Chapter 387 Congenital Disorders of the Lung
387.1 Pulmonary Agenesis and Aplasia
Bentsianov BL, Goldstein NA, Giuste R, et al. Unilateral pulmonary agenesis presenting as an airway lesion. Arch Otolaryngol Head Neck Surg. 2001;126:1386-1389.
Greenough A, Ahmed T, Broughton S. Unilateral pulmonary agenesis. J Perinat Med. 2006;34:80-81.
Krivchenya DU, Dubrovin AG, Krivchenya TD, et al. Aplasia of the right lung in a 4-year-old child: surgical stabilization of the mediastinum by diaphragm translocation leading to complete recovery from respiratory distress syndrome. J Pediatr Surg. 2000;35:1499-1502.
387.2 Pulmonary Hypoplasia
Etiology and Pathology
Pulmonary hypoplasia involves a decrease in both the number of alveoli and the number of airway generations. The hypoplasia may be bilateral in the setting of bilateral lung constraint, as in oligohydramnios or thoracic dystrophy. Pulmonary hypoplasia is usually secondary to other intrauterine disorders that produce an impairment of normal lung development (Chapter 95). Conditions such as deformities of the thoracic spine and rib cage (thoracic dystrophy), pleural effusions with fetal hydrops, cystic adenomatoid malformation, and congenital diaphragmatic hernia physically constrain the developing lung. Any condition that produces oligohydramnios (fetal renal insufficiency or prolonged premature rupture of membranes) can also lead to diminished lung growth. In these conditions, airway and arterial branching are inhibited, thereby limiting the capillary surface area. Large unilateral lesions, such as congenital diaphragmatic hernia and cystic adenomatoid malformation, can displace the mediastinum and thereby produce a contralateral hypoplasia, although usually not as severe as that seen on the ipsilateral side.
Treatment
Mechanical ventilation and oxygen may be required to support gas exchange. Specific therapy to control associated pulmonary hypertension, such as inhaled nitric oxide, may be useful. In cases of severe hypoplasia, the limited capacity of the lung for gas exchange may be inadequate to sustain life. Extracorporeal membrane oxygenation can provide gas exchange for a critical period of time and permit survival. Rib-expanding devices (vertically expansible prosthetic titanium ribs) can improve the survival of patients with thoracic dystrophies (Chapter 671).
387.3 Cystic Adenomatoid Malformation
Diagnosis
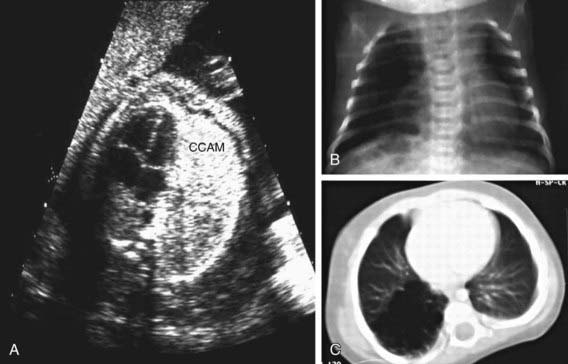
Cystic adenomatoid malformations can be diagnosed in utero by ultrasonography (Fig. 387-1). Fetal cystic lung abnormalities can include cystic adenomatoid malformations (40%), pulmonary sequestration (14%) (Chapter 387.4), or both (26%); the median age at diagnosis is usually 21 wk gestation. In 1 series, only 7% had severe signs of fetal distress including hydrops, pleural effusion, polyhydramnios, ascites, or severe facial edema; 96% of the fetuses were born alive, 2 of whom died in the neonatal period. Lesions causing fetal hydrops have a poor prognosis. Large lesions, by compressing adjacent lung, can produce pulmonary hypoplasia in nonaffected lobes (Chapter 387.2). Even lesions that appear large in early gestation can regress considerably or decrease in relative size and be associated with good pulmonary function in childhood. CT allows accurate diagnosis and sizing of the lesion.
