CHAPTER 33 Congenital Coronary Anomalies
DEFINITION
Anomalous coronary arteries can be grouped into three general categories. Angelini and colleagues1 have written one of the most complete works on this subject, and this text can serve as an excellent reference for additional study. Anomalies generally can be divided into three general classifications: (1) anomalies of origin and course (ectopic ostium within proper sinus, ostium outside normal sinus, and absent vessel); (2) anomalies of intrinsic coronary arterial anatomy (congenital stenosis of ostium, congenital aneurysms, and myocardial bridging); and (3) anomalies of coronary termination (fistulas) (Table 33-1).
| Anomalies of origin and course |
PREVALENCE AND EPIDEMIOLOGY
Congenital anomalies of the coronary arteries affect about 1.3% (range 0.2% to 5.6%) of individuals.2–10 Approximately 80% of anomalies are considered benign without significant clinical sequelae; the remaining 20% can potentially cause symptoms, and may be responsible for significant disease.3
MANIFESTATIONS OF DISEASE
Clinical Presentation
Presentation depends greatly on the specific anomaly. Most coronary artery anomalies are benign and never have any clinical symptoms. These are usually detected as incidental findings. Other anomalies are potentially significant and are sometimes ominously termed malignant or potentially lethal; these increase the risk of myocardial ischemia and sudden death because their position or orientation may lead to coronary flow compromise.11–15 Although anomalies are uncommon, they can have a substantial impact on premature cardiac morbidity and mortality among young adults. Eckart and associates16 found coronary anomalies present in more than 30% of sudden nontraumatic deaths in young individuals.
Imaging Indications and Algorithm
Noninvasive imaging has emerged as the preferred way to diagnose and evaluate coronary anomalies. Echocardiography can often detect anomalous origins and even some anomalous courses or, as in the case of larger fistulas, anomalous terminations. Electron-beam CT and MR angiography are useful for the comprehensive diagnosis of anomalous coronary arteries.17–22 More recently, multidetector CT has proved to be useful in the detection and characterization of anomalous coronary arteries.23–28
IMAGING TECHNIQUE AND FINDINGS
Computed Tomography
Cardiac multidetector CT using contrast-enhanced technique is capable of detecting and characterizing coronary artery anomalies. Most anomalies are incidental findings and are frequently detected when the coronary arteries are being evaluated for atherosclerotic stenoses. The same CT angiographic technique is used when imaging coronary anomalies and atherosclerotic coronary artery disease. This technique is discussed in depth in Chapter 9.
DIFFERENTIAL DIAGNOSIS
Anomalies of Origin and Course
Anomalous Origin of a Coronary Artery from the Opposite Sinus of Valsalva
There are several potential courses for an anomalous LCA arising from the right sinus of Valsalva or the RCA (Fig. 33-1). Diagnosis of the exact course is important because it determines if intervention is necessary. There are four possible pathways for the anomalous LCA: (1) between the aortic root and the pulmonary artery (interarterial course), (2) a transseptal (intraseptal or subpulmonic) course, (3) anterior to the right ventricular outflow tract (anterior or prepulmonic course), and (4) posterior to the aortic root (retroaortic course). Although the anterior, posterior, and septal (subpulmonic) courses are benign, an interarterial course carries a high risk for sudden cardiac death.29,30
Axial reconstructions from a multidetector CT coronary angiogram can depict the anatomic course. The exact position and course of the anomalous artery can be viewed in relation to the aortic root and pulmonary artery (Fig. 33-2). The LCA arises from the right sinus of Valsalva or directly from the RCA in 0.10% of patients, and an interarterial course is present in approximately 75% of these patients.31
Three-dimensional volume rendered multidetector CT images of the coronary arteries and aortic root can also be helpful in depicting the course and anatomic relationships. An anomalous coronary artery originating from the opposite sinus of Valsalva can cause syncope, myocardial infarction, and sudden death in the absence of critical, fixed stenosis. Patients with an anomalous LCA that takes an interarterial course have a high risk for sudden cardiac death because of the acute angle of the ostium, the stretch of the intramural segment of the anomalous artery, or the compression between the commissures of the right and left coronary cusps.1,31 This anomalous LCA may narrow the origin or proximal aspect of the vessel and limit flow. An anomalous interarterial coronary artery is frequently an underlying cause for sudden death in young athletes. Often, MRI can also show an anomalous coronary artery with an interarterial course (Fig. 33-3).
The other three types of anomalous coronary artery originating from the opposite sinus of Valsalva have predominately benign clinical outcomes. Anomalous coronary arteries originating from the opposite sinus of Valsalva can have an intraseptal (intramyocardial) course (Fig. 33-4). In this anomaly, the proximal portion of the anomalous coronary artery is completely surrounded by the gray myocardium of the interventricular septum, and is believed to be “protected” from possible compression as seen more typically with the interarterial course. Care must be taken, however, because a portion of the anomalous coronary, especially the initial proximal segment, may be extracardiac, in which case it assumes the same risks noted for the anomalous interarterial course. The intraseptal pathway is mostly located within the upper, anterior interventricular septum. Frequently, septal branches can be seen to arise from the anomalous vessel, a distinction that may help differentiate an interarterial course from an intraseptal course on conventional angiography.
