Chapter 30 Congenital Pulmonary Arteriovenous Fistula
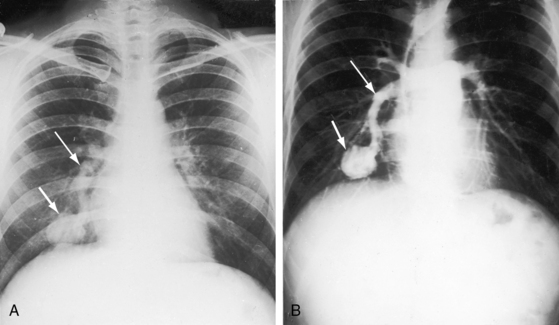
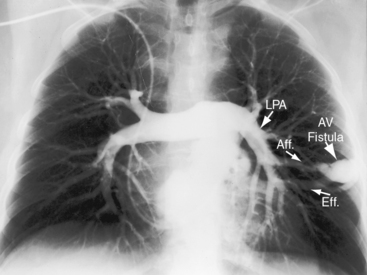
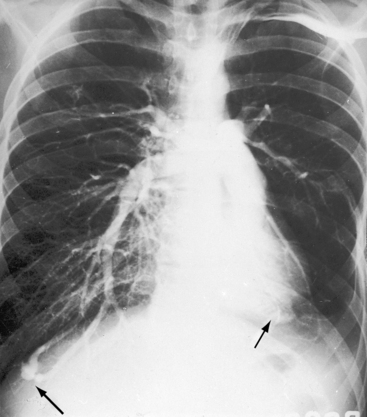
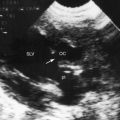
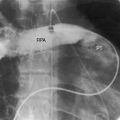
In 1897, the British Medical Journal published a necropsy description of congenital pulmonary arteriovenous fistulae,1 and four decades later, the anomaly was recognized in a living subject.2 Pulmonary arteriovenous fistulae are the result of an embryonic fault in the vascular complex that is responsible for the development of pulmonary arteries and veins.3 The fistulae can be solitary or multiple, unilateral or bilateral, or minute and diffuse throughout both lungs.4–6 Approximately 75% of congenital pulmonary arteriovenous fistulae involve the lower lobes or right middle lobe (Figures 30-1 through 30-5)4,7; they usually occur without coexisting congenital heart disease. Isolated exceptions have been reported with left isomerism (see Chapter 3)8,9 and with atrial septal defect (see Chapter 15).10 Estimated minimal prevalence rate is 1/10,000 births.11
In 1865, Babington12 called attention to familial epistaxis; and in 1876, Legg13 described recurrent epistaxes and cutaneous telangiectasia in three generations. Twenty years later, Rendu14 published his classic description of familial epistaxes and telangiectasia (cutaneous angiomatas) of the nose, cheeks, and upper lip. In 1901, Osler15 reported on a “family form of recurring epistaxis associated with multiple telangiectases of the skin and mucous membranes”; and in 1907, Weber16 reported “multiple hereditary developmental angiomata (telangiectases) of the skin and mucous membranes associated with recurring haemorrhages.” In 1909, Hanes17 referred to the disorder as hereditary hemorrhagic telangiectasia,18 but the eponym Rendu, Osler, Weber remains in use without the inclusion of Legg13 and with no consensus about the most appropriate sequence of names.19,20 The diagnosis of hereditary hemorrhagic telangiectasia (HHT) is made clinically with the Curaçao criteria, which was established in 1999 by the Scientific Advisory Board of the HHT Foundation International. The criteria include recurrent epistaxis; telangiectases of the lips, oral cavity, fingers, and nose; gastrointestinal telangiectasia; and pulmonary, hepatic, cerebral or spinal arteriovenous malformations.19,21,22
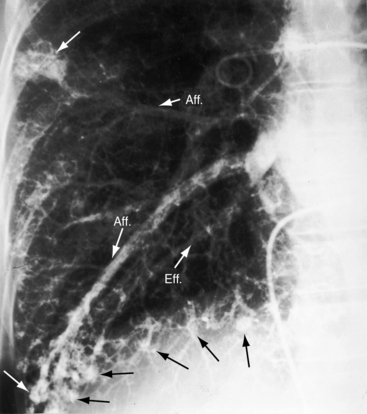
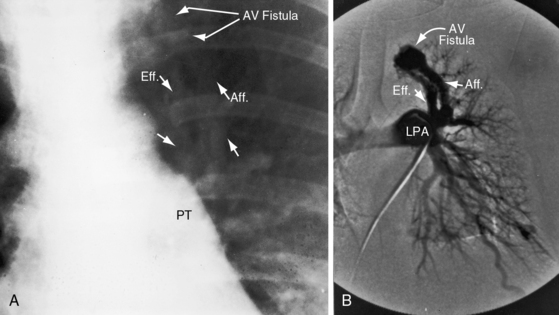
A fistula consists of either one or more relatively large vascular trunks, a thin aneurysmal sac, or a tangle of distended tortuous vascular channels (Figures 30-1 through 30-7).18 The arterial supply is through enlarged tortuous branches of a pulmonary artery, and drainage is through dilated pulmonary veins (see Figures 30-1 through 30-4 and 30-6).3,23 Fistulous rupture results in hemorrhage into the pulmonary parenchyma or into the pleural space.24 Exceptionally, the arterial supply is from a bronchial, intercostal, anomalous systemic artery or a coronary artery (see Chapter 22). The fistula is then systemic arteriovenous rather than pulmonary arteriovenous.18 A rare anatomic variation consists of a congenital connection between a pulmonary artery and the left atrium, an anomaly in which an initial connection exists between a pulmonary artery and a pulmonary vein; but during vascular development, the pulmonary vein becomes incorporated into the left atrium.25–29 Extralobar arteriovenous fistulae are represented by pulmonary sequestrations in which the arterial supply and venous drainage are systemic rather than pulmonary. Isolated congenital varicose pulmonary veins are rare and not the result of arteriovenous malformations.30
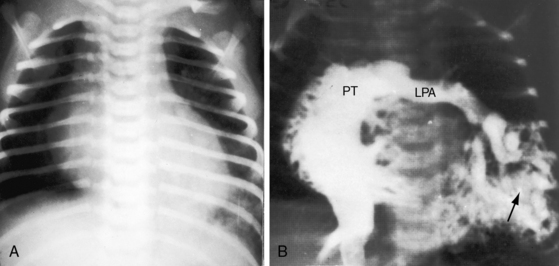
