Chapter 12 Congenital Pulmonary Valve Regurgitation
William Osler1 described pulmonary regurgitation in The Principles and Practice of Medicine (1892): “This rare affection is occasionally due to a congenital malformation, particularly fusion of the two segments. … The condition is extremely rare and of little practical significance.” The distinctive diastolic murmur of low-pressure pulmonary regurgitation was characterized in 19102; and in 1936, isolated congenital pulmonary valve regurgitation was reported with a review of the literature.3 Maude Abbott’s4 necropsy study of 1000 cases of congenital heart disease included two examples of isolated congenital pulmonary valve regurgitation. In 1955, the first clinical diagnosis was made in an asymptomatic 24-year-old medical student.5
The morphology of the congenitally malformed valve is variable.6–8 One, two, or three cusps may undergo faulty development9,10; all three cusps may be rudimentary9; one cusp may be absent11 and the other two rudimentary8; very rarely, the pulmonary cusps are dysplastic12; or no valve tissue may be found at all—congenital absence of the pulmonary valve13,14—as reported by Chevers15 in 1846. The anomaly seldom occurs in isolation16–20 but instead coexists with Fallot’s tetralogy21 (see Chapter 18) and sporadically with other malformations.8,22,23
A bicuspid pulmonary valve is rare as an isolated anomaly and is only occasionally incompetent. A quadricuspid pulmonary valve typically occurs with truncus arteriosus (see Chapter 28) and only rarely in isolation.24,25 If the four cusps are equal, the quadricuspid valve functions normally; but with cuspal inequality or a supernumerary cusp, the valve is rendered incompetent, especially if the pulmonary artery pressure is elevated.24
This chapter is concerned with pulmonary valve regurgitation as an isolated congenital malformation. Regurgitation associated with pulmonary hypertension and a structurally normal pulmonary valve is dealt with in Chapter 14 on primary pulmonary hypertension.
Significance of the diagnosis of pulmonary valve regurgitation must take into account the relatively high incidence of trivial to mild pulmonary regurgitation identified incidentally with color flow imaging and Doppler interrogation in individuals whose hearts are structurally normal.26,27 Prevalence rate from birth to age 14 years is nil under 1 year of age, with a peak incidence rate of 42% between 6 and 11 years of age.26
The mature right ventricle is a low-pressure low-resistance pump that readily adapts to augmented volume, although the degree and duration of regurgitant flow are important determinants of this response.7 The physiologic consequences of pulmonary regurgitation are especially dramatic in utero because systemic pressure in the pulmonary trunk augments regurgitant flow, more so with agenesis of the fetal ductus.9,19 Acquired elevation of pulmonary arterial pressure in adults with bronchopulmonary disease or left ventricular failure is analogous but less dramatic.28
History
Isolated congenital pulmonary valve regurgitation usually comes to light because of a murmur or because of a dilated pulmonary arterial trunk in a routine chest x-ray. The diagnosis tends to be delayed, however, because the murmur is not detected at birth9 and routine chest x-rays are seldom done before adulthood. Gender distribution is about equal.29
Clinical manifestations of isolated congenital pulmonary valve regurgitation fall into three categories. The first and largest category consists of asymptomatic children, adolescents, and young adults,6,29 who tolerate the anomaly through middle age30 and occasionally into the sixth or even eighth decade.31 Isolated congenital absence of the pulmonary valve has been reported at age 69 years32 and 73 years.18
The second and much smaller category consists of adults in whom right ventricular failure occurs after decades of stability.28,29 Severe pulmonary valve regurgitation per se can cause the right ventricle to fail,33 but moderate regurgitation produces little or no ill effect until the degree of regurgitant flow is increased by adult-acquired elevation in pulmonary artery pressure associated with bronchopulmonary disease33 or left ventricular failure.28 Infective endocarditis is a low-probability cause of increased regurgitation.