Clinical Manifestations
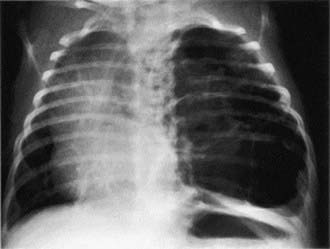
Patients can present in the newborn period or early infancy with respiratory distress, recurrent respiratory infection, and pneumothorax. The lesion may be confused with a diaphragmatic hernia (Chapter 95.8). Patients with smaller lesions are usually asymptomatic until mid-childhood, when episodes of recurrent or persistent pulmonary infection or chest pain occur. Breath sounds may be diminished, with mediastinal shift away from the lesion on physical examination. Chest radiographs reveal a cystic mass, sometimes with mediastinal shift (Fig. 387-2). Occasionally, an air-fluid level suggests a lung abscess.
Calvert JK, Boyd PA, Chamberlain PC, et al. Outcome of antenatally suspected congenital cystic adenomatoid malformation of the lung: 10 years’ experience 1991–2001. Arch Dis Child. 2006;91:F26-F28.
Davenport M, Warne SA, Cacciaguerra S, et al. Current outcome of antenatally diagnosed cystic lung disease. J Pediatr Surg. 2004;39:549-556.
Eber E. Antenatal diagnosis of congenital thoracic malformations: early surgery, late surgery, or no surgery? Semin Resir Crit Care Med. 2007;28:355-366.
Granata C, Gambini C, Balducci T, et al. Bronchioloalveolar carcinoma arising in congenital cystic adenomatoid malformation in a child: a case report and review on malignancies originating in congenital cystic adenomatoid malformation. Pediatr Pulmonol. 1998;25:62-66.
Lakhoo K. Management of congenital cystic adenomatous malformations of the lung. Arch Dis Child Fetal Neonatal Ed. 2009;94:F73-F74.
Lo AY, Jones S. Lack of consensus among Canadian pediatric surgeons regarding the management of congenital cystic adenomatoid malformation of the lung. J Pediatr Surg. 2008;43:797-799.
Riedlinger WF, Vargas SO, Jennings RW, et al. Bronchial atresia is common to extralobar sequestration, intralobar sequestration, congenital cystic adenomatoid malformation, and lobar emphysema. Pediatr Dev Pathol. 2006;9:361-373.
Sauvat F, Michel JL, Benachi A, et al. Management of asymptomatic neonatal cystic adenomatoid malformations. J Pediatr Surg. 2003;38:548-552.
Schmittenbecher P. Congenital cystic adenomatoid malformation of the lung: indications and timing of surgery. J Pediatr Surg. 2005;40:891-892.
Tsai AY, Liechty KW, Hedrick HL, et al. Outcomes after postnatal resection of prenatally diagnosed asymptomatic cystic lung lesions. J Pediatr Surg. 2008;43:513-517.
Zach MS. Adult outcome of congenital lower respiratory tract malformations. Thorax. 2001;56:65-72.
387.4 Pulmonary Sequestration
Pathophysiology
The lung tissue in a sequestration does not connect to a bronchus and receives its arterial supply from the systemic arteries (commonly off the aorta) and returns its venous blood to the right side of the heart through the inferior vena cava (extralobar) or pulmonary veins (intralobar). The sequestration functions as a space-occupying lesion within the chest; it does not participate in gas exchange and does not lead to a left-to-right shunt or alveolar dead space. Communication with the airway can occur as the result of rupture of infected material into an adjacent airway. Collateral ventilation within intrapulmonary lesions via pores of Kohn can occur. Pulmonary sequestrations can arise through the same pathoembryologic mechanism as a remnant of a diverticular outgrowth of the esophagus. Some propose that intrapulmonary sequestration is an acquired lesion primarily caused by infection and inflammation; inflammation leads to cystic changes and hypertrophy of a feeding systemic artery. This is consistent with the rarity of this lesion in autopsy series of newborns. Gastric or pancreatic tissue may be found within the sequestration. Cysts also may be present. Other associated congenital anomalies, including CCAM (Chapter 387.3), diaphragmatic hernia (Chapter 95.8), and esophageal cysts, are not uncommon. Some believe that intrapulmonary sequestration is often a manifestation of cystic adenomatoid malformation and have questioned the existence of intrapulmonary sequestration as a separate entity.