In cases with an anomalous LCA or LAD artery with an anterior course, the anomalous LCA or LAD artery arises from the proximal RCA and takes a course anterior to the pulmonary artery (Fig. 33-5). Because the anomalous vessel is long and is associated primarily with the low-pressure pulmonary outflow tract, it does not get compressed or stretched as does the interarterial course of an anomalous artery arising from the opposite coronary sinus. It is considered a benign anomaly without significant clinical sequelae. This particular anomaly is commonly seen in patients with tetralogy of Fallot. An anterior course may also be seen with an anomalous RCA that arises from the LCA or LAD artery.
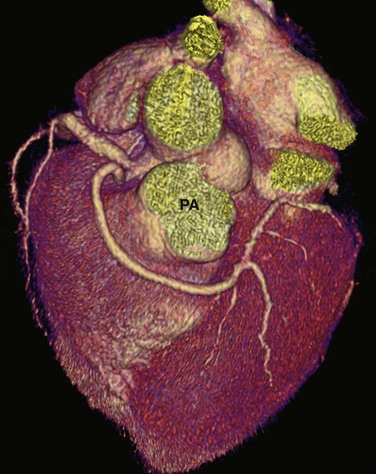
 FIGURE 33-5 An anomalous LCA arises from the proximal RCA and takes a course anterior to the pulmonary artery (PA).
FIGURE 33-5 An anomalous LCA arises from the proximal RCA and takes a course anterior to the pulmonary artery (PA).
The fourth variant of anomalous coronary artery originating from the opposite sinus of Valsalva involves a course posterior to the aorta (i.e., retroaortic course) (Fig. 33-6). In this case, an anomalous circumflex coronary artery arises with the RCA from the right sinus of Valsalva. The retroaortic course is the most common pathway for an anomalous origin of a coronary artery from the opposite sinus of Valsalva. The retroaortic left circumflex artery is seen in 0.1% to 0.9% of the population.1
Anomalous Origin of a Coronary Artery from the Pulmonary Artery
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a very serious anomaly, reported in 1 of every 300,000 live births.32 Most patients become symptomatic in infancy and early childhood. Ninety percent of untreated infants die before 1 year of age, and only a few patients survive to adulthood.33 In ALCAPA, the LCA arises from the pulmonary artery (Fig. 33-7), and the RCA arises normally from the aorta; this is also known as Bland-White-Garland syndrome.1 ALCAPA is usually an isolated anomaly, but rarely it has been described with patent ductus arteriosus, ventricular septal defect, tetralogy of Fallot, and coarctation of the aorta.
Current surgical treatment for ALCAPA is directed at establishing revascularization by recreating a two–coronary artery system. This is done via a number of methods: a left subclavian artery–coronary artery anastomosis, a saphenous vein bypass graft, Takeuchi procedure (creation of an intrapulmonary tunnel), or direct reimplantation. After surgery, most patients experience normalization of left ventricular function, improving long-term survival.32 An anomalous origin of the RCA from the pulmonary artery may also occur, but with ischemic complications affecting the RCA versus LCA vascular distribution.
Anomalous Coronary Artery Arising from the Noncoronary Sinus
Either the RCA or the LCA may arise from the noncoronary sinus of Valsalva (Fig. 33-8). Both of these anomalies are rare, and in an otherwise normal heart, they usually have no clinical relevance. These anomalies may also be seen with transposition of the great vessels.34 Although of minimal clinical importance, documentation of the anomaly would be useful before conventional coronary angiography or intervention. As patients age, the aortic root can rotate clockwise, causing the normal LCA origin to turn more posterior than usual. One needs to be careful not to misdiagnose anomalous LCA arising from a noncoronary sinus in this situation.
High Origin of a Coronary Artery
A high origin of a coronary artery refers to origin outside of the coronary sinus (Fig. 33-9), above the junctional zone between its sinus and the tubular part of the ascending aorta. High coronary ostia, located above the sinotubular junction, have been reported in 6% of adult hearts.35 A high origin of a coronary artery is generally not related to hemodynamic or clinical problems; however, it may lead to difficulty in cannulating the artery during conventional coronary arteriography. Intra-arterial cannulation of an RCA with a high origin, especially when it is above the right sinus of Valsalva, can be particularly difficult.
