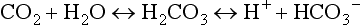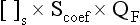Continuous Renal Replacement Therapy
Acute kidney injury (AKI) is common and serious. The incidence of AKI in hospitalized patients ranges from 5% to 7% and is rising rapidly.1–4 In a multinational study of critically ill patients, the prevalence of AKI requiring dialysis was 5.7% with a mortality rate of 60.3%.5 In addition, patients with AKI have a higher risk for developing other nonrenal comorbid conditions,6 and when present in conjunction with other conditions, AKI is associated with higher mortality rate.7–9 The use of renal replacement therapy (RRT) for treatment of AKI has been ongoing over the last 60 years.10 According to Hoste and Schurgers, 200 to 300 patients per 1 million population per year develop AKI and are treated with RRT.11 Despite this, these patients still have a mortality rate of 50% to 60%.11Advances and optimization of RRT could benefit the high mortality rate associated with AKI.
The establishment of continuous renal replacement therapy (cRRT) evolved as a treatment for the hemodynamically unstable patient unable to undergo standard intermittent hemodialysis (IHD). Although world trends for cRRT use show an increase in utilization, the majority of the world continues to treat AKI with IHD.12 Although cRRT offers many theoretical advantages such as better fluid balance, hemodynamic management, and renal recovery, the superiority of cRRT over IHD for RRT in the intensive care unit (ICU) remains controversial.13
Physiologic Principles
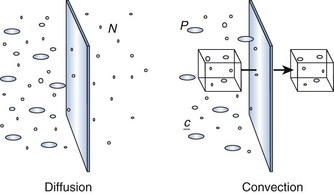
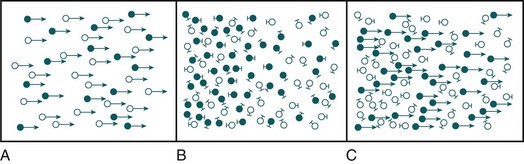
Dialysis uses a semipermeable membrane to alter the molecular composition and concentration of blood to restore the body back toward homeostatic balance. Blood flows along one side of the semipermeable membrane and a wash solution, dialysate, flows on the opposite side of the membrane (Fig. 18.1). Dialysis relies upon two physical forces—diffusion and convection—either in isolation or in combination (Fig. 18.2). In diffusion, the net movement of solute is directly dependent on the diffusivity of the solute and solvent, permeability of the membrane, surface area across the membrane, and concentration gradient. Other membrane characteristics also play a role: thickness, pore size, and electrostatic charge. In order to maximize the concentration gradient between the blood and dialysate, the dialysate runs countercurrent to the flow of blood. Any substance that is in higher concentration in the blood than the dialysate flows “down” its concentration gradient and leaves the blood and flows into the dialysate. Conversely, any solute that is in higher concentration in the dialysate (e.g., bicarbonate) will leave the dialysate, cross the semipermeable membrane, and enter the blood. The movement of molecules down their concentration gradient from one solution to another continues until equilibrium is achieved in both the blood and dialysate. Diffusion is more efficient in the clearance of small-molecular-weight substances (less than 500 daltons [D]). This is particularly useful in correcting the imbalance in small molecule electrolytes (e.g., K, Ca, Mg, PO4) (see Fig. 18.1). Thus, thoughtful manipulation of dialysate allows the clinician to decide what will be removed from the blood and what will be added to the blood during a dialysis session.
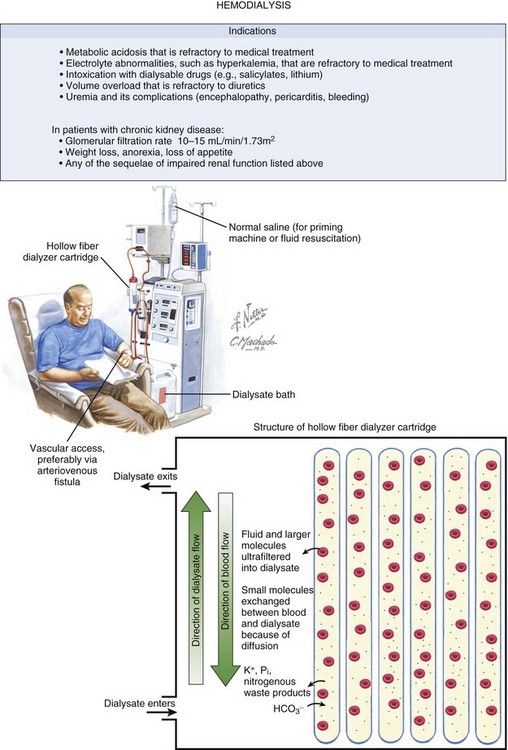
Figure 18.1 Hemodialysis and the filter at a microscopic level. Netter illustration from www.netterimages.com @ Elsevier Inc. All rights reserved. (Netter Plate 10-9 Membrane and Dialysis).
In convection, solutes move across a membrane in response to or following solvent flux or drag: solutes are moving along with the solvent containing them (Fig. 18.3). This is similar to a wave pushing seashells onto the shore. Solvent drag is limited only by the pore size or electrostatic charge of any semipermeable membrane that is applied across the passage of the solution. In convection, the concentration of a solute is similar on either side of the membrane. Convection is more efficient at the clearance of large-molecular-weight substances (500-5000 D). Convective removal of plasma water from blood across a large-pore, semipermeable membrane results in an ultrafiltrate with a solute composition equivalent to plasma water.
