Chapter 9 Congenital Obstruction to Left Atrial Flow
Mitral Stenosis, Cor Triatriatum, Pulmonary Vein Stenosis
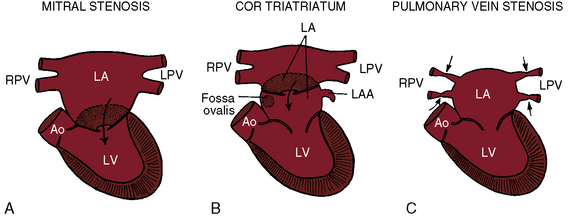
Congenital obstruction to left atrial flow can originate at or near the junction of the pulmonary veins and the left atrium (pulmonary vein stenosis), within the left atrium (cor triatriatum), immediately above the mitral valve (supravalvular stenosing ring), or within the mitral apparatus (mitral stenosis; Figure 9-1; Box 9-1). Pure or relatively pure forms of each defect are emphasized in this chapter, although a variety of anomalies often coexist.1–3 Pulmonary veno-occlusive disease is covered in Chapter 14, total anomalous pulmonary venous connection with obstruction is covered in Chapter 15, and hypoplastic left heart with a hypoplastic mitral orifice is covered in Chapter 31.
Box 9-1 Congenital Obstruction To Left Atrial Flow With A Functionally Adequate Left Ventricle
Congenital mitral stenosis
The incidence rate has been estimated at 0.6% of necropsy cases of congenital heart disease and 0.21% to 0.42% of clinical cases.4 Congenital mitral stenosis with a functionally adequate left ventricle includes the following malformations in approximate order of frequency (see Box 9-1):5–9
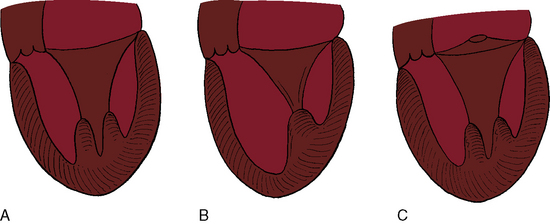
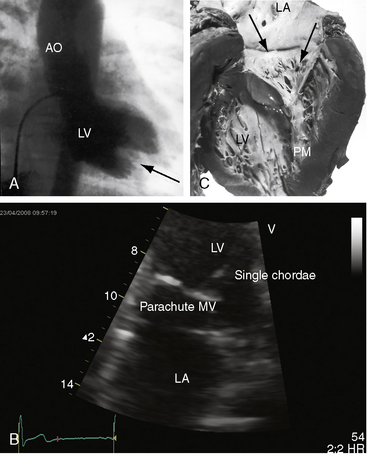
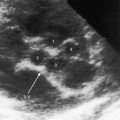
Flow across a normal mitral orifice is between the leaflets (interleaflet) and the chordae tendineae (interchordal). In a parachute mitral valve, all chordae tendineae insert into a single papillary muscle and the interchordal spaces are reduced or obliterated,10 so flow cannot be interchordal (see Figures 9-2B and 9-3).
A parachute mitral valve is usually one of the components of Shone’s complex, a developmental combination of four obstructive lesions: namely, supravalvular stenosing ring, parachute mitral valve, subaortic stenosis, and coarctation of the aorta (Figures 9-3 and 9-10).1,2,15,16 All four lesions are not always present; if they are present, they may not be functionally significant.2,10,20 The supravalvular mitral ring can be rudimentary (see Figure 9-3C), or can bridge the mitral orifice as a stenosing diaphragm (see Figure 9-3B).8,9,18
History
There is a male predilection in congenital mitral stenosis,1 in contrast to rheumatic mitral stenosis, which has a female predilection. Familial recurrence has not been reported. If stenosis is severe, symptoms begin shortly after birth when pulmonary blood flow commences and suddenly enters the obstructed left atrium. Fifty percent of symptomatic infants die within 6 months, but an occasional infant is asymptomatic and a few remain relatively free of symptoms for years.21 With a parachute mitral valve or a supravalvular mitral ring, longevity is better (median, 10 years and 5.5 years, respectively), but short chordae tendineae and obliterated interchordal spaces result in death at a median age of 6 months.6,10 Anomalous mitral arcade or double-orifice mitral valve permits adult survival when the mitral apparatus functions normally or is purely regurgitant.5,14
Orthopnea, dyspnea, tachypnea, and paroxysmal cough are results of pulmonary edema that is punctuated by lower respiratory infections.1,21–23 Congenital mitral stenosis is occasionally associated with syncope21 but seldom with hemoptysis.23 Aphonia has been attributed to compression of the recurrent laryngeal nerve by a dilated hypertensive pulmonary trunk, analogous to hoarseness in adults with pulmonary hypertension and rheumatic mitral stenosis. Infective endocarditis is rare.1
