117 Contrast-Induced Nephropathy
The use of intravascular iodinated radiocontrast media is very prevalent. After injection of these contrast agents, a mild transient kidney function impairment can be detected by sensitive tests.1 However, clinically important kidney injury, known as contrast-induced nephropathy (CIN) or contrast-induced acute kidney injury (CIAKI), is less common, especially with normal preexisting kidney function.
CIN is usually defined as an acute kidney function impairment within 72 hours of intravascular injection of iodinated radiocontrast media, in the absence of other etiology. For research purposes, CIN is commonly defined as a 25% increase or an absolute increase in serum creatinine of 0.5 mg/dL (44 µmol/L) relative to precontrast values. Acute kidney injury (AKI) markers such as serum cystatin C, urinary neutrophil gelatinase-associated lipocalin (NGAL), or interleukin (IL)-18 will possibly be used in upcoming studies of CIN and may predict later CIN-associated morbidity and mortality.1
Awareness of the nephrotoxicity of contrast and the factors predisposing to it have improved over time to the point that clinicians may now overestimate the risk associated with some specific medical conditions.2 However, the increasing use of radiographic contrast media, possibly combined with increasing age and comorbidity of the treated population, contribute to the continuing importance of contrast nephropathy. In reality, given the mild and transient nature of the AKI in most CIN cases, it is the association with later more momentous clinical adverse events that drives current interest in preventing CIN.
 Epidemiology
Epidemiology
The exact incidence of CIN is not clear, ranging from 1% to 30%. This variability is due to lack of consistent definitions, variation in patient risk, contrast dose, and likely route of injection (intraarterial versus intravenous [IV]).2–4
Typically, about 15% of patients undergoing coronary angiography have serum creatinine rise by more than 25%, but the risk for dialysis is less than 1%.3 In the recent Cardiac Angiography in Renally Impaired Patients (CARE) Study,5 CIN defined by serum creatinine rise occurred in 11.1% of the 414 enrolled patients, while smaller increments in creatinine or rise in cystatin C occurred more frequently.5
The frequency of similar kidney function impairment after IV contrast injection appears to be many-fold less common than after cardiac angiography.6,7 In several studies, IV injection of nonionic low-osmolality contrast media (LOCM) in patients with chronic kidney disease was associated with a low risk of CIN.8
The fluctuation in serum creatinine due to other causes makes control groups not receiving contrast necessary to truly judge the risk to the kidney from IV contrast. In a small study, Langner et al. found a similar pattern of kidney function in a group having multiple contrast-enhanced studies with IV iodixanol as in a control group receiving no contrast media.9
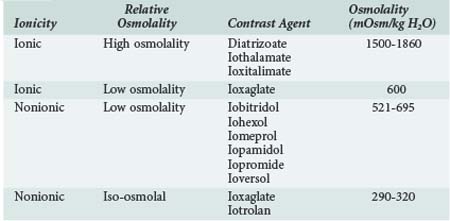
A variety of contrast media are available for use, and certain media are recommended ahead of others if a contrast study is required in a patient at risk for CIN. Contrast media are often classified according to osmolality and as ionic or nonionic (Table 117-1), but these factors are not necessarily the most important in determining nephrotoxicity. High-osmolality contrast agents such as diatrizoate are not commonly used nowadays and were associated with greater risk to the kidney. The relative toxicity of low and iso-osmolal contrast agents is controversial. Recent analyses