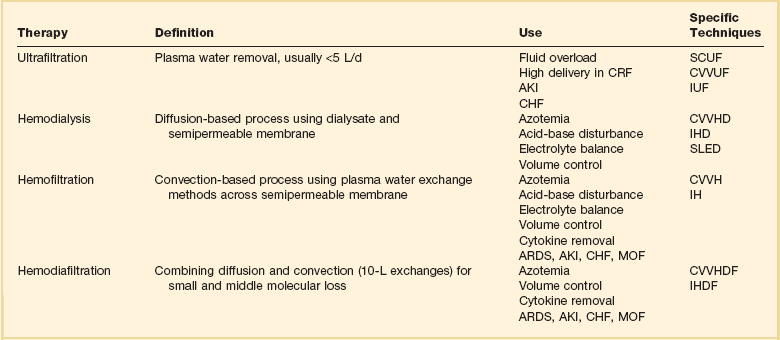
Fluid removal is termed ultrafiltration (UF). UF utilizes hydrostatic pressure, which is applied across a semipermeable membrane. This is a form of convective removal of solute. The clearance of molecules in UF is dependent on the volume of fluid removed. It may be applied in isolation (in volumes usually <5 L/day) or in combination with other blood clearance techniques, such as dialysis. Table 18.1 reviews the commonly used terms in cRRT.
One other form of clearance of the blood is through a process called adsorption. This refers to molecules in the blood sticking or adhering to the semipermeable membrane. This process is dependent on the molecules contained in the blood and the composition of the semipermeable membrane. Adsorption is usually time dependent (i.e., as the semipermeable membrane is used over time, it will become saturated with a given molecule). The process begins anew when the membrane is changed (approximately every 72 hours). Some inflammatory cytokine clearance occurs through this process.14
Modalities
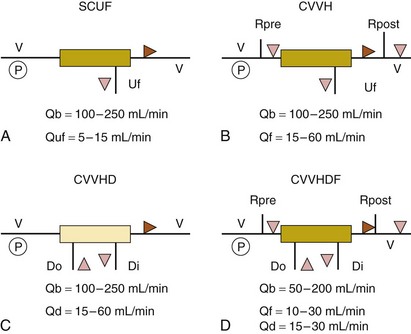
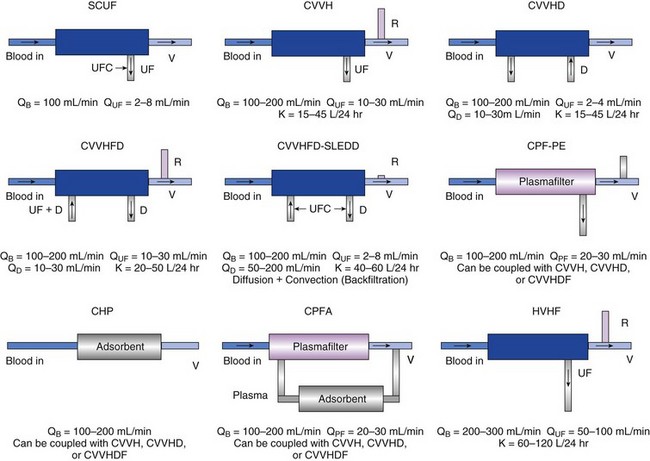
The various modalities of cRRT are depicted in Table 18.1 and Figure 18.4.
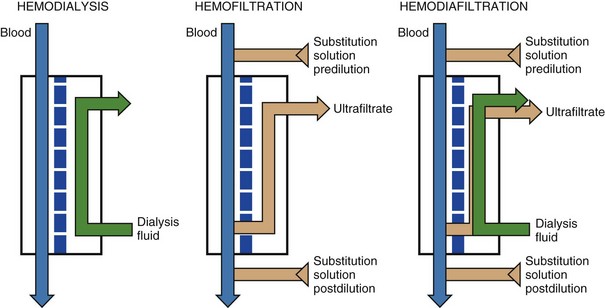
Dialysis may also be performed on an intermittent basis (IHD or sustained low-efficiency dialysis [SLED]) (see Fig. 18.1) or a continuous basis (Figs. 18.4 and 18.5). When performed continuously, this is known as continuous venovenous hemodialysis (CVVHD). The “venovenous” refers to the access employed and will be discussed in a later section. As described earlier, this is a diffusion-based process that primarily provides small molecule clearance.
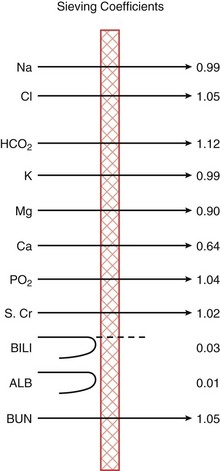
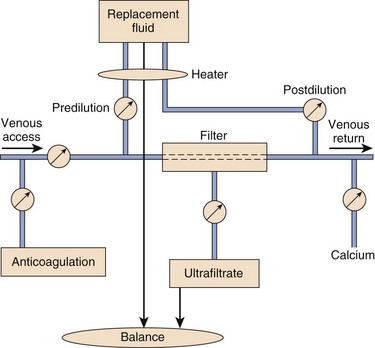
Hemofiltration (HF) relies on convective removal of plasma solute, in high fluid volumes, across a semipermeable membrane (see Fig. 18.5). Hydrostatic pressure is applied across the semipermeable membrane as a positive pressure on the blood side of the membrane or a negative pressure on the fluid collection side, or both. Fluid lost through this process is restored with replacement fluid in either a predilutional mode (before the filter containing the semipermeable membrane) or in a postdilutional mode (after the filter). The composition of the effluent fluid created by this system of plasma water exchange (hemofiltrate) depends on the membrane sieving coefficient for that particular solute and that particular semipermeable membrane. The sieving coefficient is expressed in terms of the ratio of the solute concentrations of the hemofiltrate to the plasma and is a function of membrane thickness, pore size, and electrostatic charge (Fig. 18.6). Hemofiltration is more efficient in middle-molecular-weight compound clearance, but less so for smaller solutes. When performed on a continuous basis, this is known as continuous venovenous hemofiltration (CVVH).
