Chapter 22 Congenital Coronary Arterial Fistula
Coronary arterial fistulas are the most frequent functionally significant congenital malformations of the coronary circulation; they comprise 14% of all congenital coronary artery anomalies and 0.2% to 0.4% of all congenital cardiac defects (see Chapter 32). The right and left coronary arteries arise from their appropriate aortic sinuses, but a fistulous branch of one or more than one drains into a cardiac chamber or into the pulmonary trunk, coronary sinus, vena cava, or a pulmonary vein.1 When the fistula drains into a right cardiac chamber or into the pulmonary trunk, it is arteriovenous, an appropriate term because the communication allows arterialized systemic blood—arterio—to mix with unoxygenated blood in the right side of the heart—venous. When the fistula drains into the left atrium or left ventricle, the appropriate term is coronary arterial rather than arteriovenous.
Congenital coronary arterial fistula was described by Krause2 in 1865 and confirmed by Abbott in 19083 and by Trevor in 1912.4 Approximately half of these fistulas arise from the right coronary artery, somewhat less from the left coronary artery, and only 5% from both coronary arteries.5 Even more rarely, all three coronary arteries are involved,6 or multiple fistulas arise from one coronary artery7 or from a single coronary artery.8 Isolated reports have appeared of fistulas from the conus artery to the right atrium,9 from the coronary sinus to the left ventricle,10 from the left circumflex to the coronary sinus11,12 or to the pulmonary artery,13 or from the microfistulae to the left ventricle.14 A significant minority of these fistulas are acquired (i.e., traumatic) because of intravascular, interventional, or surgical procedures.15–17 The contralateral coronary artery is absent in about 3% of congenital cases.
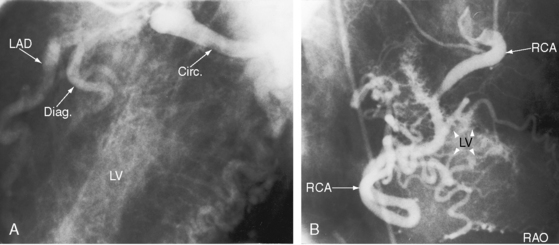
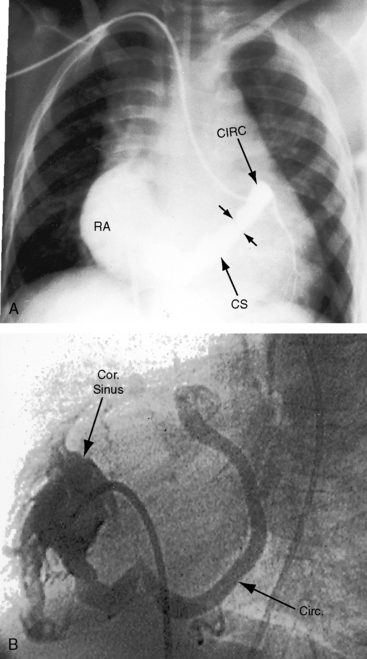
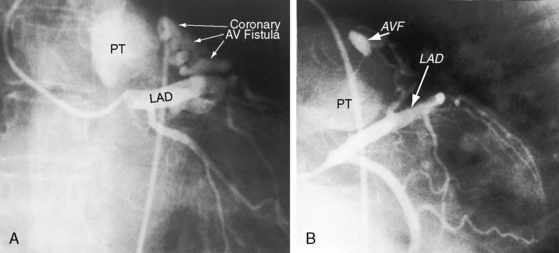
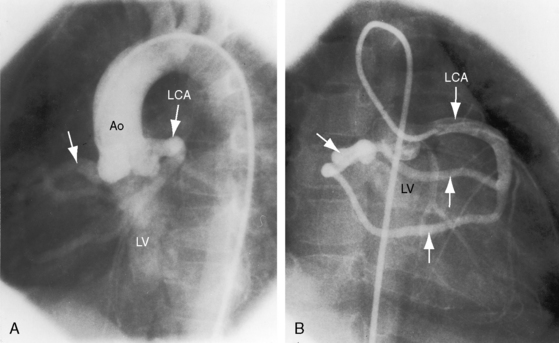
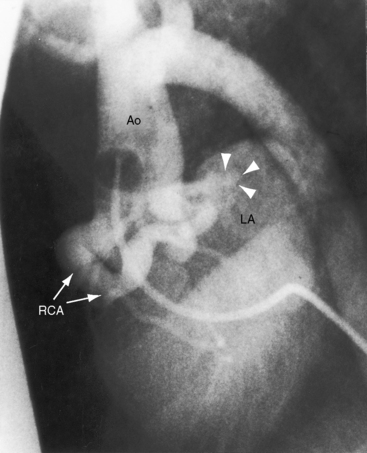
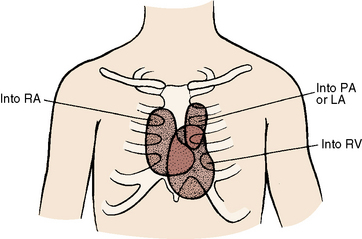
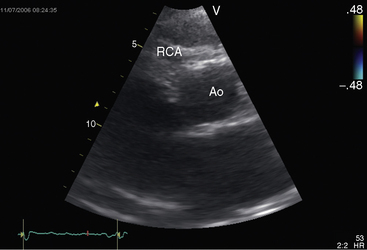
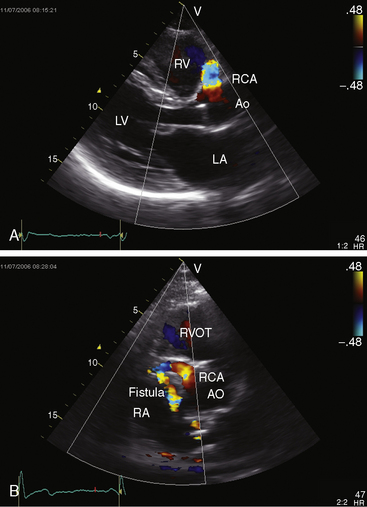
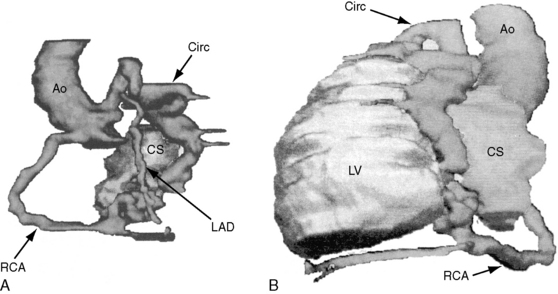
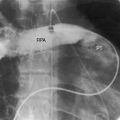