suggest that CIN incidence may be higher with iohexol than with other LOCM, but the comparisons were across rather than within studies.10 A meta-analysis of pooled data from 16 randomized controlled trials (RCTs) including 2727 patients showed that intraarterial injection of the iso-osmolar contrast medium (IOCM), iodixanol, was associated with smaller rises in serum creatinine and lower incidence of CIN relative to low-osmolar contrast media (LOCM) (1.4% versus 3.5%, P = 0.003), especially in chronic kidney disease patients with or without diabetes mellitus (3.5% versus 15.5%, P = 0.003; and 2.8% versus 8.4%, P = 0.001, respectively).11 Nonetheless, a more recent meta-analysis of pooled data from 3270 patients and 25 trials including some of the above RCTs in addition to 7 new RCTs published within the last 3 years indicated that iodixanol is not associated with a significant decrease in the incidence of CIN compared with LOCM in the general population (relative risk [RR] = 0.80; 95% confidence interval [CI]: 0.61–1.04).12 Further, in this meta-analysis, iodixanol (IOCM) was less nephrotoxic than iohexol but not noticeably superior to other LOCM.12 Based on these data, current American Heart Association (AHA) guidelines recommend that either ioxaglate or a low-osmolality medium other than iohexol or ioxaglate be used in cases at risk for CIN.13
 Risk Factors
Risk Factors
The presence or absence of risk factors, especially preexisting kidney function, in addition to the type of imaging procedure are the most relevant predictors of CIN.3 The risk of dialysis-requiring CIN will increase considerably if precontrast creatinine clearance is less than 47 mL/min (0.78 mL/sec).3 Diabetes is a major risk factor,3,14 particularly in patients with diabetic nephropathy.15 Other factors associated with variable risk for CIAKI are: age older than 75 years, periprocedure volume depletion, heart failure, hypotension, cirrhosis, proteinuria, coadministration of nephrotoxins (e.g., diuretics, nonsteroidal antiinflammatory drugs [NSAIDs]), high doses of contrast, and intraarterial injection. The tolerable contrast dose depends in part on kidney function.3,16 Exceeding a maximum recommended contrast dose derived from serum creatinine and body weight strongly predicts dialysis-requiring CIN.4,16 The risk for CIN can be predicted by counting the number of risk factors present17 or by specific risk prediction models such as that shown in Table 117-2.18,19
TABLE117-2 Risk Prediction Score for Contrast-Induced Nephropathy Following Percutaneous Coronary Intervention
| Risk Factor | Score | |
|---|---|---|
| Systolic blood pressure <80 mm Hg longer than 1 h, requiring inotropes or intraaortic balloon pump (IABP) within 24 h of procedure | 5 | |
| Utilization of intraaortic balloon pump | 5 | |
| Heart failure (NYHA class III/IV) and/or history of pulmonary edema | 5 | |
| Age >75 years | 4 | |
| Hematocrit <39% in males, <36% in females | 3 | |
| Diabetes | 3 | |
| Volume of contrast medium | 1 for every 100 mL | |
| Serum creatinine level >1.5 mg/dL (133 µmol/L) | 4 | |
| Estimated GFR (eGFR) <60 mL/min per 1.73 m2 | 2; 40-59 mL/min/1.73 m2 4; 20-39 mL/min/1.73 m2 6; < 20 mL/min/1.73 m2 |
|
| eGFR = 186 × (serum creatinine mg/dL)−1.154 × age−0.203 × (0.742 if female) × (1.21 if black) | ||
| Total Risk Score | Risk of CIN % | Risk of Dialysis % |
| ≤5 | 7.5 | 0.04 |
| 6-10 | 14.0 | 0.12 |
| 11-15 | 26.1 | 1.09 |
| ≥16 | 57.3 | 12.6 |
CIN, contrast-induced nephropathy; NYHA, New York Heart Association.
Adapted from Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 2004; 44:1393–9.