Finally, hemodiafiltration (HDF) combines both diffusive and convective solute removal (see Fig. 18.5). When performed continuously, this is known as continuous venovenous hemodiafiltration (CVVHDF). Dialysate is used in this configuration and runs countercurrent to the blood. Concurrently, replacement solution is infused either prefilter or postfilter. This allows for both efficient low-molecular-weight and enhanced middle-molecular-weight clearances. This modality has been employed for sepsis and multiorgan failure, in which removal of cytokine mediator substances is thought to be important.15
Figure 18.4 illustrates the mechanics of each of these modalities. Any modality may be described according to its frequency (I [intermittent] versus C [continuous]) and technique (H or HF [hemofiltration], HD, HDF, or UF) as shown in Table 18.2. To date, no one modality has been shown to be superior to the others. Continuous techniques are additionally described according to their vascular access: arteriovenous (AV) or venovenous (VV) (see later discussion under “Access”). Much debate has occurred regarding the superiority of intermittent therapies versus the continuous therapies. To date, the continuous therapies have not been shown to be superior in clinical outcomes to the intermittent therapies. Although the intermittent therapies (IHD and SLED) may be less costly, the continuous modalities allow for hemodynamic stability, enhanced fluid removal, and delivered dose of dialysis.16
Principles of Hemofiltration
Postdilutional fluid replacement has the advantage of being easier to perform, with lower rates of fluid exchange compared with the predilutional system. One problem with this form of replacement is the increased oncotic pressures at the venous end of the filter. With high rates of exchange or high degrees of access recirculation, blood viscosity may be increased to the extent that clotting may occur. Therefore, fluid exchange rates may be dictated by such factors as hematocrit, blood flow, and access recirculation. Table 18.3 compares predilutional and postdilutional CVVH.
Table 18.3
| Feature | Predilution | Postdilution |
| Clearance | Less clearance per milliliter | High small solute clearance per milliliter |
| Efficiency | Reduced efficiency by 10-15% and reduced filtration fraction | |
| Ultrafiltration rate limitations | Ultrafiltration rate not limited | Ultrafiltration rate limited by blood pressure and hemoconcentration |
| Blood viscosity | Low viscosity of the blood | High viscosity of the blood and increased risk of clotting |
Principles of Hemodialysis
In CVVHD, dialysate flow rates remain the most influential factor in determining urea clearance.17 Dialysate usually is delivered via pumps at rates of 15 to 40 mL per minute. Given adequate blood flow through the circuit, one can see why the limiting factor for flow-dependent transfer is the relatively low dialysate flow rate. Blood flow and filter membrane have limited effects on the diffusion of molecules compared with the potential of dialysate flow changes.
Continuous Renal Replacement Therapy Versus Intermittent Therapy
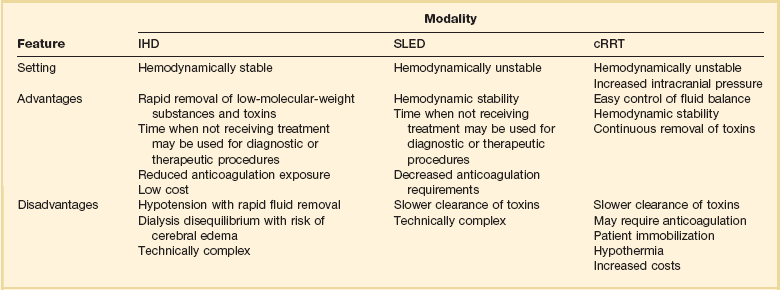
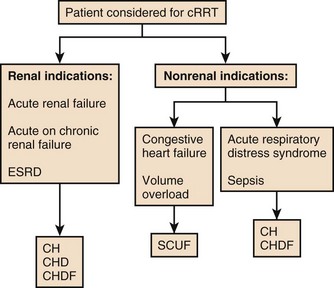
The clinical presentation and circumstances may favor either intermittent or continuous therapies (Table 18.4). There are many theoretical benefits that may favor the use of cRRT over intermittent forms of therapy, such as improved hemodynamic stability, faster resolution of fluid overload, and increased dialysis dose delivery (Table 18.5). Box 18.1 lists some nonrenal indications for using cRRT. However, clinical trials demonstrating an evidence-based assessment of these potential advantages are still lacking.
Table 18.4
Clinical Considerations in Continuous versus Intermittent Renal Support in the Intensive Care Unit
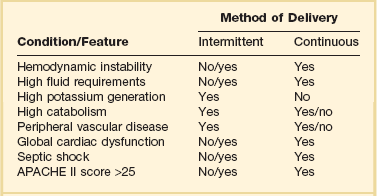
Definitive data to support many of the suspected advantages of cRRT are still lacking. In fact, Box 18.2 lists some of the disadvantages of cRRT. Interpretation of much of the published data has been hampered by retrospective analysis, the use of historical control groups, incomplete randomization, incomplete descriptions of patient populations and dialysis dose delivery, and study group–control group heterogeneity.
In the absence of a solid evidence base, how should one decide between prescribing continuous or intermittent therapy? The basic indications for delivering renal support remain unchanged (Box 18.3) and range from the most frequent request for fluid balance to more esoteric requirements such as toxin removal. Common considerations in choosing to apply intermittent or continuous support are listed in Table 18.3, being mindful of numerous relative advantages and disadvantages of each (see Table 18.5).