Physical Appearance
Mild cyanosis coincides with congestive heart failure.21,22 Recurrent lower respiratory infections and the catabolic effects of heart failure account for physical underdevelopment.21,23
Arterial Pulse, Jugular Venous Pulse, Precordial Movement, and Palpation
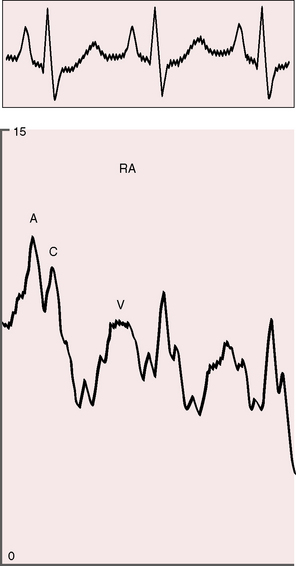
The arterial pulse is normal and confirms normal sinus rhythm, which is the rule in congenital mitral stenosis.21 The jugular venous pulse has an increased A wave because of pulmonary hypertension (Figure 9-4). A precordial bulge is common,23 and a right ventricular impulse is palpable atthe lower left sternal border and subxiphoid area.21,23
Auscultation
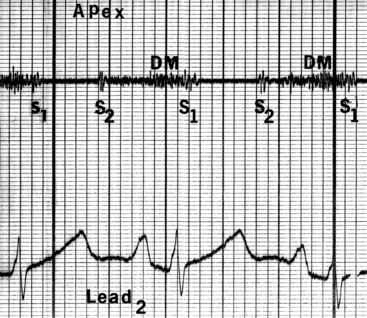
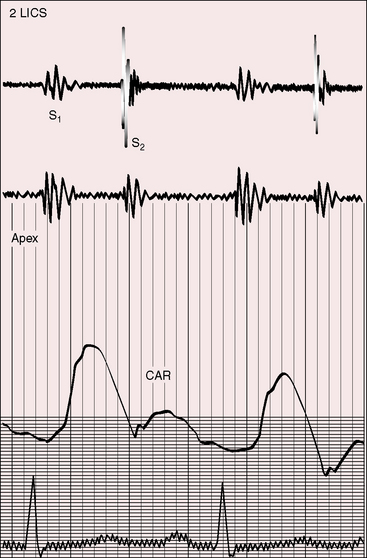
Neither a loud first heart sound nor an opening snap are heard; the necessary preconditions of these two auscultatory signs—abrupt opening and closing movements of the belly of a mobile anterior mitral leaflet—are not features of congenital mitral stenosis (Figures 9-5 and 9-6).1,10,11,23
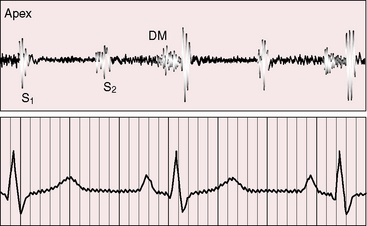
Figure 9-6 Phonocardiogram from the 3-year-old girl with congenital mitral stenosis referred to in Figure 9-4. The first heart sound (S1) is loud, but there is no opening snap. A soft mid-diastolic murmur (DM) is followed by presystolic accentuation.
In the presence of a parachute mitral valve or a supravalvular ring,17 a holosystolic murmur at the apex or lower left sternal edge is the result of mitral regurgitation or pulmonary hypertensive tricuspid regurgitation.22 An apical mid-diastolic murmur with presystolic accentuation (see Figures 9-5 and 9-6) is exceptional because the rapid heart rate in infants shortens diastole1,23 and because a dilated hypertensive right ventricle displaces the left ventricle from the apex.24,25 The pulmonary component of the second heart sound is loud because of pulmonary hypertension that sets the stage for the Graham Steell murmur of high-pressure pulmonary regurgitation.1,18
Electrocardiogram
Atrial fibrillation is exceptional in contrast to rheumatic mitral regurgitation.1 Pulmonary hypertension results in right atrial P wave abnormalities,1 right axis deviation,1 and right ventricular hypertrophy (Figure 9-7).1
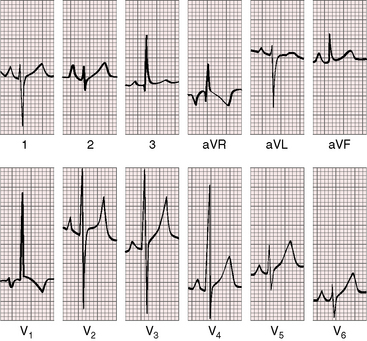
Figure 9-7 Electrocardiogram from the 2-year-old girl with Shone’s complex and pulmonary vascular disease referred to in Figure 9-5. Tall peaked right atrial P waves are present in leads 1, 2, and V2. There is marked right axis deviation. Right ventricular hypertrophy is manifested by the tall monophasic R wave in lead V1 and the deep S wave in lead V6. Large RS complexes in lead V2 and V3 suggest biventricular hypertrophy because of coarctation of the aorta.
X-Ray
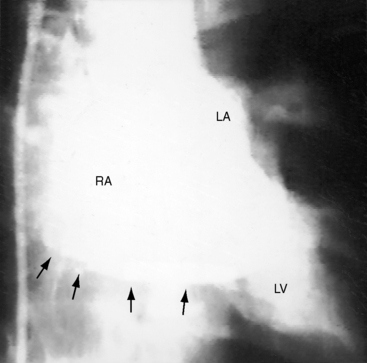
Pulmonary venous congestion includes Kerley’s lines (Figure 9-8).1,23 Mild to moderate left atrial enlargement is recognized in the lateral projection (see Figure 9-8B). Straightening of the left cardiac border (Figure 9-9) by an enlarged left atrial appendage is much less common than in rheumatic mitral stenosis.1 Calcification of the mitral valve is absent in the x-ray and absent histologically.26 The pulmonary trunk, right ventricle, and right atrium are enlarged because of pulmonary hypertension (see Figures 9-8 and 9-9).
