women with hypothalamic amenorrhoea have increased levels of cortisol in their circulation, suggesting that this is the mechanism by which stress influences the hypothalamic–pituitary–ovarian axis.
Other Neurotransmitters and Second Messengers
The intrapituitary paracrine–autocrine system
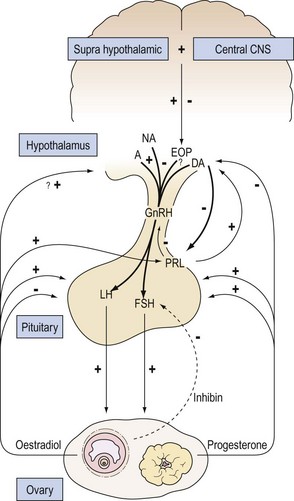
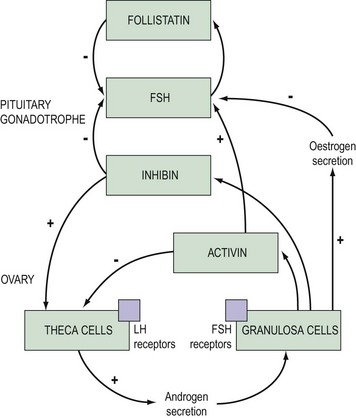
Growth factors and cytokines provide a paracrine–autocrine system which regulates cell development and replication, and pituitary hormone synthesis and secretion. As with other organs in the body, the pituitary contains epidermal growth factors, fibroblast growth factors, insulin-like growth factors, interleukins, activin, inhibin, endothelin and many others. Interactions between all of these substances constitute a complex interplay, but one group of compounds are better understood — those of the activin-inhibin and follistatin substances (see Figure 15.8).
Activin
Activin is produced by granulosa cells but is also present in pituitary gonadotropes. It contains two subunits identical to the β subunits of inhibin A and B, but some variants of the β subunits have also been identified, although their role and function are not thought to be important. Activin augments the secretion of FSH and inhibits prolactin, ACTH and growth hormone response (Corrigan et al 1991, Blumenfeld 2001). Activin increases pituitary responsiveness to GnRH by enhancing GnRH receptor formation, and its effects are blocked by inhibin and follistatin.
Follistatin
This is a peptide secreted by a variety of pituitary cells including gonodatrophes. It inhibits FSH synthesis and secretion, and the FSH response to GnRH by binding to activin and thus decreasing the activity of activin (Besecke et al 1997).
Modulatory Effect of Ovarian Steroids
Negative feedback
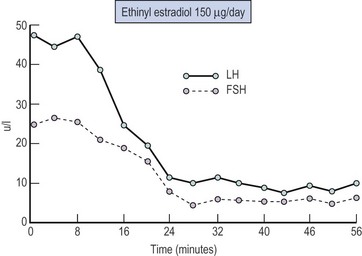
Negative feedback control or inhibition of pituitary LH and FSH release has been postulated since 1932, when Moore and Price considered that the ovary and adenohypophysis were linked in a rigid system of hormonal interactions. The quantitative relationship between ovarian steroids and gonadotrophin release can be demonstrated by disturbing the negative feedback loop by oophorectomy which produces, over a period of days, an increase in circulating LH and FSH; this reaches a plateau at approximately 3 weeks with levels which are some 10 times higher than preoperative values. Alternatively, the administration of exogenous oestrogens to oophorectomized or postmenopausal women will result in rapid suppression of elevated circulating gonadotrophin levels (Figure 15.9).
The negative feedback changes result from both a direct pituitary site of action of oestradiol, with a decrease in sensitivity of the gonadotroph to GnRH (McCann et al 1974), and an action within the hypothalamus and a decrease in GnRH secretion, possibly via increased inhibitory dopaminergic and opiate activity.
A negative feedback effect of progesterone on gonadotrophin secretion is now well established. Whilst progesterone, even in large doses, has little effect on baseline LH release, it can suppress the ovulatory surge of LH, as demonstrated in human females administered synthetic progestogens (Larsson-Cohn et al 1972), and oestrogen-induced positive feedback surges cannot be produced during the luteal phase in women (Shaw 1975). The principal negative feedback action of progesterone is thus upon the midcycle gonadotrophin surge, and it may be responsible for its short 24-h duration. It also seems likely that progesterone is an important factor in the reduced frequency of gonadotrophin pulses observed during the luteal phase of the cycle compared with their frequency in the follicular phase (see below).