Continuous Hemodialysis Versus Continuous Hemofiltration
The influence of the dialysate rate on dialyzer clearance is depicted in Figure 18.7. The higher the rate, the more effective the urea clearance, until one reaches a rate approximating the effective plasma flow of the system. The stability of continuous therapies lies in their low rates of exchange over a longer period. The increase in clearance rates must be balanced against the desire to provide stable therapy. If one desires a high rate of removal in a short time, then an intermittent therapy should be employed.
Figure 18.7 depicts an approach to determining the modality of cRRT based on the patient presentation.
Prescription Variables
Prescribed Versus Delivered Dose
It is recommended that the prescription of dialysis should exceed that which is calculated to be adequate because there is a difference between what is prescribed and what is actually delivered. In a number of cases the delivered dose of dialysis is much smaller than what is prescribed.18,19 This can be due to interruptions in treatment due to hemodynamic instability, patient testing, circuit clotting, access recirculation, or other reasons why a patient may need to be disconnected from a machine.
Technical Considerations
Circuits and Material
Access
Several venous catheters are available; the double-lumen design is the most popular because of ease of insertion and good flow characteristics (Figs. 18.8 and 18.9).20 Catheter failure, either from poor flow or clotting, is the most common cause of therapy underdelivery.21 System clotting and resistance to blood flow are some of the most frequent manifestations of catheter failure.
The most common sites for dialysis catheter placement are the femoral and internal jugular approaches. Femoral access requires the patient to remain in bed with no more than a 30-degree bend between trunk and leg.22 The internal jugular and subclavian approaches allow for mobilization, but carry with them the risk of pneumothorax or other intrathoracic trauma during placement. Femoral catheters shorter than 20 cm from hub to tip are associated with higher degrees of access recirculation. Catheters at least 24 cm in length may produce improved flow rates, presumably because their tip reaches the inferior vena cava.23 Use of the subclavian vein also includes the long-term risk of subclavian venous stenosis with repeated access and should be avoided. If a patient is to require an AV fistula in the future for end-stage renal disease, subclavian stenosis will delay maturation of the AV fistula placed on the same side.
Fluids
Solutions of fluid take the form of either dialysate or replacement fluid. When hemofiltration is performed, replacement fluid composition dictates the resultant concentration of electrolytes. Similarly, knowing the composition of dialysate is important to determine to what equilibrium the electrolytes will settle. The sieving coefficients for relevant electrolytes and blood components are listed in Figure 18.6. Elements with a negative charge that are small enough to cross the membrane do so at greater than unity. This apparent active transport is actually accomplished through a dynamic process similar to the Gibbs-Donnan effect seen in stagnant fluid balance. Because negatively charged proteins are unable to cross the membrane, chloride and bicarbonate move against a concentration gradient to maintain electrical neutrality. The exaggerated loss of these elements must be reflected in the replacement solution used.
Most fluids either contain lactate or bicarbonate at the base buffer. Other substances have also been considered, for example, the use of acetate or citrate. The use of citrate has an added advantage of being an anticoagulant. Acetate, although previously used extensively in long-term HD, may give rise to vasodilation, myocardial depression, and increased oxygen consumption.24 As a result acetate should be avoided for cRRT.
Lactate undergoes hepatic conversion to bicarbonate on an equimolar basis. Although more stable than bicarbonate, there are numerous theoretical disadvantages to its use in cRRT. Although bicarbonate-based and lactate-based solutions can correct acidosis, bicarbonate is preferred because lactate-buffered fluids may require intravenous bicarbonate supplementation to achieve the same bicarbonate level.25 The metabolism of exogenous lactate may be impaired in critical illness, with accumulation giving rise to a paradoxic metabolic acidosis.26 Hyperlactatemia carries with it potential negative hemodynamic effects and metabolic complications, including increased protein catabolism and reduced adenosine triphosphate regeneration.27 Complications of lactate overload are more likely to develop in patients with liver impairment and poor peripheral perfusion, particularly with high-volume treatment. Lactate intolerance is defined arbitrarily as a greater than 5 mmol/L increase in lactate levels during therapy.
When CO2 outgassing from the solution occurs (e.g., delivering bicarbonate using an open-top container), overall bicarbonate concentration may be reduced. Additionally, calcium and magnesium can precipitate out as insoluble carbonate compounds when sterilized with the buffer. Many bicarbonate-based solutions are produced with lower concentrations of both cations to help ameliorate this problem, with final mixing of the electrolyte and bicarbonate solutions just before use. A novel adaptation has electrolyte and bicarbonate solutions housed and sterilized in separate chambers of the same bag. A connecting valve is broken just before use to mix the two fluids. A final caveat to the use of bicarbonate is its apparent predilection to bacterial growth—at least in liquid bicarbonate concentrates used in long-term dialysis.