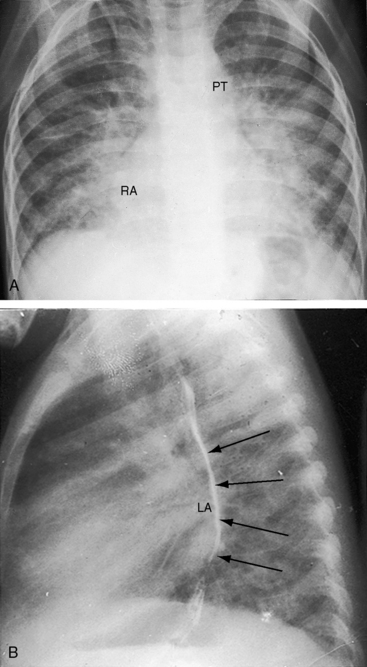
Figure 9-8 X-rays from the 2-year-old girl with Shone’s complex whose phonocardiogram is shown in Figure 9-5. A, Pulmonary venous congestion is striking. The pulmonary trunk (PT) and right atrium (RA) are dilated. B, The lateral film shows displacement of the barium esophagram by an enlarged left atrium (LA).
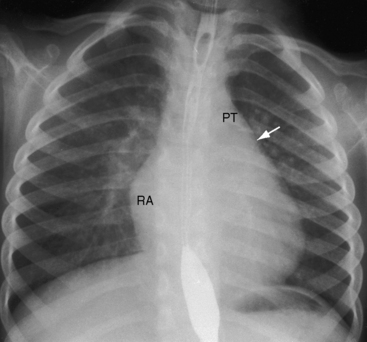
Figure 9-9 X-ray from the 3-year-old girl with congenital mitral stenosis referred to in Figures 9-4 and 9-6. There is bilateral hilar pulmonary venous congestion. The right atrium (RA) and pulmonary trunk (PT) are dilated, and the left cardiac border is straightened by an enlarged left atrial appendage (arrow).
Echocardiogram
Congenital mitral stenosis is characterized by two well-formed papillary muscles with a reduced interpapillary distance.10 A parachute mitral valve is characterized by a single papillary muscle into which all chordae tendineae converge (Figures 9-3B,C and 9-10).16,27–29 The effective orifice size cannot be determined with two-dimensional echocardiography because flow is interchordal and the valve is eccentric (see Figure 9-10B), but Doppler scan interrogation can determine the gradient and the functional orifice size (see Figure 9-10C,D). A supravalvular ring is more readily identified when it is not attached to the mitral leaflets (see Figures 9-3B and 9-10A).6 Otherwise, the ring is imaged only in diastole.6 An anomalous mitral arcade has multiple papillary muscles with few or no chordae tendineae interposed between the arcade and the leaflets.27 The relative sizes of the two orifices of a double-orifice mitral valve can be determined, the chordal insertions can be characterized, and the functional state of the valve can be established as normal, stenotic, or incompetent.7
Cor triatriatum
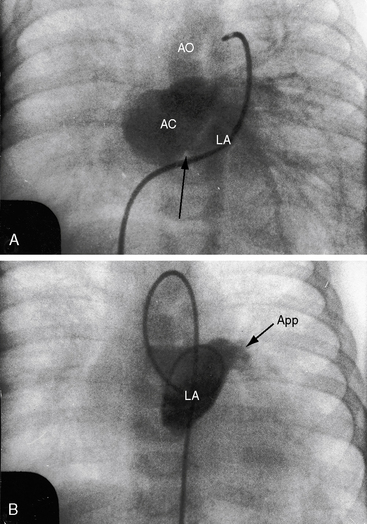
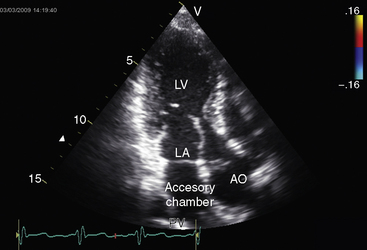
Partition of the left atrium into two compartments was recognized by Andral30 in 1829. Four decades later, Church31 published the first detailed pathologic description of the malformation that Borst32 (1905) called cor triatriatum. The anomaly is rare, with a prevalence rate of about 0.1% of cases of congenital heart disease.33 Cor triatriatum occurs typically as an isolated defect or atypically in association with other congenital cardiac anomalies.3 The anomaly is characterized by a membrane that partitions the left atrium into a proximal accessory chamber that receives the pulmonary veins and by a distal true left atrial chamber that contains the left atrial appendage and the fossa ovalis (Figures 9-1B and 9-11).4,33–37
The pathogenesis of cor triatriatum is persistence of the right valve of the embryonic sinus venosus,38 which results in failure of incorporation of the common pulmonary vein into the left atrium, a process that begins at about the fifth week of gestation and proceeds through subsequent stages of embryogenesis.36,38 The proximal accessory chamber represents persistence of the common pulmonary vein of the embryo and cannot be identified externally.35
The three anatomic varieties of cor triatriatum are referred to as diaphragmatic, hourglass, and tubular.33,35 In the diaphragmatic type, which is the most representative, the proximal accessory chamber and the distal true left atrial chamber are separated by a fibrous or fibromuscular diaphragm (see Figure 9-11) that contains either a single opening or multiple openings, the sizes of which determine the degree of obstruction.35,39 Functionally insignificant ridges of tissue go unrecognized or appear as incidental findings at necropsy.39,40 At the other extreme, no communication exists between the accessory chamber and the true left atrium—atresia of the common pulmonary vein.41
Tubular cor triatriatum is the most primitive and least common variety.35 The proximal chamber retains the shape of the common pulmonary vein, which is the anatomic basis of the tubular configuration, the distal end of which joins the left atrium directly without an intervening membrane.35
The hourglass type of cor triatriatum is developmentally intermediate between the diaphragmatic and tubular types. The constriction projects inward as an obstructing shelf, which is seen externally as an hourglass deformity at the junction of the accessory chamber and the true left atrium.35
An interatrial communication usually takes the form of a valve-incompetent foramen ovale positioned between the right atrium and the distal true left atrium.35 Alternatively, an ostium secundum atrial septal defect communicates with the true left atrium or, exceptionally, with the accessory chamber.35
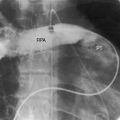
Cor triatriatum dexter is a rare type of triatrial heart (Figure 9-12).42–45 During early cardiogenesis, the right horn of the sinus venosus is guarded by two valves. The smaller left valve becomes incorporated into the septum secundum. The larger right valve initially divides the right atrium into two chambers and then regresses between the ninth and the 15th week of gestation.42 In cor triatriatum dexter, a persistent embryonic right valve of the sinus venosus becomes a septating membrane. The venae cavae and the coronary sinus are on one side of the membrane, and the right atrial appendage and tricuspid orifice are on the other side. One or more perforations in the membrane permit communication from one side to the other. Septation ranges from partial to complete.