Positive feedback
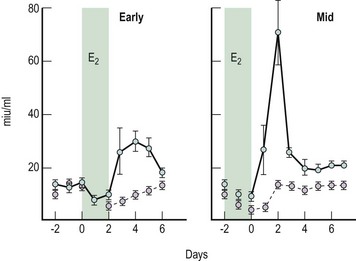
The fact that oestrogen may stimulate (positive feedback) rather than inhibit gonadotrophin release under certain circumstances was first proposed by Hohlweg and Junkmann (1932). Their proposal has since been substantiated by numerous experimental reports in animals and humans. Under physiological conditions, the positive feedback only operates in females; it is brought about by oestrogen and appears to be an essential component in producing the midcycle ovulatory surge of gonadotrophins. Administration of oestradiol-17β to females during the early or midfollicular phase of the cycle will induce a surge of gonadotrophins (Yen et al 1974, Shaw 1975) (Figure 15.10), but treatment with the same doses of oestrogen during the midfollicular phase induces a far greater release of LH than during the early follicular phase (Yen et al 1974). Studies on the dynamics of this positive feedback response to oestrogen, observed in greatest detail in the rhesus monkey, demonstrate an activation delay of some 32–48 h from the commencement of oestrogen administration until the onset of the positive-feedback-induced gonadotrophin surge, a minimum threshold level to be exceeded and a strength-duration aspect of the stimulus (Karsch et al 1973).
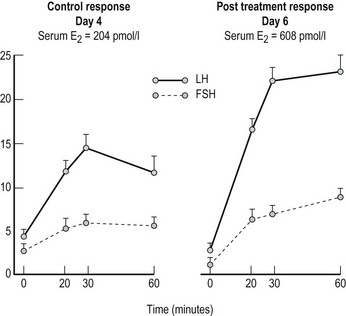
Oestrogen elicits gonadotrophin release by increasing pituitary responsiveness to GnRH and possibly by stimulating increased GnRH secretion by the hypothalamus. In the normally menstruating female, oestrogen pretreatment produces an initial suppressive action on pituitary responsiveness (Shaw et al 1975a) followed by a later augmenting action which is both concentration and duration dependent (Shaw et al 1975a, Young and Jaffe 1976). The augmenting effect of oestrogen on GnRH pituitary responsiveness is demonstrated in Figure 15.11.
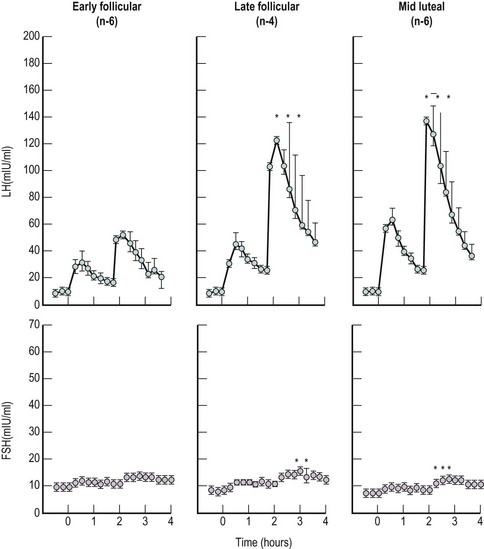
Progesterone by itself does not appear to exert a positive feedback effect. However, when administered to females in whom the pituitary has undergone either endogenously induced or exogenously administered oestrogen priming, progesterone can induce increased pituitary responsiveness to GnRH (Shaw et al 1975b; Figure 15.12). Since circulating progesterone levels are increasing significantly during the periovulatory period, this action may be of importance in determining the magnitude and duration of the midcycle gonadotrophin surge.
Self-Priming of the Pituitary Gonadotroph by GnRH
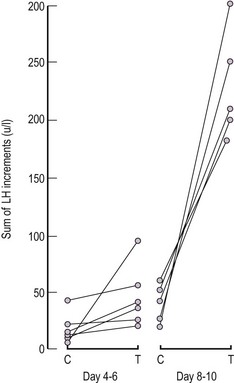
Rommler and Hammerstein (1974) first demonstrated that the response to a second injection of GnRH was greater than the initial response in females, when they were retested 1–4 h following the first exposure. This response has been termed ‘self-priming’.