The physiologic consequences of pulmonary arteriovenous fistulae depend on the amount of unoxygenated blood delivered through the malformation and on the size of the malformation, which tends to increase with age.31,32 Although the volume of blood delivered through the fistula is sufficient to cause cyanosis, it is rarely sufficient to impose a physiologic burden (see Figure 30-7). Pulmonary artery pressure is normal, with rare exception.33 In experimental pulmonary arteriovenous fistulae, cardiac output and left ventricular stroke volume are increased,34 but in congenital pulmonary arteriovenous fistulae, blood flow through the malformation is increased while flow through uninvolved lung decreases by a comparable amount. Accordingly, the net volume of blood that reaches the left side of the heart is little if at all affected, so left ventricular stroke volume and cardiac output remain normal or nearly so. Rarely, a large pulmonary arteriovenous malformation imposes an excess volume load on the left side of the heart and induces congestive heart failure (see Figure 30-7).
Blood flow through pulmonary arteriovenous fistulae is affected by mechanical factors.35 Flow through lower lobe fistulae is augmented in the upright position because of increased perfusion of dependent portions of the lungs. A decubitus position compresses the dependent lung and reduces blood flow through an ipsilateral fistula. A case in point was a large pulmonary arteriovenous fistula in an infant in whom ipsilateral chest wall compression was therapeutic, immediately decreasing the cyanosis and relieving the dyspnea (see Figure 30-7). Elevation of the diaphragm during pregnancy can compress a lower lobe fistula and abolish the accompanying murmur, which reappears after delivery.35
Acquired pulmonary arteriovenous fistulae are occasional sequelae of cavo-pulmonary shunts, especially Glenn shunts.36,37 Acquired fistulae occur in children with hepatic cirrhosis and portal hypertension, especially with biliary atresia and right isomerism (see Chapter 3),38,39 and regress after liver transplantation.38 Large hepatic arteriovenous fistulae sometimes occur without pulmonary arteriovenous fistulae in Rendu-Osler-Weber disease.40–42 Hepatic, cerebral, and pulmonary arteriovenous fistulae may coexist.43
In 1917, telangiectasia and epistaxes were reported in a patient who died of massive hemothorax and at necropsy had three pulmonary arteriovenous fistulae.44 The association of hemorrhagic telangiectasias with pulmonary arteriovenous fistulae has been amply confirmed.4,5,18,19 Hereditary hemorrhagic telangiectasis is an autosomal dominant vasculopathy.11 Clinical diagnostic criteria have changed remarkably little in the last century.19
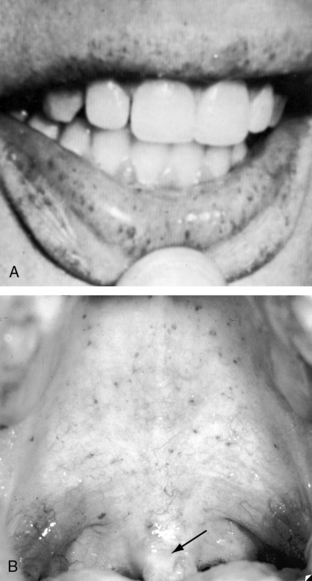
Pulmonary arteriovenous fistulae occur in 5% to 30% of patients with telangiectasia, and telangiectasia occur in 30% to 60% of patients with pulmonary arteriovenous fistulae.45 The incidence rate of pulmonary arteriovenous fistulae with telangiectasia is 1:50,000 with autosomal dominant transmission and a 20% mutation rate. The mucocutaneous lesions are tiny localized arteriovenous fistulae composed of thin dilated vascular membranes with a layer of endothelium and no muscular or elastic coat.18,46 The lesions are fragile and rupture easily.18 Telangiectasia are found on the skin, the lips (Figure 30-8), and the nasal, oral and vaginal mucous membranes; beneath the nails; and in the gastrointestinal tract, liver, central nervous system, kidney, and retina.18,47–49
History
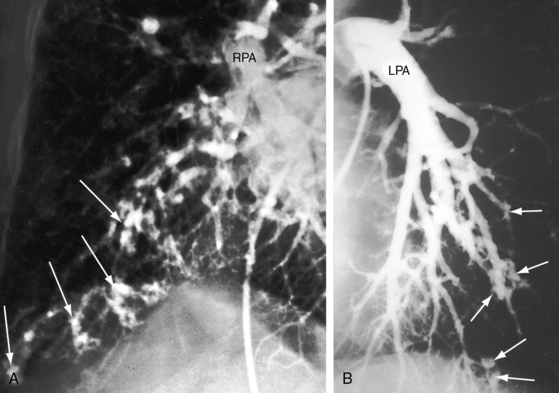
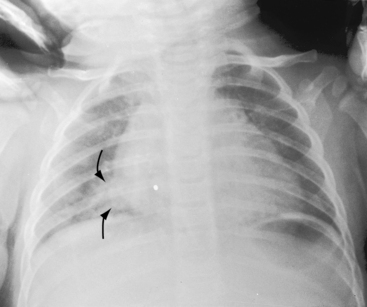
Pulmonary arteriovenous fistulae and hereditary hemorrhagic telangiectasia afflict males and females with equal frequency.18,50,51 The fistulae tend to increase in size and number with the passage of time31,32 and are seldom recognized until adulthood.50,52 Symptoms are the same whether the malformations are multiple or single (see Figures 30-1, 30-2, and 30-3), except for minute diffuse fistulous channels (see Figures 30-4 and 30-5). Mean patient age in a large series was 39 years (range, 3 years to 73 years), with a distinct majority over age 20 years. However, the first description of a pulmonary arteriovenous fistula was at necropsy in a 12-year-old boy (see previous).1 Despite adult prevalence, cyanosis is occasionally present shortly after birth,53 and the malformation is occasionally overtly manifest in childhood (Figure 30-9).53