The drainage site of a coronary arterial fistula is more important than its site of origin and consists of a single vascular channel, multiple channels, or a maze of fine channels that form a diffuse network or plexus (spongy myocardium), a pattern especially likely when the left ventricle receives the fistula (see Figure 22-6B).14 More than 90% of congenital coronary arterial fistulas drain into the right side of the heart and are therefore arteriovenous. A substantial majority, in approximate order of frequency, enter the right ventricle (40%) or right atrium (25%) (Figure 22-1A); less commonly, the pulmonary trunk (15%; Figures 22-2 and 22-3) or coronary sinus (7%; Figure 22-1B)18; and rarely, the hepatic vein19 or superior vena cava.20–22 A dual right coronary artery has been accompanied by a fistulous communication.23 The coronary sinus that receives a fistula can be aneurysmal, especially if it receives fistulas from two coronary arteries (see Figure 22-7).5 A giant right coronary artery–to–superior vena caval fistula has been reported,24 as has an aneurysmal coronary artery fistula in which the left main coronary connected to the right atrium.25 Bilateral coronary arterial fistulas usually drain into the pulmonary trunk. The relatively few that do not communicate with the right side of the heart drain into the left atrium (5%; Figure 22-4), left ventricle (3%; Figures 22-5 and 22-6),26,27 pulmonary veins, or both ventricles. A coronary artery–to–left ventricular fistula is not the same as an aortic–to–left ventricular tunnel (see Chapter 6).27,28 Small coronary arterial fistulas without clinical evidence of their presence have been unexpectedly discovered during routine echocardiography (see Figure 22-18) and are incidental findings in 0.1% to 0.26 % of patients undergoing routine coronary angiography (see Figure 22-3B).29 In a series of 14,708 coronary arteriograms, 19 congenital coronary arterial fistulas were found; and in a series of 11,000 coronary angiograms, 13 fistulas were identified.30 These incidentally found fistulas are characterized by one or more small channels that originate from the left anterior descending coronary artery and form networks that communicate at sites in the pulmonary trunk (see Figure 22-3B).
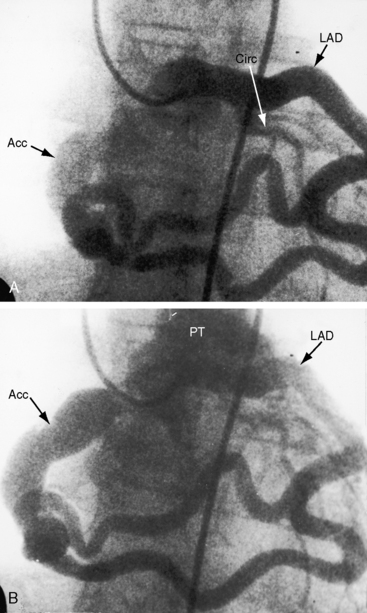
The coronary artery that gives rise to the fistula is characteristically dilated, elongated, and tortuous (see Figure 22-2),31 and the coronaries distal to the fistula are of normal caliber (see Figure 22-3A). A fistulous coronary artery may contain saccular aneurysms that reach an astonishing size and may rupture (see previous).32,33
An estimated 1% to 2% of coronary arterial fistulas close spontaneously in infants, children, and adults.34,35 Occlusion of an atherosclerotic coronary artery proximal to the fistula was responsible for closure in a 44-year-old woman. Occasionally, calcification of the wall of the fistula and thrombi with embolization are seen.