 Pathogenesis
Pathogenesis
Although debate remains about the exact pathogenesis in humans and the relevance of animal models, pathogenetic considerations inspire most efforts to prevent CIN. In vitro and animal studies suggest CIN results from direct toxic injury to renal tubular cells and medullary ischemic injury secondary to subcorticomedullary congestion.20,21
Injection of a contrast agent induces a biphasic renal hemodynamic change, resulting initially in a transient increase and then a more prolonged decrease in global renal blood flow.21 Cortical vasoconstriction and outer medullary vasodilation and congestion occurred during the hypoperfusion phase.20 Vasoactive substances including endothelin, vasopressin, prostacyclin, nitric oxide, and adenosine are involved in the cortical vasoconstriction.22–25
In humans, the pathogenesis of CIN is still unclear, and there is no specific diagnostic marker for CIN. Contrast may be a contributory rather than a sole cause of AKI in specific cases of CIN. Concomitant insults may include intravascular volume depletion, surgery, atheroembolic disease, or coadministration of other nephrotoxins (e.g., NSAIDs). The mechanism of cellular injury may also vary by contrast viscosity, dose and concentration, associated ions, concomitant hypoxemia, and oxygen free radicals.21,26
 Clinical Features and Diagnosis
Clinical Features and Diagnosis
Patients with CIN are generally asymptomatic but have an acute rise in serum creatinine concentration 24 to 72 hours after administration of the contrast agent. The renal failure is usually nonoliguric, but it may be oliguric, especially if there is significant preexisting renal impairment.27,28 Serum creatinine level typically peaks at 3 days and returns to baseline within 10 days.29 Clinically significant deterioration is unlikely if the serum creatinine concentration does not increase by more than 0.5 mg/dL within 24 hours.30 In a minority of cases, the renal failure is severe enough to require dialysis, or renal function does not recover to precontrast values. To make an unequivocal diagnosis of contrast nephropathy, other potential causes of acute renal failure must be ruled out. Prerenal factors, atheroembolic disease, and other nephrotoxic insults should be excluded. The relatively rapid onset and typical course may help differentiate CIN from other causes of AKI. Urinalysis may be unremarkable or may show granular casts, tubular cells, or proteinuria. Fractional excretion of sodium can be low.27,29
 Prognosis
Prognosis
Most episodes of CIN are self-limiting and resolve within 10 days, but CIN is consistently associated with increased morbidity, prolonged hospital stay, major adverse cardiac events, and early death.3,31 In the United States and Europe, CIN is the third leading cause of AKI in hospitalized patients, accounting for 10% of all causes of hospital-acquired renal failure.32 Less than 1% of CIN cases may require dialysis, and 13% to 50% of such cases may become permanently dialysis dependent.3,33
Although the association of CIN with adverse clinical outcomes other than requirement for dialysis has been clearly and consistently shown, it is not yet known whether CIN events are causally linked to early death and adverse cardiovascular events.5 If in fact CIN is causally related to these later events, efforts to prevent CIN become even more important. However, if CIN does not cause early death or major adverse events, it may be a less important health issue. Future trials using a variety of interventions with different mechanisms of action showing parallel diminution in CIN and adverse events are required to establish some evidence for causality.
 Preventive Interventions
Preventive Interventions
As outlined in Box 117-1, the risk of CIN can be reduced by general and specific measures. The first step is to assess the presence of risk factors and indications for use of a contrast agent. Most risk factors can be detected with a routine history and physical examination. It is not practical or necessary to measure serum creatinine concentration on every patient before use of a contrast agent, but this should be done in those patients with other risk factors.34 The following specific prophylactic measures have been studied and should be considered for high-risk patients.
Box 117-1
Recommendations to Reduce the Risk of Contrast-Induced Nephropathy
Fluid Administration and Bicarbonate
Dehydration is one of the risk factors for CIN, so fluid restriction and diuretic use prior to contrast administration should be avoided unless necessary for other reasons. Although hydration is recommended in guidelines to reduce the risk for CIN, the optimal fluid type and regimen remain unclear.35 Prolonged IV fluid regimens (12 hours before and after contrast injection) are the best supported but are impractical for ambulatory procedures. In a large RCT, isotonic saline was found to be superior to 0.45% saline in patients with preserved kidney function.36
In an initial trial that was prematurely terminated, Merten et al. found that alkalinizing the urine using IV isotonic sodium bicarbonate reduced CIN.37 Since then, several further trials and meta-analyses have been completed. In a recent meta-analysis of 23 published and unpublished trials involving 3563 patients and 396 CIN events, the pooled RR of CIN with isotonic sodium bicarbonate as compared to other fluids was 0.62 (95% CI: 0.45–0.86).38 However, as in other meta-analyses on this question, there was evidence of both heterogeneity and publication bias, suggesting that the true effect of bicarbonate has yet to be fully established.38 In an effort to reduce the influence of publication bias, Brar et al. analyzed the protective effects of sodium bicarbonate in three large trials (n = 1145) out of 14 total trials (n = 2290) and reported a non-significant RR of 0.85 (95% CI: 0.63–1.16) without evidence of heterogeneity (I2 = 0%; P = 0.89).39 Furthermore, several meta-analyses showed no significant effects of sodium bicarbonate on the risk of post-CIN dialysis, heart failure, and total mortality.38–40
At this time, it is practical to use either IV isotonic saline or IV isotonic sodium bicarbonate as described by Merten et al.37 to diminish the risk of CIN. Meanwhile, patients should be observed for signs of volume overload.