The physiologic consequences of cor triatriatum are analogous to other forms of congenital obstruction to left atrial flow with the following unique qualification.35,46 In cor triatriatum, the pressure is elevated in the accessory chamber that is proximal to the obstruction and is normal in true left atrium that is distal to the obstruction. Accordingly, the stenotic orifice remains open throughout the cardiac cycle, so blood flow across the obstructing partition is continuous.
History
Mild asymptomatic cor triatriatum is diagnosed incidentally during routine echocardiography or is discovered incidentally at necropsy. An echocardiographic diagnosis was made in a 70-year-old woman during routine investigation of a murmur,46 and the condition was inadvertently discovered in an elderly asymptomatic patient.47 Severe cor triatriatum announces itself in neonates and young children because of dyspnea, tachypnea, paroxysmal cough, irritability, poor feeding, and failure to thrive.34 However, symptoms may be delayed until adolescence or adulthood, and severe obstruction occasionally remains virtually asymptomatic until announced by acute pulmonary edema.35,46 Sudden deterioration may follow years of good health. Massive recurrent hemoptysis can be the precipitating cause of death,46 in contrast to congenital mitral stenosis, in which hemoptysis is uncommon (see previous discussion).
Arterial Pulse, Jugular Venous Pulse, Precordial Movement, and Palpation
Atrial fibrillation is uncommon if not rare.48 The jugular venous pulse reflects pulmonary hypertension and an increased A wave. With the advent of tricuspid regurgitation and right ventricular failure, a large V wave appears. Pulmonary hypertension is responsible for a palpable right ventricle and a palpable pulmonary component of the second sound.
Auscultation
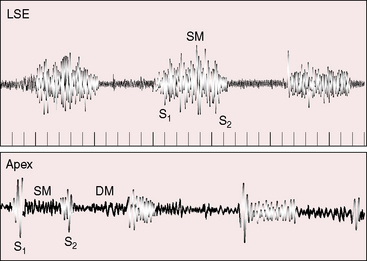
The first heart sound is not increased because diastolic pressure is not exerted against the belly of the anterior mitral leaflet, so its systolic excursion is not brisk (Figure 9-13). The second heart sound is closely split or single with a loud pulmonary component that reflects pulmonary hypertension (see Figure 9-13). An opening snap is rare for the same reason that the first sound is not loud.48
The stenosing diaphragm of cor triatriatum is responsible for systolic, diastolic, or continuous murmurs or may be accompanied by no murmur at all (Figures 9-13 and 9-14).48,49 The undulating membrane moves toward the mitral valve during diastole, reflecting a diastolic gradient, and moves away from the mitral valve during systole, reflecting reversal of the gradient as left ventricular contraction exerts pressure on the membrane through the closed mitral valve.48 Systolic and diastolic thrills over the left atrium at surgery are in accord with these pressure/flow relationships and coincide with the systolic/diastolic murmurs heard at the bedside.50 A continuous murmur reflects continuous flow across the partitioning diaphragm and is the result of a systolic murmur that continues into diastole and is reinforced as left ventricular pressure falls below the pressure in the true left atrium. Pulmonary hypertension is responsible for the high-frequency holosystolic murmur of tricuspid regurgitation (see Figure 9-14). When the enlarged right ventricle occupies the apex, the tricuspid systolic murmur is heard at the apex and is mistaken for mitral regurgitation. A high-frequency early diastolic murmur at the mid-left sternal edge is a Graham Steell murmur.
Electrocardiogram
Peaked right atrial P waves are common (Figure 9-15). Broad notched left atrial P waves have been ascribed to prolonged conduction in the proximal accessory chamber.50 Right axis deviation and right ventricular hypertrophy are typical features of the electrocardiogram (see Figure 9-15).
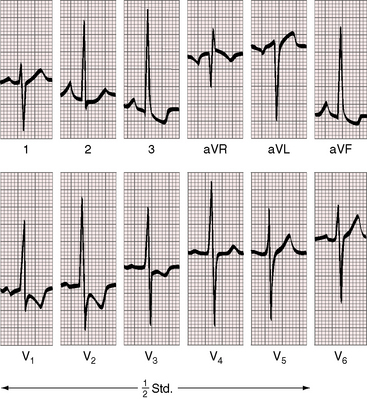
Figure 9-15 Electrocardiogram from an 18-year-old man with cor triatriatum and suprasystemic pulmonary vascular resistance (see Figure 9-13). Pulmonary hypertension is reflected in the tall peaked right atrial P wave in lead 2, right axis deviation, and striking evidence of right ventricular hypertrophy with tall R waves and ST-T wave abnormalities in right precordial leads and deep S waves in the left precordium.