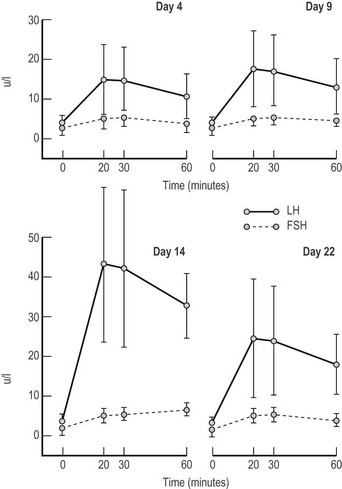
Wang et al (1976) published more intensive studies, carried out throughout the menstrual cycle, and were able to demonstrate that self-priming had a definite cycle relationship which was greatest in the late follicular phase and around midcycle (i.e. at times of increased circulating oestrogen levels), and that oestrogen preferentially induces LH rather than FSH release (Figure 15.13).
Pulsatile Nature of Gonadotrophin Release
This pulsatile pattern of GnRH concentration has been reported in the pituitary stalk effluent of the rhesus monkey (Carmel et al 1976). This suggests that the pulsatile pattern of gonadotrophin release from the anterior pituitary is probably causally related to the periodic increase in the hypothalamic GnRH system.
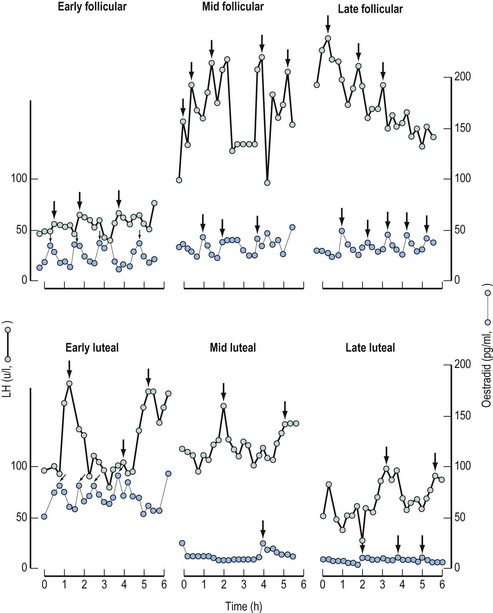
Comparison of the pulsatile pattern of gonadotrophin release at different phases of the menstrual cycle demonstrates profound modulation by ovarian steroids (Figure 15.14).
In females with normal cycles, a characteristic low-amplitude, high-frequency pulse pattern is observed during the follicular phase. This suggests that oestrogen appears to be most effective in reducing the amplitude of gonadotrophin pulses, more markedly in FSH than LH. In contrast, the pulse pattern during the luteal phase is one of high amplitude and low frequency, probably modified by progesterone effects, on the catecholaminergic and GnRH neurone systems (Figure 15.14).
Integrative Control of the Hypothalamic–Pituitary Unit during the Menstrual Cycle
How can the complex inter-related changes in ovarian steroids and pituitary gonadotrophins that occur within each menstrual cycle be explained on the basis of our present understanding of the control of the hypothalamic pituitary unit (see Figure 15.15 for overview).
Mechanism for preovulatory gonadotrophin surge
During the late follicular phase, there is an increase in the amount of oestradiol secreted by the ovary. Under this influence, the sensitivity of the gonadotroph to GnRH eventually reaches a phase when GnRH can exert its full self-priming action. The consequence is the transference of gonadotrophins from the secondary reserve pool to the releasable pool. The increased pituitary responsiveness to GnRH may be further enhanced by the slight progesterone rise which can affect its action on a fully oestrogen-primed anterior pituitary. These changes culminate in the production of the ovulatory gonadotrophin surge. It is possible that these events could occur even if the gonadotrophs were exposed to a constant level of GnRH. However, an increased secretion of GnRH, as reported in rhesus monkeys and rats (Sarker et al 1976), at midcycle, which would act synergistically with the changes in pituitary sensitivity, also seems likely in the human.