N-Acetylcysteine
In the earliest trial, Tepel et al. showed a significantly lower incidence rate of CIN with N-acetylcysteine (NAC) compared with placebo (CIN occurred in 2% versus 21%; P = 0.01).41 However, the rate of CIN in the placebo group was unexpectedly high. Numerous further trials and meta-analyses have been completed since.
More recent meta-analyses generally find evidence of heterogeneity that is not easily explained.42,43 In one of these, Gonzales et al. divided the trials into two groups.42 The first group showed no benefit (RR = 0.87; 95% CI: 0.68–1.12; P = 0.28), whereas in the second group which contained relatively early, small, and lower-quality trials, NAC was extremely beneficial (RR = 0.15; 95% CI: 0.07–0.33; P < 0.0001). Marenzi showed a dose-dependent effect of NAC on CIN risk after intraarterial contrast injection and a positive effect on in-hospital mortality.44 This latter finding was recently confirmed in a meta-analysis of 16 RCTs with a total sample size of 1677 patients and no significant heterogeneity (I2 = 34%; P = 0.09).45 The odds ratio for CIN was 54% lower in patients assigned to high-dose NAC (95% CI: 0.33–0.63).45
While there remains uncertainty about the benefit of NAC, and the results of ongoing trials such as the Acetylcysteine for Contrast-Induced Nephropathy Trial (ACT)46 are pending, the drug appears safe, and it would be reasonable to use it giving at least 1200 mg orally (PO) or IV prior to contrast and repeated 12 hourly for the following 24 hours.
Prophylactic Renal Replacement Therapy
Lee et al. in a recent trial showed that prophylactic hemodialysis immediately post coronary angiography in patients with baseline creatinine clearance around 13 mL/min lessened the decrease in creatinine clearance on the fourth day post contrast injection (0.4 ± 0.9 versus 2.2 ± 2.8 mL/min/1.73 m2; P < 0.001).47 Additionally, the risk for further or permanent dialysis was also reduced. However, the same benefit was not seen in several other trials, and the procedure carries its own inherent risks.48–50 Prophylactic hemofiltration before and after contrast, but not post contrast alone, was associated with a lower rate of CIN in patients with advanced kidney disease as reported by Marenzi et al.51,52 These trials are challenging to interpret insofar as it is hard to judge the effect of contrast on kidney function from trends in serum creatinine in patients undergoing hemofiltration. In-hospital mortality was also lower in those exposed to hemofiltration, but the mechanism by which hemofiltration led to better outcomes is unclear. The invasive nature of both prophylactic dialysis and hemofiltration suggests that these should only be considered in patients with existing advanced kidney disease.
Other Pharmacologic Agents
The volume contraction associated with forced diuresis with furosemide, mannitol, dopamine, or a combination of these agents at the time of contrast exposure has been associated with equal or higher rates of CIN when compared to prophylactic fluids alone.53–56 A recent meta-analysis of three published trials including 251 patients found that forced euvolemic diuresis with furosemide and mannitol was associated with a significant risk of CIN (pooled RR = 2.15; 95% CI: 1.37–3.37; I2 = 0%).57
The vasodilatory effects of calcium channel blockers, dopamine, fenoldopam, atrial natriuretic peptide (ANP), prostaglandin E1, and a nonselective endothelin receptor antagonist failed to reduce the CIN risk compared with fluid hydration in several small trials.53,58–62 In a more recent trial, the incidence of CIN was significantly lower in the ANP group than in the control group within 48 hours (3.2% versus 11.7%, respectively; P = 0.015) and at 1 month (P = 0.006) following contrast.63
Two small trials using captopril as a prophylactic agent for CIN had conflicting results, and no conclusion can be reached about the efficacy of this approach.64,65 Similarly, ascorbic acid seemed promising in one small trial66 but was inferior to NAC in another trial.67
Although lipid-lowering drugs such as high-dose simvastatin or probucol failed to show protective effects against CIN,68,69 a recent trial compared the protective effects of simvastatin 80 mg versus 20 mg on renal function in 228 patients with good kidney function undergoing percutaneous coronary intervention (PCI).70 The results favored the 80-mg dose, but the differences were not really clinically significant.70
