Chapter 15 Congenital Elbow Disorders
Introduction
Congenital elbow disorders can involve either the soft tissues, e.g. arthrogryposis, bone e.g. radio-ulnar synostosis, or the joint itself, producing instability. Ogino and Hikino1 classified congenital elbow deformities into three types based on the presence of associated anomalies:
Congenital elbow synostosis
Background and aetiology
A single mesodermal condensation is seen along the long axis of the limb bud at week 4. Pre-chondrogenic cells in these condensations then differentiate into chondrocytes under the influence of bone morphogenetic proteins (BMPs). Sequential chondrification and ossification subsequently follow, resulting in the formation of bones and joints in the upper limb. The elbow becomes visible at 34 days. The humerus, radius and ulna are continuous with each other and are joined by a common perichondrium at 5 weeks of gestation. The forearm is in a neutral position and subsequently by the 6th week condensation of the tissue separates the cartilaginous anlage of the three bones. The forearm pronates at the 8th week due to growth discrepancy between the arterial tree and the radius.2
Although most cases are sporadic, a positive family history in some cases of congenital radio-ulnar synostosis has suggested a genetic component. Inheritance may be autosomal dominant or recessive. Some of these cases, especially humeroradial synostosis may be syndromic as they are associated with other congenital syndromes like Carpenter syndrome, Apert syndrome, Antley–Bixler syndrome, Roberts syndrome,3,4 arthrogryposis and Cornelia de Lange syndrome. Chromosomal abnormalities such as Klinefelter syndrome5 may be associated with radio-ulnar synostosis.
Classification
Classically, synostosis has been divided anatomically into humeroradial, humero-ulnar, humeroradio-ulnar and radio-ulnar. Further subdivisions in the humeroradial group have been added based on the anatomical differences, namely classes 1 and 2:6
| Anatomical | Humeroradial |
| Humero-ulnar | |
| Radio-ulnar | |
| Aetiological | Class 1 – bone hypoplasia group with synostosis of elbow |
| Class 2 – joint maldevelopment group with synostosis of elbow |
Proximal radio-ulnar synostosis has also been classified on the basis of the radiological appearance. Mital7 presented a two-group classification system:
This was modified by Cleary and Omer8 to a four-group system:
An aetiological classification of elbow synostosis was proposed by McIntyre and Benson.9 They divided synostosis around the elbow into two classes:
All the three anatomical types can occur within each class, may be sporadic or familial and, if familial, may be inherited as an autosomal dominant or recessive trait. The class I group is associated with musculoskeletal abnormalities outside the affected limb in 25–55% of cases.10 Class II familial cases may be syndromic and associated with multiple synostosis syndrome, Pfeiffer syndrome, Apert syndrome and Antley–Bixler syndrome.
Presentation, investigations and treatment options
Proximal radio-ulnar synostosis
Two major surgical procedures are available for this condition. One involves a mobilization procedure to separate the radio-ulnar synostosis and restore forearm rotation,11,12 while the other is an osteotomy to correct the forearm position, making it more suitable for the patient’s activities of daily living.
Another alternative that has been proposed to achieve and maintain motion is the swivel prosthesis designed by Kelikian and Doumanian.13 This is a stainless steel implant that is inserted into the intramedullary canal of the radius to restore motion. However, although it has been used in four cases of post-traumatic proximal radio-ulnar synostosis with good results, it has been shown to have poor results in cases of congenital radio-ulnar synostosis.14
Surgical techniques and rehabilitation
Mobilization procedure11
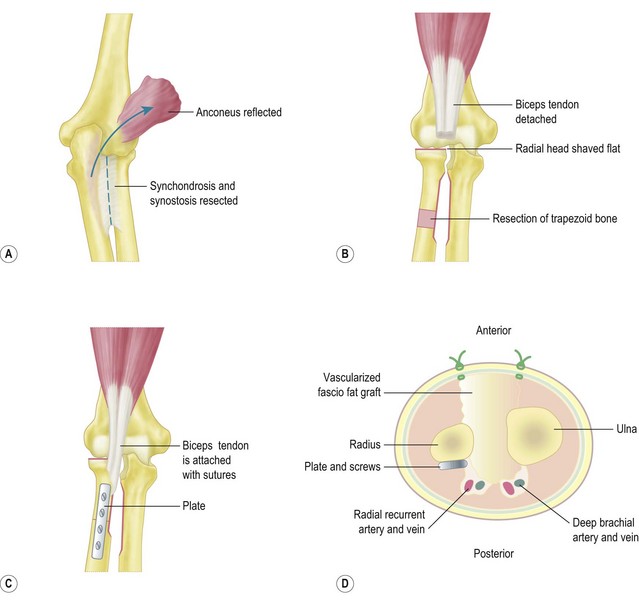
Kanaya described this procedure in the following four steps:
Vascularized fascio-fat graft
The wounds are closed and an above-elbow cast is applied with the forearm in neutral rotation and the elbow in 90° of flexion. The cast is maintained for 4 weeks (Fig. 15.1).