X-Ray
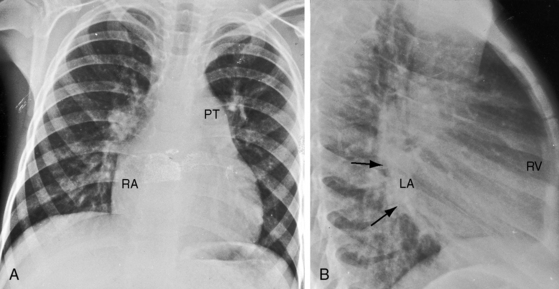
The lung fields exhibit pulmonary venous congestion with a diffuse ground-glass appearance (Figures 9-16 through 9-18), and Kerley’s lines (see Figure 9-17). Radiologic hemosiderosis has been verified at necropsy and is in accord with a history of hemoptysis (see previous discussion).51 The configuration of the heart varies from normal or nearly so to conspicuous enlargement of the right ventricle, right atrium, and pulmonary trunk in response to pulmonary hypertension (see Figures 9-16 through 9-18). The radiologic appearance of the left atrium is of special interest because the size of the true distal left atrial chamber is normal. The combination of pulmonary venous congestion without left atrial enlargement is therefore an important radiologic feature of cor triatriatum. Slight enlargement of the proximal accessory compartment should not be mistaken for enlargement of the left atrium proper (see Figure 9-16).46 Because the left atrial appendage resides in the distal low-pressure compartment, it is not seen in the x-ray (see Figures 9-11, 9-16, and 9-17).46 Calcium has been found in specimens of the stenosing diaphragm but not in the x-ray.46
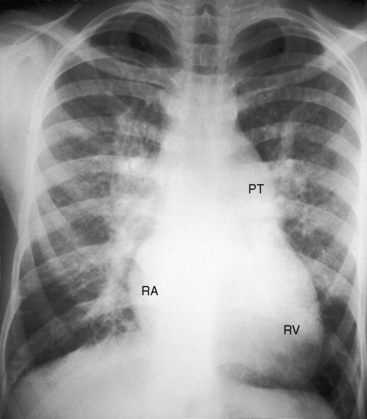
Figure 9-17 X-ray from an 18-year-old man with cor triatriatum and suprasystemic pulmonary vascular resistance. The phonocardiogram is shown in Figure 9-13. Pulmonary venous congestion, especially hilar, is striking. The pulmonary trunk (PT), right atrium (RA), and right ventricle (RV) are enlarged. The left atrial appendage is conspicuous by its absence.
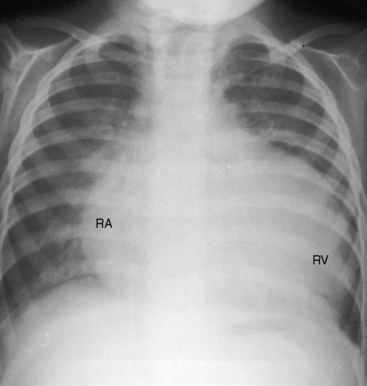
Figure 9-18 X-ray from a 17-month-old boy with cor triatriatum and suprasystemic vascular resistance (phonocardiogram is shown in Figure 9-14). There is moderate pulmonary venous congestion. The right atrium (RA) is huge. The pulmonary trunk is obscured by the dilated outflow tract of a huge right ventricle (RV). A left atrial appendage is not seen.
Echocardiogram
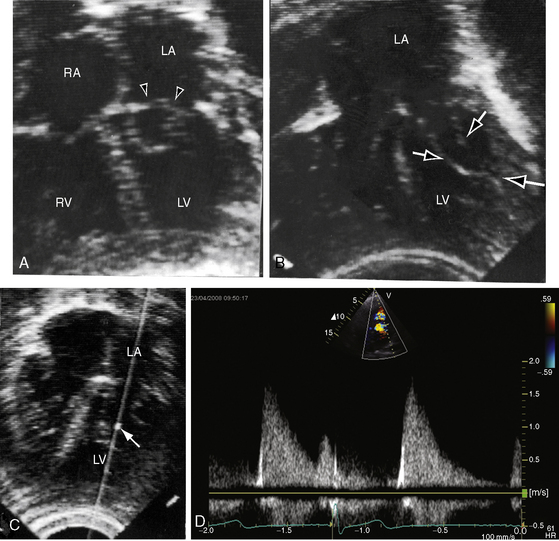
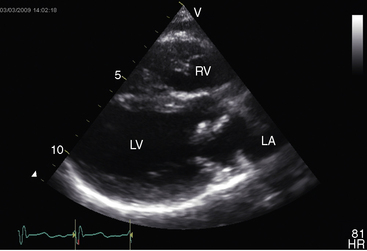
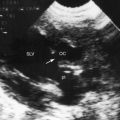
Echocardiography is used to identify an essential anatomic feature of cor triatriatum: the thin undulating intra-atrial membrane, which is characterized by diastolic movement toward the mitral funnel and systolic movement away (Figures 9-19 and 9-20 and Videos 9-2 and 9-3).48,49,52
The left atrial appendage lies within the distal compartment (see Figure 9-20), thus distinguishing cor triatriatum from a supravalvular mitral ring.28,48 The position of the left atrial appendage is best determined during ventricular systole when the membrane moves away from the mitral orifice (see Figure 9-20). The mitral valve itself is normal in cor triatriatum, except for high-frequency diastolic oscillations,49 in contrast to a supravalvular stenosing ring, which is accompanied by a deformed mitral valve (see previous discussion). Color flow imaging and continuous wave Doppler scan interrogation disclose continuous flow across the membrane with peak flow during diastole.53 The true distal left atrium is normal, but the proximal accessory chamber may be slightly dilated (see Figure 9-19).