Luteal phase
KEY POINTS
Backstrom CT, McNeilly AS, Leak RM, Baird DT. Pulsatile secretion of LH, FSH, prolactin, oestradiol and progesterone during the human menstrual cycle. Clinical Endocrinology. 1982;17:29-42.
Besecke LM, Guendner MJ, Sluss PA, et al. Pituitary follistatin regulates activin mediated production of follicle-stimulating hormone during the rat estrous cycle. Endocrinology. 1997;132:2841-2848.
Bick D, Franco B, Sherins RJ, et al. Brief report: intragenic deletion of the KALIG-1 gene in Kallmann’s syndrome. New England Journal of Medicine. 1992;326:1752-1755.
Blumenfeld Z. Response of human fetal pituitary cells to activin, inhibin, hypophysiotrophe and neuroregulatory factors in-vitro. Early Pregnancy. 2001;5:41-42.
Carmel PD, Araki S, Ferin M. Prolonged stalk portal blood collection in rhesus monkeys. Pulsatile release of gonadotrophin-releasing hormone (GnRH). Endocrinology. 1976;99:243-248.
Clayton RN. Cellular actions of gonadotrophin-releasing hormone: the receptor and beyond. In: Shaw RW, Marshall JC, editors. LHRH and its Analogues — their Use in Gynaecological Practice. London: Wright; 1989:19-34.
Corrigan AZ, Bilezikjian LM, Carroll RS, et al. Evidence for an autocrine role of activin B within rat anterior pituitary cultures. Endocrinology. 1991;128:1682-1684.
Hohlweg W, Junkmann K. Die hormonal-nervosae Regulierung der Funktion des Hypophysenvorderlappens. Klinische Wochenschrift. 1932;11:321-323.
Karsch FJ, Weick RF, Butler WR, et al. Induced LH surges in the rhesus monkey: strength-duration characteristics of the oestrogen stimulus. Endocrinology. 1973;92:1740-1747.
Knobil E. The electrophysiology of the GnRH pulse generator. Journal of Steroid Biochemistry. 1989;33:669-671.
Lachelin GCL, LeBlanc H, Yen SSC. The inhibitory effect of dopamine agonists on LH release in women. Journal of Clinical Endocrinology and Metabolism. 1977;44:728-732.
Larsson-Cohn V, Johansson EDB, Wide L, Gemzell C. Effects of continuous daily administration of 0.1 mg of norethindrone on the plasma levels of progesterone and on the urinary excretion of luteinizing hormone and total oestrogens. Acta Endocrinologica (Copenhagen). 1972;71:551-556.
LeBlanc H, Lachelin GCL, Abu-Fadil S, Yen SSC. Effects of dopamine infusion on pituitary hormone secretion in humans. Journal of Clinical Endocrinology and Metabolism. 1976;43:668-674.
Matsuo H, Arimura A, Nair RMG, Schally AV. Synthesis of the porcine LH- and FSH releasing hormone by the solid phase method. Biochemical and Biophysical Research Communications. 1971;45:822-827.
Matsuo H, Baba Y, Nair RMG, Anmura A, Schally AV. Structure of porcine LH- and FSH-releasing hormone. I The proposed amino acid sequence. Biochemical and Biophysical Research Communications. 1971;43:1334-1339.
McCann SM, Ojeda SR. Synaptic transmitters involved in the release of hypothalamic releasing and inhibiting hormones. In: Ehrenpreis S, Kopin IJ, editors. Reviews of Neuroscience, Vol 2. New York: Raven Press; 1976:91-110.
McCann SM, Ojeda SR, Fawcett CP, Krulich L. Catecholaminergic control of gonadotrophin and prolactin secretion with particular reference to the possible participation of dopamine. Advances in Neurology. 1974;5:435-445.
Moore CR, Price D. Gonad hormone function and the reciprocal influence between gonads and hypophysis. American Journal of Anatomy. 1932;50:13-72.
Ojeda SR, McCann SM. Control of LH and FSH release by LHRH: influence of putative neurotransmitters. Clinics in Obstetrics and Gynaecology. 1978;5:283-303.
Pau KF, Berria M, Hess DL, Spies HG. Hypothalamic site-dependent effects of neuropeptide Y on gonadotropin-releasing hormone secretion in the rhesus macaques. Journal of Neuroendocrinology. 1995;7:63-67.