Rotational osteotomy
An alternative procedure to mobilize the synostosis is a rotational osteotomy.15,16
These have been described at two sites in the diaphysis of the radius and ulna17,18 or at one site in the distal diaphysis of the radius.19,20 An osteotomy at the synostosis is extremely complex, whereas an osteotomy at two sites in the diaphysis of the radius and ulna is easier, with fewer reported complications.
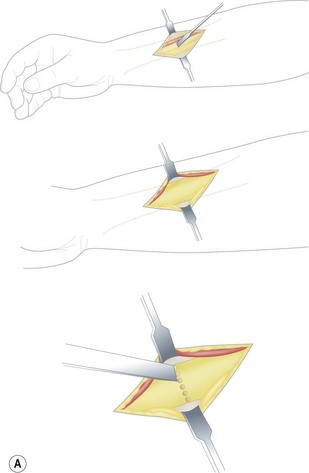
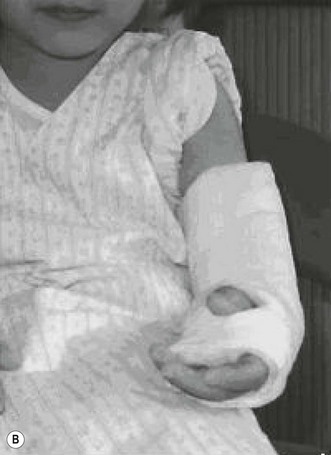
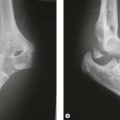
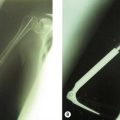
A single osteotomy of the distal radius is performed through a 4 cm skin incision made distally on the radiovolar aspect of the distal forearm. The diaphysis of the radius is exposed and the superficial radial nerve and the radial artery are identified and retracted ulnarward. A sharp longitudinal incision is made on the periosteum and reflected atraumatically from the radius to preserve the longitudinal continuity of the periosteum. Several drill holes are made in the transverse line to the radius using a K-wire and a transverse osteotomy is performed using a chisel (Fig. 15.2A). A meticulous periosteal repair is then performed and the distal forearm maximally supinated and immobilized in an above-elbow plaster cast (Fig. 15.2B).
Outcome including literature review
Operative procedures used in the treatment of congenital radio-ulnar synostosis fall into two major groups. Group 1 procedures are designed to restore rotation and remove the synostosis, while group 2 procedures restore the forearm to a fixed functional position. Several authors have reported poor results after separation of the synostosis and interposition of fat, muscle or silicone.11,21–23
Kanaya et al performed the mobilization procedure in seven boys (average age 8 years 2 months) who had isolated proximal radio-ulnar synostosis. All seven children had a dislocation of the radial head preoperatively. A shortening-wedge osteotomy of the radius was performed in four patients at the time of the index procedure, while in three patients the radial head was manually reduced without an osteotomy. The radial head subsequently redislocated in the latter three patients and one of them had an osteotomy 2 years and 8 months after the index procedure to reduce and maintain the radial head alignment. At follow-up the total range of forearm rotation achieved averaged 71° with average supination of 26° and average pronation of 45°. There were no cases of reankylosis at an average follow-up of 4 years 4 months.12 The group who had a radial osteotomy in addition to the index procedure had an average arc of 83° of forearm rotation compared to the second group, who had an average arc of rotation of 40°.
Kamineni et al24 have described a new technique of restoring forearm rotation in post-traumatic proximal radio-ulnar synostosis. This involves resecting a 1 cm thick section of bone from the proximal radial shaft distal to the synostosis. They performed this surgery on seven patients and reported an improvement in forearm rotation from an average fixed pronation of 5° to an average arc of 98°.
Osteotomies to achieve a more functional forearm position are suitable for patients with marked pronation deformity. Green and Mital16 have reported the best results with derotational osteotomies through the fusion mass. This has the advantages of achieving a better correction and rapid healing of the osteotomy owing to the good coaptation of the divided ends. In their series results were excellent in 50%, good in 33%, fair in 6% and poor in 6%. Ogino and Hikino1 also favoured an osteotomy through the fusion mass, but recommended resection of 0.5 cm of bone at the osteotomy site in order to shorten the forearm. All the patients in their series had complete relief of symptoms.