Asymptomatic acyanotic pulmonary arteriovenous fistulae usually come to light because of abnormal shadows on a routine chest x-ray. Symptoms and complications are related to the pulmonary malformation per se or to coexisting hereditary hemorrhagic telangiectasia.34,54 Congestive heart failure is reserved for infants with a rare large fistula (see Figure 30-7). The right-to-left shunt inherent in the fistulous communication results in cyanosis but seldom causes significant symptoms.53 Dyspnea and fatigue are the result of anemia provoked by bleeding telangiectasia. Rupture of fistulae into a contiguous bronchus causes hemoptysis that varies from mild and occasional to recurrent and massive.55,56 Fistulae are rarely substrates for infective endocarditis.23 Chest pain accompanies pleural involvement. Hemothorax results from rupture of a subpleural fistula.18,23,24,55,57 Intrapulmonary hemorrhage can be massive and fatal.58 Pregnancy is accompanied by adverse effects that resolve after delivery, including hemothorax,59 hemoptysis,60,61 enlargement of existing fistulae, increasing cyanosis,59,61 and expansion of occult fistulae.62 However, pregnancy exerts favorable effects, such as compression of lower lobe fistulae, because of elevation of the diaphragm.35
Epistaxes are the most frequent and usually the first overt hemorrhagic event.18 Cutaneous lesions bleed easily, especially when exposed to sunlight.18 Tracheobronchial telangiectases set the stage for hemoptysis,18 and appropriately placed lesions elsewhere cause melena, hematuria, intraocular hemorrhage, vaginal bleeding, and cerebrovascular accidents.18,48,46
Gastrointestinal endoscopy detects mucosal telangiectases.22 Cerebral events special deserve comment with reference to a peculiar constellation of symptoms, including dizziness, vertigo, paresthesiae, tinnitus, faintness, visual disturbances, speech defects, headache, weakness of the limbs, hemiplegia, mental confusion, and convulsions.35,18 Cerebral episodes may be brief or prolonged, isolated or recurrent, and tend to manifest similar patterns with subsequent attacks.18 Pathogenesis has not been established, but occurrence in acyanotic patients without pulmonary arteriovenous fistulae incriminates telangiectasias,18 which have been described with a cerebral arteriovenous malformation characterized by a plexiform nidus with an afferent arterial pedicle and a draining vein.63
Pulmonary arteriovenous fistulae set the stage for paradoxical emboli, stroke, and brain abscess (see Figure 30-6).18,32,63–68 Intracranial malformations induce grand mal seizures, which can be fatal.69 Sudden death results from massive intrapulmonary hemorrhage and massive hemothorax.18,24,58 A 41-year-old woman whose case was reported in 1906 died of intractable epistaxes.70 Platypnea-orthodeoxia has been described with pulmonary arteriovenous fistula and a patent foramen ovale.71
Physical appearance
Cyanosis and clubbing are intense when the fistulous shunt is large.18,72 Anemia caused by bleeding telangiectasia can virtually abolish the cyanosis, but clubbing persists.18,72 Cyanosis is absent when systemic arteries rather than pulmonary arteries feed the fistulae.18
However, the most distinctive physical appearance in patients with pulmonary arteriovenous fistulae is the coexisting mucocutaneous telangiectases—clusters of tiny ruby lesions on the nasal and oral mucous membranes (see Figure 30-8) and on the face, tongue, skin, retina, and nail beds.18,20 The lesions blanch with the slightest pressure and bleed with the slightest provocation. Unlike spider angioma, blanching with pressure is usually incomplete. Telangiectases are rarely evident in infants and young children but may be the first evidence of a pulmonary arteriovenous fistula.