Clinical Manifestations and Diagnosis
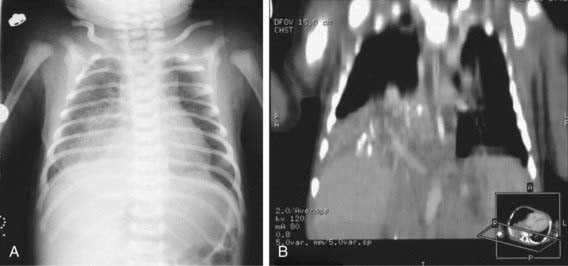
Physical findings in patients with sequestration include an area of dullness to percussion and decreased breath sounds over the lesion. During infection, crackles may also be present. A continuous or purely systolic murmur may be heard over the back. If findings on routine chest radiographs are consistent with the diagnosis, further delineation is indicated before surgical intervention (Fig. 387-3). CT with contrast can demonstrate both the extent of the lesion and its vascular supply. Magnetic resonance angiography (MRA) is also useful. Ultrasonography can help rule out a diaphragmatic hernia and demonstrate the systemic artery. Surgical removal is recommended. Identifying the blood supply before surgery avoids inadvertently severing its systemic artery.
Carpentieri DF, Guttenberg M, Quinn TM, et al. Subdiaphragmatic pulmonary sequestration: a case report with review of the literature. J Perinatol. 2000;20:60-62.
Garcia-Pena P, Lucaya J, Hendry GM, et al. Spontaneous involution of pulmonary sequestration in children: a report of two cases and review of the literature. Pediatr Radiol. 1998;28:266-270.
Nicolette LA, Kosloske AM, Bartow SA, Murphy S. Intralobar pulmonary sequestration: a clinical and pathological spectrum. J Pediatr Surg. 1993;28:802-805.
Riedlinger WF, Vargas SO, Jennings RW, et al. Bronchial atresia is common to extralobar sequestration, intralobar sequestration, congenital cystic adenomatoid malformation, and lobar emphysema. Pediatr Dev Pathol. 2006;9:361-373.
Zach MS. Adult outcome of congenital lower respiratory tract malformations. Thorax. 2001;56:65-72.
387.5 Bronchogenic Cysts
Clinical Manifestations and Treatment
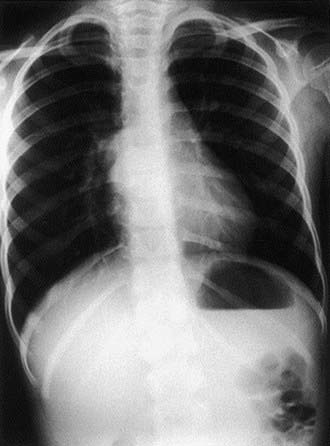
Fever, chest pain, and productive cough are the most common presenting symptoms. Dysphagia may be present; some bronchogenic cysts are asymptomatic. A chest radiograph reveals the cyst, which can contain an air-fluid level (Fig. 387-4). CT scan or MRI is obtained in most cases to better demonstrate anatomy and extent of lesion before surgical resection. Treatment of symptomatic cysts is surgical excision after appropriate antibiotic management. Asymptomatic cysts are generally excised in view of the high rate of infection.
387.6 Congenital Pulmonary Lymphangiectasia
387.7 Lung Hernia
Etiology and Pathology
A lung hernia is a protrusion of the lung beyond its normal thoracic boundaries. About 20% are congenital, with the remainder being noted after chest trauma or thoracic surgery or in patients with pulmonary diseases such as cystic fibrosis (Chapter 395) or asthma (Chapter 138), which cause frequent cough and generate high intrathoracic pressure. A congenital weakness of the suprapleural membrane (Sibson’s fascia) or musculature of the neck can play a role in the appearance of a lung hernia. More than half of congenital lung hernias and almost all acquired hernias are cervical. Congenital cervical hernias usually occur anteriorly through a gap between the scalenus anterior and sternocleidomastoid muscles. Cervical herniation is usually prevented by the trapezius muscle (posteriorly, at the thoracic inlet) and by the three scalene muscles (laterally).