The embryogenesis of coronary arterial fistulas is uncertain. Fistulas that enter the right ventricle have been related to persistence of primitive intramyocardial sinusoids36 or to the development of a rectiform vascular network in the distal branches of the involved coronary artery.18 Fistulas that enter the left ventricle are thought to result from direct flow through thebesian venous channels.37 Interestingly, the veins of Thebesius were cited as evidence of direct passage of blood from one side of the heart to the other before William Harvey discovered the circulation (see Chapter 32).
Of the six coronary anlagen in the embryo, three are in the developing aorta and three are in the developing pulmonary artery (see Chapter 21).38 These anlagen are normally involute, except for the two from the right and left aortic sinuses. The relatively high incidence of bilateral coronary arterial–to–pulmonary arterial fistulas is in accord with this observation. A coronary arterial–to–pulmonary artery fistula may result from persistence of one or more of the pulmonary arterial anlagen, hence the term accessory coronary artery, which is either a single large channel (see Figure 22-2), one or more smaller channels, multiple tortuous channels, or a plexiform arrangement.
The physiologic consequences of coronary arterial fistulas depend on the volume of blood flowing through them, the chamber or vascular bed into which they drain, and the myocardial ischemia that results from a coronary steal caused by low-resistance vascular channels. About 10% of blood from the aortic root normally enters the coronary circulation, but in the presence of a coronary arterial fistula, the volume is considerably larger. A fistula that drains into the right atrium, right ventricle, or coronary sinus constitutes a left-to-right shunt. If drainage is into the right ventricular outflow tract, pulmonary trunk (see Figures 22-2 and 22-3), left atrium (see Figure 22-6), or left ventricle (see Figures 22-4 and 22-5), the hemodynamic burden is borne by the left ventricle alone.26 A fistulous coronary artery receives blood during systole when its stoma is large.26,39 If the fistula drains into the inflow tract of the right ventricle, volume overload of the right ventricle coexists. If drainage is directly into the right atrium or indirectly through the coronary sinus (see Figure 22-1), volume overload of right ventricle exists in addition to overload of the left side of the heart.
Pulmonary-to-systemic flow ratios are typically small, even negligible, regardless of patient age. Shunts in excess of 2:1 are unusual, but an occasional neonate experiences congestive heart failure (see Figure 22-17) when an exceptionally large fistula drains into the left or right side of the heart.36,39
Myocardial ischemia is incurred when a coronary arterial fistula functions as a low-resistance pathway that constitutes a coronary steal.26 The coronary artery gives rise to the fistula and then assumes an important role because steal from a major branch of the left coronary artery is more significant than steal from a smaller right coronary artery. Acquired coronary artery stenosis distal to a congenital coronary arterial fistula aggravates the perfusion deficit because the fistula acts as a low-resistance alternative to the acquired obstruction.
History
The initial suspicion of a congenital coronary arterial fistula in an asymptomatic child or young adult is likely to be a continuous murmur. A small acoustically silent coronary arterial fistula is usually discovered during routine echocardiography or coronary angiography (see previous). When a coronary arterial fistula drains into the low-pressure right or left atrium, the continuous murmur dates from birth because the pressure gradient responsible for the murmur is present in utero. Conversely, coronary arterial fistulas that drain into the right ventricle or pulmonary trunk do not generate continuous murmurs until after the neonatal fall in pulmonary vascular resistance. The continuous murmur is overlooked when it is soft and localized to an atypical site. An isolated diastolic murmur may be heard but misinterpreted. Coronary arterial fistulas are mistaken for patent ductus arteriosus (Figure 22-8), which occasionally coexists.40,41 A coronary arteriovenous fistula may present with angina,26,42 and occasionally with a pericardial effusion13 or infective endocarditis.22
The male:female ratio is about equal. Survival into adulthood is expected, but lifespan is not normal.18 Longevity has been reported in the sixth to ninth decade (see Figure 22-16),13,43 and the diagnosis has been made as late as the seventh to ninth decade.44 A 68-year-old professional athlete was undiagnosed until acquired coronary artery disease prompted coronary angiography, which disclosed bilateral coronary arterial fistulas.
Most patients, especially those less than 20 years of age, are asymptomatic when the coronary arterial fistula is first diagnosed.18 An uncommon, if not rare, exception is the infant with an exceptionally large fistula (see Figure 22-17).36,39 Symptoms and complications, in approximate order of frequency, include dyspnea, fatigue, myocardial ischemia,26 congestive heart failure, sudden death,45 infective endocarditis,22,46 and rupture.47 Obstruction of the superior vena cava has been caused by a large fistulous saccular aneurysm, one of which ruptured at age 82 years.48 Atrial fibrillation that accompanies drainage into the right or left atrium or coronary sinus heralds congestive heart failure (see Figure 22-16).