An annotation in the Lancet vividly described the classic pattern of mucocutaneous telangiectases:
“Every large general hospital is certain to have on its list of frequent attenders a small group of unfortunate adults who come to the casualty department complaining of recurrent bleeding from the nose, lips or mouth. The blood is seen to stem from an insignificant leak in the center of a small ruby patch, many of which are usually to be found scattered here and there on the mucous membrane (Figure 30-8). Although the flow of blood is seldom vigorous it may eventually, by its persistence, cause some concern. Its arrest can be infuriatingly difficult. Each ruby patch marks the position of a tiny arteriovenous communication at the capillary level. Presumably these vessels are very close to the surface or else they are abnormally fragile. Whatever the cause, they can be induced to bleed by the most trivial of injuries.”
Arterial pulse and jugular venous pulse
The arterial pulse and the jugular venous pulse are normal because left ventricular stroke volume is normal or only modestly increased, and congestive heart failure is rare. Isolated examples of an increased arterial pulse occur with a massive pulmonary arteriovenous fistula (see Figure 30-7) and when coexisting intrahepatic arteriovenous fistulae cause a hyperdynamic circulatory state.40,42
Precordial movement and palpation
The extra amount of blood pumped by the right or left ventricle is relatively small (see Physiologic Consequences), so the impulses of both ventricles are normal. Precordial impulses are hyperdynamic in the rare event of a massive pulmonary arteriovenous fistula (see Figure 30-7), and hepatic arteriovenous fistulae are accompanied by hyperdynamic ventricular impulses, hepatomegaly, and an hepatic thrill.40,42
Auscultation
Pulmonary arteriovenous fistulae transmit murmurs to overlying chest wall sites because the fistulae are close to the periphery of the lungs (Figures 30-1, 30-2, and 30-3).53 Murmurs are absent when fistulae lie deeply within the lungs or when they are small and diffuse (see Figures 30-4 and 30-5).10,18 Murmurs are missed when they are faint and are assigned to nonprecordial sites.53 The clinical diagnosis is untenable in acyanotic patients with no murmur and no telangiectases.
The abdominal location of the murmur is responsible for oversight when arteriovenous fistulae are hepatic.40,41
Murmurs are typically less than grade 3 and are therefore rarely accompanied by a thrill.23 Loud harsh murmurs are exceptional. Auscultation should be carried out in a quiet room with the patient comfortable and the thorax relaxed to avoid the interference of muscle tremor. The entire chest should be examined, in addition to the liver and the cranium. Respiration should be quiet and should include gentle held exhalation. Because 75% of pulmonary arteriovenous fistulae are in the lower lobes and right middle lobe, the relevant overlying chest wall sites warrant special auscultatory attention.7 Murmurs overlying the lingula of the left lung can be mistaken for intracardiac murmurs (see Figure 30-3).
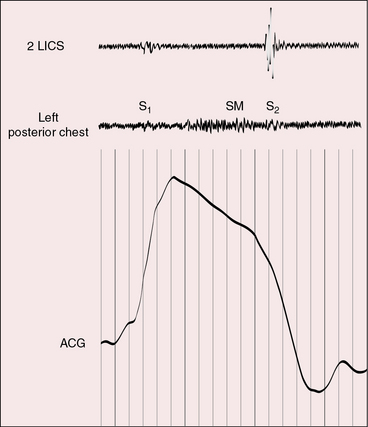
Pulmonary arteriovenous fistulae generate systolic or continuous murmurs (Figures 30-10 and 30-11).18,23,73 In the first clinically diagnosed case, the murmur was continuous.2 The major pressure gradient across the fistula is systolic.23,73 Diastolic gradients are negligible or absent, so the diastolic portion of the murmur is negligible or absent, which accounts for the high incidence rate of murmurs that are confined to systole (see Figure 30-10). Isolated diastolic murmurs are rare.73 Fistulae are downstream from the right ventricle, so an interval elapses from the onset of right ventricular ejection to blood flow through the fistula. This interval is responsible for the delayed onset and late crescendo exhibited by the murmurs, which begin late and finish after the second heart sound (see Figure 30-11)
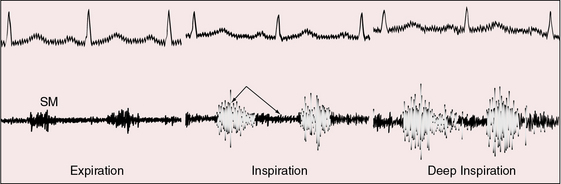
Figure 30-10 Phonocardiogram from the left mid axillary line of the 35-year-old woman whose solitary left lower lobe pulmonary arteriovenous fistula is shown in Figure 30-2. The systolic murmur (SM) recorded during expiration becomes continuous and increases appreciably during deep inspiration.