Echocardiography is used to diagnose cor triatriatum dexter, together with the location, size, and attachment site of the anomalous remnant of the valve of the right sinus venosus (see Figure 9-12).42,43,45
Congenital pulmonary vein stenosis
The incidence rate of congenital pulmonary vein stenosis is estimated at 0.4% to 0.6% of pediatric cardiac necropsies.54 Isolated pulmonary vein stenosis is far more rare.55 The abnormality is characterized by hypoplasia of one or more pulmonary veins or by focal narrowing at or near their left atrial junction (see Figure 9-1C).39,54–56 Hypoplasia varies from slight narrowing to atresia of individual pulmonary veins or of a common pulmonary vein.41,57,58 Focal narrowing is caused by a circumferential collar of fibrous intimal thickening or by a membranous diaphragm.59,60 Focal stenosis, hypoplasia, and atresia may coexist with involvement of most or all of the pulmonary veins.61 Congenital pulmonary vein stenosis is believed to be a developmental fault of the common pulmonary vein, a structure that is normally incorporated into the left atrium as four separate venous channels (see previous discussion).57,58
The following discussion deals with pulmonary vein stenosis as an isolated cause of congenital obstruction to left atrial inflow, although the disorder usually coexists with a number of other congenital malformations.55,56,59 The physiology of the circulation resembles congenital mitral stenosis or cor triatriatum, but left atrial pressure is normal. Except for stenosis or atresia of the common pulmonary vein,57 high pulmonary venous pressure is not distributed uniformly within the lungs because of variation in the location and severity of individual sites of stenosis.39
Clinical Features
Pulmonary vein stenosis causes dyspnea, orthopnea, cough, hemoptysis, and lower respiratory infections.54,56,59,60 Lifespan occasionally extends into the middle or late teens, but only a minority of patients survive childhood.56 Precordial palpation detects a right ventricular and pulmonary trunk impulse and a loud pulmonary component of the second heart sound.54 Murmurs are absent except for a Graham Steell murmur or tricuspid regurgitation.59,60 The electrocardiogram reflects pulmonary hypertension with right atrial P wave abnormalities, right axis deviation, and right ventricular hypertrophy.54,60 Left atrial P wave abnormalities are absent. Pulmonary vascular patterns in the x-rays are determined by which of the four pulmonary veins are stenosed and by the degree of stenosis.58 Regional differences are characterized by asymmetry between the right and left lungs and by nonuniform distribution within each lung.58 Left atrial size remains normal because pulmonary vein stenosis is proximal to the left atrium.39,60 The heart tends to shift toward the side of major involvement, which is usually the left hemithorax.62 The echocardiogram excludes congenital mitral stenosis or cor triatriatum as the cause of obstruction to left atrial flow. Color flow imaging and Doppler scan interrogation disclose continuous turbulent flow with normal velocities in the involved pulmonary veins.55 Interrogation of each pulmonary vein is required because focal and long-segment stenoses are associated with different flow patterns.55 Atresia of the common pulmonary vein has been diagnosed with fetal echocardiography.63
1 Collins-Nakai R.L., Rosenthal A., Castaneda A.R., Bernhard W.F., Nadas A.S. Congenital mitral stenosis. A review of 20 years’ experience. Circulation. 1977;56:1039-1047.
2 Tandon R., Moller J.H., Edwards J.E. Anomalies associated with the parachute mitral valve: a pathologic analysis of 52 cases. Can J Cardiol. 1986;2:278-281.
3 Alphonso N., Norgaard M.A., Newcomb A., D’udekem Y., Brizard C.P., Cochrane A. Cor triatriatum: presentation, diagnosis and long-term surgical results. Ann Thorac Surg. 2005;80:1666-1671.
4 Godoy I., Tantibhedhyangkul W., Karp R., Lang R. Images in cardiovascular medicine. Cor triatriatum. Circulation. 1998;98:2781.
5 Bano-Rodrigo A., Van Praagh S., Trowitzsch E., Van Praagh R. Double-orifice mitral valve: a study of 27 postmortem cases with developmental, diagnostic and surgical considerations. Am J Cardiol. 1988;61:152-160.
6 Sullivan I.D., Robinson P.J., De Leval M., Graham T.P.Jr. Membranous supravalvular mitral stenosis: a treatable form of congenital heart disease. J Am Coll Cardiol. 1986;8:159-164.
7 Trowitzsch E., Bano-Rodrigo A., Burger B.M., Colan S.D., Sanders S.P. Two-dimensional echocardiographic findings in double orifice mitral valve. J Am Coll Cardiol. 1985;6:383-387.
8 Collison S.P., Kaushal S.K., Dagar K.S., et al. Supramitral ring: good prognosis in a subset of patients with congenital mitral stenosis. Ann Thorac Surg. 2006;81:997-1001.