Trimetazidine, a cellular antiischemic and antioxidant agent, restrains the cellular and mitochondrial ischemia/reperfusion toxic effects and inhibits the release of oxygen free radicals in various tissues.71 In a single trial in 82 patients, 72 hours of 20 mg trimetazidine, 3 times daily starting 48 hours prior to coronary angiography together with IV saline, reduced the incidence rate of CIN from 16.6% to 2.5% compared with IV saline alone (P < 0.05).71
Theophylline and aminophylline antagonize adenosine-mediated vasoconstriction and have been used as a means to prevent CIN. However, the benefit was quite modest, and there was a potential for harm shown in meta-analysis.72
 Management and Outcome
Management and Outcome
In most instances, CIN never becomes clinically evident, and renal function returns to baseline. In more severe cases, management is no different than that for acute renal failure of any other cause. Careful control of fluid and electrolyte balance, avoidance of further nephrotoxic insults, attention to nutrition, and surveillance for complications are generally all that is required, although dialysis may be necessary in the occasional patient.4,73 Prophylactic hemodialysis soon after administration of a contrast agent in patients with high serum creatinine concentrations has had inconsistent effects as previously noted. Dialysis does not have to be done for routine removal of contrast medium after imaging in previously dialysis-dependent cases.74
 Conclusion
Conclusion
CIN remains a concern, especially with interventions involving intraarterial contrast. CIN is not common in the absence of risk factors, and these are generally detectable with a history and physical examination plus or minus determination of a serum creatinine concentration. Because CIN can be associated with other adverse clinical outcomes, preventive measures are advisable, especially with advanced preexisting renal disease when there is a risk the patient may require dialysis. Although CIN or CIAKI is associated with later adverse events, causality has not been proven, and the efficacy of preventive measures directed at CIN in preventing these associated events has not been established. Future research is needed in this area. At this time, the optimal approach to prevent CIN is unclear. Minimizing contrast dose, using either iodixanol or a LOCM other than iohexol, use of isotonic sodium bicarbonate or saline, and possibly NAC are the main components of our approach, which is summarized in Box 117-1. Finally, supportive care is indicated if contrast nephropathy occurs.
Key Points
Heinrich MC, Häberle L, Müller V, Bautz W, Uder M. Nephrotoxicity of iso-osmolar iodixanol compared with nonionic low-osmolar contrast media: meta-analysis of randomized controlled trials. Radiology. 2009;250:68-86.
Gruberg L, Mintz GS, Mehran R, Gangas G, Lansky AJ, Kent KM, et al. The prognostic implications of further renal function deterioration within 48 hours of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542-1548.
Thomsen HS, Morcos SK. Members of the Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). In which patients should serum creatinine be measured before iodinated contrast medium injection? Eur Radiol. 2005;15:749-754.
Brar SS, Hiremath S, Dangas G, Mehran R, Brar SK, Leon MB. Sodium bicarbonate for the prevention of contrast-induced acute kidney injury: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4:1584-1592.
Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773-2782.
1 Bachorzewska-Gajewska H, Malyszko J, et al. Could neutrophil-gelatinase-associated lipocalin and cystatin c predict the development of contrast-induced nephropathy after percutaneous coronary interventions in patients with stable angina and normal serum creatinine values? Kidney Blood Press Res. 2007;30:408-415.
2 Katzberg RW, Haller C. Contrast-induced nephrotoxicity: Clinical landscape. Kidney Int. 2006;69(Suppl S100):53-57.
3 McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368-375.
4 Freeman RV, O’Donnell MO, Share D, et al. Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of an adjusted contrast dose. for the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. Am J Cardiol. 2002;90:1068-1073.
5 Solomon RJ, Mehran R, Natarajan MK, Doucet S, Katholi RE, Staniloae CS, et al. Contrast-induced nephropathy and long-term adverse events: cause and effect? Clin J Am Soc Nephrol. 2009;4:1162-1169.