Anticoagulation
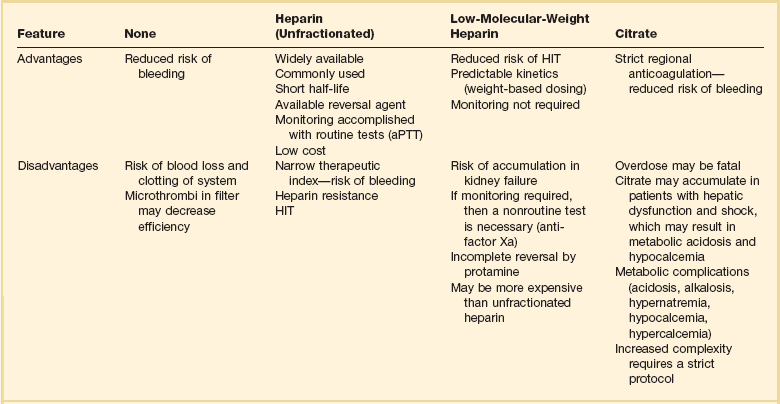
Circuit clotting is the most frequent cause of therapy interruption in cRRT. Various factors may account for the hypercoagulable state in a patient receiving cRRT.28 Anticoagulation during cRRT is necessary to preserve the life of the extracorporeal circuit, to maximize the cRRT dose, and to minimize blood loss caused by clotting during cRRT. The ideal anticoagulant should have no effect on systemic hemostasis, no increase in hemorrhagic risk, only be limited to the extracorporeal circuit, optimize filter performance in circuit life, have a short half-life, be easily monitored, be easily reversible, and be inexpensive.29 Options for anticoagulation of the circuit include using no anticoagulation, unfractionated heparin, low-molecular-weight heparin, citrate, and in rare circumstances thrombin antagonists or prostaglandins. Box 18.4 lists current strategies to prevent circuit clotting. According to a world survey, 44% of those surveyed preferred unfractionated heparin for anticoagulation.30
The option of no anticoagulation is usually used in patients with intrinsic coagulopathies such as hepatic failure or low platelet count. Circuits are usually primed with saline or heparin. Intermittent normal saline flushes may be used as well. The rates of filter clotting using this method vary widely. The mean filter life lies between 16 and 70 hours if the patient is coagulopathic. In cases of severe coagulopathy, shorter filter life may be seen. The disadvantages of no anticoagulation include the need for increased UF, the risk of dialyzer fiber rupture, and the extra nursing workload.31 The hemoconcentration induced by high UF volumes in CVVH and CVVHDF promotes clotting but may be minimized by predilutional fluid replacement. This comes at the price, however, of the inefficiency of ultrafiltering a mixture of just-infused replacement fluid and plasma, the proportions of which are important considerations in the cRRT prescription.
HIT may develop during heparin therapy.32 HIT begins with heparin exposure stimulating the formation of heparin-platelet factor 4 antibodies, usually 5 to 12 days after starting therapy. This triggers the release of procoagulant platelet particles. Both thrombosis and thrombocytopenia ensue and cause significant vascular complications. The prevalence of HIT varies among several subgroups, with greater incidence in surgical as compared with medical populations. HIT must be acknowledged for its intense predilection for thrombosis and suspected whenever thrombosis occurs after heparin exposure. In cRRT, this is manifested as recurrent filter clotting. The treatment of HIT mandates the cessation of all heparin exposure and the institution of an antithrombotic therapy, most commonly using a direct thrombin inhibitor. Current “diagnostic” tests, which primarily include functional and antigenic assays, have more of a confirmatory than diagnostic role in the management of HIT. Platelet aggregation studies are highly specific but lack sensitivity, so if they and heparin-induced platelet activation tests yield negative results, an enzyme-linked immunosorbent assay should be performed. Direct thrombin inhibitors are appropriate, evidence-based alternatives to heparin in patients with a history of HIT.
Direct thrombin inhibitors such as recombinant hirudin (lepirudin), danaparoid, and argatroban have been used in response to HIT.33,34 Bleeding resulting from overdosage of lepirudin or argatroban may be treated with the administration of fresh-frozen plasma. Hemofiltration with high-flux dialyzers also can reduce the plasma levels of hirudin.
Citrate regional anticoagulation works by chelating free calcium in the extracorporeal circuit and prevents the activation of calcium-dependent procoagulants. The anticoagulant effect of citrate is measured by ionized calcium levels. Anticoagulation is reversed by a calcium infusion. Normal blood levels of citrate are approximately 0.05 mmol/L. The bleeding time for a patient with citrate levels of 4 to 6 mmol/L can be infinite. Levels of 12 to 15 mmol/L are required for storing blood products for transfusion therapy. Citrate levels are usually performed in another facility or reference laboratory. Citrate has a plasma half-life of 5 minutes and is rapidly metabolized by the liver, kidney, and muscle cells. The extracorporeal clearance of citrate is the same as that of urea. The sieving coefficient ranges between 0.87 and 1.0. The clearance of citrate is the same for both CVVH and CVVHD.35 The advantage of using citrate is that it is regional and avoids bleeding complications. It also doubles as a buffer. It is more effective than heparin in studies,36,37 and it does not cause thrombocytopenia. Two early trials have shown that the use of citrate for regional anticoagulation yields no additional bleeding risk and leads to longer filter life.36,37 The largest citrate randomized controlled trial used citrate for anticoagulation in a postdilutional CVVH modality with blood flows of 220 mL per minute and citrate concentration of 3 mmol/L.38 The study showed a lower mortality rate in patients receiving citrate regional anticoagulation. It is hypothesized that citrate inhibits leukocytes esterase, which may yield immunologic benefits. The major disadvantage of citrate stems from its metabolic complications and the complex protocols that are involved in its administration. Metabolic consequences of citrate use include metabolic alkalosis from citrate overdose or toxicity, metabolic acidosis in a setting of severe liver disease or hypoperfusion, hypernatremia from hyperosmolar citrate solutions (4% sodium citrate), and hypocalcemia and hypercalcemia from inappropriate calcium supplementation. The risk factors for citrate toxicity include liver disease, nursing or pharmacy errors leading to overdose, shock liver, and severe hypoperfusion states. The detection of citrate toxicity then becomes extremely important. One should expect citrate toxicity when noticing a rising anion gap, worsening metabolic acidosis, a falling systemic ionized calcium, escalating calcium infusion requirements, or a total calcium/systemic ionized calcium ratio greater than 2.5 : 1.39 Strategies to manage citrate toxicity include decreasing the citrate infusion rate, decreasing the blood pump speed, and increasing the dialysate flow rate.
