Chapter 11 Congenital Pulmonary Stenosis
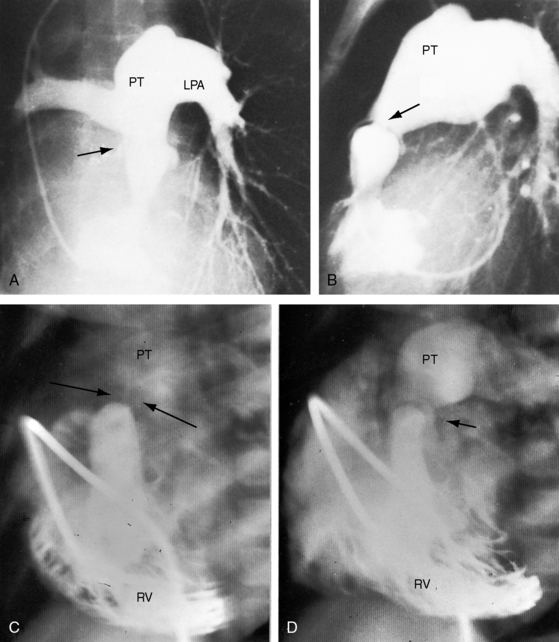
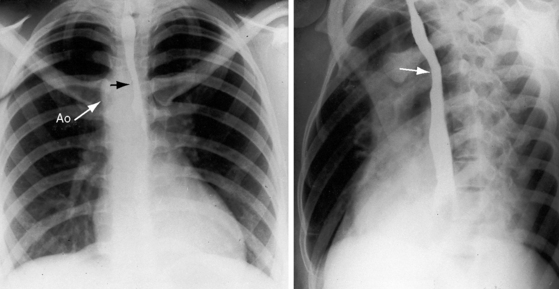
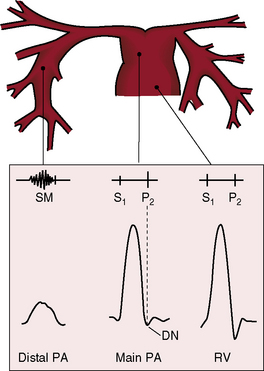
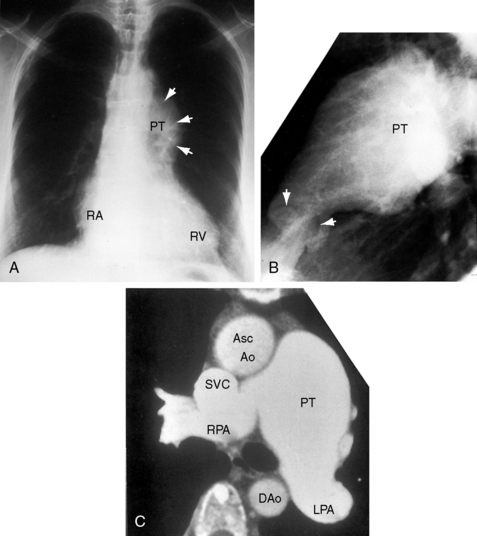
Congenital obstruction to right ventricular outflow in hearts with two noninverted ventricles originates in, below, or above the pulmonary valve. Three morphologic types of pulmonary stenosis involve the pulmonary valve: (1) typical mobile dome-shaped; (2) dysplastic; and (3) bicuspid. Dome-shaped pulmonary valve stenosis was described in 1761 by John Baptist Morgagni1 and is characterized by a thin mobile valve mechanism with a narrow central opening at its apex (Figure 11-1). Three rudimentary raphes extend from the central opening to the wall of the pulmonary artery, but separate leaflets and separate commissures cannot be identified. Pinpoint dome-shaped pulmonary valve stenosis in neonates is sometimes referred to as functional pulmonary atresia (see Figure 11-1A,B, lower). The pulmonary trunk is consistently dilated because of an inherent medial abnormality2 that is coupled with the mobile dome-shaped valve but not with its functional state (see Figure 11-1). The jet from the stenotic valve breaks up on striking the apex of the pulmonary trunk. The pressure component of total energy increases with a proportionate increase in pressure in the left branch (see Figure 11-35A).3 The physics of jet dispersion is believed to account for the larger size of the left branch. Calcification of a dome-shaped stenotic pulmonary valve is exceptional and is reserved for older patients (see section X-Ray).4 Dysplastic pulmonary valve stenosis is much less common and is characterized by myomatous thickening of three separate but poorly mobile leaflets without commissural fusion (Figure 11-2; Box 11-1).5,6 Bicuspid pulmonary valve stenosis is a feature of Fallot’s tetralogy (see Chapter 18). Isolated bicuspid pulmonary valves are rare and are of little or no functional significance.
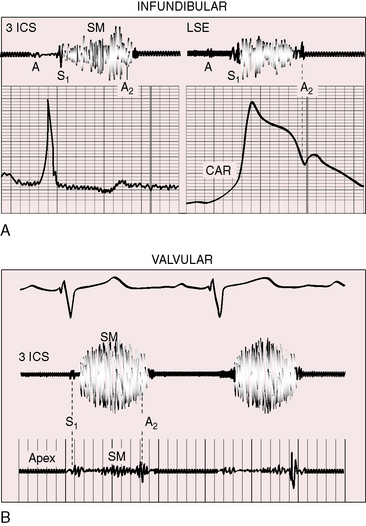
Subvalvular pulmonary stenosis can be infundibular or subinfundibular. The infundibular variety is caused by anterior and rightward deviation (malalignment) of the infundibular septum and is dealt with in Chapter 18. Secondary hypertrophic infundibular pulmonary stenosis accompanies—is secondary to—severe pulmonary valve stenosis (Figure 11-3 and see also Figure 11-38). Stenosis of the ostium of the infundibulum is a rare form of fixed obstruction to right ventricular outflow (see Figures 11-32 and 11-37). Subinfundibular stenosis or double-chambered right ventricle, described by Thomas Peacock7 in 1858, is a rare form of congenital obstruction to right ventricular outflow. Obstructing muscle bundles within the right ventricular cavity were the subject of Sir Arthur Keith’s8 Hunterian lecture in 1909. The double-chambered right ventricle is divided into a high-pressure inlet portion and a low-pressure outlet portion by normal or anomalous muscle bundles9,10 or by apical trabecular muscle sequestered from the rest of the right ventricle.11 The degree of obstruction varies from nil to severe to virtually complete.10 Double-chambered right ventricle usually coexists with a ventricular septal defect.9,10
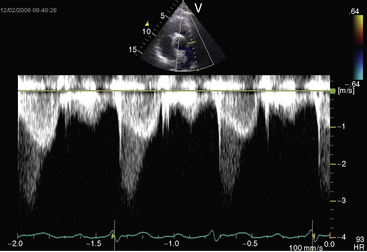
Figure 11-38 Continuous wave Doppler scan from a 32-year-old man with severe mobile dome-shaped pulmonary valve stenosis (PS) and secondary hypertrophic subpulmonary stenosis (compare with Figure 11-3). The asymmetric profile within the envelope of the continuous wave Doppler signal represents the gradient across the zone of secondary hypertrophic subpulmonary stenosis.
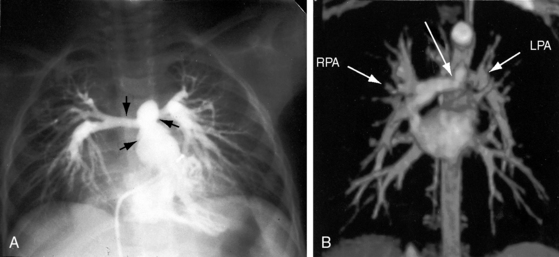
Pulmonary artery stenosis (supravalvular), described by Oppenheimer12 in 1938, is caused by narrowing of the pulmonary trunk, its bifurcation, or its primary or intrapulmonary branches (Figures 11-4 and 11-5).13 Stenosis of the pulmonary artery and its branches usually occurs as an isolated malformation13 and can be unilateral or bilateral, single or multiple, and segmental or tubular (see Figures 11-4 and 11-5).13 Intrapulmonary arteries distal to the stenoses tend to be dilated (see Figures 11-4 and 11-5). Rarely, a membranous form of obstruction occurs immediately above the valve.13
Neonates, especially premature, normally exhibit a disparity in size between the pulmonary trunk and its proximal branches. Angulations at the origins of the branches cause a drop in systolic pressure in the absence of morphologic pulmonary artery stenosis (see Chapter 2, Figure 2-4).14,15 The small pulmonary trunk and small proximal branches in Fallot’s tetralogy are discussed in Chapter 18. In Williams syndrome, bilateral stenosis of pulmonary artery branches is associated with supravalvular aortic stenosis (see Chapter 7).16 Experimental constriction of the pulmonary trunk in fetal lambs results in thin-walled intrapulmonary resistance vessels.17
In 1941, McAlister Gregg,18 an Australian ophthalmologist, described a relationship between maternal rubella and congenital abnormalities in offspring. What came to be known as the rubella syndrome consists of stenosis of the pulmonary artery and its branches with patent ductus arteriosis.19,20 Maternal rubella can have serious noncardiac effects that include spontaneous abortion, stillbirth, mental retardation, cataracts, and deafness. Viremia with placental and fetal infection during initial exposure is a prerequisite for the rubella syndrome. Fetal risk is small when infection occurs later than the 16th week of gestation.20 A worldwide rubella epidemic in 1962 was followed in 1964 and 1965 by an epidemic in the United States that affected approximately 12.5 million patients and caused 11,000 fetal deaths.19 Of an estimated 20,000 infants born with the rubella syndrome, approximately 2,100 died as neonates.19
The physiologic consequences of pulmonary stenosis result from increased resistance to right ventricular discharge. Systolic pressure is elevated proximal to the obstruction and is normal or reduced distally. Pulmonary artery stenosis causes an increase in systolic pressure in the pulmonary trunk, which is proximal to the obstruction (see Figure 11-19). Valvular and subvalvular pulmonary stenosis increase the systolic pressure in the right ventricle, which is proximal to the obstruction. The gross morphologic response of the right ventricle to pressure overload is new sarcomeres in parallel, hence an increase in thickness of the free wall and ventricular septum, adaptive responses appropriate for developing power.21 Cavity size remains normal.22 In neonates with pinpoint pulmonary stenosis, cavity size is reduced.