Goldner and Lipton recommended derotation in the distal forearm to minimize circulatory problems.25 The osteotomy was done in the distal radius alone for younger patients and in both the radius and ulna in older patients.
A single osteotomy technique distal to the synostosis was reported by Kashiwa et al in 199919 and subsequently modified by Fujimoto et al.20 This osteotomy is performed at the distal radial diaphysis. Fujimoto presented the results of this osteotomy in three patients (four forearms) with congenital radio-ulnar synostosis. Bony union was achieved in all cases. The period of immobilization in plaster varied from 6 to 9 weeks. All patients achieved good correction, with functional improvement in activities of daily living.
Bolano26 has shown good results with an osteotomy and gradual correction of the rotation using a ring fixator. This technique is useful as there is less risk to the neurovascular structures and it has the added advantage of allowing the patients to choose the most functional position of their forearm.
The ideal position to place the forearm after the osteotomy depends on several factors. If the synostosis is bilateral and both hands are in pronation, Simmons and Waters27 recommended 20° of pronation in the dominant hand and a neutral position for the non-dominant. In unilateral cases, Green and Mital16 recommended 20–25° of supination as the supinated forearm can attain some amount of functional pronation by internal rotation, flexion and abduction at the shoulder. Ogino and Hikino1 from Japan recommend at least 70° of palmar supination, especially in the non-dominant hand, to enable the use of chopsticks by their patients. Hence the amount of angular correction is dependent on customs, dominant hand and bilaterality.
Complications
Following excision of a synostosis and mobilization of the forearm, reankylosis can occur, giving poor results21,22 and a risk of radial head dislocation and posterior interosseous nerve palsy.
Complications reported following derotational osteotomies include neurovascular compromise. Green and Mital reported one case of severe Volkmann’s contracture and one case of posterior interosseous nerve palsy from their series of 13 patients. Ogino and Hikino1 reported two posterior interosseous nerve palsies in 13 operations, both of which spontaneously recovered.
Simmons and Waters27 reported a 36% complication rate with loss of correction in three out of 21 children and an 18% incidence of vascular compromise. A decreased range of elbow movement has also been reported.
Arthrogryposis affecting the elbow
Introduction
The disorder was first described in 1841 by Otto28 and was later called multiple congenital contractures by Schanz. The term arthrogryposis was coined by Rosencranz29 and Stern in 1923 proposed the term arthrogryposis multiplex congenita,30 which is the current name for this condition.
Background and aetiology
The typical pathological features of arthrogryposis – thin, atrophic extremities without skin creases and fibrosis of joints with fatty accumulations about the joints – can be explained by fetal akinesia, which may result from maternal or fetal abnormalities. There is a direct correlation between the duration of akinesia and the severity of contractures at birth.31 The cause of fetal akinesia may be neurological or muscle abnormalities. Hence two pathological forms of arthrogryposis have been identified, namely a neuropathic form and a myopathic form. The neuropathic form is more common, accounting for over 90% of all cases, and is caused by failure of development of the anterior horn cells at the spinal level. The myopathic form is caused by defective myogenic regulatory genes,32 resulting in defective somites and absent myocytes that are replaced by adipose cells.
Classification
Presentation, investigation and treatment options
The elbow joint is the key for rehabilitation of the upper extremity.
Treatment options
The order of priorities includes:
Non-operative management
Palmer et al34 recommended stretching and serial splinting from the age of 3 months. They reported an improvement of 38% for elbow mobility and 43% for wrist mobility in their group of 63 patients who had treatment at an age younger than 18 months. Corrective splints and serial casts have been used with variable success.