The decision as to which citrate protocol to use depends on the available citrate solutions, the method of citrate delivery, and the cRRT circuit options. Available commercial citrate solutions include concentrations of sodium citrate between 1.32% and 4%. Regional citrate anticoagulation may be performed using 4% trisodium citrate or with ACD-A solution (anticoagulant citrate dextrose solution, form A) containing 3% combined trisodium citrate (2.2 g/100 mL), citric acid (0.73 g/100 mL), and dextrose (2.45 g/100 mL) (Baxter-Fenwal Healthcare Corp., Deerfield, IL). ACD-A solution is preferred over trisodium citrate for routine regional citrate anticoagulation because it is less hypertonic and commercially prepared, potentially reducing mixing errors and the complications associated with overinfusion. Regional citrate anticoagulation protocols differ in the type of citrate preparation used, the mode of dialysis, and the ability to customize dialysis solutions. There is a fixed relationship between the blood flow in citrate delivery. The titration of citrate delivery should be based on the ionized calcium level. The amount of citrate delivered to achieve a blood citrate concentration of 4 mmol/L depends on the blood flow.40
In a typical circuit (Fig. 18.10) arterial blood leading from the patient is first infused with citrate, which chelates the free ionized calcium. This blood then enters the filter where a calcium-free dialysate is used. Postfilter ionized calcium is monitored and used to titrate the citrate rate to ensure anticoagulation. The goal is to keep the ionized calcium at less than 0.35 mmol/L. The returning blood combines with the venous blood in the body, which normalizes the ionized calcium and prevents systemic anticoagulation. Calcium is infused through a separate central line to replace the calcium lost in the ultrafiltrate. Citrate is metabolized primarily in the liver to bicarbonate and the bound calcium is released. For CVVH, citrate may or may not be a component of the replacement fluid.
A number of citrate protocols have been published. Protocols vary based on the number of fluid solutions utilized and commercial versus hospital specific fluid options. Published protocols include those from Massachusetts General Hospital,41 Gainesville,42 University of Alabama at Birmingham,43 Sunnybrook,44 and San Diego.45
Low-molecular-weight heparins (such as enoxaparin, dalteparin, and nadroparin) have also been examined for their effect on circuit life.46 Prostacyclin (prostaglandin I2), a potent, short-acting, endogenous inhibitor of platelet aggregation, has also been studied.47
Clinical experience with various agents and strategies influence choice. Table 18.6 reviews the advantages and disadvantages of each anticoagulation option.
Therapeutic Considerations
Patient Selection
The question as to whether a patient needs renal replacement therapies is often difficult. There is no consensus as to the indication to start RRT, the criteria to start RRT, or the appropriate time for initiation of RRT. The conventional indications include volume overload, metabolic acidosis, hyperkalemia, uremia, azotemia without uremic manifestations, and drug overdose. The risks of dialysis include hypotension, complications of placing vascular access, and air embolism (extremely rare). Additional risks and complications are listed in Box 18.5 Patients in the ICU may have a number of conditions that impact the decision to initiate and prescribe RRT. In general, if patients have insufficient renal function, cRRT can be utilized to provide organ support much in the way that the ventilator is used to provide pulmonary support. Potential indications for renal support include nutrition, fluid removal in CHF, cancer chemotherapy, the treatment of respiratory acidosis in acute respiratory distress syndrome (ARDS), and fluid management in multiorgan failure.48
Pharmacokinetics During Continuous Renal Replacement Therapy
Body clearance of drugs without significant tubular secretion or reabsorption is a linear function of creatinine clearance. Estimation of drug clearance by dialytic modalities is a more complex proposition and has to take account of dialysate and blood flow rates; the molecular weight of the drug; and membrane surface area, thickness, and composition. Nomograms used in the prediction of dialytic drug clearance may be used,49 but these provide only a general guideline. The impact of molecular weight differs between dialytic techniques and may be lower in CVVHD, which usually uses high flux membranes at a low dialysate flow rate (QD). Under these circumstances, the QD may approximate QF in the previous equations.50 Drug clearance during CVVHDF may be estimated by combining calculations of filtrative removal with calculations of dialytic removal, but the complex interplay between convection and diffusion is not fully appreciated by this approach.
A final confounder of pharmacokinetic predictability is the impact of membrane adsorption of the drug, which may be substantial with PAN/AN69 materials and may vary depending on the frequency of filter changes. Such filters that remain in situ for long enough can start to release their adsorbed drug back into the circulation. In adjusting dosing of a drug that is significantly cleared by cRRT, a choice must be made either to shorten the dosing interval (to maintain plasma levels) or to increase the dose (to optimize peak concentrations) depending on that agent’s mode of action. Formulas have been developed to estimate adjustments of dose and dosing interval.50,51
Complications of Therapy
Perhaps the most frequent problems encountered are electrolyte derangements (Table 18.7). With the use of hemofiltration, as we have seen earlier, the replacement solution determines the final electrolyte outcome. The replacement solution must reflect the sodium, chloride, and bicarbonate concentrations that one would like to achieve in patient serum and the relative loss via the hemofilter. The substance’s particular sieving coefficient can be used to determine therapeutic losses, following the formula:
where [ ]s is the incoming blood concentration of the substance, Scoef is its sieving coefficient, and QF is the UF rate (see Fig. 18.6). Not only the actual replacement fluid, but all fluid must be taken into consideration. Frequently, drug vehicles with water or hypotonic hyperalimentation solutions are calculated in the fluid exchange, but not in the electrolyte balance. The resultant loss of sodium (in the hemofiltrate) and the replacement with hypotonic solution produce a true hyponatremia. The exaggerated loss of bicarbonate and chloride also produces variations if these balances are not considered in the total composition delivered to the patient.