9 Mychaskiw G.2nd, Sachdev V., Braden D.A., Heath B.J. Supramitral ring: an unusual cause of congenital mitral stenosis. Case series and review. J Cardiovasc Surg. 2002;43:199-202.
10 Ruckman R.N., Van Praagh R. Anatomic types of congenital mitral stenosis: report of 49 autopsy cases with consideration of diagnosis and surgical implications. Am J Cardiol. 1978;42:592-601.
11 Castaneda A.R., Anderson R.C., Edwards J.E. Congenital mitral stenosis resulting from anomalous arcade and obstructing papillary muscles. Report of correction by use of ball valve prosthesis. Am J Cardiol. 1969;24:237-240.
12 Parr G.V., Fripp R.R., Whitman V., Bharati S., Lev M. Anomalous mitral arcade: echocardiographic and angiographic recognition. Pediatr Cardiol. 1983;4:163-165.
13 Layman T.E., Edwards J.E. Anomalous mitral arcade. A type of congenital mitral insufficiency. Circulation. 1967;35:389-395.
14 Perez J.A., Herzberg A.J., Reimer K.A., Bashore T.M. Congenital mitral insufficiency secondary to anomalous mitral arcade in an adult. Am Heart J. 1987;114:894-895.
15 Shone J.D., Sellers R.D., Anderson R.C., Adams P.Jr, Lillehei C.W., Edwards J.E. The developmental complex of “parachute mitral valve,” supravalvular ring of left atrium, subaortic stenosis, and coarctation of aorta. Am J Cardiol. 1963;11:714-725.
16 Smallhorn J., Tommasini G., Deanfield J., Douglas J., Gibson D., Macartney F. Congenital mitral stenosis. Anatomical and functional assessment by echocardiography. Br Heart J. 1981;45:527-534.
17 Isner J.M., Salem D.N., Seaver P.R., Payne D.D., Cleveland R.J. Supravalvular stenosing ring of the left atrium associated with bilateral atrioventricular valvular regurgitation. Am Heart J. 1983;106:1150-1152.
18 Davachi F., Moller J.H., Edwards J.E. Diseases of the mitral valve in infancy. An anatomic analysis of 55 cases. Circulation. 1971;43:565-579.
19 Shrivastava S., Moller J.H., Tadavarthy M., Fukuda T., Edwards J.E. Clinical pathologic conference. Am Heart J. 1976;91:513-519.
20 Rosenquist G.C. Congenital mitral valve disease associated with coarctation of the aorta: a spectrum that includes parachute deformity of the mitral valve. Circulation. 1974;49:985-993.
21 Daoud G., Kaplan S., Perrin E.V., Dorst J.P., Edwards F.K. Congenital mitral stenosis. Circulation. 1963;27:185-196.
22 Elliott L.P., Anderson R.C., Amplatz K., Lillehei C.W., Edwards J.E. Congenital mitral stenosis. Pediatrics. 1962;30:552-562.
23 Singh S.P., Gotsman M.S., Abrams L.D., Astley R., Parsons C.G., Roberts K.D. Congenital mitral stenosis. Br Heart J. 1967;29:83-90.
24 Perloff J.K. Auscultatory and phonocardiographic manifestations of pulmonary hypertension. Prog Cardiovasc Dis. 1967;9:303-340.
25 Perloff J.K. Physical examination of the heart and circulation, 4th ed. Shelton, Connecticut: People’s Medical Publishing House; 2009.
26 Rodan B.A., Chen J.T., Kirks D.R., Benson D.W.Jr. Mitral valve calcification in congenital mitral stenosis. Am Heart J. 1983;105:514-515.
27 Grenadier E., Sahn D.J., Valdes-Cruz L.M., Allen H.D., Oliveira Lima C., Goldberg S.J. Two-dimensional echo Doppler study of congenital disorders of the mitral valve. Am Heart J. 1984;107:319-325.
28 Snider A.R., Roge C.L., Schiller N.B., Silverman N.H. Congenital left ventricular inflow obstruction evaluated by two-dimensional echocardiography. Circulation. 1980;61:848-855.
29 Vitarelli A., Landolina G., Gentile R., Caleffi T., Sciomer S. Echocardiographic assessment of congenital mitral stenosis. Am Heart J. 1984;108:523-531.
30 Andral G. Precis d’anatomie pathologique. Paris: Gabon; 1829.
31 Church W.S. Congenital malformation of the heart: abnormal septum in the left auricle. Transactions of the Pathology Society of London. 1868;19:188-190.
32 Borst M. Ein cor triatriatum. Zentralbl f Path. 1905;16:812.
33 Thilenius O.G., Bharati S., Lev M. Subdivided left atrium: an expanded concept of cor triatriatum sinistrum. Am J Cardiol. 1976;37:743-752.
34 Gheissari A., Malm J.R., Bowman F.O.Jr, Bierman F.Z. Cor triatriatum sinistrum: one institution’s 28-year experience. Pediatr Cardiol. 1992;13:85-88.
35 Marin-Garcia J., Tandon R., Lucas R.V.Jr, Edwards J.E. Cor triatriatum: study of 20 cases. Am J Cardiol. 1975;35:59-66.
36 Van Praagh R., Corsini I. Cor triatriatum: pathologic anatomy and a consideration of morphogenesis based on 13 postmortem cases and a study of normal development of the pulmonary vein and atrial septum in 83 human embryos. Am Heart J. 1969;78:379-405.