The normal inspiratory increase in right ventricular volume is available for forward flow through the fistula, and inspiratory depression of the diaphragm reduces compression of lower lobe fistulae. Accordingly, the murmur decreases during expiration and increases during inspiration, especially during deep held inspiration (see Figure 30-10).18,23,35,73 The murmur may be heard only at the end of an exaggerated inspiratory effort, so unless this maneuver is performed, the response of the murmur will be missed. Inspiration may also cause a systolic murmur to become continuous (see Figure 30-10). To detect these respiratory effects, auscultation should be carried out at the end of normal expiration and at the height of deep momentarily held inspiration. These maneuvers serve two purposes: namely, to minimize respiratory interference and to provide time for right ventricular blood to reach the fistula. The Valsalva’s and Müller’s maneuvers exaggerate these effects.23 Flow through the fistula diminishes during the Valsalva’s maneuver, so the murmur softens or vanishes. When straining is released, flow abruptly accelerates and the murmur abruptly intensifies. The Müller’s maneuver (forced inspiration against a closed glottis) increases flow through the fistula and increases the intensity of the fistulous murmur. Standing increases perfusion of the dependent portions of the lungs and increases audibility of lower lobe murmurs.35 A lateral decubitus position on the side of a fistula compresses the ipsilateral lung and decreases the murmur.35 Pregnancy elevates the diaphragm, compresses lower lobe fistulae, and decreases or abolishes the murmur (see previous).35
Electrocardiogram
The electrocardiogram is typically normal because the hemodynamic burden is modest. Flow through the fistula is associated with a reciprocal decrease in flow through the uninvolved lung, so right ventricular output is normal, and the volume entering the left side of the heart is normal. The increment of blood shunted through the fistula seldom imposes volume load on either ventricle. These circulatory adjustments are less effective when fistulae are large, which may account for the occasional presence of right or left axis deviation.23,73 When a large pulmonary arteriovenous fistula or a large pulmonary arterial–to–left atrial fistula exists in utero, right ventricular blood preferentially flows through the low-resistance fistula and imposes volume overload on the left side of the heart. This mechanism is held responsible for left ventricular hypertrophy and left atrial P wave abnormalities.
X-ray
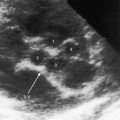
The rare large fistula is associated with an increase in heart size (see Figure 30-7),23 but otherwise, the size and shape of the heart are normal (see Figures 30-1 and 30-2). The most important feature of the x-ray is the abnormal density cast by the fistula itself (see Figures 30-1A, 30-3, 30-6A, 30-7A, and 30-9). Lateral views should be examined to disclose a density hidden by the heart or diaphragm (see Figure 30-3).4 Multiple small fistulae are hazy radiodensities rather than circumscribed lesions and are difficult to identify in the x-ray.3,6,18,23 Fistulae in infants are usually poorly defined in the x-ray (see Figure 30-9) but tend to increase in size and radiodensity as the child grows.31,32 Large fistulae are exceptional (see Figure 30-7).
Pulmonary arteriovenous fistulae can be single or multiple, unilateral or bilateral, and typically involve the lower lobes and right middle lobe (see Figures 30-1 through 30-4; see previous). Upper lobe fistulae are uncommon (see Figure 30-6). The densities vary in size and shape from small focal opacities (see Figures 30-4 and 30-5) to large homogeneous densities that can be mistaken for coin lesions or metastatic carcinoma (see Figures 30-1 and 30-2). The densities are hazy and ill-defined or round or lobulated with well-demarcated edges (see Figures 30-1, 30-2, 30-7,A, and 30-9).18,20,23 Lateral projections confirm that the opacities are within the lung parenchym.18 Calcification is exceptional.74 Hemorrhage may obscure the lesion. Intrapleural rupture is accompanied by painful hemothorax.18,24
Vascular shadows that connect the parenchymal density to the hilus establish the lung lesion as pulmonary arteriovenous (see Figures 30-1 through 30-6).18,23,75 The linear vascular shadows correspond to dilated afferent pulmonary arterial channels that join the fistula and efferent channels that leave the fistula (see Figures 30-1 through 30-4 and 30-6). Rarely, localized notching of ribs is caused by dilated tortuous intercostal arteries that enter the fistula.23 Subtle variations in size of a fistula can be induced by changes in intrathoracic pressure (see previous).23 The Valsalva’s maneuver diminishes the fistula by decreasing flow. The Müller’s maneuver enlarges the fistula by increasing flow.