The ultrastructural response to mechanical stress depends on myocyte maturity or immaturity at the time the inciting stimulus of overload becomes operative.21 In pulmonary stenosis, the inciting stimulus is present at birth when cardiomyocytes are immature and capable of replication, which is accompanied by capillary angiogenesis. Accordingly, each replicated normal-sized myocyte is paired with its own capillary, so the myocyte:capillary ratio (capillary density) is normal. However, a morphologic right ventricle is perfused by a morphologic right coronary artery, which imposes an inherent limitation.
Children and young adults with mild to moderate pulmonary valve stenosis have normal or near normal right ventricular function and can increase cardiac output with exercise.23 In severe pulmonary valve stenosis, however, stroke volume and cardiac output are fixed.24 Systolic contraction of hypertrophied infundibular muscle (see Figure 11-3) is reinforced by exercise.23
Pressure overload of the right ventricular can result in systolic and diastolic dysfunction of the left ventricle.25,26 Systolic dysfunction has been ascribed to chronic underfilling of the left ventricle, and diastolic dysfunction has been ascribed to displacement of the hypertrophied septum into the left ventricular cavity.
History
Typical mobile dome-shaped pulmonary valve stenosis is relatively common, with a prevalence rate as high as 10% of cases of congenital heart disease.27,28 Gender distribution is equal or with female prevalence.28 This is also the case in dysplastic pulmonary valve stenosis.29
The murmur of pulmonary stenosis is usually discovered at birth because the anatomic and physiologic conditions necessary for its production are present at birth. Parents are told that their newborn has congenital heart disease before discharge. However, the murmur of pinpoint pulmonary stenosis (see Figure 11-1, lower) is disarmingly soft. Murmurs at nonprecordial thoracic sites accompany pulmonary artery stenosis and in neonates and infants are easily overlooked or lost in the rapid breath sounds. A history of first trimester maternal rubella arouses suspicion of pulmonary artery stenosis.
Familial recurrence of isolated pulmonary valve stenosis is uncommon if not rare (Figure 11-6).30–33 In dysplastic pulmonary valve stenosis, the converse is the case.5 Some family members have a dysplastic valve, and others have a mobile dome-shaped valve.5 Similar observations have been made in the hereditary canine pulmonary valve dysplasia of beagles,34 in whom dysplastic pulmonary stenosis and dome-shaped pulmonary stenosis occur in litter mates.34 Familial Noonan’s syndrome frequently occurs with dysplastic pulmonary valve stenosis and has appeared in three generations.35,36 Successful pregnancy is possible in females with Noonan’s syndrome.37
Normal birth weights and normal growth and development are characteristic of mobile dome-shaped pulmonary valve stenosis. However, in Noonan’s syndrome with dysplastic pulmonary valve stenosis, growth and development are poor.5,29,38 Pulmonary artery stenosis is associated with low birth weights and retarded physical and mental development in the rubella syndrome and in Williams syndrome (see Chapter 7).19,39
Neonates with pinpoint pulmonary valve stenosis experience rapidly progressive cardiac failure and early death. However, most patients with mobile dome-shaped pulmonary valve stenosis experience little or no difficulty in infancy and childhood.40 In a review of 69 cases, the average age at death was 26 years; seven patients survived to age 50 years, and three survived to 70 and 75 years. In 21 adults, the average follow-up period was 50 years.41 Examples are found of survival into the sixth, seventh, and eighth decades,42–46 with one patient reaching 78 years.42 Longevity depends on three variables: 1, the initial severity of stenosis; 2, whether a given degree of stenosis remains constant or progresses; and 3, whether the function of the afterloaded right ventricle is preserved.24,40,47
The normal pulmonary valve orifice increases linearly with age and body surface area.48 The orifice of a stenotic mobile dome-shaped pulmonary valve increases with age, but not necessarily at the rate of somatic growth.40,48–50 Mild pulmonary valve stenosis in infancy usually remains mild,51 but moderate to severe pulmonary stenosis tends to progress.44 Stenosis of the pulmonary artery and its branches is not progressive. Fibrous thickening and occasionally calcification are responsible for increasing the degree of stenosis in older adults.
Equivalent degrees of pulmonary stenosis may handicap one patient in childhood but leave another relatively free of symptoms in adulthood. That mild pulmonary stenosis is asymptomatic is not surprising, but it is surprising that an appreciable number of patients with moderate to severe pulmonary stenosis claim to be virtually asymptomatic. Patients with right ventricular systolic pressures between 50 and 100 mm Hg include a New Zealand long-distance swimmer, a long-distance runner, and an English hockey captain.52 The author’s patients have included a 17-year-old boy who played baseball despite a right ventricular systolic pressure of nearly 200 mm Hg, a 32-year-old man who had run the quarter mile in high school despite a right ventricular systolic pressure of 75 mm Hg, and a woman with a right ventricular pressure of nearly 200 mm Hg who worked fulltime and had recurrent ascites for 7 years before death at age 60 years. Dyspnea and fatigue are mild as long as the right ventricle maintains a normal stroke volume at rest and augments its stroke volume with exercise.24,47 However, relatively asymptomatic patients can have rapid deterioration. Cardiac output is inadequate even at rest when the hemodynamic burden imposed on the right ventricle leads to right ventricular failure, which is the commonest cause of death.43
Giddiness and light-headedness with effort prefigure syncope.52 Children and adults occasionally experience the chest pain of right ventricular myocardial ischemia.46,53 A 3-year-old with severe pulmonary artery stenosis died during an episode of chest pain and at necropsy had infarction of the right ventricular free wall and interventricular septum.53 Sudden death has been associated with right ventricular infarction and an abnormal right coronary artery.54 Dilated thin-walled intrapulmonary artery aneurysms distal to the stenoses of pulmonary artery branches (see Figure 11-5B) are sources of hemoptyses that can be intermittent and mild or recurrent and brisk.13
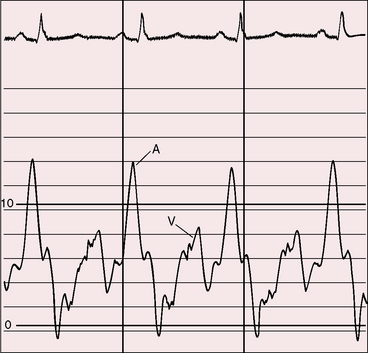
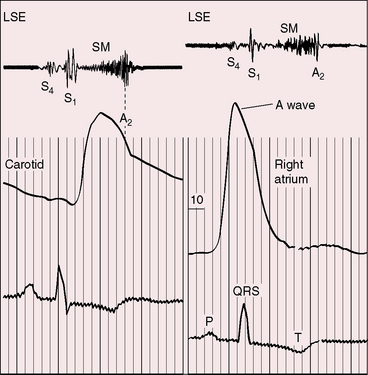
Severe pulmonary stenosis is accompanied by giant jugular venous A waves (Figure 11-7) of which patients are subjectively aware, especially during effort or excitement. These neck pulsations can be seen in the mirror when a young man shaves or when a young woman sits at her vanity. A 13-year-old girl with pulmonary valve stenosis and congenital complete heart block was aware of intermittent amplification of A waves as her right atrium randomly contracted against a closed tricuspid valve, and a 15-year-old boy was unpleasantly aware of intermittent amplification of jugular venous A waves caused by premature ventricular beats.
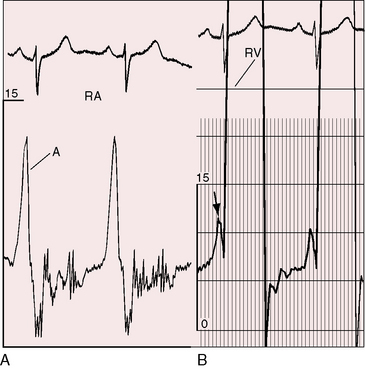
Figure 11-7 Pressure pulses from the 47-year-old woman with severe pulmonary valve stenosis referred to in Figure 11-1. A, Large right atrial (RA) A wave. B, Presystolic distention (arrow) of the right ventricle (RV) coincided with the large right atrial A wave.
Mobile dome-shaped pulmonary valve stenosis is a substrate for infective endocarditis44 that can induce a medical valvotomy when tissue is interrupted and orifice size increases.52 Jet lesions in pulmonary artery stenosis can serve as substrates for infective endarteritis.13
Physical appearance
Infants with mobile dome-shaped pulmonary valve stenosis occasionally have chubby, round, bloated facies with highly colored cheeks (Figure 11-8A) and well-developed fat deposits.52 The digits may be erythematous or frankly red in response to a small or intermittent right-to-left shunt through a patent foramen ovale (see Chapter 16).36,55
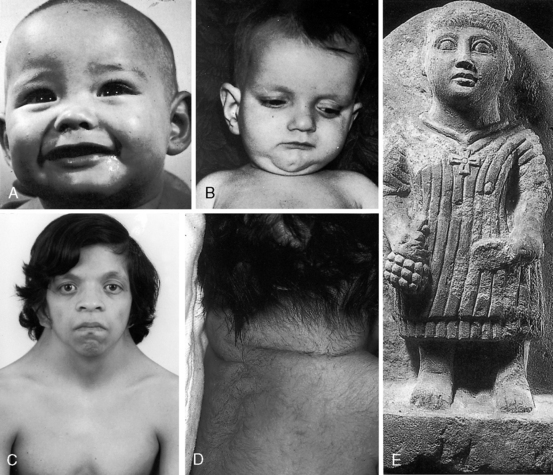
Noonan’s syndrome (Figure 11-8C)37,55,56 is characterized by short stature, webbed neck, pterygium colli, ptosis,hypertelorism, lymphedema, low-set ears, low anterior and posterior hairlines, flat or shield chest, pectus excavatum or carinatum, hyperelastic skin, inguinal hernia, nevi, dystrophic nails, micrognathia, hypospadias, and small undescended or cryptorchid testes.