A third and rare category of congenital pulmonary valve regurgitation occurs in the fetus19 or neonate9,29 when elevated pulmonary arterial pressure augments the volume of regurgitant flow across a congenitally malformed or absent pulmonary valve.19,29In utero patency of the ductus is desirable because ductal agenesis increases resistance to right ventricular discharge and increases regurgitant flow. The converse is the case with ductal patency after birth, which adds to the burden of the right ventricle by allowing diastolic flow to proceed from the aorta through the ductus into the pulmonary artery and across the incompetent pulmonary valve into the right ventricle.17,19,29,34
Precordial movement and palpation
When pulmonary regurgitation is mild to moderate, the right ventricular impulse is gentle and is detected only in the subzyphoid area during held inspiration. Severe regurgitation that accompanies congenital absence of the pulmonary valve generates a hyperdynamic impulse at the left sternal border and subzyphoid area, in addition to an impulse in the second left intercostal space caused by systolic expansion of the dilated pulmonary trunk (see Figure 12-7).29,31
Auscultation
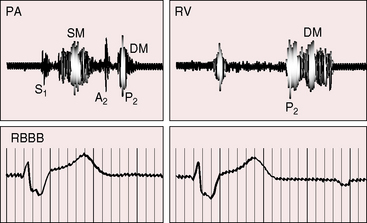
A normal first heart sound is occasionally followed by an ejection sound into the dilated pulmonary trunk. The pulmonary component of the second heart sound is absent when the leaflets are absent or rudimentary29,35 and soft when the cusps are present but defective, although audibility per se indicates the presence of valve tissue.36 The split tends to be wide because of a delay in the pulmonary component caused by increased capacitance of the pulmonary vascular bed and slow elastic recoil.36–38 The occasional occurrence of complete right bundle branch block also delays the pulmonary component of the second sound (Figure 12-1). Inspiration increases the degree of splitting unless the right ventricle has failed.36 Narrow splitting sometimes occurs because rapid right ventricular ejection coupled with brisk decline in the descending limb of the pulmonary artery pressure pulse cancels a potential delay in pulmonary valve closure.
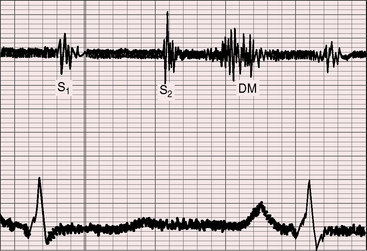
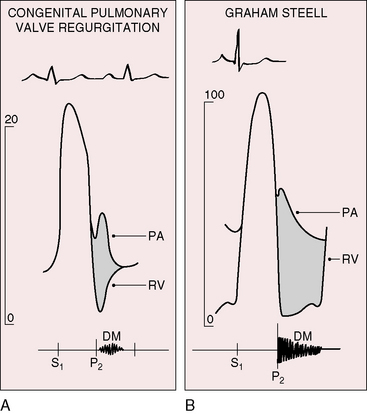
The hallmark of congenital pulmonary valve regurgitation is the distinctive diastolic murmur. The murmur of low-pressure low-velocity regurgitant flow is maximal in the second or third left intercostal space, medium-pitched to low-pitched, crescendo-decrescendo, delayed in onset, and short in duration; and it ends well before the subsequent first heart sound (Figures 12-1 and 12-2).29,30,33,39 The murmur begins immediately after the right ventricular and pulmonary arterial pressure pulses diverge in early diastole, and is loudest at the timing of an early diastolic dip when the diastolic gradient is maximal (Figure 12-3A).33,39 If the pulmonary component of the second heart sound is soft or absent, a silent interval exists between the aortic component and the onset of the diastolic murmur (see Figure 12-2).39 Equilibration of pulmonary arterial and right ventricular pressures in latter diastole reduces or eliminates the regurgitant gradient and eliminates the murmur (Figures 12-3A, 12-4, and 12-5).39 The normally low diastolic pressure in the pulmonary trunk is responsible for the low rate of regurgitant flow and the correspondingly low to medium pitch of the murmur.39 Conversely, maximal instantaneous diastolic flow velocity in pulmonary hypertensive pulmonary regurgitation is maintained at about the same signal strength throughout diastole, which is appropriate for the high-frequency holodiastolic Graham Steell murmur (Figure 12-3B).40 The velocity of low-pressure pulmonary regurgitation peaks in early diastole and is followed by a gradual decline toward end-diastole. Accordingly, the low-frequency to medium-frequency diastolic murmur ends well before the subsequent first heart sound (see Figure 12-3A). Mild congenital pulmonary regurgitation permits the diastolic pressure in the pulmonary trunk to remain higher than the diastolic pressure in the right ventricle, so the low-frequency to medium-frequency murmur extends throughout diastole (see Figure 12-5).