A coronary steal may be the cause of myocardial ischemia and angina pectoris (see previous), and ischemia has an undesirable effect on left ventricular function.26,49 Spontaneous closure of a coronary arterial fistula is uncommon but not rare (see previous).34,35
Arterial pulse
The arterial pulse is normal because flow through the fistula is usually small. However, when the right ventricle, right atrium, or left atrium receives a large fistula, the arterial pulse is brisk and the pulse pressure is wide because a fall in aortic diastolic pressure caused by flow into low-pressure drainage sites is accompanied by a rise in systolic pressure caused by an increase in left ventricular stroke volume and ejection velocity (Figure 22-9). When a large fistula drains into the left ventricle, the hemodynamic response is analogous to aortic regurgitation.
Jugular venous pulse
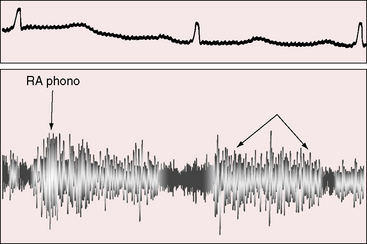
The jugular pulse is normal even when a small or moderate-sized fistula drains directly into the right atrium. With the advent of congestive heart failure induced by a large fistula (see Figure 22-16B), the mean right atrial pressure rises. Obstruction of the superior vena by the saccular aneurysm of a fistula that drained into the right atrium elevated the mean jugular venous pressure and damped the A and V waves. A giant left atrium caused by a large coronary arterial–to–left atrial fistula in a 64-year-old man contributed to inferior vena caval obstruction.
Auscultation
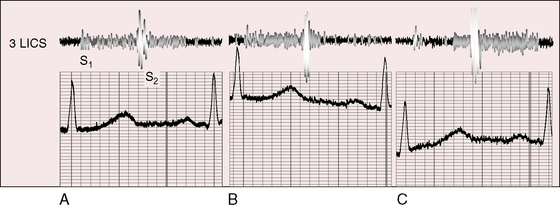
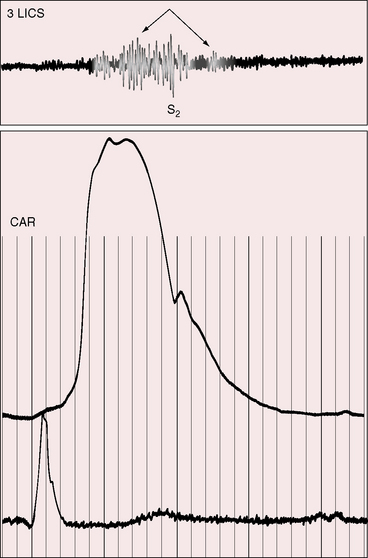
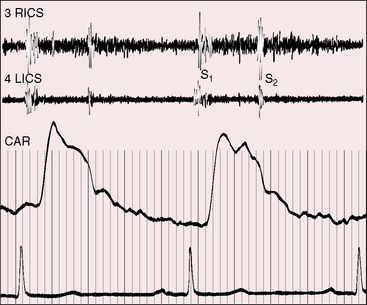
A continuous murmur that is an auscultatory hallmark of coronary arterial fistulas (Figures 22-10, 22-11, and 22-12)50–53 may be mistaken for the continuous murmur of patent ductus arteriosus.40,41 The distinction is based on the configuration of the murmur (see Figure 22-8) and on its precordial location that is determined by the drainage site of the fistula, not by its coronary artery of origin (Figure 22-13). Whether the fistula drains directly into the right atrium or drains into the right atrium indirectly through the coronary sinus (see Figure 22-1), intracardiac phonocardiography records the murmur within the right atrial cavity (see Figure 22-10) and the thoracic wall site is topographically appropriate at the right upper or lower sternal border or over the sternum (see Figure 22-12).35,40 When a left circumflex coronary arterial fistula drains into the coronary sinus (see Figure 22-1A), the continuous murmur is heard in the back between the spine and left scapula. When the fistula drains into the inflow tract of the right ventricle, the murmur sites are along the mid to lower left sternal border, over the lower sternum, or subxyphoid (see Figure 22-13). When the fistula drains into the outflow tract of the right ventricle, the murmur is maximal along the upper to mid left sternal border (see Figures 22-8 and 22-13).51 With drainage into the pulmonary trunk, the continuous murmur is prominent at the upper left sternal border (see Figure 22-13).50 A small or plexiform pulmonary arterial fistula discovered incidentally at coronary angiography (see Figure 22-3B) is not accompanied by a chest wall murmur. When a fistula drains into the left atrium, the murmur is maximal along the upper left sternal border (see Figure 22-13) and may radiate toward the left anterior auxiliary line. A fistula that drains into a left superior vena cava is accompanied by a continuous murmur at the upper to mid left sternal edge.21,22 The coronary arterial fistulas that are especially mistaken for patent ductus arteriosus are those that drain into the pulmonary trunk, left atrium, or right ventricular outflow tract. Because most coronary arterial fistulas drain into the body of the right ventricle or into the right atrium, the accompanying continuous murmur is heard at sites remote from the ductus location. Spontaneous closure of a coronary arterial fistula (see previous) is accompanied by diminution or disappearance of the murmur.34
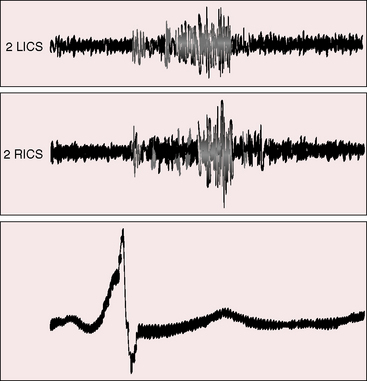
Figure 22-10 Phonocardiograms from an 8-year-old boy with a coronary arterial fistula from the circumflex coronary artery to the coronary sinus (see angiogram, Figure 22-3A). The continuous murmur was louder in systole and maximal in the second and third right intercostal spaces (2 RICS), which was topographically appropriate for the drainage site. (2 LICS = second left intercostal space). The electrocardiogram shows an incidental delta wave.