6 Katzberg R, Barrett B. Risk of contrast-induced nephropathy with the intravenous administration of iodinated contrast media. Radiology. 2007;243:622-628.
7 Rao QA, Newhouse JH. Risk of nephropathy after intravenous administration of contrast material: a critical literature analysis. Radiology. 2006 May;239(2):392-397.
8 Garcia-Ruiz C, Martinex-Vea A, Sempere T, et al. Low risk of contrast nephropathy in high-risk patients undergoing spiral computed tomography angiography with the contrast medium iopromide and prophylactic oral hydration. Clin Nephrol. 2004;61:170-176.
9 Langner S, Stumpe S, Kirsch M, Petrik M, Hosten N. No increased risk for contrast-induced nephropathy after multiple CT perfusion studies of the brain with a nonionic, dimeric, iso-osmolal contrast medium. AJNR Am J Neuroradiol. 2008;29:1525-1529.
10 Solomon R, DuMouchel W. Contrast media and nephropathy: findings from systematic analysis and food and drug administration reports of adverse effects. Invest Radiol. 2006;41:651-660.
11 McCullough PA, Bertrand ME, Brinker JA, Stacul F. A meta-analysis of the renal safety of isoosmolar iodixanol compared with low-osmolar contrast media. J Am Coll Cardiol. 2006;48:692-699.
12 Heinrich MC, Häberle L, Müller V, Bautz W, Uder M. Nephrotoxicity of iso-osmolar iodixanol compared with nonionic low-osmolar contrast media: meta-analysis of randomized controlled trials. Radiology. 2009 Jan;250(1):68-86.
13 Kushner FG, Hand M, Smith SC, et al. 2009 Focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update). Circulation. 2009;120:2271-2306.
14 Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259-2264.
15 Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast-material induced renal failure in patients with diabetes mellitus, renal insufficiency, or both: a prospective controlled study. N Engl J Med. 1989;320:143-149.
16 Cigarroa RG, Lange RA, Williams RH, Hillis LD. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. Am J Med. 1989;86:649-652.
17 Rich MW, Crecelius CA. Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older. Arch Intern Med. 1995;150:1237-1242.
18 Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393-1399.
19 Dangas G, Iakovou I, Nikolsky E, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95:13-19.
20 Heyman SN, Brezis M, Epstein FH, Spokes K, Silva P, Rosen S. Early renal medullary hypoxic injury from radiocontrast and indomethacin. Kidney Int. 1991;40:632-642.
21 Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int. 2005;68:14-22.
22 Arakawa K, Suzuki H, Naitoh M, et al. Role of adenosine in the renal responses to contrast medium. Kidney Int. 1996;49:1199-1206.
23 Heyman SN, Goldfarb M, Carmeli F, et al. Effect of radiocontrast agents on intrarenal nitric oxide (NO) and NO synthase activity. Exp Nephrol. 1998;6:557-562.
24 Heyman SN, Reichman J, Brezis M. Pathophysiology of radiocontrast nephropathy: A role for medullary hypoxia. Invest Radiol. 1999;34:685-691.
25 Agmon Y, Peleg H, Greenfeld Z, et al. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J Clin Invest. 1994;94:1069-1075.
26 Katholi RE, Woods WT, Taylor GJ, Deitrick CL, Womack KA, Katholi CR, et al. Oxygen free radicals and contrast nephropathy. Am J Kidney Dis. 1998;32:64-71.
27 Fang LS, Sirota RA, Ebert TH, et al. Low fractional excretion of sodium with contrast media-induced acute renal failure. Arch Intern Med. 1980;140:531-533.
28 Manske CL, Sprafka JM, Strony JT, et al. Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am J Med. 1990;89:615-620.
29 Solomon R. Nephrology forum: Contrast medium–induced acute renal failure. Kidney Int. 1998;53:230-242.
30 Guiterrez NV, Diaz A, Timmis GC, et al. Determinants of serum creatinine trajectory in acute contrast nephropathy. J Intervent Cardiol. 2002;15:349-354.
31 Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275:1489-1494.
32 Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930-936.
33 Gruberg L, Mintz GS, Mehran R, et al. The prognostic implications of further renal function deterioration within 48 hours of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542-1548.