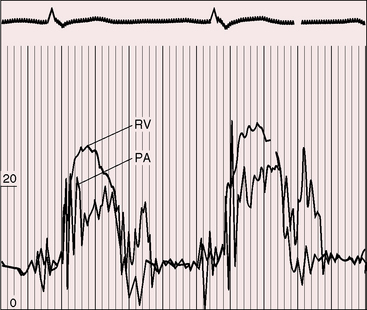
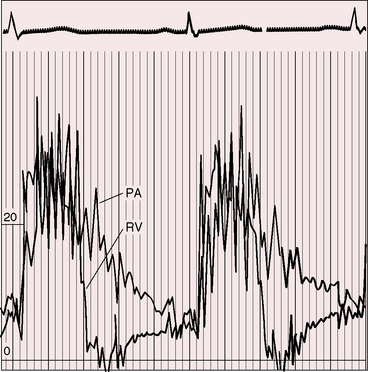
Figure 12-4 Right ventricular (RV) and pulmonary artery (PA) pressure pulses from an 18-year-old man with appreciable congenital pulmonary valve regurgitation. The regurgitant gradient appears immediately after the pressure pulses diverge and is rapidly abolished as the pressure pulses equilibrate (compare with Figure 12-3A).
Intensity of the diastolic murmur varies from grade 1/6 to 3/6, but exceptionally it is loud and rough enough to generate a thrill.30,31 During inspiration, the early diastolic dip in the right ventricular pressure pulse falls more rapidly than the diastolic pressure in the pulmonary trunk, so the regurgitant gradient transiently increases and the murmur becomes correspondingly louder.19
A midsystolic murmur with congenital pulmonary valve regurgitation is the result of rapid ejection of an increased right ventricular stroke volume into a dilated pulmonary trunk (see Figure 12-1).29 The systolic murmur is short and crescendo-decrescendo and ends well before both components of the second heart sound (see Figure 12-1). Rarely, a low-frequency presystolic murmur assigned to vibrations of the tricuspid valve is thought to represent a right-sided Austin Flint.29
Electrocardiogram
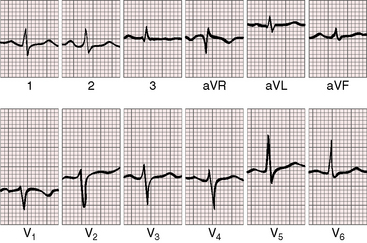
The electrocardiogram is normal when congenital pulmonary regurgitation is mild to moderate.18,30,31 Atrial fibrillation is exceptional even with congenital absence of the pulmonary valve.32 The most common change in the QRS reflects the pattern of volume overload of the right ventricle and is represented by terminal r waves in leads V1 and aVR, and S waves in leads 1 and V5-6 (Figure 12-6).29,32,41 Right bundle branch block is uncommon (see Figure 12-1).6,32
X-ray
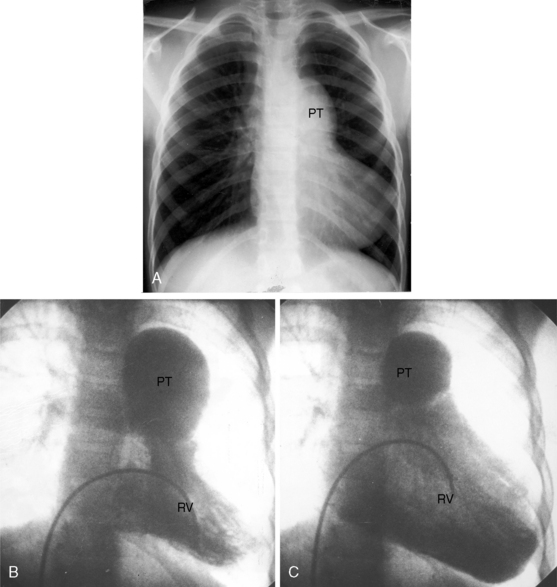