About one third of patients with Noonan’s syndrome are mentally retarded, and approximately two thirds have congenital heart disease, especially dysplastic pulmonary valve stenosis (60%) and hypertrophic cardiomyopathy (20%).36,55,57–59
The rubella syndrome is characterized by cataracts, retinopathy, deafness, hypotonia, dermatoglyphic abnormalities, and mental retardation.60 Height and weight are usually normal for age despite intrauterine growth retardation. Stenosis of the pulmonary artery and its branches and patent ductus arteriosis (see Chapter 20) are the most frequent types of coexisting congenital heart disease.60
Williams syndrome includes a small chin, large mouth, patulous lips, blunt upturned nose, wide-set eyes, broad forehead, baggy cheeks, and malformed teeth (see Chapter 7). The most frequent coexisting congenital heart disease is pulmonary artery stenosis with supravalvular aortic stenosis (see Chapter 7).
Alagille syndrome, also called arteriohepatic dysplasia or the Alagille-Watson syndrome, is an autosomal dominant disorder with abnormalities of liver, eyes, kidneys, and skeleton.61,62 Facial appearance is characterized by a prominent overhanging forehead, deep-set eyes, and a small pointed chin (Figure 11-8B).61–63 The most frequent coexisting congenital heart disease is stenosis of the pulmonary artery and its branches.62
Cornelia de Lange’s syndrome expresses itself with low birth weight, slow growth, small stature, microcephaly, thin eyebrows that meet at midline, long eyelashes, short upturned nose, thin downturned lips, hirsutism, small hands and feet, incurved fifth fingers, cleft palate, and missing limbs or portions of limbs. The incidence rate of pulmonary valve stenosis is reportedly 39%.64
Pulmonary stenosis also occurs in LEOPARD syndrome, which is characterized by multiple lentigines and café-au-lait spots.65
Arterial pulse
When severe pulmonary stenosis is accompanied by right ventricular failure, especially with coexisting left ventricular dysfunction, the arterial pulse is reduced. Asymmetry of right and left brachial and carotid arterial pulses (right greater than left) is a feature of supravalvular aortic stenosis that may accompany stenosis of the pulmonary artery and its branches (see Chapter 7).
Jugular venous pulse
The jugular venous A wave is distinctive and increases progressively as the stenosis increases (Figures 11-8A and 11-9), culminating in a giant A wave that leaps to the eye, towering above and dwarfing the other waves of the venous pulse.27 Powerful right atrial contraction generates a giant A wave via the superior vena cava and a presystolic liver pulse via the inferior cava. With the advent of right ventricular failure and tricuspid regurgitation, the large A wave is accompanied by an increase in the V wave. The liver then manifests presystolic and systolic pulsations.
Precordial movement and palpation
The thrill associated with pulmonary valve stenosis is maximal in the second left intercostal space, with radiation upward and to the left because the intrapulmonary jet is directed upward and toward the left pulmonary artery (see Figure 11-35A).3 When secondary hypertrophic subpulmonary stenosis coexists, the thrill is maximal in the third or fourth left intercostal space (see Figure 11-3).
Palpation of the right ventricular impulse is best achieved with applying the fingertips between the ribs along the left sternal edge during full held exhalation or with applying a single fingertip against the diaphragm in the subzyphoid area.66,67 The subzyphoid technique is especially useful in infants because rapid respiratory excursions interfere with parasternal palpation.
The gentle right ventricular impulse of mild pulmonary valve stenosis is more readily palpated after exercise or in infants after the stress of feeding or crying. In severe pulmonary valve stenosis, the right ventricular impulse is forceful and sustained and is detected up to and including the third intercostal space.66–68 Infundibular pulmonary stenosis relegates the high-pressure zone to the inflow portion of the right ventricle, so the accompanying impulse is assigned to the fourth and fifth left intercostal spaces. Subinfundibular stenosis is accompanied by a deceptively unimpressive right ventricular impulse confined to the fifth interspace or subxyphoid area. The pulmonary trunk is necessarily distal to the stenosis and cannot be palpated despite conspicuous dilation.
A presystolic impulse along the lower left sternal border and in the subxyphoid area (see Figure 11-7B) indicates presystolic distention of the right ventricle in response to the increased force of right atrial contraction that is also responsible for the large jugular venous A wave (see Figure 11-7A). A prominent pulmonary ejection sound is occasionally palpable in the second left intercostal space during held exhalation.
Auscultation
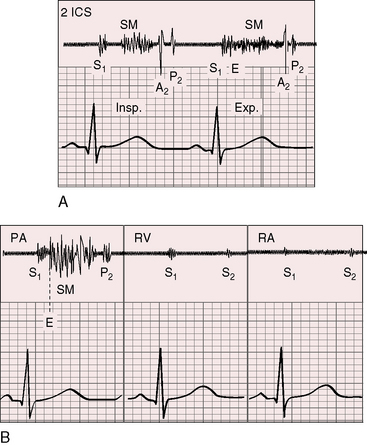
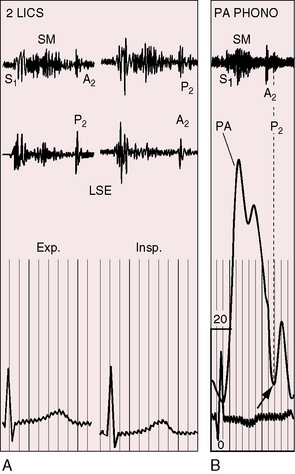
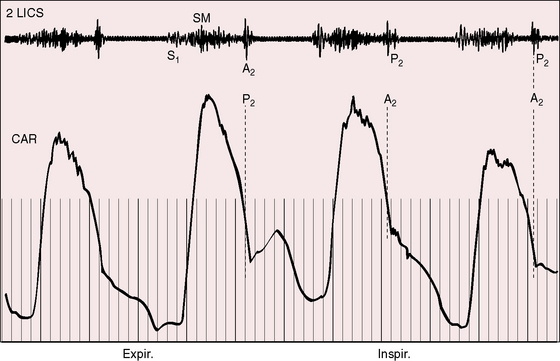
A pulmonary ejection sound coincides with abrupt cephalad movement of the mobile dome-shaped pulmonary valve (see Figures 11-1B, upper, and 11-3) and is therefore an important sign in the auscultatory characterization of right ventricular outflow obstruction (Figure 11-10).67,69 An ejection sound does not occur with dysplastic pulmonary valves, which move poorly if at all,5 or with fixed subvalvular or pulmonary artery stenosis.67 An ejection sound can also originate in a functionally normal bicuspid pulmonary valve.70 The sound is recognized by its high-pitched clicking quality, by its maximal intensity in the second left intercostal space, and by its distinctive selective decrease during inspiration (Figures 11-11 through 11-13).67,71,72 The inspiratory decrease is related to the reduced cephalad movement available to the mobile valve as the right ventricle contracts.71 The inspiratory increase in right atrial contractile force is transmitted into the right ventricle and onto the undersurface of the mobile pulmonary valve, which moves into a relatively cephalad position.73 Additional cephalad movement is necessarily reduced, so the ejection sound is reduced or vanishes altogether.71 Conversely, right atrial contractile force decreases during expiration, leaving the mobile pulmonary valve in a slack position, so right ventricular contraction induces maximal cephalad movement and maximal intensity of the ejection sound.71
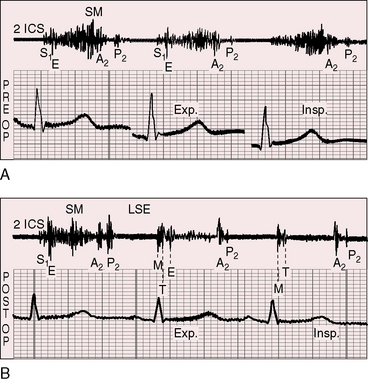
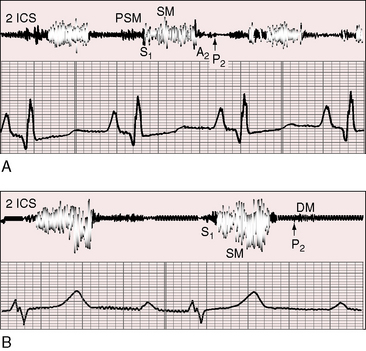
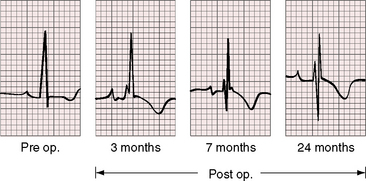
Figure 11-13 Phonocardiograms from a 21-year-old woman with mobile dome-shaped pulmonary valve stenosis and a gradient of 80 mm Hg. A, Before pulmonary valvotomy (PRE OP), the systolic murmur (SM) peaked late and enveloped the aortic component of the second sound (A2). The pulmonary component (P2) was soft and delayed. A pulmonary ejection sound (E) was recorded during expiration (Exp) but was absent during inspiration (Insp). B, After valvotomy (POST OP), the gradient fell to 20 mm Hg, and the phonocardiogram resembled mild pulmonary valve stenosis (see Figures 11-10 and 11-11). (M = mitral component of the first sound; T = tricuspid component).