34 Thomsen HS, Morcos SK. Members of the Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). In which patients should serum creatinine be measured before iodinated contrast medium injection? Eur Radiol. 2005;15:749-754.
35 Solomon R, Deray G. How to prevent contrast-induced nephropathy and manage risk patients: practical recommendations. Kidney Int. 2006;69(Suppl S100)):S51-S53.
36 Mueller C, Buettner HJ, Petersen J, et al. Prevention of contrast media-associated nephropathy. Randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329-336.
37 Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328-2334.
38 Zoungas S, Ninomiya T, Huxley R, Cass A, Jardine M, Gallagher M, et al. Systematic review: sodium bicarbonate treatment regimens for the prevention of contrast-induced nephropathy. Ann Intern Med. 2009;151:631-638.
39 Brar SS, Hiremath S, Dangas G, Mehran R, Brar SK, Leon MB. Sodium bicarbonate for the prevention of contrast induced-acute kidney injury: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4:1584-1592.
40 Meier P, Ko DT, Tamura A, Tamhane U, Gurm HS. Sodium bicarbonate-based hydration prevents contrast-induced nephropathy: a meta-analysis. BMC Med. 2009;7:23.
41 Tepel M, van der Giet M, Schwarzfeld C, et al. Prevention of radiographic contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180-184.
42 Gonzales DA, Norsworthy KJ, Kern SJ, et al. A meta-analysis of N-acetylcysteine in contrast-induced nephrotoxicity: unsupervised clustering to resolve heterogeneity. BMC Med. 2007;5:32.
43 Zagler A, Azadpour M, et al. N-acetylcysteine and contrast-induced nephropathy: A meta-analysis of 13 randomized trials. Am Heart J. 2006;151:140-145.
44 Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006 Jun 29;354(26):2773-2782.
45 Trivedi H, Daram S, Szabo A, Bartorelli AL, Marenzi G. High-dose N-acetylcysteine for the prevention of contrast-induced nephropathy. Am J Med. 2009;122:874.e9-874.e15.
46 Acetylcysteine for Contrast-Induced nephropathy Trial (ACT). Available at: www.clinicaltrials.gov (NCT00736866)
47 Lee PT, Chou KJ, Liu CP, Mar GY, Chen CL, Hsu CY, et al. Renal protection for coronary angiography in advanced renal failure patients by prophylactic hemodialysis. A randomized controlled trial. J Am Coll Cardiol. 2007;50:1015-1020.
48 Frank H, Werner D, Lorusso V, Klinghammer L, Daniel WG, Kunzendorf U, Ludwig J. Simultaneous hemodialysis during coronary angiography fails to prevent radiocontrast-induced nephropathy in chronic renal failure. Clin Nephrol. 2003;60:176-182.
49 Vogt B, Ferrari P, Schonholzer C, Marti HP, Mohaupt M, Wiederkehr M, et al. Prophylactic hemodialysis after radiocontrast media in patients with renal insufficiency is potentially harmful. Am J Med. 2001;111:692-698.
50 Sterner G, Frennby B, Kurkus J, Nyman U. Does post-angiographic hemodialysis reduce the risk of contrast-medium nephropathy? Scand J Urol Nephrol. 2000;34:323-326.
51 Marenzi G, Marana I, Lauri G, et al. The prevention of radiocontrast-agent-induced nephropathy by hemofiltration. N Engl J Med. 2003;349:1333-1340.
52 Marenzi G, Lauri G, Campodonico J, Marana I, Assanelli E, De Metrio M, et al. Comparison of two hemofiltration protocols for prevention of contrast-induced nephropathy in high-risk patients. Am J Med. 2006 Feb;119(2):155-162.
53 Weisberg LS, Kurnik PB, Kurnik BRC. Risk of radiocontrast nephropathy in patients with and without diabetes mellitus. Kidney Int. 1994;45:259-265.
54 Weinstein J-M, Heyman S, Brezis M. Potential deleterious effect of furosemide in radiocontrast nephropathy. Nephron. 1992;62:413-415.
55 Solomon R, Werner C, Mann D, et al. Effects of saline, mannitol, and furosemide on acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416-1420.