Echocardiogram
Pulmonary arteriovenous fistulae are seldom imaged with echocardiography because the lesions are within the lung parenchyma. An exception is a solitary large paracardiac fistula.25 Pulsed Doppler echocardiography can record bidirectional flow into and out of the fistula,76 and the diagnosis has been made with color Doppler scan and amplitude ultrasound angiography.77 “Bubble echocardiography” can be a useful diagnostic tool.78 Contrast echocardiography detects relatively small right-to-left fistulous shunts and establishes the shunts as extracardiac.25,79,80 Agitated saline solution injected into a peripheral vein, vena cava, or pulmonary artery is promptly detected in pulmonary veins or the left atrium with transthoracic and transesophageal echocardiograpy.25,32,79,80
1 Churton T. Multiple aneurysms of pulmonary artery. Br Med J. 1897;1:1223.
2 Smith H.L., Horton B.T. Arteriovenous fistula of the lung associated with polycythemia vera: report of a case in which the diagnosis was made clinically. Am Heart J. 1939;18:589-592.
3 Anabtawi I.N., Ellison R.G. Maldevelopment of the pulmonary veins and pulmonary arteriovenous aneurysms. Am Surg. 1964;30:770-773.
4 Boczko M.L. Pulmonary arteriovenous fistulas. Mayo Clin Proc. 1999;74:1305.
5 Brian C.A., Payne R.M., Link K.M., Hundley W.G., Warner J.G.Jr. Pulmonary arteriovenous malformation. Circulation. 1999;100:e29-e30.
6 Hales M.R. Multiple small arteriovenous fistulae of the lungs. Am J Pathol. 1956;32:927-943.
7 Sahn S.H., Bluth I., Schub H. Pulmonary arteriovenous fistula: a report of two unusual cases. Dis Chest. 1963;44:542-546.
8 Amodeo A., Marino B. Pulmonary arteriovenous fistulas in patients with left isomerism and cardiac malformations. Cardiol Young. 1998;8:283-284.
9 Kapur S., Rome J., Chandra R.S. Diffuse pulmonary arteriovenous malformation in a child with polysplenia syndrome. Pediatr Pathol Lab Med. 1995;15:463-468.
10 Sanders J.S., Martt J.M. Multiple small pulmonary arteriovenous fistulas. Diagnosis by cardiac catheterization. Circulation. 1962;25:383-389.
11 Franzen O., Lund C., Baldus S. Pulmonary arteriovenous fistula in hereditary hemorrhagic telangiectasis. Clin Res Cardiol. 2009;98:749-750.
12 Babington B.G. Hereditary epistaxis. Lancet. 1865;2:362.
13 Legg J.W. A case of haemophilia complicated with multiple naevi. Lancet. 1876;2:856.
14 Rendu H. Epistaxis répetée chez un sujet porteur de petits angiomes cutanés et muqueaux. Bull Mem Soc Med Hop Paris. 1896;13:731.
15 Osler W. On a family form of recurring epistaxis, associated with multiple telangiectases of skin and mucous membranes. Bull Johns Hopkins Hosp. 1901;12:333.
16 Weber F.P. Multiple hereditary developmental angiomata (telangiectases) of the skin and mucous membranes associated with recurring haemorrhages. Lancet. 1907;2:160-162.
17 Hanes F.M. Multiple hereditary telangiectases causing hemorrhage (hereditary hemorrhagic) telangiectasia. Bull Johns Hopkins Hosp. 1909;20:63-73.
18 Hodgson C.H., Burchell H.B., Good C.A., Clagett O.T. Hereditary hemorrhagic telangiectasia and pulmonary arteriovenous fistula: survey of a large family. N Engl J Med. 1959;261:625-636.
19 Shovlin C.L., Guttmacher A.E., Buscarini E., et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet. 2000;91:66-67.
20 Steinberg I., Mcclenahan J. Pulmonary arteriovenous fistula; angiocardiographic observations in nine cases. Am J Med. 1955;19:549-568.
21 Hu S.-Y., Tsai C.-H., Tsan Y.-T. Pulmonary arteriovenous malformation in Osler-Weber-Rendu syndrome. Eur J Cardiothorac Surg. 2009;36:395.
22 Yunoki K., Naruko T., Komiyama M., et al. Images in cardiovascular medicine. Hereditary hemorrhagic telangiectasia with pulmonary arteriovenous fistulas. Circulation. 2006;114:e48-e49.
23 Le Roux B.T. Pulmonary arteriovenous fistulae. Q J Med. 1959;28:1.
24 Dalton M.L.Jr, Goodwin F.C., Bronwell A.W., Rutledge R. Intrapleural rupture of pulmonary arteriovenous aneurysm. Report of a case. Dis Chest. 1967;52:97-100.
25 Jimenez M., Fournier A., Choussat A. Pulmonary artery to the left atrium fistula as an unusual cause of cyanosis in the newborn. Pediatr Cardiol. 1989;10:216-220.
26 Kroeker E.J., Adams H.D., Leon A.S., Pouget J.M. Congenital communication between a pulmonary artery and the left atrium. Physiologic observations and review of the literature. Am J Med. 1963;34:721-725.