Rommler A, Hammerstein J. Time-dependent alterations in pituitary responsiveness caused by LH-RH stimulations in man. Acta Endocrinologica (Copenhagen). 1974;21(Suppl):184.
Ropert JR, Quigley ME, Yen SSC. Endogenous opiates modulate pulsatile LH release in humans. Journal of Clinical Endocrinology and Metabolism. 1981;52:584-585.
Sahu A, Phelps CP, White JD, Crowley WR, Kalra SP, Kalra PS. Steroidal regulation of neuropeptide Y release and gene expression. Endocrinology. 1992;130:3331-3336.
Sarker DK, Chiappa SA, Fink G. Gonadotrophin releasing hormone surge in proestrus rats. Nature. 1976;264:461-463.
Schally AV, Arimura A, Kastin A. Gonadotrophin releasing hormone — one polypeptide regulates secretion of LH and FSH. Science. 1971;173:1036-1038.
Schwanzel-Fukunda M, Bick D, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH) — expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Molecular Brain Research. 1989;6:311-315.
Schwanzel-Fukunda M, Pfaff DW. Origin of luteinizing hormone releasing hormone neurons. Nature. 1989;338:161.
Seeburg PH, Adelman JP. Characterisation of cDNA for precursor of human luteinizing hormone-releasing hormone. Nature. 1984;311:666-669.
Shaw RW. A Study of Hypothalamic–Pituitary–Gonadal Relationships in the Female. Birmingham: MD Thesis, Birmingham University; 1975.
Shaw RW, Butt WR, London DR, Marshall JC. Variation in response to synthetic luteinizing hormone-releasing hormone (LHRH) at different phases of the same menstrual cycle in normal women. Journal of Obstetrics and Gynaecology of the British Commonwealth. 1974;81:632-639.
Shaw RW, Butt WR, London DR. Effect of oestrogen pretreatment on subsequent response to luteinizing hormone-releasing hormone (LH-RH) in normal women. Clinical Endocrinology. 1975;4:297-304.
Shaw RW, Butt WR, London DR. The effect of progesterone on FSH and LH response to LH-RH in normal women. Clinical Endocrinology. 1975;4:543-550.
Waldstreicher J, Seminara SB, Jameson JL, et al. The genetic and clinical heterogeneity of gonadotropin-releasing hormone deficiency in the human. Journal of Clinical Endocrinology and Metabolism. 1996;81:4388.
Wang CF, Lusley BL, Lein A, Yen SSC. The functional changes of the pituitary gonadotrophs during the menstrual cycle. Journal of Clinical Endocrinology and Metabolism. 1976;42:718-724.
Wildt L, Leyendecker G, Sir-Petermann T, Waibel-Treber S. Treatment with naltrexone and hypothalamic ovarian failure: induction of ovulation and pregnancy. Human Reproduction. 1993;8:350-358.
Xiao E, Luckhaus J, Niemann W, et al. Acute inhibition of gonadotrophin secretion by corticotrophin-releasing hormone in the primate. Are adrenal glands involved? Endocrinology. 1989;124:1632-1639.
Yen SSC, van den Berg G, Rebar R, Ehara Y. Variations of pituitary responsiveness to synthetic LRF during different phases of the menstrual cycle. Journal of Clinical Endocrinology and Metabolism. 1972;35:931-934.
Yen SSC, van den Berg G, Tsai CC, Siler T. Causal relationship between the hormonal variables in the menstrual cycle. In: Ferin M, et al, editors. Biorhythms and Human Reproduction. New York: John Wiley; 1974:219-238.
Yen SSC, Quigley ME, Reid RL, Cetel NS. Neuroendocrinology of opioid peptides and their role in the control of gonadotrophin and prolactin secretion. American Journal of Obstetrics and Gynecology. 1985;152:485-493.
Young JR, Jaffe RB. Strength duration characteristics of estrogen effects on gonadotrophin response to gonadotrophin-releasing hormone in women. II Effects of varying concentrations of oestradiol. Journal of Clinical Endocrinology and Metabolism. 1976;42:432-442.

 , follicle-stimulating hormone.
, follicle-stimulating hormone.