56 Stevens MA, McCullough PA, Tobin KJ, et al. A prospective randomized trial of prevention measures in patients at high risk for contrast nephropathy. J Am Coll Cardiol. 1999;33:403-411.
57 Majumdar SR, Kjellstrand CM, Tymchak WJ, Hervas-Malo M, Taylor DA, Teo KK. Forced euvolemic diuresis with mannitol and furosemide for prevention of contrast-induced nephropathy in patients with CKD undergoing coronary angiography: a randomized controlled trial. Am J Kidney Dis. 2009;54:602-609.
58 Khoury Z, Schlicht JR, Como J, et al. The effect of prophylactic nifedipine on renal function in patients administered contrast media. Pharmacotherapy. 1995;15:59-65.
59 Stone GW, McCullough PA, Tumlin JA, et al. Fenoldopam mesylate for the prevention of contrast-induced nephropathy: a randomized controlled trial. JAMA. 2003;290:2284-2291.
60 Kurnik BR, Allgren RL, Genter FC, et al. Prospective study of atrial natriuretic peptide for the prevention of radiocontrast-induced nephropathy. Am J Kidney Dis. 1998;31:674-680.
61 Sketch MH, Whelton A, Schollmayer E, et al. Prevention of contrast media-induced renal dysfunction with prostaglandin E1: a randomized, double-blind, placebo-controlled study. Am J Ther. 2001;8:155-162.
62 Wang A, Holcslaw T, Bashore TM, et al. Exacerbation of radiocontrast nephrotoxicity by endothelin receptor antagonism. Kidney Int. 2000;57:1675-1680.
63 Morikawa S, Sone T, Tsuboi H, et al. Renal protective effects and the prevention of contrast-induced nephropathy by atrial natriuretic peptide. J Am Coll Cardiol. 2009;53:1040-1046.
64 Gupta RK, Kapoor A, Tewari S, et al. Captopril for prevention of contrast-induced nephropathy in diabetic patients: a randomised study. Indian Heart J. 1999;51:521-526.
65 Toprak O, Cirit M, Bayata S, Yesil M, Aslan SL. [The effect of pre-procedural captopril on contrast-induced nephropathy in patients who underwent coronary angiography] [Article in Turkish]. Anadolu Kardiyol Derg. 2003 Jun;3(2):98-103.
66 Spargias K, Alexopoulos E, Kyrzopoulos S, et al. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation. 2004;110:2837-2842.
67 Jo SH, Koo BK, Park JS, et al. N-acetylcysteine versus ascorbic acid for preventing contrast-induced nephropathy in patients with renal insufficiency undergoing coronary angiography: NASPI study—a prospective randomized controlled trial. Am Heart J. 2009;157:576-583.
68 Jo SH, Koo BK, Park JS, et al. Prevention of radiocontrast medium–induced nephropathy using short-term high-dose simvastatin in patients with renal insufficiency undergoing coronary angiography (PROMISS) trial—a randomized controlled study. Am Heart J. 2008;155:499.e1-499.e8.
69 Li G, Li Yin L, Liu T, et al. Role of probucol in preventing contrast-induced acute kidney injury after coronary interventional procedure. Am J Cardiol. 2009;103:512-514.
70 Xinwei J, Xianghua F, Jing Z, Xinshun G, Ling X, Weize F, et al. Comparison of usefulness of simvastatin 20 mg versus 80 mg in preventing contrast-induced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol. 2009;104:519-524.
71 Onbasili AO, Yeniceriglu Y, Agaoglu P, Karul A, Tekten T, Akar H, Discigil G. Trimetazidine in the prevention of contrast-induced nephropathy after coronary procedures. Heart. 2007;93:698-702.
72 Bagshaw SM, Ghali WA. Theophylline for prevention of contrast-induced nephropathy. Arch Intern Med. 2005;165:1087-1093.
73 Barrett JB, Parfery PS. Preventing Nephropathy induced by contrast medium. N Engl J Med. 2006;354:379-386.
74 Morcos SK, Thomsen HS, Webb JA. Dialysis and contrast media. Contrast Media Safety Committee of the European Society of Urogenital Radiology (ESUR). Eur Radiol. 2002;12:3026-3030.