Surgical technique and rehabilitation
Operative technique and postoperative management
Surgical anatomy of pectoralis major
The pectoralis major has two parts, namely the sternocostal and the clavicular part. The former is innervated by the lateral pectoral nerves and the latter by the medial pectoral nerve. The length of the sternocostal part and the muscle power are comparable to that of the biceps. The segmental anatomy and neurovascular supply of this muscle make it suitable for segmental splitting.35–37
Bipolar latissimus dorsi transfer
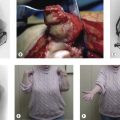
Gagnon et.al38 have described in detail the use of the latissimus dorsi muscle to restore elbow flexion in arthrogryposis. It involves the following steps:
Incision
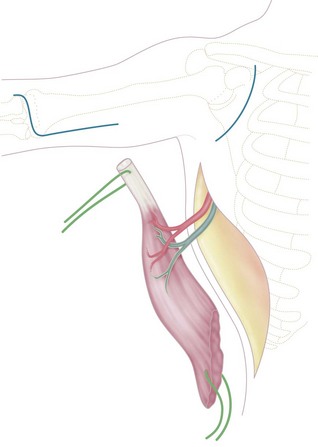
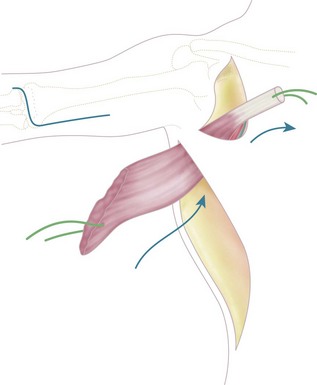
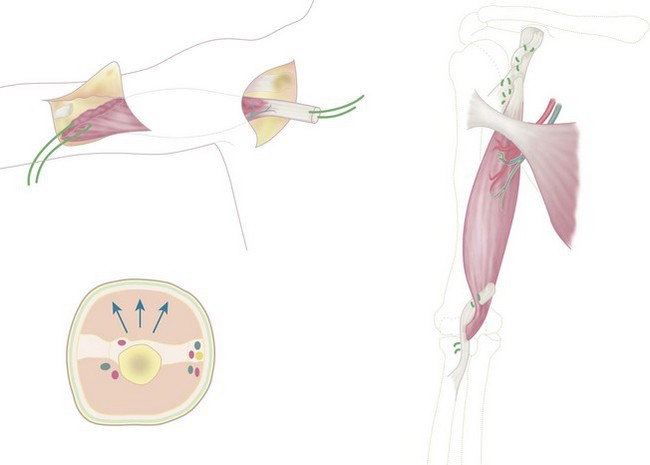
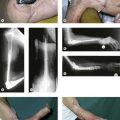
An incision is made along the anterolateral aspect of the latissimus dorsi muscle, extending from the posterior border of the axilla down to the last ribs (Fig. 15.3). The entire muscle is exposed and mobilized, proceeding from caudal to cephalad and taking care to free the neurovascular pedicle (thoracodorsal vessels and nerve) up to its origin in the axilla. This is necessary to allow transposition of the muscle to the arm. The latissimus dorsi muscle is detached from its origin and insertion, preserving the dorsolumbar fascia (Fig. 15.4). The coracoid process in the shoulder is then exposed through a deltopectoral incision (Fig. 15.3). The pectoralis major is mobilized to make a channel for the latissimus dorsi muscle. The biceps insertion at the elbow is then exposed through a bayonet incision. Following this, the paralysed biceps muscle is resected between these two incisions, taking care to preserve the neurovascular bundle in the arm along with the musculocutaneous nerve.
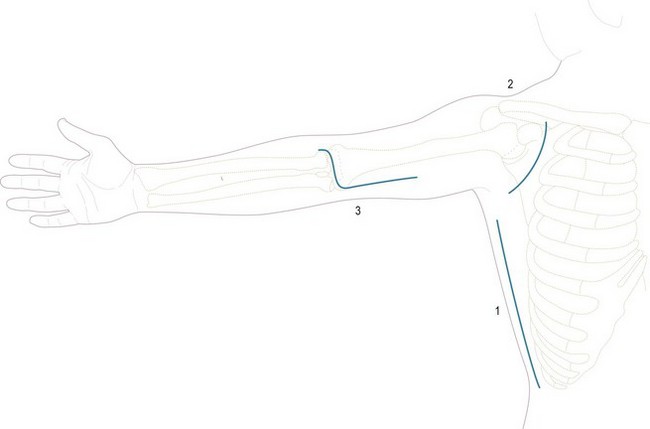
Figure 15.3 Skin incisions to expose: (1) latissimus dorsi; (2) coracoid process; (3) biceps tendon.
The latissimus dorsi muscle is then passed under the skin bridge of the axilla into the anterior brachial compartment (Fig. 15.5). The insertional tendon of the latissimus is passed under the pectoralis major tendon and secured to the coracoid process (Fig. 15.6) using non-absorbable interrupted sutures. This simulates the origin of the short head of the biceps brachii. An insertional tendon for the new biceps can be fashioned from the dorsolumbar fascia of the latissimus and secured to the radius (Fig. 15.6). Fixation can be achieved by drilling a 4–5 mm hole through the radial tuberosity (Fig. 15.7). The muscle should be anchored with adequate tension (the elbow should remain spontaneously at 100° of flexion, with the forearm in supination) to avoid laxity in the muscle unit. Laxity will result in limitation of active flexion, necessitating further surgery to shorten the tendon. Gagnon et al suggested that imbricating the tendinous origin at the coracoid process and thus shortening the tendon by approximately 1 cm should retension the muscle.