The interval between the first heart sound and the ejection sound varies inversely with the degree of stenosis (see Figure 11-10).69,74 As severity increases, the velocity of right ventricular contraction increases, the pulmonary valve opens earlier, and the ejection sound occurs earlier and merges with the first heart sound (see Figure 11-10) but retains its distinctive respiratory variation that distinguishes it from a loud first heart sound.74,75 Accordingly, a pulmonary ejection sound that occurs at a well-defined interval after the first heart sound is a sign of mild pulmonary valve stenosis and an ejection sound that merges with the first heart sound is a sign of severe pulmonary stenosis. Fibrosis and calcification in older patients impair mobility, so the ejection sound decreases or disappears altogether.
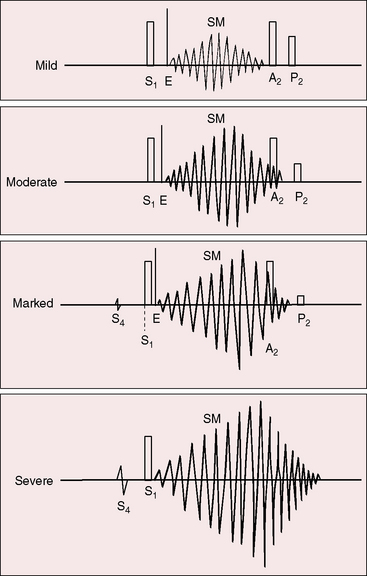
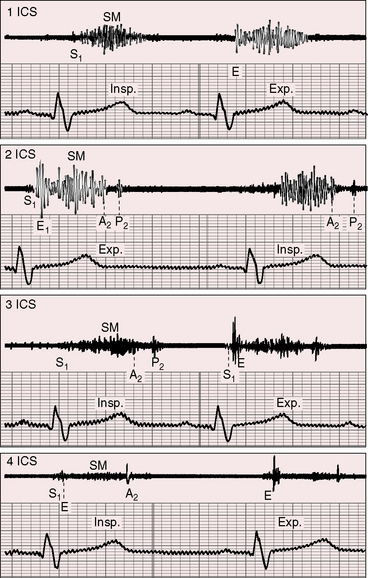
The murmur of pulmonary valve stenosis was recognized in 1858 by Peacock.7 The maximum precordial site is the second left intercostal space (see Figures 11-11 through 11-13), which topographically overlies the pulmonary trunk and coincides with the location of the murmur recorded with intracardiac phonocardiography (see Figure 11-11B).67 Prominence of the murmur in the third left intercostal space occurs with secondary hypertrophic subpulmonary stenosis (Figures 11-3 and 11-14B). The murmur radiates upward and to the left (see Figure 11-12) because the intrapulmonary jet is directed upward and to the left (see Figure 11-35A). Loud systolic murmurs radiate into the suprasternal notch and the base of the neck, especially the left side. Intensity varies directly with the degree of obstruction, but intensity per se is not necessarily an index of severity. In neonates with pinpoint pulmonary stenosis, the murmur is deceptively soft (see Figure 11-1A,B, lower). Grade 3/6 murmurs are features of mild pulmonary stenosis (see Figure 11-11), and grade 3/6 to 6/6 murmurs are features of moderate to severe pulmonary stenosis (see Figures 11-12 through 11-14).
The severity determines the duration of right ventricular ejection, which in turn determines the duration of the systolic murmur. The length of the murmur relative to the aortic component of the second heart sound permits comparison of the duration of right and left ventricular ejection (see Figure 11-10). When pulmonary stenosis is mild, right ventricular remains normal, so a symmetric systolic murmur ends before the aortic component of the second sound (see Figures 11-10 and 11-11).75 When stenosis is moderate, the murmur ends at or slightly after the aortic component of the second sound, which remains audible (see Figures 11-10 and 11-12).75 When stenosis is severe, the murmur extends beyond the aortic component, which is partially or completely obscured (see Figures 11-10, 11-13 and 11-14).75 With a still greater increase in severity, the murmur lengthens further, peaks later in systole, and assumes an asymmetric kite shape.74,75 Figure 11-10 illustrates the relationship between the duration and configuration of the systolic murmur and the degree of pulmonary stenosis. The holosystolic murmur of tricuspid regurgitation accompanying right ventricular failure is distinguished by its high frequency, its location along the lower left or right sternal edge, and its selective increase during inspiration.
Stenosis of the ostium of the infundibulum (see Figures 11-32 and 11-37) is accompanied by a murmur that is maximal in the third and fourth left intercostal spaces (see Figure 11-14).27,75 Subinfundibular stenosis assigns the murmur still lower—to the fourth or fifth left intercostal space.76
Several types of diastolic murmurs occur, although rarely, in pulmonary valve stenosis. A low-intensity atrial systolic murmur is attributed to presystolic flow across the pulmonary valve (Figure 11-15A).69 Powerful right atrial contraction generates a giant A wave that exceeds the diastolic pressure in the pulmonary trunk, so the pulmonary valve opens before the onset of ventricular contraction, permitting presystolic flow and a presystolic murmur (see Figure 11-38).67,69,75 Presystolic murmurs are occasionally generated by prolonged vibrations of a fourth heart sound,67 and soft diastolic murmurs have been attributed to low-pressure pulmonary regurgitation (Figures 11-15B, 11-35B, and 11-38).69,75 Dysplastic or calcific pulmonary valves77 are rarely incompetent.
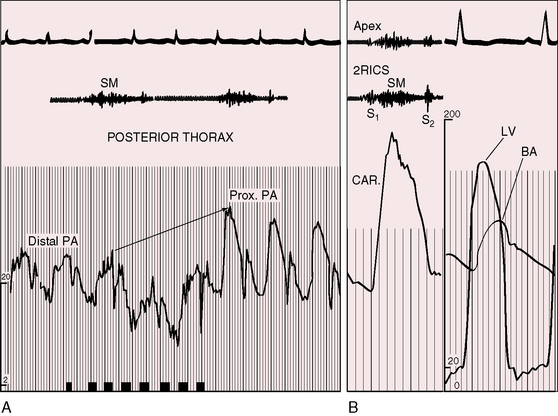
Murmurs associated with pulmonary artery stenosis are typically confined to systole (Figures 11-16 and 11-17) but exceptionally continue into diastole.13,78 Thoracic distribution is determined by the locations of the stenotic pulmonary artery segments, which are usually multiple and involve the pulmonary trunk and the proximal and distal branches (see Figures 11-4 and 11-5B). The accompanying murmurs are heard anteriorly in the vicinity of the second left and right intercostal spaces and are heard peripherally in the axillae and back (Figure 11-18).13 An attempt should be made to distinguish murmurs that originate peripherally from a loud central murmur that radiates widely. Peripheral pulmonary systolic murmurs from high flow accompany uncomplicated atrial septal defect and are difficult to distinguish from murmurs of coexisting pulmonary artery stenois.78 Healthy neonates, especially when premature, experience a physiologic drop in systolic pressure from pulmonary trunk to proximal branches. This pressure drop is accompanied by peripheral pulmonary systolic murmurs that vanish as the lungs mature (see Chapter 2).14,15
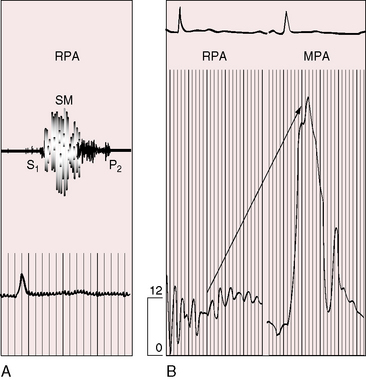
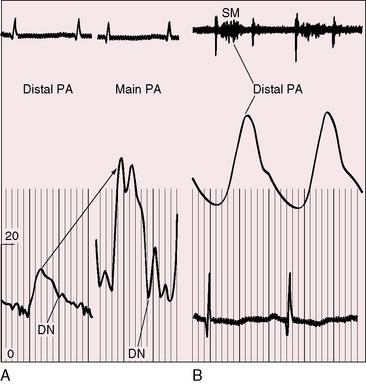
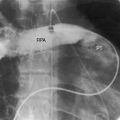
Figure 11-17 Tracings from a 19-year-old man with pulmonary artery stenosis. A, Intracardiac phonocardiogram from the distal right pulmonary artery (RPA) recorded a delayed crescendo-decrescendo systolic murmur (SM). B, As the catheter was withdrawn from the distal right pulmonary artery (RPA) into the main pulmonary artery (MPA), a gradient was recorded (oblique arrow) together with the distinctive contour of the main pulmonary artery pressure pulse (see Figure 11-16A). The systolic gradient did not continue into diastole, so the murmur was confined to systole.
Systolic murmurs of pulmonary artery stenosis are crescendo-decrescendo (see Figures 11-16 and 11-17) with delayed onset because of their relatively distal origins (see Figure 11-17). Stenoses that originate at or near the bifurcation of the pulmonary trunk generate murmurs that occasionally reach grade 4/6 in the second left intercostal space, but as a rule, the central precordial murmur is grade 3/6 and the peripheral murmurs are softer. When the intensities of precordial and nonprecordial murmurs are equal, one can assume that the widespread nonprecordial murmurs originate peripherally. Intrapulmonary phonocardiograms confirm that systolic murmurs originate distal to stenoses in the pulmonary trunk and proximal pulmonary arterial branches (see Figures 11-16 through 11-18). Peripheral pulmonary systolic murmurs are overlooked because of their low intensity and because auscultation is not routinely conducted at nonprecordial sites. The difficulty is compounded in infants because of rapid respiratory rates and because the frequency composition of breath sounds is close to the frequency of composition of peripheral pulmonary systolic murmurs. The diaphragm of the stethoscope should be applied during held respiration with the patient supine, prone, and sitting comfortably.