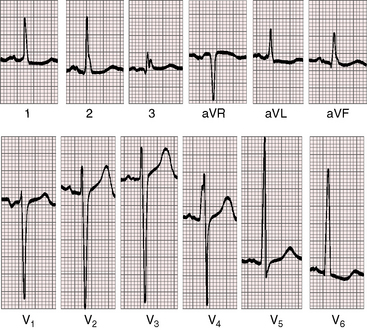
Figure 22-13 Electrocardiogram from a 47-year-old man with a right coronary arterial fistula that entered the outflow tract of the right ventricle. The phonocardiogram and carotid pulse are shown in Figure 22-8. P waves are slightly bifid in leads 1, 2, and aVR, and the P terminal force is abnormal in lead V1. There are voltage and repolarization criteria for left ventricular hypertrophy in leads V5-6.
Coronary arterial fistulas that drain into the right atrium, left atrium, or right ventricle generate continuous murmurs because pressure differences between the aortic root and the low-pressure receiving chambers are continuous (i.e., without interruption from systole into diastole). The configurations of continuous murmurs from these different fistulous sources differ from each other and differ from the continuous murmur of patent ductus arteriosus. When the fistula drains into the right atrium, coronary sinus, or left atrium, pressure gradients between aortic root and receiving chamber are much larger during systole than during diastole, so the murmur is louder in systole (see Figure 22-11). When the fistula communicates with the pulmonary trunk, the continuous murmur may envelope the second heart sound as in patent ductus, but there no eddy sounds.52,53 Flow patterns are more complex when the fistula drains into the right ventricle because right ventricular contraction compresses the fistula during its transmural course (see Figure 22-8). Compression that is sufficient to reduce systolic flow softens the systolic portion of the murmur (see Figure 22-8C). Less compression increases the intramural systolic gradient, so the systolic portion of the continuous murmur is louder (see Figures 22-8B, and 22-9).54 A wide aortic pulse pressure (high systolic, low diastolic) reinforces the systolic portion of the continuous murmur (see Figure 22-9). When congestive heart failure elevates right ventricular diastolic pressure, a continuous murmur becomes only systolic because elevated diastolic pressure decreases diastolic flow.
An early diastolic murmur is generated when a fistula drains into the left ventricle. Blood flow into the fistula during systole may generate a systolic murmur when a large fistulous stoma remains widely patent during left ventricular contraction. When drainage is through thebesian venous channels, a coronary arterial–to–left ventricular fistula is silent.37
The second heart sound splits normally during respiration even when the right atrium receives the fistula. This is so for two reasons: first, the shunt volume is shared equally by the right and left sides of the heart because shunted blood must traverse both ventricles on its way back to the aorta; second, inspiration results in an increase in right ventricular stroke volume and a decrease in left ventricular stroke volume because the shunt into the right atrium occurs with an intact atrial septum (see Chapter 15).
Electrocardiogram
Electrocardiographic abnormalities are related chiefly to the chamber receiving the shunt and to the volume of blood flowing through the fistula. Fistulas that drain into the right atrium or coronary sinus result in biatrial P wave abnormalities; drainage into the right ventricle, pulmonary trunk, or left atrium results in left atrial P wave abnormalities (Figure 22-14); and drainage into the right atrium or coronary sinus results in right atrial P wave abnormalities. Atrial fibrillation occasionally occurs in older patients when fistulas drain into the right atrium, left atrium, or coronary sinus (see Figure 22-16B).5 Fistulas into the right ventricular outflow tract, pulmonary trunk, left atrium, or left ventricle cause left ventricular hypertrophy (see Figure 22-14). Biventricular hypertrophy occurs with drainage into the right atrium or into the body of the right ventricle because shunted blood circulates through both ventricles. A coronary steal (see previous) induces ischemic ST segment and T wave changes at rest or during exercise stress testing. An ischemic pattern is more likely to occur if the coronary steal involves a major branch of the left coronary artery. Myocardial infarction is rarely a feature of the electrocardiogram, although a fistula may aggravate the perfusion deficit of coexisting acquired coronary artery disease (see previous).