Operative technique and postoperative management
Incision
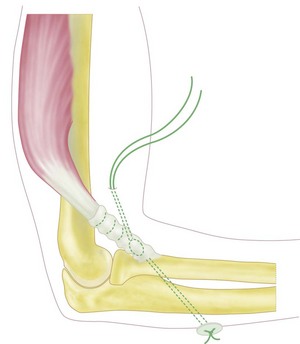
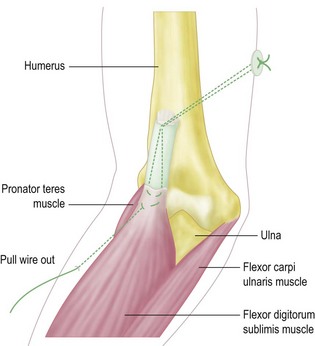
Through a curvilinear incision on the ulnar aspect of the elbow the medial epicondyle is exposed. The ulnar nerve is identified and traced distally to its entrance into the flexor carpi ulnaris muscle. The median nerve is also identified and traced to its entry under the fibrous arch of the flexor digitorum superficialis. The common flexor origin is then mobilized with a thin wafer of bone (Fig. 15.8) and the brachialis muscle identified and elevated subperiosteally from the anteromedial surface of the distal humerus.
Outcome including literature review
Williams39 published his results of surgery on 23 arthrogrypotic elbows. Tricepsplasties were performed on four, while 19 had both tricepsplasty followed by triceps transfer. The average age of the patients at the time of operation was 6 years. The range of elbow motion improved from 7.5–26° before surgery to 42.5–108° after surgery in the four patients who underwent tricepsplasty alone. There was an average increase in the range of forearm rotation of 137° in the19 patients who had both procedures. However, the results of gain in flexor power were variable, with the best results occurring in children aged between 4 and 6 years. Younger children had a higher incidence of weak transfers. All 23 elbows achieved good functional results after the procedure. Subsequent reports, however, have condemned triceps transfers owing to the development of a flexion contracture secondary to unopposed elbow flexion.
Lahoti and Bell40 have reported their long-term results of six pectoralis major transfer in arthrogryposis to restore elbow flexion. They noted good early results in five of the six patients. However, an increase in flexion deformity developed over time in four of the five patients who showed initial good results, resulting in deterioration of elbow flexion. Only one patient had good function 10 years after the transfer. Lahoti and Bell recommend this transfer only for patients who maintain a good passive range of movement in the elbow following triceps lengthening and posterior capsulotomy.
Complications
Traction injuries to the neurovascular pedicle can complicate any of the flexorplasties.
Congenital radial head dislocation
Introduction
Congenital dislocation of the radial head is the most common congenital anomaly of the elbow.41,42 It can exist as an isolated defect or may be associated with other anomalies or syndromes.
Background, aetiology and classification
Hypoplasia of the capitellum in the developing embryo has been proposed as the cause of congenital radial head dislocation. The cause for capitellar hypoplasia, however, is unknown.43 In addition, it is not clear whether capitellar hypoplasia is the cause or effect of a dislocated radial head since the removal of one bone from a developing joint can result in relative hypoplasia of the other bone. The underlying aetiology of this anomaly has been attributed to a germplasm defect.
Congenital radial head dislocation may be anterior, posterior or lateral. Posterior dislocations constitute 65% of all congenital dislocations, while anterior dislocations account for 18% and lateral for the remaining 17%.42 Anterior dislocations exist as an isolated anomaly and show an autosomal dominant inheritance. They can be classified based on aetiology as congenital, dysplastic (osteochondromatosis, fibrous dysplasia) and traumatic. Congenital dislocation of the radial head may be familial, especially on the paternal side, and may be associated with chondro-osteodystrophy. Like other elbow abnormalities, an association between congenital radial head dislocation and sex chromosomal abnormality (XYY syndrome) has been reported.44
Presentation, investigations and treatment options
Presentation
Congenital dislocation of the radial head is bilateral in 30–40% of cases.42,45,46 Bilateral involvement is often associated with other abnormalities42,46 and is usually posterior. Anterior dislocations are more common with isolated congenital radial head dislocation. Although occasionally congenital radial head dislocation may be diagnosed at birth, most cases are identified later during childhood when they present with limitation in the range of elbow movement. Full flexion of the elbow is limited in anterior dislocations and full extension in posterior dislocations. However, forearm rotation is restricted in all cases. The average reduction in forearm rotation reported in one series was 99°.42
Mardam-Bey and Ger42 suggested additional clinical factors that help differentiate a congenital from a traumatic radial head dislocation: (1) bilateral involvement; (2) associated other congenital anomalies; (3) familial occurrence; (4) lack of a history of trauma; (5) not reducible by closed methods; (6) dislocation seen at birth.