Continuous murmurs imply continuous gradients across the stenotic sites, but recordings of pressure pulses proximal and distal to the zones of stenosis show that systolic gradients seldom continue into diastole.79 The central pulmonary arterial pulse is characterized by an elevated systolic pressure and a rapid fall to diastolic levels that are normal or nearly so (Figures 11-17 and 11-19). Gradients are confined to systolic, so the accompanying murmur is confined to systole (see Figures 11-17 and 11-19).79 Why, then, do continuous murmurs occur at all? It has been postulated that systolic expansion of the high-pressure pulmonary trunk proximal to the stenosis sets the stage for brisk diastolic flow across the distal segments as the expanded proximal segments recoil.79 Modest diastolic flow following a large systolic gradient explains the occasional occurrence of a soft low-frequency diastolic murmur that precedes a prominent systolic murmur. Pulmonary artery stenosis may also give rise to increased flow through bronchial arterial collaterals.80 Angiographically demonstrated bronchial collaterals are believed to be responsible for continuous murmurs.81
Splitting of the second heart sound and the intensity of the pulmonary component are important signs in the auscultatory characterization of obstruction to right ventricular outflow. In typical mobile dome-shaped pulmonary valve stenosis, the intensity of the pulmonary component varies from normal to inaudible as severity increases (see Figures 11-10 through 11-15). However, timing is more important than intensity in assessment of severity.69,74 The more severe the stenosis, the longer the duration of right ventricular ejection, the later the timing of pulmonary valve closure, and the wider the split (see Figure 11-10). With mild stenosis, splitting is mild (see Figure 11-11).82 With severe stenosis, splitting may be as great as 120 to 140 msec (see Figure 11-15) and, if heard at all, is fixed because right ventricular stroke volume is fixed.82 The long systolic murmur of severe pulmonary stenosis obscures the aortic component of the second sound, and the soft pulmonary component may be inaudible (see Figures 11-10 and 11-14).69,82,83 When stenosis is mild, the pulmonary component may be delayed by the increased capacitance of the pulmonary vascular bed associated with dilation of the pulmonary trunk and its proximal branches.75,82–84 These qualifications notwithstanding, a slight increase in splitting with normal intensity of the pulmonary component favors mild stenosis (see Figure 11-11) and a marked increase in splitting with a faint or inaudible pulmonary component favors severe stenosis (see Figures 11-14 and 11-15).
In pulmonary artery stenosis, factors that influence the behavior of the second heart sound differ from those that influence the second sound in valvular or subvalvular stenosis.78 Stenosis of the pulmonary artery and its branches is unique among causes of pulmonary hypertension because it elevates systolic but not diastolic pressure in the pulmonary trunk. Accordingly, the valve closes at a normal or near normal pressure, so the intensity of the pulmonary component of the second sound is normal (Figures 11-19 through 11-21).13,78,85 The abruptness with which the leaflets seat affects the intensity of the pulmonary component of the second heart sound. The cusps might bulge abruptly toward the right ventricle during isometric relaxation, suddenly increasing the volume capacity of the pulmonary trunk. In pulmonary artery stenosis, the intensity of the pulmonary second sound remains unchanged as severity increases78 in contrast to mobile dome-shaped pulmonary valve stenosis or fixed subvalvular stenosis in which the intensity of the pulmonary component varies inversely with the degree of stenosis. The timing of pulmonary valve closure cannot be judged with criteria that apply to valvular or fixed subvalvular pulmonary stenosis, both of which prolong right ventricular ejection. Potential prolongation of right ventricular ejection in pulmonary artery stenosis is countered by the rapidity with which the pulmonary artery pressure falls from its systolic peak (see Figure 11-17). The second heart sound is therefore normally split even in the presence of appreciable obstruction10; the degree of splitting varies appropriately with respiration (see Figures 11-20 and 11-21).78
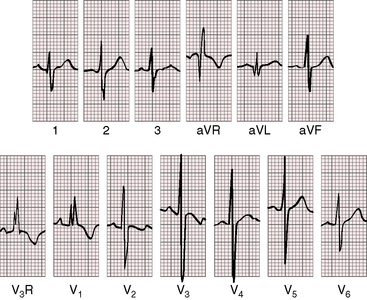
Abrahams and Wood27 drew attention to the significance of fourth heart sounds in pulmonary stenosis, reasoning that “strong right atrial contraction in presystole helps the hypertrophied right ventricle generate a high systolic pressure.” This was considered “an example of Starling’s law of the heart, strong atrial systole augmenting late diastolic distention of the right ventricle so that the chamber contracts more forceably.”27 Presystolic distension of the right ventricle and fourth heart sounds are features of severe pulmonary stenosis because these physiologic conditions prevail when obstruction to right ventricular outflow is severe (Figures 11-7B, 11-10, and 11-22).69
Electrocardiogram
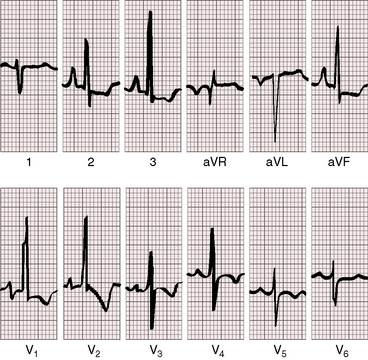
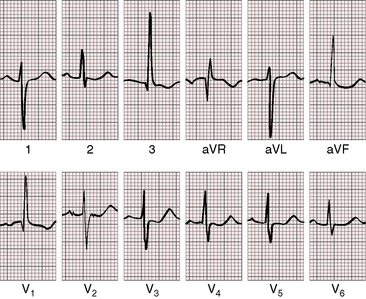
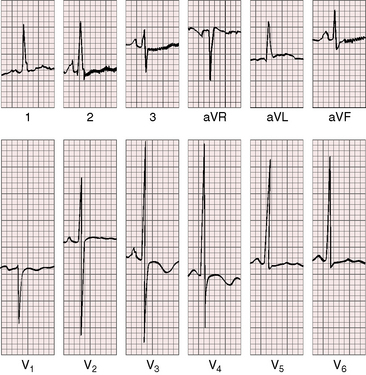
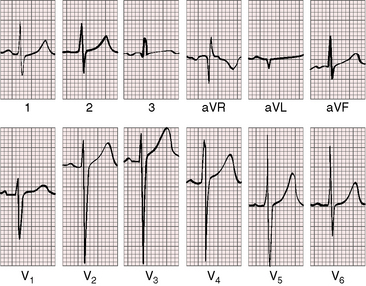
As severity increases, P waves become tall and peaked (Figure 11-23).86 Giant P waves occasionally appear especially in lead 2 (Figure 11-24). The P wave in lead V1 may be entirely negative because the P terminal force is written by a dilated right atrium (see Figure 11-24).87 PR interval prolongation has been attributed to an increase in right atrial size that prolongs the transit time from sinus node to atrioventricular node (Figure 11-25).22 Right axis deviation tends to vary with right ventricular systolic pressure (Figures 11-23 through 11-26).49 Superior orientation of the QRS axis occurs in infants with pulmonary artery stenosis and the rubella syndrome.88
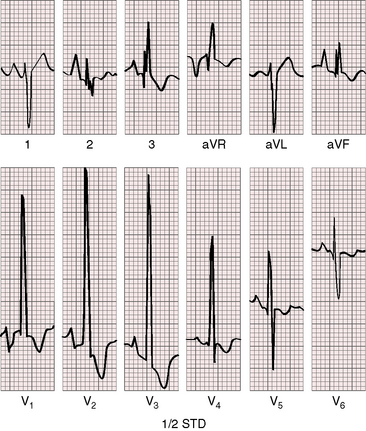
R wave amplitude in lead V1 and R/S ratio in leads V1 and V6 are closely coupled with severity (Figures 11-23 through 11-27).49,89 After infancy, the R wave in lead V1 is normally less than 8 mm; and in children, an R wave amplitude greater than 10 mm is exceptional. With severe pulmonary stenosis, the R wave in lead V1 is tall and monophasic, and lead V6 exhibits a reciprocally deep S wave (see Figures 11-23 and 11-24). Children tend to generate higher R waves in lead V1 than adults with similar gradients.
The configuration of the QRS in lead V1 varies (see Figure 11-27). In mild pulmonary stenosis, an rSr′ is difficult to distinguish from normal.80,90,91 With greater degrees of stenosis, the terminal R wave becomes taller, writing an rsR pattern (see Figure 11-26),80 or a small r wave appears as a notch on the upstroke of a tall R prime (see Figures 11-26 and 11-27). In severe pulmonary stenosis, the pattern in lead V1 is either a monophasic R wave (see Figures 11-23 and 11-24) or a qR, with the q wave reflecting right atrial enlargement (see Figure 11-25). The neonate with pinpoint pulmonary stenosis and a small right ventricular cavity has an electrocardiogram that resembles pulmonary atresia with intact ventricular septum, namely a normal QRS axis and adult progression in precordial leads (Figure 11-28).92 In subinfundibular pulmonary stenosis (double-chambered right ventricle), a smaller portion of right ventricle is in the high-pressure compartment, so the degree of right ventricular hypertrophy in leads V1-3 is appreciably less than expected based on the severity of stenosis alone.76
In healthy neonates, upright right precordial T waves become inverted by 4 days of age. In infants and children with mild pulmonary stenosis, upright right precordial T waves may persist as the only electrocardiographic sign of elevated right ventricular systolic pressure (Figure 11-29).93 In severe pulmonary stenosis, the T wave direction shifts leftward, superiorly and posteriorly, as the QRS axis shifts to the right and anteriorly. The QRS-T angle widens, so inverted T waves appear in leads 2, 3, and aVF and in right precordial leads (see Figures 11-23 and 11-24). Deeply inverted T waves that cove upward on their descending limbs and are accompanied by ST segment depressions extending beyond lead V2 are features of severe pulmonary stenosis (see Figures 11-23 and 11-24).