Table 18.7
Common Electrolyte Problems During Continuous Renal Replacement Therapy with Their Solutions
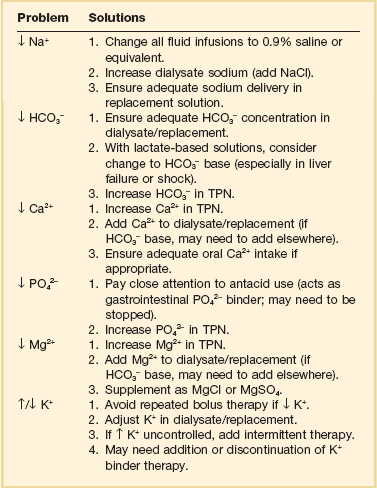
Continuous HD procedures also require frequent electrolyte monitoring. Serum values generally reflect the dialysate concentration of that particular solute. Establishing a potassium floor of 4 mEq/L merely requires that the dialysate concentration of potassium also be set at 4 mEq/L. Common electrolyte problems seen in patients receiving continuous therapies are listed in Table 18.7. Box 18.5 lists other possible complications.
Nonrenal Application of Continuous Therapies
The hemodynamic stability of continuous renal therapies has contributed to their use in situations in which fluid loss is desired but patient status has restricted more standard dialytic or other therapeutic interventions. The ability to manipulate a continuous extracorporeal blood circuit also has opened wide opportunities to assess a variety of different nonrenal applications (Fig. 18.11). The basic circuit has been incorporated into liver assist devices (currently undergoing clinical testing), and the ability to either warm or cool circulating blood has applications in clinical and experimental medicine. The adsorptive nature of different membrane types and structures also has been the focus for potential therapeutic interventions.
References
1. Nickolas, TL, Barasch, J, Devarajan, P. Biomarkers in acute and chronic kidney disease. Curr Opin Nephrol Hypertens. 2008; 17(2):127–132.
2. Hou, SH, Bushinsky, DA, Wish, JB, et al. Hospital-acquired renal insufficiency: A prospective study. Am J Med. 1983; 74(2):243–248.
3. Shusterman, N, Strom, BL, Murray, TG, et al. Risk factors and outcome of hospital-acquired acute renal failure. Clinical epidemiologic study. Am J Med. 1987; 83(1):65–71.
4. Nash, K, Hafeez, A, Hou, S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002; 39(5):930–936.
5. Uchino, S, Kellum, JA, Bellomo, R, et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005; 294(7):813–818.
6. Chertow, GM, Soroko, SH, Paganini, EP, et al. Mortality after acute renal failure: Models for prognostic stratification and risk adjustment. Kidney Int. 2006; 70(6):1120–1126.
7. Bellomo, R. The epidemiology of acute renal failure: 1975 versus 2005. Curr Opin Crit Care. 2006; 12(6):557–560.
8. Ympa, YP, Sakr, Y, Reinhart, K, Vincent, JL. Has mortality from acute renal failure decreased? A systematic review of the literature. Am J Med. 2005; 118(8):827–832.
9. Chertow, GM, Burdick, E, Honour, M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005; 16(11):3365–3370.
10. Scribner, BH, Buri, R, Caner, JE, et al. The treatment of chronic uremia by means of intermittent hemodialysis: A preliminary report. Trans Am Soc Artif Intern Organs. 1960; 6:114–122.
11. Hoste, EA, Schurgers, M. Epidemiology of acute kidney injury: How big is the problem? Crit Care Med. 2008; 36(4 Suppl):S146–S151.
12. Ishani, A, Xue, JL, Himmelfarb, J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009; 20(1):223–228.
13. Hoyt, DB. cRRT in the area of cost containment: Is it justified? Am J Kidney Dis. 1997; 30(5 Suppl 4):S102–S104.
14. De Vriese, AS, Colardyn, FA, Philippe, JJ, et al. Cytokine removal during continuous hemofiltration in septic patients. J Am Soc Nephrol. 1999; 10(4):846–853.
15. Page, B, Vieillard-Baron, A, Chergui, K, et al. Early veno-venous haemodiafiltration for sepsis-related multiple organ failure. Crit Care. 2005; 9(6):R755–763.
16. Parikh, A, Shaw, A. The economics of renal failure and kidney disease in critically ill patients. Crit Care Clin. 2012; 28(1):99–111.
17. Bellomo, R. Do we know the optimal dose for renal replacement therapy in the intensive care unit? Kidney Int. 2006; 70(7):1202–1204.
18. Venkataraman, R, Kellum, JA, Palevsky, P. Dosing patterns for continuous renal replacement therapy at a large academic medical center in the United States. J Crit Care. 2002; 17(4):246–250.
19. Vesconi, S, Cruz, DN, Fumagalli, R, et al. Delivered dose of renal replacement therapy and mortality in critically ill patients with acute kidney injury. Crit Care. 2009; 13(2):R57.
20. Tapson, JS, Hoenich, NA, Wilkinson, R, Ward, MK. Dual lumen subclavian catheters for haemodialysis. Int J Artif Organs. 1985; 8(4):195–200.
21. Vijayan, A. Vascular access for continuous renal replacement therapy. Semin Dial. 2009; 22(2):133–136.
22. Kim, IB, Fealy, N, Baldwin, I, Bellomo, R. Insertion side, body position and circuit life during continuous renal replacement therapy with femoral vein access. Blood Purif. 2011; 31(1-3):42–46.
23. Abidi, SM, Khan, A, Fried, LF, et al. Factors influencing function of temporary dialysis catheters. Clin Nephrol. 2000; 53(3):199–205.
24. Maccariello, E, Rocha, E, Dalboni, MA, et al. Customized bicarbonate buffered dialysate and replacement solutions for continuous renal replacement therapies: Effect of crystallization on the measured levels of electrolytes and buffer. Artif Organs. 2001; 25(11):870–875.