Investigations
The classic article on this condition by McFarland47 in 1936 describes the radiological criteria for diagnosing congenital radial head dislocation and differentiating it from traumatic dislocations. These criteria relate to the shape of the radial head, hypoplasia of the capitellum and the shape of the posterior border of the proximal ulna. They were subsequently confirmed by Almquist et al.48
Arthrography has been used to differentiate traumatic from congenital radial head dislocations.49 In congenital dislocations the radial head is displaced from the capitellum but well covered by the capsule of the elbow joint, suggesting that the lesion is an intra-articular dislocation of the head, whereas traumatic dislocations show no capsule covering the radial head suggesting an extra-articular dislocation.
Treatment options
The literature on the surgical management of congenital radial head dislocation is very sparse. Most of the studies on surgical intervention are simple case series without control groups and hence do not provide enough data to make firm conclusions. Asymptomatic children with minimal restriction of activities of daily living should be observed. For symptomatic patients either open reduction of the radiocapitellar joint41,45,50,51 or radial head excision42,45,52,53 should be considered.
Surgical technique and rehabilitation
Open reduction of the radial head dislocation
Open reduction of the radial head with an ulnar osteotomy and annular ligament reconstruction has been reported in the management of congenital radial head dislocation. However, this technique is associated with a high recurrence rate, due to loss of the bony buttress resulting from the capitellum being hypoplastic or an oblong radial head. It has also been reported to be ineffective at improving the range of motion at the elbow joint.45,54 Improvement in forearm rotation has, however, been noted if the procedure is performed in very young children (younger than 2 years). Sachar and Mih41 postulated that early reduction of a radial head dislocation resulted in the normal formation of the radiocapitellar joint in a similar way to that seen with developmental hip dysplasia.
Rotational osteotomy of the radius
Since the radial head has its maximum stability with the forearm externally rotated, a rotational osteotomy of the radius may help to maintain the reduction of the radial head. It works by releasing the tension on the biceps, which is the factor responsible for recurrent anterior dislocation and increasing the tension of pronator teres and pronator quadratus. This osteotomy of the radius is usually done at the mid-diaphyseal level. Occasionally radial shortening may have to be done if there is radial overgrowth.55 By undertaking a rotational osteotomy of the radius Futami et al achieved anatomical radiographic reduction of the radial head in five patients with congenital radial head dislocation and one post-traumatic dislocation.
Outcome including literature review
There is, however, universal agreement to avoid surgery if there is little or no restriction in activities of daily living.41,42,52
Although radial head excision relieves pain, it does not improve the range of forearm rotation. Yamazaki and Kato51 have reported good long-term results following open radial head reduction undertaken in a patient with bilateral congenital radial head dislocation. However, their case lacked the typical radial head morphology characteristic of congenital dislocation. Annular ligament reconstruction was performed using the fascia of extensor carpi ulnaris.
Kim et al50 published their results of open reduction and reconstruction of 15 elbows with chronic radial head dislocations. Three of these were congenital and the rest were post-traumatic in aetiology. The average age of the children was 9.5 years. Two of the three congenital cases showed persistent subluxation on the follow-up radiographs.
Complications
Complications reported with radial head excision include proximal migration of the radius causing wrist pain, valgus instability of the elbow,48 valgus deformity of the elbow48,53 with tardy ulnar nerve palsy, radio-ulnar synostosis52 and regrowth of the radial head.52 Open reduction of the radial head has been associated with high recurrence rates.
The elbow in longitudinal growth arrest of the upper limb
Ulnar club hand
The elbow can be severely affected in ulnar club hand and one of the earlier classifications of this condition was based on the severity of elbow involvement.56 Dobyns et al57 classified ulnar club hand into four types:
1 Ogino T, Hikino K. Congenital radioulnar synostosis: compensatory rotation around the wrist and rotation osteotomy. J Hand Surg (Br). 1987;12:173.
2 Lovell WW, Winter RB, editors. Pediatric orthopaedics. Philadelphia, PA: Lippincott, 1978.
3 Bottero L, Cinalli G, Labrune P, et al. Antley–Bixler syndrome: description of two new cases and a review of the literature. Childs Nerv Syst. 1997;13:275-281.