Noonan’s syndrome with dysplastic pulmonary valve stenosis has an electrocardiogram that differs from the patterns just described. Axis deviation may be extreme,5,94 the QRS tends to be splintered and prolonged, and a QS pattern appears in inferior and left precordial leads.29 However, the pattern of left axis deviation, abnormal R/S ratio over the left precordium with abnormal Q waves is related to the syndrome per se and not to a specific accompanying cardiac defect.95
x-ray
Pulmonary vascularity remains normal, even in severe pulmonary stenosis, until the right ventricle fails and blood flow to the lungs declines (Figure 11-30). Flow to the right lung is occasionally less than flow to the left lung (unequal distribution).96 Radiologic signs of pulmonary artery stenosis include zones of segmental hypovascularity that correspond to locations of the arterial obstructions and areas of fusiform intrapulmonary dilation beyond the zones of stenosis (Figures 11-4, 11-5B, and 11-31).13
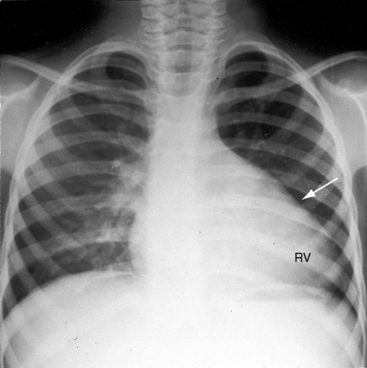
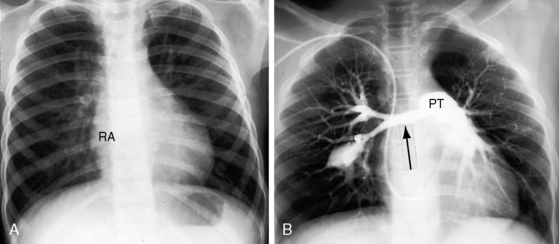
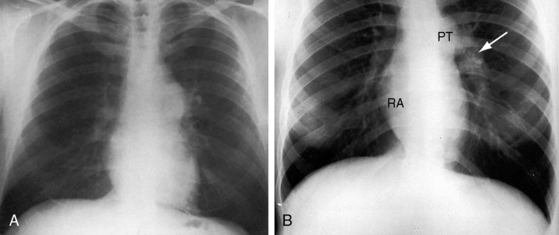
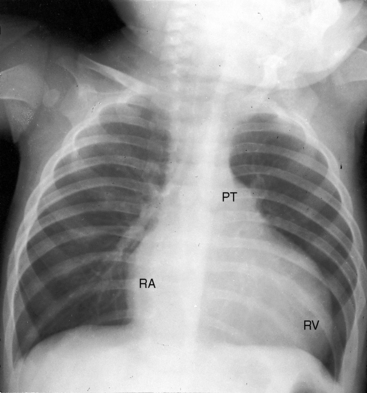
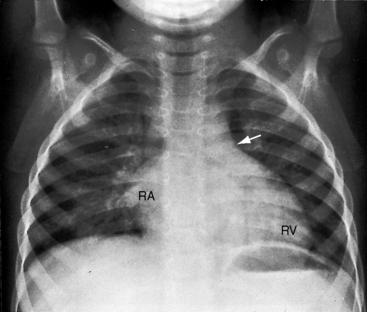
Conspicuous increases in right atrial and right ventricular size are radiologic signs of severity, especially when the enlargement appears at an early age (see Figure 11-30). The dilated right ventricle is rounded rather than boot-shaped, and its apex lies above the left hemidiaphragm (see Figure 11-30). The aortic arch is left-sided, with rare exceptions (Figure 11-32).97 In Noonan’s syndrome, in contrast to Turner’s syndrome, ascending aortic dilation is rare (see Chapter 7).98
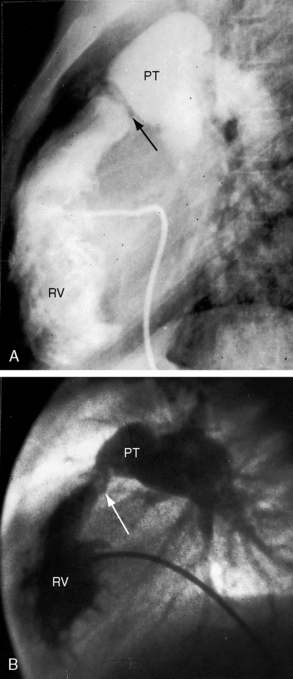
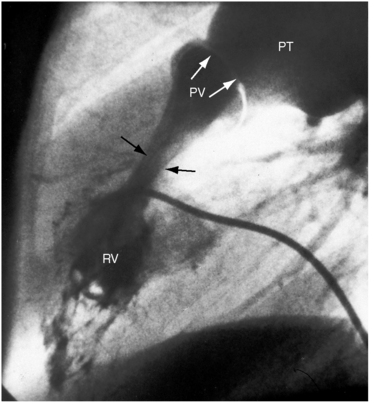
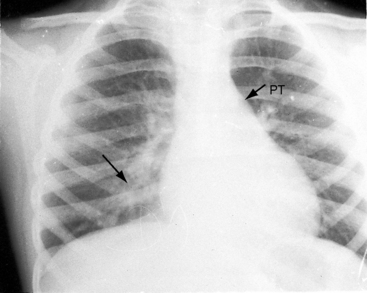
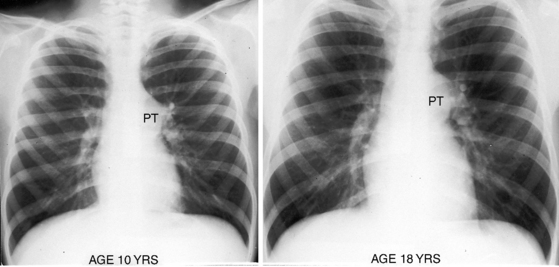
Typical mobile dome-shaped pulmonary valve stenosis is accompanied by conspicuous dilation of the pulmonary trunk (Figures 11-1, 11-3, 11-6, 11-30, and 11-33).27 Dilation is present at an early age (see Figures 11-1 and 11-30) and may reach aneurysmal proportions (Figure 11-34) but without risk of rupture.27,99,100 Dilation of the pulmonary trunk above a mobile dome-shaped pulmonary valve is related to intrinsic abnormalities of the media associated with the specific dome-shaped congenital valve morphology, not with its functional state.2 Disproportionate dilation of the left branch often accompanies dilation of the pulmonary trunk (see Figure 11-1,upper).101 The jet from the stenotic valve breaks up as it strikes the dome of the pulmonary trunk (Figure 11-35A and video11-1) whose left branch is a geometric continuation of the dilated trunk.3 Calcification of a dome-shaped stenotic pulmonary valve is rare and is reserved for older adults,4,102 although calcific deposits can be sequelae of infective endocarditis.77 Dysplastic pulmonary valve stenosis is accompanied by comparatively little dilation of the pulmonary trunk (Figure 11-36), an observation that discounts turbulent flow as an cause of dilation.2,5,6 Localized stenosis of the ostium of the infundibulum is characterized by slight indentation at that site, above which the infundibular chamber is moderately dilated (Figure 11-37).
Echocardiogram
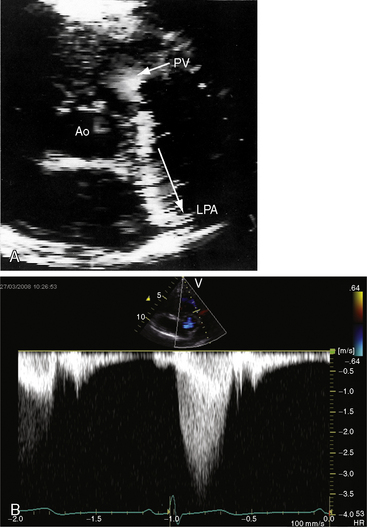
Color flow imaging in typical mobile dome-shaped pulmonary valve stenosis discloses a high-velocity jet directed toward the left pulmonary artery (see Figure 11-35A).103 Continuous wave Doppler scan establishes the velocity across the stenotic pulmonary valve from which the peak instantaneous gradient can be estimated (see Figure 11-35B).103
During right ventricular systole, the mobile pulmonary valve domes briskly cephalad as a single unit. A bicuspid pulmonary valve can occasionally be identified.104 In severe pulmonary stenosis, the increased force of right atrial contraction is transmitted into the right ventricle onto the under surface of the mobile pulmonary valve, which opens in presystole, generating a Doppler signal (Figure 11-38; see previous discussion). Secondary hypertrophic subpulmonary stenosis is recognized as an envelope within the major continuous wave Doppler signal (see Figure 11-38). Mild pulmonary regurgitation is occasionally detected in the presence of pulmonary valve stenosis (see Figures 11-35B and 11-38).