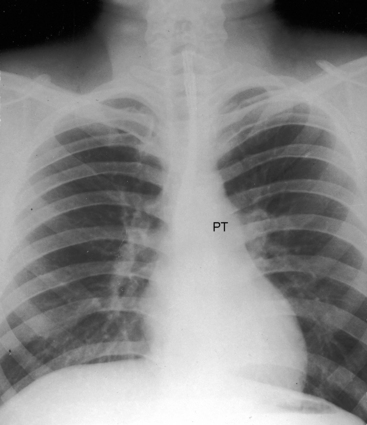
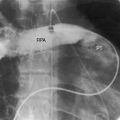
Dilation of the pulmonary trunk is the most prominent and consistent feature of the x-ray (Figure 12-7).30,31,41 The dilation has been ascribed to coexisting medial abnormalities42and varies considerably in size, sometimes reaching aneurysmal proportions (see Figure 12-7).13 Vigorous pulsations of the pulmonary trunk and its proximal branches are seen with fluoroscopy29,30 and with real-time echocardiography (see subsequent section). Occasionally, the chest x-ray fortuitously records striking intermittent changes in size of the dilated pulmonary trunk (Figures 12-7 and 12-8).31 Right ventricular enlargement corresponds to the degree of volume overload.
Echocardiogram
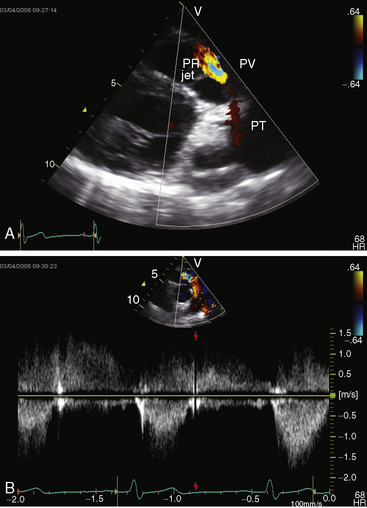
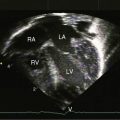
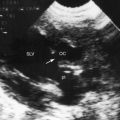
Color flow imaging and Doppler interrogation are used to establish the diagnosis of congenital pulmonary valve regurgitation and to establish the depth, width, duration, and peak velocity of the diastolic jet (Figure 12-9), which differs from pulmonary hypertensive pulmonary regurgitation (Figure 12-10 and Video 12-1). Mild pulmonary regurgitation detected in healthy subjects is not accompanied by a diastolic murmur.26,27
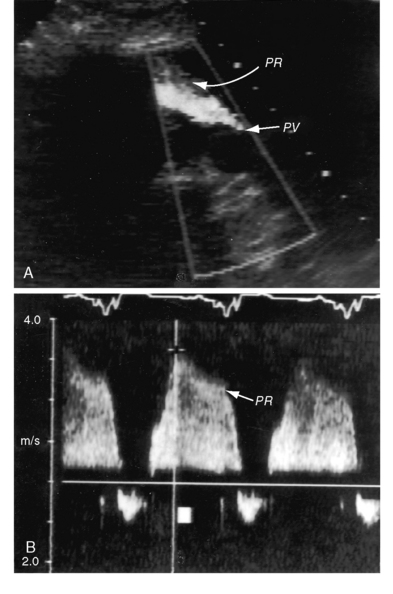
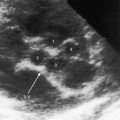
Figure 12-10 Echocardiograms from an 18-year-old woman with primary (idiopathic) pulmonary hypertension. A, Short axis shows the high-velocity jet of pulmonary hypertensive pulmonary regurgitation (PR) beginning at the pulmonary valve (PV). B, Continuous-wave Doppler scan across the right ventricular outflow tract shows high-pressure, high-velocity pulmonary regurgitation (PR) with a peak velocity just over 3 m/sec. Compare with Figure 12-9 (Video 12-1).