27 Sheikhzadeh A., Hakim H., Ghabusi P., Ataii M., Tarbiat S. Right pulmonary artery-to-left atrial communication: recognition and surgical correction. Am Heart J. 1984;107:396-398.
28 Tirilomis T., Busch T., Aleksic I., et al. Pulmonary arteriovenous fistula drainage into the left atrium. Thorac Cardiovasc Surg. 2000;48:37-39.
29 Tuncali T., Aytac A. Direct communication between right pulmonary artery and left atrium. Report of a case and proposal of a new entity. J Pediatr. 1967;71:384-389.
30 Nelson W.P., Hall R.J., Garcia E. Varicosities of the pulmonary veins simulating arteriovenous fistulas. JAMA. 1966;195:13-17.
31 Swanson K.L., Prakash U.B., Stanson A.W. Pulmonary arteriovenous fistulas: Mayo Clinic experience, 1982-1997. Mayo Clin Proc. 1999;74:671-680.
32 Teragaki M., Akioka K., Yasuda M., et al. Hereditary hemorrhagic telangiectasia with growing pulmonary arteriovenous fistulas followed for 24 years. Am J Med Sci. 1988;295:545-547.
33 Sapru R.P., Hutchison D.C., Hall J.I. Pulmonary hypertension in patients with pulmonary arteriovenous fistulae. Br Heart J. 1969;31:559-569.
34 Waldhausen J.A., Abel F.L. The circulatory effects of pulmonary arteriovenous fistulas. Surgery. 1966;59:76-80.
35 Hazlett D.R., Medina J. Postural effects on the bruit and right-to-left shunt of pulmonary arteriovenous fistula. Chest. 1971;60:89-92.
36 Bernstein H.S., Brook M.M., Silverman N.H., Bristow J. Development of pulmonary arteriovenous fistulae in children after cavopulmonary shunt. Circulation. 1995;92:II309-II314.
37 Premsekar R., Monro J.L., Salmon A.P. Diagnosis, management, and pathophysiology of post-Fontan hypoxaemia secondary to Glenn shunt related pulmonary arteriovenous malformation. Heart. 1999;82:528-530.
38 Barbe T., Losay J., Grimon G., et al. Pulmonary arteriovenous shunting in children with liver disease. J Pediatr. 1995;126:571-579.
39 Hoffbauer F.W., Rydell R. Multiple pulmonary arteriovenous fistulas in juvenile cirrhosis. Am J Med. 1956;21:450-460.
40 Burckhardt D., Stalder G.A., Ludin H., Bianchi L. Hyperdynamic circulatory state due to Osler-Weber-Rendu disease with intrahepatic arteriovenous fistulas. Am Heart J. 1973;85:797-800.
41 Danchin N., Thisse J.Y., Neimann J.L., Faivre G. Osler-Weber-Rendu disease with multiple intrahepatic arteriovenous fistulas. Am Heart J. 1983;105:856-859.
42 Razi B., Beller R.M., Ghidoni J., et al. Hyperdynamic circulatory state due to intrahepatic fistula in Osler-Weber-Rendu disease. Am J Med. 1971;50:809-815.
43 Maruyama J., Watanabe M., Onodera S., et al. A case of Rendu-Osler-Weber disease with cerebral hemangioma, multiple pulmonary arteriovenous fistulas and hepatic arteriovenous fistula. Jpn J Med. 1989;28:651-656.
44 Wilkens G.D. Ein fall von multiplen pulmonalis aneurysmen. Beitr Klin Tuberk. 1917;38:1-10.
45 Ehrenhaft J.L., Taber R.E. Arteriovenous fistulae and arterial aneurysms of the pulmonary arterial tree. AMA Arch Surg. 1956;73:567-577.
46 Mestre J.R., Andres J.M. Hereditary hemorrhagic telangiectasia causing hematemesis in an infant. J Pediatr. 1982;101:577-579.
47 Boston L.N. Gastric hemorrhage due to familial telangiectasis. Am J Med Sci. 1930;180:798.
48 Humphries J.E., Frierson H.F.Jr, Underwood P.B.Jr. Vaginal telangiectasias: unusual presentation of the Osler-Weber-Rendu syndrome. Obstet Gynecol. 1993;81:865-866.
49 Reilly P.J., Nostrant T.T. Clinical manifestations of hereditary hemorrhagic telangiectasia. Am J Gastroenterol. 1984;79:363-367.
50 Mckusick V.A. A genetical view of cardiovascular disease. The Lewis A. Conner memorial lecture. Circulation. 1964;30:326-357.
51 Peery W.H. Clinical spectrum of hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease). Am J Med. 1987;82:989-997.
52 Standefer J.E., Tabakin B.S., Hanson J.S. Pulmonary arteriovenous fistulas. Case report with cine-angiographic studies. Am Rev Respir Dis. 1964;89:95-99.