The position of the atrial septum is imaged as concave to the left, reflecting the high pressure in the right atrium. Color flow detects a right-to-left interatrial shunt across a patent foramen ovale. Right atrial size can be estimated, and the size, function, and degree of right ventricular hypertrophy can be established.103 When pulmonary stenosis is severe, the ventricular septum thickens and flattens.103 Color flow imaging determines the presence and degree of tricuspid regurgitation from which right ventricular systolic pressure can be estimated by the velocity of the regurgitant jet. Systolic and diastolic function of the left ventricle can and should be assessed because right ventricular pressure overload influences the function of the left ventricle (see previous discussion).25,26
Dysplastic pulmonary valve stenosis is imaged as thickened immobile leaflets. The pulmonary trunk is not significantly dilated. Stenosis of the ostium of the infundibulum and subinfundibular stenosis (double-chambered right ventricle) can be identified and physiologically assessed. Pulmonary artery stenosis is accompanied by a normal-sized pulmonary trunk and small proximal pulmonary arteries. Doppler scan interrogation records the velocities across the sites of branch stenosis and distinguishes congenital pulmonary artery stenosis from the normal neonatal physiologic drop in systolic pressure between the pulmonary trunk and its proximal branches.15
1 Morgagni G.B. The seats and causes of diseases investigated by anatomy. London: Millar, Cadell, Johnson and Payne; 1769.
2 Niwa K., Perloff J.K., Bhuta S.M., et al. Structural abnormalities of great arterial walls in congenital heart disease: light and electron microscopic analyses. Circulation. 2001;103:393-400.
3 Muster A.J., Van Grondelle A., Paul M.H. Unequal pressures in the central pulmonary arterial branches in patients with pulmonary stenosis. The influence of blood velocity and anatomy. Pediatr Cardiol. 1982;2:7-14.
4 Roberts W.C., Mason D.T., Morrow A.G., Braunwald E. Calcific pulmonic stenosis. Circulation. 1968;37:973-978.
5 Koretzky E.D., Moller J.H., Korns M.E., Schwartz C.J., Edwards J.E. Congenital pulmonary stenosis resulting from dysplasia of valve. Circulation. 1969;40:43-53.
6 Schneeweiss A., Blieden L.C., Shem-Tov A., Goor D., Milo S., Neufeld H.N. Diagnostic angiocardiographic criteria in dysplastic stenotic pulmonic valve. Am Heart J. 1983;106:761-762.
7 Peacock T.B. On malformations of the human heart. London: J. Churchill and Sons; 1866.
8 Keith A. The Hunterian lectures on malformations of the heart. Lancet. 1909;174:359-363.
9 Lucas R.V.Jr, Varco R.L., Lillehei C.W., Adams P.Jr, Anderson R.C., Edwards J.E. Anomalous muscle bundle of the right ventricle. Hemodynamic consequences and surgical considerations. Circulation. 1962;25:443-455.
10 Perloff J.K., Ronan J.A.Jr, De Leon A.C.Jr. Ventricular septal defect with the “two-chambered right ventricle”. Am J Cardiol. 1965;16:894-900.
11 Yoo S.J., Kim Y.M., Bae E.J., Sohn S., Ko J.K., Park I.S. Rare variants of divided right ventricle with sequestered apical trabecular component. Int J Cardiol. 1997;60:249-255.
12 Oppenheimer E.H. Partial atresia of the main branches of the pulmonary artery occurring in infancy and accompanied by calcification of the pulmonary artery and aorta. Bulletin of the Johns Hopkins Hospital. 1938;63:261.
13 Franch R.H., Gay B.B.Jr. Congenital stenosis of the pulmonary artery branches. A classification, with postmortem findings in two cases. Am J Med. 1963;35:512-529.
14 Danilowicz D.A., Rudolph A.M., Hoffman J.I., Heymann M. Physiologic pressure differences between main and branch pulmonary arteries in infants. Circulation. 1972;45:410-419.
15 Rodriguez R.J., Riggs T.W. Physiologic peripheral pulmonic stenosis in infancy. Am J Cardiol. 1990;66:1478-1481.
16 Beuren A.J., Schulze C., Eberle P., Harmjanz D., Apitz J. The syndrome of supravalvular aortic stenosis, peripheral pulmonary stenosis, mental retardation and similar facial appearance. Am J Cardiol. 1964;13:471-483.
17 Levin D.L., Heymann M.A., Rudolph A.M. Morphological development of the pulmonary vascular bed in experimental pulmonic stenosis. Circulation. 1979;59:179-182.
18 Gregg N.M. Congenital cataract following German measles in the mother. Trans Ophthalmol Soc Aust. 1941;3:35-46.
19 Cochi S.L., Edmonds L.E., Dyer K., et al. Congenital rubella syndrome in the United States, 1970–1985. On the verge of elimination. Am J Epidemiol. 1989;129:349-361.
20 Munro N.D., Sheppard S., Smithells R.W., Holzel H., Jones G. Temporal relations between maternal rubella and congenital defects. Lancet. 1987;2:201-204.
21 Perloff J.K. Myocardial growth and the development and regression of increased ventricular mass. In Perloff J.K., Child J.S., Aboulhosn J., editors: Congenital heart disease in adults, 3rd ed, Philadelphia: Saunders/Elsevier, 2009.
22 Nakazawa M., Marks R.A., Isabel-Jones J., Jarmakani J.M. Right and left ventricular volume characteristics in children with pulmonary stenosis and intact ventricular septum. Circulation. 1976;53:884-890.
23 Truccone N.J., Steeg C.N., Dell R., Gersony W.M. Comparison of the cardiocirculatory effects of exercise and isoproterenol in children with pulmonary or aortic valve stenosis. Circulation. 1977;56:79-82.
24 Moller J.H., Rao S., Lucas R.V.Jr. Exercise hemodynamics of pulmonary valvular stenosis. Study of 64 children. Circulation. 1972;46:1018-1026.
25 Kelly D.T., Spotnitz H.M., Beiser G.D., Pierce J.E., Epstein S.E. Effects of chronic right ventricular volume and pressure loading on left ventricular performance. Circulation. 1971;44:403-412.
26 Stenberg R.G., Fixler D.E., Taylor A.L., Corbett J.R., Firth B.G. Left ventricular dysfunction due to chronic right ventricular pressure overload. Resolution following percutaneous balloon valvuloplasty for pulmonic stenosis. Am J Med. 1988;84:157-161.
27 Abrahams D.G., Wood P. Pulmonary stenosis with normal aortic root. Br Heart J. 1951;13:519-548.
28 Grech V. History, diagnosis, surgery and epidemiology of pulmonary stenosis in Malta. Cardiol Young. 1998;8:337-343.
29 Sanchez-Cascos A. The Noonan syndrome. Eur Heart J. 1983;4:223-229.
30 Campbell M. Factors in the aetiology of pulmonary stenosis. Br Heart J. 1962;24:625-632.
31 Hsu J.-H., Dai Z.-K., Wu J.-R., et al. Critical pulmonary stenosis in two successive siblings. Int J Cardiol. 2003;87:297-299.
32 Klinge T., Laursen H.B. Familial pulmonary stenosis with underdeveloped or normal right ventricle. Br Heart J. 1975;37:60-64.
33 Mccarron W.E., Perloff J.K. Familial congenital valvular pulmonic stenosis. Am Heart J. 1974;88:357-359.
34 Patterson D.F., Haskins M.E., Schnarr W.R. Hereditary dysplasia of the pulmonary valve in beagle dogs. Pathologic and genetic studies. Am J Cardiol. 1981;47:631-641.
35 Baird P.A., De Jong B.P. Noonan’s syndrome (XX and XY Turner phenotype) in three generations of a family. J Pediatr. 1972;80:110-114.
36 Mendez H.M., Opitz J.M. Noonan syndrome: a review. Am J Med Genet. 1985;21:493-506.
37 Cullimore A.J., Smedstad K.G., Brennan B.G. Pregnancy in women with Noonan syndrome: report of two cases. Obstet Gynecol. 1999;93:813-816.
38 Noonan J.A. Hypertelorism with Turner phenotype. A new syndrome with associated congenital heart disease. Am J Dis Child. 1968;116:373-380.
39 Wasserman M.P., Varghese P.J., Rowe R.D. The evolution of pulmonary arterial stenosis associated with congenital rubella. Am Heart J. 1968;76:638-644.
40 Hayes C.J., Gersony W.M., Driscoll D.J., et al. Second natural history study of congenital heart defects. Results of treatment of patients with pulmonary valvar stenosis. Circulation. 1993;87:I28-I37.
41 Johnson L.W., Grossman W., Dalen J.E., Dexter L. Pulmonic stenosis in the adult. Long-term follow-up results. N Engl J Med. 1972;287:1159-1163.
42 Genovese P.D., Rosenbaum D. Pulmonary stenosis with survival to the age of 78 years. Am Heart J. 1951;41:755-761.
43 Geraci J.E., Burchell H.B., Edwards J.E. Cardiac clinics. 140. Congenital pulmonary stenosis with intact ventricular septum in persons more than 50 years of age: report of 2 cases. Proc Staff Meet Mayo Clin. 1953;28:346-352.
44 Mody M.R. The natural history of uncomplicated valvular pulmonic stenosis. Am Heart J. 1975;90:317-321.
45 White P.D., Hurst J.W., Fennell R.H. Survival to the age of seventy-five years with congenital pulmonary stenosis and patent foramen ovale. Circulation. 1950;2:558-564.
46 Wild J.B., Eckstein J.W., Van Epps E.F., Culbertson J.W. Three patients with congenital pulmonic valvular stenosis surviving for more than fifty-seven years; medical histories and physiologic data. Am Heart J. 1957;53:393-403.
47 Stone F.M., Bessinger F.B.Jr, Lucas R.V.Jr, Moller J.H. Pre- and postoperative rest and exercise hemodynamics in children with pulmonary stenosis. Circulation. 1974;49:1102-1106.
48 Lueker R.D., Vogel J.H., Blount S.G.Jr. Regression of valvular pulmonary stenosis. Br Heart J. 1970;32:779-782.
49 Danilowicz D., Hoffman J.I., Rudolph A.M. Serial studies of pulmonary stenosis in infancy and childhood. Br Heart J. 1975;37:808-818.