1 Osler W. The principles and practice of medicine. New York: D. Appleton And Company; 1892.
2 Hirschfelder A. Diseases of the heart and aorta. Philadelphia: J.B. Lippincott Company; 1910.
3 Kissin M. Pulmonary insufficiency with supernumerary cusp in pulmonary valve; report of a case and review of the literature. Am Heart J. 1936;12:206.
4 Abbott M.E. Atlas of congenital cardiac disease. New York: American Heart Association; 1936.
5 Kezdi P., Priest W.S., Smith J.M. Pulmonic regurgitation. Q Bull Northwest Univ Med Sch. 1955;29:368-373.
6 Cortes F.M., Jacoby W.J. Isolated congenital pulmonary valvular insufficiency. Am J Cardiol. 1962;10:287-290.
7 Lau K.C., Cheung H.H., Mok C.K. Congenital absence of the pulmonary valve, intact interventricular septum, and patent ductus arteriosus: management in a newborn infant. Am Heart J. 1990;120:711-714.
8 Wennevold A., Jacobsen J.R., Efsen F. Spontaneous change from pulmonic stenosis to pulmonic regurgitation. Am Heart J. 1984;108:608-609.
9 Berman W.Jr, Fripp R.R., Rowe S.A., Yabek S.M. Congenital isolated pulmonary valve incompetence: neonatal presentation and early natural history. Am Heart J. 1992;124:248-251.
10 Tanabe Y., Takahashi M., Kuwano H., et al. Long-term fate of isolated congenital absent pulmonary valve. Am Heart J. 1992;124:526-529.
11 Sayger P., Lewis M., Arcilla R., Ilbawi M. Isolated congenital absence of a single pulmonary valve cusp. Pediatr Cardiol. 2000;21:487-489.
12 Mori K., Hayabuchi Y., Kuroda Y. Diagnosis and natural history of isolated congenital pulmonary regurgitation in fetal life. Cardiol Young. 2000;10:162-165.
13 Attie F., Rijlaarsdam M., Chuquiure E. Images in cardiovascular medicine. isolated congenital absence of the pulmonary valve. Circulation. 1999;99:455-456.
14 Pouget J.M., Kelly C.E., Pilz C.G. Congenital absence of the pulmonic valve. report of a case in a seventy-three-year-old man. Am J Cardiol. 1967;19:732-740.
15 Chevers N. A collection of facts illustrative of the morbid conditions of the pulmonary artery, as bearing upon the treatment of cardiac and pulmonary diseases. London Medical Gazette. 1846;38:828-835.
16 Laneve S.A., Uesu C.T., Taguchi J.T. Isolated pulmonic valvular regurgitation. Am J Med Sci. 1962;244:446-458.
17 Lendrum B.L., Shaffer A.B. Isolated congenital pulmonic valvular regurgitation. Am Heart J. 1959;57:298-308.
18 Price B.O. Isolated incompetence of the pulmonic valve. Circulation. 1961;23:596-602.
19 Takao S., Miyatake K., Izumi S., et al. Clinical implications of pulmonary regurgitation in healthy individuals: detection by cross sectional pulsed doppler echocardiography. Br Heart J. 1988;59:542-550.
20 Venables A.W. Absence of the pulmonary valve with ventricular septal defect. Br Heart J. 1962;24:293-296.
21 Chaturvedi R.R., Redington A.N. Pulmonary regurgitation in congenital heart disease. Heart. 2007;93:880-889.
22 Baker W.P., Kelminson L.L., Turner W.M.Jr, Blount S.G. Absence of pulmonic valve associated with double-outlet right ventricle. Circulation. 1967;36:452-455.