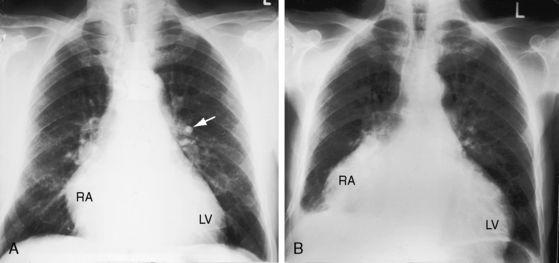
Figure 22-14 X-ray from a 23-year-old woman with a right coronary arterial fistula that drained into the right atrium (RA). The fistula reveals itself as a shadow to the right of the vertebral column (white and black arrows). The pulmonary trunk (PT) is moderately dilated. The phonocardiogram is shown in Figure 22-11.
X-ray
The radiologic features of coronary arterial fistulas reflect the volume and duration of flow and the site of drainage. Young patients with small fistulas have normal x-rays. Infants with large fistulas and congestive heart failure have appreciable cardiomegaly.36,39 A large fistula that drains into the right atrium or coronary sinus is accompanied by increased pulmonary vascularity and a convex pulmonary trunk with biventricular and biatrial enlargement (Figures 22-15 and 22-16 and Video 22-1).18 Drainage into the right ventricle results in a similar picture but without dilation of the right atrium. When a coronary arterial fistula drains into the pulmonary trunk or left atrium, chamber enlargement is confined to the left ventricle and left atrium. A giant left atrium was reported in a 64-year-old man with a right coronary arterial–to–left atrial fistula.
Multiple saccular aneurysms of an enlarged tortuous coronary arterial fistula are occasionally recognized in the x-ray as an irregular silhouette along the right or left cardiac border. A giant aneurysm associated with a left coronary artery–to–pulmonary artery fistula presented as a calcified mediastinal mass.55 Calcification in the wall of a fistula is only occasionally visible.
Echocardiogram
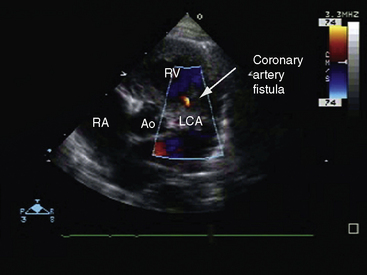
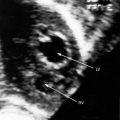
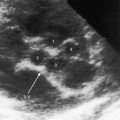
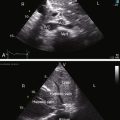
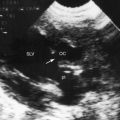
Transthoracic and transesophageal echocardiography with color flow imaging and Doppler interrogation are used to identify the origin and drainage site of coronary arterial fistulas and assess their functional consequences.56–58 The stoma of a fistulous coronary artery considerably exceeds in size the origin of a normal coronary artery (Figure 22-17 and Video 22-2A and 22-2B).57 The drainage site can be identified with color flow imaging (Figures 22-18 and 22-19),56 and Doppler interrogation is used to establish systolic and diastolic flow patterns57 that shed light on the configuration of accompanying murmurs.
Coronary arterial fistulas drain into the pulmonary trunk immediately distal to the pulmonary valve, and flow proceeds upward along the medial wall (see Figure 22-18). Alternatively, a small fistula that drains into the pulmonary artery at a site more distal to the pulmonary valve may exhibit only diastolic flow (see Figure 22-18).
Color flow imaging distinguishes a coronary arterial fistula that drains into the right atrium or right ventricle from a ruptured sinus of Valsalva aneurysm (see Chapter 23). A coronary arterial fistula that drains into the pulmonary trunk can be distinguished from a patent ductus arteriosus (see Chapter 20) and from anomalous origin of the left coronary artery from the pulmonary trunk (see Chapter 21), and a fistula that drains into the left ventricle can be distinguished from an aortic–to–left ventricular tunnel (see Chapter 6).
Echocardiography characterizes the hemodynamic response to flow through a coronary arterial fistula and characterizes the response of the left ventricle to potential ischemic effects of a coronary steal. The coronary sinus is dilated when it receives one or more fistulas (see Figures 22-1B, and 22-7). Left ventricular ejection fraction reflects the volume overload delivered through the fistula, and global and regional wall motion reflect potentially adverse ischemic effects of a coronary steal.
1 Liberthson R.R., Sagar K., Berkoben J.P., Weintraub R.M., Levine F.H. Congenital coronary arteriovenous fistula. Report of 13 patients, review of the literature and delineation of management. Circulation. 1979;59:849-854.
2 Krause W. Ueber den ursprung einer accessorischen A. Coronaria Cordis ausder. A Pulmonis Z Rationelle Med. 1865;24:225.
3 Abbott M. Anomalies of the coronary arteries. In: Osier W., editor. Modern medicine. Philadelphia: Lea & Febiger, 1908.
4 Trevor R.S. Aneurysm of the descending branch of the right coronary artery, situated in the wall of the right ventricle, and opening into the cavity of the ventricle, associated with great dilatation of the right coronary artery and non-valvular infective endocarditis. Proc R Soc Med. 1912;5:20-26.
5 Duerinckx A.J., Perloff J.K., Currier J.W. Arteriovenous fistulas of the circumflex and right coronary arteries with drainage into an aneurysmal coronary sinus. Circulation. 1999;99:2827-2828.