50 Moller J.H., Adams P.Jr. The natural history of pulmonary valvular stenosis. Serial cardiac catheterizations in 21 children. Am J Cardiol. 1965;16:654-664.
51 Ardura J., Gonzalez C., Andres J. Does mild pulmonary stenosis progress during childhood? A study of its natural course. Clin Cardiol. 2004;27:519-522.
52 Wood P. Diseases of the heart and circulation, 2nd ed. Philadelphia: J.B. Lippincott; 1956.
53 Genkins G., Lasser R.P. Chest pain in patients with isolated pulmonic stenosis. Circulation. 1957;15:258-266.
54 Shirani J., Zafari A.M., Roberts W.C. Sudden death, right ventricular infarction, and abnormal right ventricular intramural coronary arteries in isolated congenital valvular pulmonic stenosis. Am J Cardiol. 1993;72:368-370.
55 Burch M., Sharland M., Shinebourne E., Smith G., Patton M., Mckenna W. Cardiologic abnormalities in Noonan syndrome: phenotypic diagnosis and echocardiographic assessment of 118 patients. J Am Coll Cardiol. 1993;22:1189-1192.
56 Noonan J.A., Ehmke D.A. Associated noncardiac malformations in children with congenital heart disease. J Pediatr. 1963;63:468-470.
57 Ucar T., Atalay S., Tekin M., Tutar E. Bilateral coronary artery dilatation and supravalvular pulmonary stenosis in a child with Noonan syndrome. Pediatr Cardiol. 2005;26:848-850.
58 Collins E., Turner G. The Noonan syndrome—a review of the clinical and genetic features of 27 cases. J Pediatr. 1973;83:941-950.
59 Wong C.K., Cheng C.H., Lau C.P., Leung W.H. Congenital coronary artery anomalies in Noonan’s syndrome. Am Heart J. 1990;119:396-400.
60 Freij B.J., South M.A., Sever J.L. Maternal rubella and the congenital rubella syndrome. Clin Perinatol. 1988;15:247-257.
61 Berrocal T., Gamo E., Navalon J., et al. Syndrome of Alagille: radiological and sonographic findings. A review of 37 cases. Eur Radiol. 1997;7:115-118.
62 Krantz I.D., Piccoli D.A., Spinner N.B. Alagille syndrome. J Med Genet. 1997;34:152-157.
63 Levin S.E., Zarvos P., Milner S., Schmaman A. Arteriohepatic dysplasia: association of liver disease with pulmonary arterial stenosis as well as facial and skeletal abnormalities. Pediatrics. 1980;66:876-883.
64 Selicorni A., Colli A.M., Passarini A., et al. Analysis of congenital heart defects in 87 consecutive patients with Brachmann-de Lange syndrome. Am J Med Genet. 2009;149A:1268-1272. Part A
65 Digilio M.C., Sarkozy A., De Zorzi A., et al. LEOPARD syndrome: clinical diagnosis in the first year of life. Am J Med Genet. 2006;140:740-746. Part A
66 Holt J.H.Jr, Eddleman E.E.Jr. The precordial movements in adults with pulmonic stenosis. Circulation. 1967;35:492-500.
67 Perloff J.K. Physical examination of the heart and circulation, 4th ed. Shelton, Connecticut: People’s Medical Publishing House; 2009.
68 Nagle R.E., Tamara F.A. Left parasternal impulse in pulmonary stenosis and atrial septal defect. Br Heart J. 1967;29:735-741.
69 Leatham A., Weitzman D. Auscultatory and phonocardiographic signs of pulmonary stenosis. Br Heart J. 1957;19:303-317.
70 Mehlman D.J., Troncoso P., Hay R., Glagov S. Midsystolic click accompanying isolated bicuspid pulmonic valve. Am Heart J. 1982;103:145-147.
71 Hultgren H.N., Reeve R., Cohn K., Mcleod R. The ejection click of valvular pulmonic stenosis. Circulation. 1969;40:631-640.
72 Leatham A., Vogelpoel L. The early systolic sound in dilatation of the pulmonary artery. Br Heart J. 1954;16:21-33.
73 Riggs T.W., Weinhouse E. Respiratory influence on Doppler estimation of valvar gradients in congenital pulmonic stenosis. Am J Cardiol. 1992;70:956-958.
74 Gamboa R., Hugenholtz P.G., Nadas A.S. Accuracy of the phonocardiogram in assessing severity of aortic and pulmonic stenosis. Circulation. 1964;30:35-46.
75 Vogelpoel L., Schrire V. Auscultatory and phonocardiographic assessment of pulmonary stenosis with intact ventricular septum. Circulation. 1960;22:55.
76 Patel R., Astley R. Right ventricular obstruction due to anomalous muscle bands. Br Heart J. 1973;35:890-893.
77 Alday L.E., Moreyra E. Calcific pulmonary stenosis. Br Heart J. 1973;35:887-889.
78 Perloff J.K., Lebauer E.J. Auscultatory and phonocardiographic manifestations of isolated stenosis of the pulmonary artery and its branches. Br Heart J. 1969;31:314-321.
79 Eldridge F., Selzer A., Hultgren H. Stenosis of a branch of the pulmonary artery; an additional cause of continuous murmurs over the chest. Circulation. 1957;15:865-874.
80 Ellison R.C., Miettinen O.S. Interpretation of RSR’ in pulmonic stenosis. Am Heart J. 1974;88:7-10.
81 Lees M.H., Dotter C.T. Bronchial circulation in severe multiple peripheral pulmonary artery stenosis; case report illustrating the origin of continuous murmur. Circulation. 1965;31:759-761.
82 Singh S.P. Unusual splitting of the second heart sound in pulmonary stenosis. Am J Cardiol. 1970;25:28-33.
83 Shaver J.A., Nadolny R.A., O’toole J.D., et al. Sound pressure correlates of the second heart sound. An intracardiac sound study. Circulation. 1974;49:316-325.
84 Schrire V., Vogelpoel L. The role of the dilated pulmonary artery in abnormal splitting of the second heart sound. Am Heart J. 1962;63:501-507.
85 Perloff J.K. Auscultatory and phonocardiographic manifestations of pulmonary hypertension. Prog Cardiovasc Dis. 1967;9:303-340.
86 Deverall P.B., Roberts N.K., Stark J. Arrhythmias in children with pulmonary stenosis. Br Heart J. 1970;32:472-476.
87 Macruz R., Perloff J.K., Case R.B. A method for the electrocardiographic recognition of atrial enlargement. Circulation. 1958;17:882-889.
88 Halloran K.H., Sanyal S.K., Gardner T.H. Superiorly oriented electrocardiographic axis in infants with the rubella syndrome. Am Heart J. 1966;72:600-606.
89 Rasmussen K., Sorland S.J. Prediction of right ventricular systolic pressure in pulmonary stenosis from combined vectorcardiographic data. Am Heart J. 1973;86:318-328.
90 Camerini F., Davies L.G. Secondary R waves in right chest leads. Br Heart J. 1955;17:28-32.
91 Fowler R.S. Terminal QRS conduction delay in pulmonary stenosis in children. Am J Cardiol. 1968;21:669-672.
92 Miller G.A., Restifo M., Shinebourne E.A., et al. Pulmonary atresia with intact ventricular septum and critical pulmonary stenosis presenting in first month of life. Investigation and surgical results. Br Heart J. 1973;35:9-16.
93 Celermajer J.M., Izukawa T., Varghese P.J., Rowe R.D. Upright T wave in V1: a useful diagnostic sign of mild pulmonic valve stenosis in children. J Pediatr. 1969;74:413-415.
94 Rasmussen K., Sorland S.J. Electrocardiogram and vectorcardiogram in Turner phenotype with normal chromosomes and pulmonary stenosis. Br Heart J. 1973;35:937-945.
95 Raaijmakers R., Noordam C., Noonan J.A., Croonen E.A., Van Der Burgt C.J.A.M., Draaisma J.M.T. Are ECG abnormalities in Noonan syndrome characteristic for the syndrome? Eur J Pediatr. 2008;167:1363-1367.
96 Chen J.T., Robinson A.E., Goodrich J.K., Lester R.G. Uneven distribution of pulmonary blood flow between left and right lungs in isolated valvular pulmonary stenosis. Am J Roentgenol Radium Ther Nucl Med. 1969;107:343-350.
97 Bressie J.L. Pulmonary valvular stenosis with intact ventricular septum and right aortic arch. Br Heart J. 1964;26:154-156.
98 Shachter N., Perloff J.K., Mulder D.G. Aortic dissection in Noonan’s syndrome (46 XY turner). Am J Cardiol. 1984;54:464-465.
99 Shindo T., Kuroda T., Watanabe S., Hojo Y., Sekiguchi H., Shimada K. Aneurysmal dilatation of the pulmonary trunk with mild pulmonic stenosis. Intern Med. 1995;34:199-202.
100 Tami L.F., Mcelderry M.W. Pulmonary artery aneurysm due to severe congenital pulmonic stenosis. Case report and literature review. Angiology. 1994;45:383-390.
101 Gay B.B.Jr, Franch R.H. Pulsations in the pulmonary arteries as observed with roentgenoscopic image amplification. Observations in patients with isolated pulmonary valvular stenosis. Am J Roentgenol Radium Ther Nucl Med. 1960;83:335-344.
102 Gabriele O.F., Scatliff J.H. Pulmonary valve calcification. Am Heart J. 1970;80:299-302.
103 Nishimura R.A., Pieroni D.R., Bierman F.Z., et al. Second natural history study of congenital heart defects. Pulmonary stenosis: echocardiography. Circulation. 1993;87:I73-I79.
104 Mcaleer E., Kort S., Rosenzweig B.P., et al. Unusual echocardiographic views of bicuspid and tricuspid pulmonic valves. J Am Soc Echocardiogr. 2001;14:1036-1038.