4 Buck-Gramcko D. Ulnar deficiency. In: Saffar P, Foucher G, Amadio P, editors. Current practice in hand surgery. London: Martin Dunitz; 1997:371-390.
5 Simmons BP, Southmayd WW, Riseborough EJ. Congenital radioulnar synostosis. J Hand Surg. 1983;8:829-838.
6 Wood VE. Congenital elbow contractures. In: Buck-Gramcko D, editor. Congenital malformations of the hand and forearm. Hong Kong: Churchill Livingstone; 1998:463-486.
7 Mital MA. Congenital radioulnar synostosis with congenital dislocation of radial head. Orthop Clin North Am. 1976;7:375.
8 Cleary JE, Omer GE. Congenital proximal radio-ulnar synostosis: natural history and functional assessment. J Bone Joint Surg Am. 1985;67:539-545.
9 McIntyre JD, Benson MK. An aetiological classification for developmental synostoses at the elbow. J Pediatr Orthop B. 2002;11(4):313-319.
10 Card RY, Strachman J. Congenital ankylosis of the elbow. J Pediatr. 1955;46:81-85.
11 Kanaya F, Ibaraki K. Mobilization of a congenital proximal radioulnar synostosis with use of a free vascularized fascio-fat graft. J Bone Joint Surg (Am). 1998;80:1186-1192.
12 Funakoshi T, Kato H, Minami A, et al. The use of pedicled posterior interosseous fat graft for mobilization of congenital radioulnar synostosis: a case report. J Shoulder Elbow Surg. 2004;13(2):230-234.
13 Kelikian H, Doumanian A. Swivel for proximal radioulnar synostosis. J Bone Joint Surg. 1957;39:945-951.
14 Tachdjian MO. Pediatric orthopaedics: congenital radioulnar synostosis. Philadelphia, PA: WB Saunders; 1990.
15 Khalil I, Vizkelety T. Osteotomy of the synostosis mass for the treatment of congenital radio-ulnar synostosis. Arch Orthop Trauma Surg. 1993;113(1):20-22.
16 Green WT, Mital MA. Congenital radio-ulnar synostosis: surgical treatment. J Bone Joint Surg Am. 1979;61(5):738-743.
17 Lin HH, Strecker WB, Manske PR, et al. A surgical technique of radioulnar osteoclasis to correct severe forearm rotation deformities. J Pediatr Orthop. 1995;15:53-58.
18 Murase T, Tada K, Yoshida T, et al. Derotational osteotomy at the shafts of the radius and ulna for congenital radioulnar synostosis. J Hand Surg (Am). 2003;28:133-137.
19 Kashiwa H, Ogino T, Tsuchida H, et al. Simple rotation osteotomy of the radius for congenital radioulnar synostosis. Congenit Anom (Kyoto). 1999;39:167.
20 Fujimoto M, Kato H, Minami A. Rotational osteotomy at the diaphysis of the radius in the treatment of congenital radioulnar synostosis. J Pediatr Orthop. 2005;25:676-679.
21 Sacher K, Akelman E, Ehrlich MG. Radioulnar synostosis. Hand Clinic. 1994;10:399-404.
22 Hankin FM, Smith PA, Kling TFJr, Louis DS. Ulnar nerve palsy following rotational osteotomy of congenital radioulnar synostosis. J Pediatr Orthop. 1987;7(1):103-106.
23 Miura T, Nakamura R, Horri E. Congenital hand anomalies in Japan: a family study. J Hand Surg. 1990;15A(3):439-444.
24 Kamineni S, Maritz NG, Morrey BF. Proximal radial resection for posttraumatic radioulnar synostosis: a new technique to improve forearm rotation. J Bone Joint Surg Am. 2002;84:745-751.
25 Goldner JL, Lipton MA. Congenital radioulnar synostosis: diagnosis and treatment based on anatomic and functional assessment. Orthop Trans. 1982;6:466.
26 Bolano LE. Congenital radioulnar synostosis: treatment with Ilizarov method. J Hand Surg. 1984;19A(6):977-978.
27 Simmons BP, Waters PM. Congenital abnormalities of the elbow region. In: Peimer C, editor. Surgery of the hand and upper extremity. New York: McGraw-Hill; 1996:2052.
28 Otto AW. Monstrum humanum extremitatibus incurvatus: monstrorum sexcentorum descripto anatomica in Vratislaviae Museum (English translation). Clin Orthop. 1985;194:4.