6 Reddy K., Gupta M., Hamby R.I. Multiple coronary arteriosystemic fistulas. Am J Cardiol. 1974;33:304-306.
7 Schamroth C.L., Sareli P., Curcio A., Barlow J.B. Multiple coronary artery-right ventricle fistulas. Am Heart J. 1985;109:1388-1390.
8 Gupta P.D., Rahimtoola S.H., Miller R.A. Single coronary artery–right ventricle fistula. Br Heart J. 1972;34:755-757.
9 Lipoff J.I. Anomalous origin of the left main coronary artery from the right sinus of Valsalva with coronary AV fistula of the conus artery. Chest. 1988;93:203-204.
10 Gnanapragasam J.P., Houston A.B., Lilley S. Congenital fistula between the left ventricle and coronary sinus: elucidation by colour Doppler flow mapping. Br Heart J. 1989;62:406-408.
11 Aoyagi S., Fukunaga S., Ishihara K., Egawa N., Hosokawa Y., Nakamura E. Coronary artery fistula from the left circumflex to the coronary sinus. Int Heart J. 2006;47:147-152.
12 Hayabuchi Y. Coronary arteriovenous fistula: direct connection of the proximal circumflex artery to the coronary sinus. Pediatr Cardiol. 2010;31:168-169.
13 Bellisarii F.I., Marchetti M., Caputo M., De Caterina R. Coronary arteriovenous fistula presenting as chronic pericardial effusion. J Cardiovasc Med. 2006;7:449-453.
14 Cartoni D., Salvini P., De Rosa R., Cortese A., Nazzaro M.S., Tanzi P. Images in cardiovascular medicine. Multiple coronary artery-left ventricle microfistulae and spongy myocardium: the eagerly awaited link? Circulation. 2007;116:e81-e84.
15 Tabrah F., Aintablian A., Hamby R.I. Coronary arteriovenous fistula complicating aortocoronary bypass surgery. Am Heart J. 1973;85:534-537.
16 Wexberg P., Gottsauner-Wolf M., Kiss K., Steurer G., Glogar D. An iatrogenic coronary arteriovenous fistula causing a steal phenomenon: an intracoronary Doppler study. Clin Cardiol. 2001;24:630-632.
17 Renard V.P., Vandenbogaerde J. Fistula between the left internal thoracic artery and the coronary sinus. N Engl J Med. 2000;343:149-150.
18 Ogden J.A. Congenital anomalies of the coronary arteries. Am J Cardiol. 1970;25:474-479.
19 Gorgulu S., Nurkalem Z., Eren M. Right coronary artery hepatic vein fistula: a case report. Echocardiography. 2006;23:869-871.
20 Galbraith A.J., Werner D., Cutforth R.H. Fistula between left coronary artery and superior vena cava. Br Heart J. 1981;46:99-100.
21 Marcus B., Sivazlian K., Gordon L.S. Echocardiographic detection of left circumflex coronary artery to left superior vena cava fistula by use of Doppler color flow mapping. J Am Soc Echocardiogr. 1991;4:405-407.
22 Stansel H.C.Jr, Fenn J.E. Coronary arteriovenous fistula between the left coronary artery and persistent left superior vena cava complicated by bacterial endocarditis. Ann Surg. 1964;160:292-296.
23 Huang Z.-Q., Chen S.-J., Chen J. Dual right coronary artery associated coronary artery fistula. Eur Heart J. 2008;29:968.
24 Lapenna E., Torracca L., De Bonis M., Alfieri O. A giant right coronary artery-to-superior vena cava fistula. Eur J Cardiothorac Surg. 2007;31:546.
25 Shiga Y., Tsuchiya Y., Yahiro E., et al. Left main coronary trunk connecting into right atrium with an aneurysmal coronary artery fistula. Int J Cardiol. 2008;123:e28-e30.
26 Cheng T.O. Left coronary artery-to-left ventricular fistula: demonstration of coronary steal phenomenon. Am Heart J. 1982;104:870-872.
27 Takeda K., Okuda Y., Matsumura K., Sakuma H., Tagami T., Nakagawa T. Giant fistula between the right coronary artery and the left ventricle: diagnostic significance of right posterior oblique chest radiograph. AJR Am J Roentgenol. 1992;159:1087-1090.
28 Sung C.S., Leachman R.D., Zerpa F., Angelini P., Lufschanowski R. Aortico-left ventricular tunnel. Am Heart J. 1979;98:87-93.
29 Phillips P.A., Libanoff A.J. Arteriovenous communication associated with obstructive arteriosclerotic coronary artery disease and myocardial infarction. Chest. 1974;65:106-108.
30 Said S.A., Landman G.H. Coronary-pulmonary fistula: long-term follow-up in operated and non-operated patients. Int J Cardiol. 1990;27:203-210.
31 Muir C.S. Coronary arterio-cameral fistula. Br Heart J. 1960;22:374-384.
32 Braudo J.L., Javett S.N., Zion M.M., Adler D.I. Congenital coronary arteriovenous fistula. Br Med J. 1962;1:601-604.
33 Lim C.H., Tan N.C., Tan L., Seah C.S., Tan D. Giant congenital aneurysm of the right coronary artery. Am J Cardiol. 1977;39:751-753.