25. Kierdorf, HP, Leue, C, Arns, S. Lactate- or bicarbonate-buffered solutions in continuous extracorporeal renal replacement therapies. Kidney Int Suppl. 1999; 72:S32–S36.
26. Tan, HK, Uchino, S, Bellomo, R. The acid-base effects of continuous hemofiltration with lactate or bicarbonate buffered replacement fluids. Int J Artif Organs. 2003; 26(6):477–483.
27. Cole, L, Bellomo, R, Baldwin, I, et al. The impact of lactate-buffered high-volume hemofiltration on acid-base balance. Intensive Care Med. 2003; 29(7):1113–1120.
28. Bouman, CS, de Pont, AC, Meijers, JC, et al. The effects of continuous venovenous hemofiltration on coagulation activation. Crit Care. 2006; 10(5):R150.
29. Oudemans-van Straaten, HM, Fiaccadori, E, Baldwin, I. Anticoagulation for renal replacement therapy: Different methods to improve safety. Contrib Nephrol. 2010; 165:251–262.
30. Uchino, S, Bellomo, R, Morimatsu, H, et al. Continuous renal replacement therapy: A worldwide practice survey. The beginning and ending supportive therapy for the kidney (B. E. S. T. kidney) investigators. Intensive Care Med. 2007; 33(9):1563–1570.
31. Ramesh Prasad, GV, Palevsky, PM, Burr, R, et al. Factors affecting system clotting in continuous renal replacement therapy: Results of a randomized, controlled trial. Clin Nephrol. 2000; 53(1):55–60.
32. Hong, MS, Amanullah, AM. Heparin-induced thrombocytopenia: A practical review. Rev Cardiovasc Med. 2010; 11(1):13–25.
33. Gajra, A, Vajpayee, N, Smith, A, et al. Lepirudin for anticoagulation in patients with heparin-induced thrombocytopenia treated with continuous renal replacement therapy. Am J Hematol. 2007; 82(5):391–393.
34. Link, A, Girndt, M, Selejan, S, et al. Argatroban for anticoagulation in continuous renal replacement therapy. Crit Care Med. 2009; 37(1):105–110.
35. Chadha, V, Garg, U, Warady, BA, Alon, US. Citrate clearance in children receiving continuous venovenous renal replacement therapy. Pediatr Nephrol. 2002; 17(10):819–824.
36. Kutsogiannis, DJ, Gibney, RT, Stollery, D, Gao, J. Regional citrate versus systemic heparin anticoagulation for continuous renal replacement in critically ill patients. Kidney Int. 2005; 67(6):2361–2367.
37. Monchi, M, Berghmans, D, Ledoux, D, et al. Citrate vs. heparin for anticoagulation in continuous venovenous hemofiltration: A prospective randomized study. Intensive Care Med. 2004; 30(2):260–265.
38. Oudemans-van Straaten, HM, Bosman, RJ, Koopmans, M, et al. Citrate anticoagulation for continuous venovenous hemofiltration. Crit Care Med. 2009; 37(2):545–552.
39. Meier-Kriesche, HU, Gitomer, J, Finkel, K, DuBose, T. Increased total to ionized calcium ratio during continuous venovenous hemodialysis with regional citrate anticoagulation. Crit Care Med. 2001; 29(4):748–752.
40. Flanigan, MJ, Pillsbury, L, Sadewasser, G, Lim, VS. Regional hemodialysis anticoagulation: Hypertonic tri-sodium citrate or anticoagulant citrate dextrose-A. Am J Kidney Dis. 1996; 27(4):519–524.
41. Palsson, R, Niles, JL. Regional citrate anticoagulation in continuous venovenous hemofiltration in critically ill patients with a high risk of bleeding. Kidney Int. 1999; 55(5):1991–1997.
42. Munjal, S, Ejaz, AA. Regional citrate anticoagulation in continuous venovenous haemofiltration using commercial preparations. Nephrology (Carlton). 2006; 11(5):405–409.
43. Tolwani, AJ, Prendergast, MB, Speer, RR, et al. A practical citrate anticoagulation continuous venovenous hemodiafiltration protocol for metabolic control and high solute clearance. Clin J Am Soc Nephrol. 2006; 1(1):79–87.
44. Tobe, SW, Aujla, P, Walele, AA, et al. A novel regional citrate anticoagulation protocol for cRRT using only commercially available solutions. J Crit Care. 2003; 18(2):121–129.
45. Mehta, RL, McDonald, BR, Aguilar, MM, Ward, DM. Regional citrate anticoagulation for continuous arteriovenous hemodialysis in critically ill patients. Kidney Int. 1990; 38(5):976–981.
46. Tsang, DJ, Tuckfield, A, Macisaac, CM. Audit of safety and quality of the use of enoxaparin for anticoagulation in continuous renal replacement therapy. Crit Care Resusc. 2011; 13(1):24–27.
47. Davies, H, Leslie, G. Anticoagulation in cRRT: Agents and strategies in Australian ICUs. Aust Crit Care. 2007; 20(1):15–26.
48. Mehta, RL. Indications for dialysis in the ICU: Renal replacement vs. renal support. Blood Purif. 2001; 19(2):227–232.
49. Schetz, M. Drug dosing in continuous renal replacement therapy: General rules. Curr Opin Crit Care. 2007; 13(6):645–651.
50. Golper, TA. Update on drug sieving coefficients and dosing adjustments during continuous renal replacement therapies. Contrib Nephrol. 2001; 132:349–353.
51. Bohler, J, Donauer, J, Keller, F. Pharmacokinetic principles during continuous renal replacement therapy: Drugs and dosage. Kidney Int Suppl. 1999; 72:S24–S28.