29 Rosencranz E. Ueber kongenitale Kontracturen der oberen Extremitaten. Z Orthop Chir. 1905;14:52.
30 Stern WG. Athrogryposis multiplex congenita. JAMA. 1923;81:1507.
31 Moessinger AC. Fetal akinesia deformation sequence: an animal model. Paediatrics. 1983;72:857-863.
32 Sarnat HB. New insights into the pathogenesis of congenital myopathies. J Child Neurol. 1994;9:193.
33 Hall JG. Arthrogryposis multiplex congenita: etiology, genetics, classification, diagnostic approach, and general aspects. J Pediatr Orthop B. 1997;6:159.
34 Palmer PM, MacEwen GD, Bowen JR, et al. Passive motion therapy for infants with arthrogryposis. Clin Orthop Relat Res. 1985;April(194):54-59.
35 Manktelow RT, McKee NH, Vettese T. An anatomical study of the pectoralis major muscle as related to functioning free muscle transplantation. Plast Reconstr Surg. 1980;65(5):610-615.
36 Moosman DA. Anatomy of the pectoral nerves and their preservation in modified mastectomy. Am J Surg. 1980;139:883-886.
37 Wei WI, Lam KH, Wong J. The true pectoralis major myocutaneous island flap: an anatomical study. Br J Plast Surg. 1984;37:568-573.
38 Gagnon E, Fogelson N, Seyfer AE. Use of the latissimus dorsi muscle to restore elbow flexion in arthrogryposis. Plast Reconstr Surg. 2000;106(7):1582-1585.
39 Williams PF. The elbow in arthrogryposis. J Bone Joint Surg (Br). 1973;55-B:834-840.
40 Lahoti O, Bell MJ. Transfer of pectoralis major in arthrogryposis to restore elbow flexion: deteriorating results in the long term. J Bone Joint Surg (Br). 2005;87(6):858-860.
41 Sachar K, Mih DA. Congenital radial head dislocations. Hand Clinics. 1998;14:39-47.
42 Mardam-Bey T, Ger E. Congenital radial head dislocation. J Hand Surg (Am). 1979;4:316-320.
43 Loehr JF, Uhthoff HK. The development of the elbow. In: Uhthoff HK, editor. The embryology of the human locomotor system. New York: Springer; 1990:83-93.
44 Butler D, Offiah AC, Wilson L. Congenital radial head dislocation in XYY-syndrome: case report and review of the literature. Curr Orthop Pract. 2009;20(2):203-208.
45 Mital MA. Congenital radioulnar synostosis and congenital dislocation of the radial head. Orthop Clin North Am. 1976;7:375-383.
46 Miura T. Congenital dislocation of the radial head. J Hand Surg (Br). 1990;15:477-481.
47 McFarland B. Congenital dislocation of the head of the radius. Br J Surg. 1936;24:41-44.
48 Almquist EE, Gordon LH, Blue AI. Congenital dislocation of the head of the radius. J Bone Joint Surg. 1969;51A:1118-1127.
49 Mizuno K, Usui Y, Kohyama K, et al. Familial congenital unilateral anterior dislocation of the radial head: differentiation from traumatic dislocation by means of arthrography. J Bone Joint Surg. 1991;73A:1086-1090.
50 Kim HT, Park BG, Suh JT, et al. Chronic radial head dislocation in children. Part 2. Results of open treatment and factors affecting final outcome. J Pediatr Orthop. 2002;22:591-597.
51 Yamazaki H, Kato H. Open reduction of the radial head with ulnar osteotomy and annular ligament reconstruction for bilateral congenital radial head dislocation: a case with long-term follow-up. J Hand Surg (Br). 2007;32:93-97.
52 Kelly DW. Congenital dislocation of the radial head: spectrum and natural history. J Pediatr Orthop. 1981;1:295-298.
53 Campbell CC, Waters PM, Emans JB, et al. Excision of the radial head for congenital dislocation. J Bone Joint Surg. 1992;74(A):726-733.
54 Kim HT, Park BG, Suh JT, et al. Chronic radial head dislocation in children. Part 2. Results of open treatment and factors affecting final outcome. J Pediatr Orthop. 2002;22:591-597.
55 Futami T, Tsukamoto Y, Fujita T. Rotation osteotomy for dislocation of the radial head: 6 cases followed for 7 (3–10) years. Acta Orthop Scand. 1992;63(4):455-456.
56 Kummel W. Die Missbildungen Der extremitaten Durch Defeckt. Ver Wach sung and Uberzahl (in German). Bibl Med Kassel.. 3, 1895.
57 Dobyns JH, Wood VE, Bayne LG. Congenital hand deformities. In: Green DP, editor. Operative hand surgery, vol. 1. New York: Churchill Livingstone; 1993:251-548.