53 Husson G.S. Pulmonary arteriovenous aneurysm in childhood. Pediatrics. 1956;18:871-879.
54 Kraemer N., Krombach G.A. Images in clinical medicine. Pulmonary arteriovenous fistula. N Engl J Med. 2009;360:1769.
55 Brummelkamp W.H. Unusual complication of pulmonary arteriovenous aneurysm: Intrapleural rupture. Dis Chest. 1961;39:218.
56 Lyons H.A., Mannix E.P.Jr. Successful resections for bilateral pulmonary arteriovenous fistulas. N Engl J Med. 1956;254:969-974.
57 Livingston S.O., Carr R.E. Hereditary hemorrhagic telangiectasia; report of a case with hemothorax. J Thorac Surg. 1956;31:497-503.
58 Ference B.A., Shannon T.M., White R.I.Jr, Zawin M., Burdge C.M. Life-threatening pulmonary hemorrhage with pulmonary arteriovenous malformations and hereditary hemorrhagic telangiectasia. Chest. 1994;106:1387-1390.
59 Freixinet J., Sanchez-Palacios M., Guerrero D., et al. Pulmonary arteriovenous fistula ruptured to pleural cavity in pregnancy. Scand J Thorac Cardiovasc Surg. 1995;29:39-41.
60 Bradshaw D.A., Murray K.M., Mull N.H.T. Massive hemoptysis in pregnancy due to a solitary pulmonary arteriovenous malformation. West J Med. 1994;161:600-602.
61 Esplin M.S., Varner M.W. Progression of pulmonary arteriovenous malformation during pregnancy: case report and review of the literature. Obstet Gynecol Surv. 1997;52:248-253.
62 Wilmshurst P., Jackson P. Arterial hypoxemia during pregnancy caused by pulmonary arteriovenous microfistulas. Chest. 1996;110:1368-1369.
63 Preston D.C., Shapiro B.E. Pulmonary arteriovenous fistula and brain abscess. Neurology. 2001;56:418.
64 Brydon H.L., Akinwunmi J., Selway R., Ul-Haq I. Brain abscesses associated with pulmonary arteriovenous malformations. Br J Neurosurg. 1999;13:265-269.
65 Chambers W.R. Brain abscess associated with pulmonary arterio-venous fistula. Ann Surg. 1955;141:276-277.
66 Sajeev C.G., Fasaludeen M., Venugopal K. Pulmonary arteriovenous fistula presenting as brain abscess. Int J Cardiol. 2005;98:153.
67 Kawano H., Hirano T., Ikeno K., Fuwa I., Uchino M. Brain abscess caused by pulmonary arteriovenous fistulas without Rendu-Osler-Weber disease. Intern Med. 2009;48:485-487.
68 Tomelleri G., Bovi P., Carletti M., et al. Paradoxical brain embolism in a young man with isolated pulmonary arteriovenous fistula. Neurol Sci. 2008;29:169-171.
69 Byard R.W., Schliebs J., Koszyca B.A. Osler-Weber-Rendu syndrome–pathological manifestations and autopsy considerations. J Forensic Sci. 2001;46:698-701.
70 Kelly A.B. Multiple telangiectases of the skin and mucous membranes of the nose and mouth. Glas Med J. 1906;65:411-422.
71 Ohara T., Nakatani S., Hashimoto S., et al. A case of platypnea-orthodeoxia syndrome in a patient with a pulmonary arteriovenous fistula and a patent foramen ovale. J Am Soc Echocardiogr. 2007;20:439-440.
72 Dhillon B.S., Fawcett A.W. Pulmonary arterio-venous fistula; review of world literature and report on two additional cases. Postgrad Med J. 1956;32:353-356.
73 Yater W.M., Finnegan J., Giffin H.M. Pulmonary arteriovenous fistula; review of the literature and report of two cases. J Am Med Assoc. 1949;141:581-589.
74 Sloan R.D., Cooley R.N. Congenital pulmonary arteriovenous aneurysm. Am J Roentgenol Radium Ther Nucl Med. 1953;70:183-210.
75 Singleton E.B., Leachman R.D., Rosenberg H.S. Congenital abnormalities of the pulmonary arteries. Am J Roentgenol Radium Ther Nucl Med. 1964;91:487-499.
76 Kataoka H., Matsuno O. Rendu-Osler-Weber Disease: transthoracic Doppler ultrasonographic findings. Circulation. 2001;103:E36-E38.
77 Wang H.C., Kuo P.H., Liaw Y.S., et al. Diagnosis of pulmonary arteriovenous malformations by colour Doppler ultrasound and amplitude ultrasound angiography. Thorax. 1998;53:372-376.
78 Sands A., Dalzell E., Craig B., Shields M. Multiple intrapulmonary arteriovenous fistulas in childhood. Pediatr Cardiol. 2000;21:493-496.
79 Ozkutlu S., Saraclar M. Two-dimensional contrast echocardiography in pulmonary arteriovenous fistula. Jpn Heart J. 1989;30:425-430.
80 Shub C., Tajik A.J., Seward J.B., Dines D.E. Detecting intrapulmonary right-to-left shunt with contrast echocardiography. Observations in a patient with diffuse pulmonary arteriovenous fistulas. Mayo Clin Proc. 1976;51:81-84.