37 Gahide G., Barde S., Francis-Sicre N. Cor triatriatum sinister: a comprehensive anatomical study on computed tomography scan. J Am Coll Cardiol. 2009;54:487.
38 Lee Y.-S., Kim K.-S., Lee J.-B., Kean-Ryu J., Choi J.-Y., Chang S.-G. Cor triatriatum dexter assessed by three-dimensional echocardiography reconstruction in two adult patients. Echocardiography. 2007;24:991-994.
39 Nakib A., Moller J.H., Kanjuh V.I., Edwards J.E. Anomalies of the pulmonary veins. Am J Cardiol. 1967;20:77-90.
40 Patel A.K., Ninneman R.W., Rahko P.S. Surgical resection of cor triatriatum in a 74-year-old man. Review of echocardiographic findings with emphasis on Doppler and transesophageal echocardiography. J Am Soc Echocardiogr. 1990;3:402-407.
41 Lucas R.V.Jr, Woolfrey B.F., Andersonrc L.R.G., Edwards J.E. Atresia of the common pulmonary vein. Pediatrics. 1962;29:729-739.
42 Alboliras E.T., Edwards W.D., Driscoll D.J., Seward J.B. Cor triatriatum dexter: two-dimensional echocardiographic diagnosis. J Am Coll Cardiol. 1987;9:334-337.
43 Burton D.A., Chin A., Weinberg P.M., Pigott J.D. Identification of cor triatriatum dexter by two-dimensional echocardiography. Am J Cardiol. 1987;60:409-410.
44 Hansing C.E., Young W.P., Rowe G.G. Cor triatriatum dexter. Persistent right sinus venosus valve. Am J Cardiol. 1972;30:559-564.
45 Trakhtenbroit A., Majid P., Rokey R. Cor triatriatum dexter: antemortem diagnosis in an adult by cross sectional echocardiography. Br Heart J. 1990;63:314-316.
46 Mcguire L.B., Nolan T.B., Reeve R., Dammann J.F.Jr. Cor triatriatum as a problem of adult heart disease. Circulation. 1965;31:263-272.
47 Almendro-Delia M., Trujillo-Berraquero F., Araji O., De Vinuesa P.G.-G., Fernandez J.M.C. Cor triatriatum sinistrum in an elderly man. Int J Cardiol. 2008;125:e27-e29.
48 Schluter M., Langenstein B.A., Thier W., et al. Transesophageal two-dimensional echocardiography in the diagnosis of cor triatriatum in the adult. J Am Coll Cardiol. 1983;2:1011-1015.
49 Ludomirsky A., Erickson C., Vick G.W.3rd, Cooley D.A. Transesophageal color flow Doppler evaluation of cor triatriatum in an adult. Am Heart J. 1990;120:451-455.
50 Ehrich D.A., Vieweg W.V., Alpert J.S., Folkerth T.L., Hagan A.D. Cor triatriatum: report of a case in a young adult with special reference to the echocardiographic features and etiology of the systolic murmur. Am Heart J. 1977;94:217-221.
51 Darke C.S., Emery J.L., Lorber J. Triatrial heart. Br Heart J. 1961;23:329-332.
52 Vuocolo L.M., Stoddard M.F., Longaker R.A. Transesophageal two-dimensional and Doppler echocardiographic diagnosis of cor triatriatum in the adult. Am Heart J. 1992;124:791-793.
53 Alwi M., Hamid Z.A., Zambahari R. A characteristic continuous wave Doppler signal in cor triatriatum? Br Heart J. 1992;68:6-8.
54 Geggel R.L., Fried R., Tuuri D.T., Fyler D.C., Reid L.M. Congenital pulmonary vein stenosis: structural changes in a patient with normal pulmonary artery wedge pressure. J Am Coll Cardiol. 1984;3:193-199.
55 Webber S.A., De Souza E., Patterson M.W. Pulsed wave and color Doppler findings in congenital pulmonary vein stenosis. Pediatr Cardiol. 1992;13:112-115.
56 Driscoll D.J., Hesslein P.S., Mullins C.E. Congenital stenosis of individual pulmonary veins: clinical spectrum and unsuccessful treatment by transvenous balloon dilation. Am J Cardiol. 1982;49:1767-1772.
57 Hawker R.E., Celermajer J.M., Gengos D.C., Cartmill T.B., Bowdler J.D. Common pulmonary vein atresia. Premortem diagnosis in two infants. Circulation. 1972;46:368-374.
58 Nasrallah A.T., Mullins C.E., Singer D., Harrison G., Mcnamara D.G. Unilateral pulmonary vein atresia: diagnosis and treatment. Am J Cardiol. 1975;36:969-973.
59 Mortensson W., Lundstrom N.R. Congenital obstruction of the pulmonary veins at their atrial junctions. Review of the literature and a case report. Am Heart J. 1974;87:359-362.
60 Shone J.D., Amplatz K., Anderson R.C., Adams P.Jr, Edwards J.E. Congenital stenosis of individual pulmonary veins. Circulation. 1962;26:574-581.
61 Mori K., Dohi T. Mitral and pulmonary vein blood flow patterns in cor triatriatum. Am Heart J. 1989;117:1167-1169.
62 Cullen S., Deasy P.F., Tempany E., Duff D.F. Isolated pulmonary vein atresia. Br Heart J. 1990;63:350-354.
63 Samuel N., Sirotta L., Bar-Ziv J., Dicker D., Feldberg D., Goldman J.A. The ultrasonic appearance of common pulmonary vein atresia in utero. J Ultrasound Med. 1988;7:25-28.