34 Mahoney L.T., Schieken R.M., Lauer R.M. Spontaneous closure of a coronary artery fistula in childhood. Pediatr Cardiol. 1982;2:311-312.
35 Farooki Z.Q., Nowlen T., Hakimi M., Pinsky W.W. Congenital coronary artery fistulae: a review of 18 cases with special emphasis on spontaneous closure. Pediatr Cardiol. 1993;14:208-213.
36 Verani M.S., Lauer R.M. Echocardiographic findings in right coronary arterial-right ventricular fistula. Report of a neonate with fatal congestive heart failure. Am J Cardiol. 1975;35:444-447.
37 Cha S.D., Singer E., Maranhao V., Goldberg H. Silent coronary artery-left ventricular fistula: a disorder of the thebesian system? Angiology. 1978;29:169-173.
38 Heifetz S.A., Robinowitz M., Mueller K.H., Virmani R. Total anomalous origin of the coronary arteries from the pulmonary artery. Pediatr Cardiol. 1986;7:11-18.
39 Starc T.J., Bowman F.O., Hordof A.J. Congestive heart failure in a newborn secondary to coronary artery-left ventricular fistula. Am J Cardiol. 1986;58:366-367.
40 Bosher L.H.Jr, Vasli S., Mc C.C., Belter L.F. Congenital coronary arteriovenous fistula associated with large patent ductus. Circulation. 1959;20:254-261.
41 Shaffer A.B., St Ville J., Mackler S.A. Coronary arteriovenous fistula with patent ductus arteriosus. Am Heart J. 1963;65:758-765.
42 Said SaM, Van Der Werf T. Dutch survey of coronary artery fistulas in adults: congenital solitary fistulas. Int J Cardiol. 2006;106:323-332.
43 Brack M.J., Hubner P.J., Firmin R.K. Successful operation on a coronary arteriovenous fistula in a 74 year old woman. Br Heart J. 1991;65:107-108.
44 Ben-Gal T., Herz I., Solodky A., Snir E., Birnbaum Y. Coronary artery-main pulmonary artery fistula. Clin Cardiol. 1999;22:310.
45 Lau G. Sudden death arising from a congenital coronary artery fistula. Forensic Sci Int. 1995;73:125-130.
46 Tsagaris T.J., Hecht H.H. Coronary artery aneurysm and subacute bacterial endarteritis. Ann Intern Med. 1962;57:116-121.
47 Habermann J.H., Howard M.L., Johnson E.S. Rupture of the coronary sinus with hemopericardium. A rare complication of coronary arteriovenous fistula. Circulation. 1963;28:1143-1144.
48 Bauer H.H., Allmendinger P.D., Flaherty J., Owlia D., Rossi M.A., Chen C. Congenital coronary arteriovenous fistula: spontaneous rupture and cardiac tamponade. Ann Thorac Surg. 1996;62:1521-1523.
49 Said SaM, Van Der Werf T. Dutch survey of congenital coronary artery fistulas in adults: coronary artery-left ventricular multiple micro-fistulas multi-center observational survey in the Netherlands. Int J Cardiol. 2006;110:33-39.
50 Biorck G., Crafoord C. Arteriovenous aneurysm on the pulmonary artery simulating patent ductus arteriosus botalli. Thorax. 1947;2:65-74.
51 Davis C.Jr, Dillon R.F., Fell E.H., Gasul B.M. Anomalous coronary artery simulating patent ductus arteriosus. J Am Med Assoc. 1956;160:1047-1050.
52 Ernst C.B., Klassen K.P., Ryan J.M. Vascular malformation overlying the pulmonary artery simuating a patent ductus arteriosus. Circulation. 1961;23:759-761.
53 Nunn D.B., Thrower W.B., Boone J.A., Lipton M. Coronary arteriovenous fistula simulating patent ductus arteriosus. Am Surg. 1962;28:476-482.
54 Puyau F.A., Collins H.A. Congenital coronary arteriovenous fistula. Am J Dis Child. 1963;106:65-72.
55 Okita Y., Miki S., Kusuhara K., et al. Aneurysm of coronary arteriovenous fistula presenting as a calcified mediastinal mass. Ann Thorac Surg. 1992;54:771-773.
56 Trask J.L., Bell A., Usher B.W. Doppler color flow imaging in detection and mapping of left coronary artery fistula to right ventricle and atrium. J Am Soc Echocardiogr. 1990;3:131-134.
57 Velvis H., Schmidt K.G., Silverman N.H., Turley K. Diagnosis of coronary artery fistula by two-dimensional echocardiography, pulsed Doppler ultrasound and color flow imaging. J Am Coll Cardiol. 1989;14:968-976.
58 Zahn E.M., Smallhorn J.F., Egger G., Burrows P.E., Rebecca I.M., Freedom R.M. Echocardiographic diagnosis of fistula between the left circumflex coronary artery and the left atrium. Pediatr Cardiol. 1992;13:178-180.