23 Vlad P. Congenital pulmonary regurgitation: a report of 6 autopsied cases. Am J Dis Child. 1960;100:640.
24 Becker A.E. Quadricuspid pulmonary valve. Anatomical observations in 20 hearts. Acta Morphol Neerl Scand. 1972;10:299-309.
25 Fernandez-Armenta J., Villagomez D., Fernandez-Vivancos C., Vazquez R., Pastor L. Quadricuspid pulmonary valve identified by transthoracic echocardiography. Echocardiography. 2009;26:288-290.
26 Brand A., Dollberg S., Keren A. The prevalence of valvular regurgitation in children with structurally normal hearts: a color Doppler echocardiographic study. Am Heart J. 1992;123:177-180.
27 Choong C.Y., Abascal V.M., Weyman J., et al. Prevalence of valvular regurgitation by Doppler echocardiography in patients with structurally normal hearts by two-dimensional echocardiography. Am Heart J. 1989;117:636-642.
28 Fish R.G., Takaro T., Crymes T. Prognostic considerations in primary isolated insufficiency of the pulmonic valve. N Engl J Med. 1959;261:739-742.
29 Ansari A. Isolated pulmonary valvular regurgitation: current perspectives. Prog Cardiovasc Dis. 1991;33:329-344.
30 Collins N.P., Braunwald E., Morrow A.G. Isolated congenital pulmonic valvular regurgitation. Diagnosis by cardiac catheterization and angiocardiography. Am J Med. 1960;28:159-164.
31 Goldberg E., Katz I. Isolated pulmonic regurgitation with intermittent pulmonary artery dilatation. Am J Cardiol. 1962;9:619-625.
32 Thanopoulos B.D., Fisher E.A., Hastreiter A.R. Large ductus arteriosus and intact ventricular septum associated with congenital absence of the pulmonary valve. Br Heart J. 1986;55:602-604.
33 Hamby R.I., Gulotta S.J. Pulmonic valvular insufficiency: etiology, recognition, and management. Am Heart J. 1967;74:110-125.
34 Ettedgui J.A., Sharland G.K., Chita S.K., Cook A., Fagg N., Allan L.D. Absent pulmonary valve syndrome with ventricular septal defect: role of the arterial duct. Am J Cardiol. 1990;66:233-234.
35 Sanyal S.K., Hipona F.A., Browne M.J., Talner N.S. Congenital insufficiency of the pulmonary valve. J Pediatr. 1964;64:728-734.
36 Jacoby W.J.Jr, Tucker D.H., Sumner R.G. The second heart sound in congenital pulmonary valvular insufficiency. Am Heart J. 1965;69:603-609.
37 Shaver J.A., Nadolny R.A., O’toole J.D., et al. Sound pressure correlates of the second heart sound. An intracardiac sound study. Circulation. 1974;49:316-325.
38 Sloman G., Wee K.P. Isolated congenital pulmonary valve incompetence. Am Heart J. 1963;66:532-537.
39 Maciel B.C., Simpson I.A., Valdes-Cruz L.M., et al. Color flow Doppler mapping studies of “physiologic” pulmonary and tricuspid regurgitation: evidence for true regurgitation as opposed to a valve closing volume. J Am Soc Echocardiogr. 1991;4:589-597.
40 Perloff J.K. Physical examination of the heart and circulation, 4th ed. Shelton, Connecticut: People’s Medical Publishing House; 2009.
41 Schrire V., Vogelpoel L. The role of the dilated pulmonary artery in abnormal splitting of the second heart sound. Am Heart J. 1962;63:501-507.
42 Niwa K., Perloff J.K., Bhuta S.M., et al. Structural abnormalities of great arterial walls in congenital heart disease: light and electron microscopic analyses. Circulation. 2001;103:393-400.