Multiple Ostia
Another commonly encountered benign anomaly is absence of the left main coronary artery resulting in separate origins of the LAD artery and left circumflex artery (Fig. 33-10). Separate origins of the LCA and left circumflex artery are reported in a few (0.41%) patients and are unrelated to other anomalies or cardiac disease.36 The clinical relevance of separate origins of the LAD artery and left circumflex artery is limited because there are no known adverse hemodynamic effects. This anomaly may present difficulty for the angiographer because multiple ostia need to be recognized and require separate cannulation during selective diagnostic catheterization studies. Separate origins may have some protective effects because this configuration allows for alternative collateral sources in patients with proximal coronary stenoses.34
Arterial Duplication
Arterial duplication is another form of coronary anomaly and often involves the LAD artery (Fig. 33-11). Clinically this anomaly had been termed duplication of the LAD artery, and it is seen in 0.13% to 1% of the general population.37 This anomaly consists of a short LAD artery, which courses and terminates in the anterior interventricular sulcus, and a long LAD artery, which originates from either the LAD artery or the RCA and enters the distal interventricular sulcus and courses to the apex.37 Because the LAD artery is frequently bypassed surgically, it is important to recognize this anomaly before surgical revascularization so that the surgical arteriotomy is correctly placed.37
Single Coronary Artery
A single coronary artery (Fig. 33-12) is a rare anomaly in which only one coronary artery arises from the aortic trunk by a single ostium. The artery supplies the blood to the entire heart. The presence of a single coronary artery is rare, reported in approximately 0.024% to 0.066% of the population.1,38 A single coronary artery has many different patterns of distribution. It may follow the pattern of a normal artery, divide into two branches with distributions of the RCA and the LCA, or have a distribution different from that of the normal coronary arterial tree.39 Single coronary artery is a very heterogeneous group of anomalies in which the single artery can arise from the right, left, or noncoronary sinus of Valsalva, and the pathways of the branches can vary greatly, taking retrocardiac, retroaortic, interarterial, intraseptal, and anterior courses.
Anomalies of Intrinsic Coronary Arterial Anatomy
Congenital Ostial Stenosis
Coronary arteries may have an ostial stenosis that is not due to atherosclerosis or other type of acquired disease. Congenital ostial stenoses are due to a membrane or fibrotic ridge.1 This membrane or ridge is sometimes found with anomalous coronary arteries, and is believed to be due to the tangential orientation of the anomalous artery as it passes through the aortic wall. Ostial stenoses may be present in some cases where the RCA arises ectopically from the left sinus of Valsalva and runs in an interarterial path (Fig. 33-13).
Congenital Coronary Aneurysms
Ectasia, or aneurysm formation, may be localized to a single coronary segment or can involve the coronaries diffusely. Patients with either focal or diffuse coronary ectasia, not related to atherosclerosis, have abnormal arterial walls, with medial degeneration, intimal thickening, and eventual ulceration and mural thrombus formation.40 Congenital coronary aneurysms are often associated with Kawasaki disease (Fig. 33-14).
Myocardial Bridging
Normal coronary arteries traverse the epicardial fat before entering the myocardium. In complete myocardial bridging, a short segment of the artery, known as a tunneled segment, passes into and through the myocardium for a short segment before re-entering the epicardial fat. It then branches normally and terminates within the myocardium (Figs. 33-15 and 33-16). Complete myocardial bridging is seen in 20% of asymptomatic patients and is a very rare cause of ischemia secondary to spasm. In incomplete myocardial bridging, the involved artery extends down to and touches the myocardium, but does not completely enter before extending back up into the myocardium.
Myocardial bridging typically involves the middle portion of the LAD artery, but the circumflex artery, diagonal arteries, and RCA are occasionally involved.41 The prevalence of myocardial bridging varies greatly in reported series, ranging from 0.5% to 2.5% on angiography examinations to 15% to 85% at pathologic analysis.41 Multidetector CT coronary angiography depicts the intramyocardial path of the involved coronary arterial segment. Although most patients with myocardial bridging are asymptomatic, in rare cases myocardial bridging is responsible for angina pectoris, myocardial infarction, life-threatening arrhythmias, or death.41
Anomalies of Termination: Coronary Artery Fistula
A coronary fistula is an abnormal connection between a coronary artery and the pulmonary artery (Fig. 33-17), coronary sinus, cardiac chamber, or superior vena cava. Fistulas are present in approximately 0.1% to 0.2% of patients at coronary angiography.42 Small fistulas are often incidental findings; however, large fistulas have serious hemodynamic and clinical consequences. Fistulas represent a left-to-right or a left-to-left shunt, depending on where they communicate, and they result in dilation of the coronary artery to varying degrees, depending on the shunt volume. Myocardial ischemia may develop in the portion of the myocardium supplied by the abnormally connecting coronary artery. The right ventricle is the most common site of drainage (45% of cases), followed by the right atrium (25%) and the pulmonary artery (15%).43
Angelini P, Flamm SD. Newer concepts for imaging anomalous aortic origin of the coronary arteries in adults. Catheter Cardiovasc Interv. 2007;69:942-954.
Cademartiri F, Runza G, Luccichenti G, et al. Coronary artery anomalies: incidence, pathophysiology, clinical relevance and role of diagnostic imaging. Radiol Med (Torino). 2006;111:376-391.
Dodd JD, Ferencik M, Liberthson RR, et al. Congenital anomalies of coronary artery origin in adults: 64-MDCT appearance. AJR Am J Roentgenol. 2007;188:W138-W146.
Kim SY, Seo JB, Do KH, et al. Coronary artery anomalies: classification and ECG-gated multi-detector row CT findings with angiographic correlation. RadioGraphics. 2006;26:317-334.
Manghat NE, Morgan-Hughes GJ, Marshall AJ, et al. Multidetector row computed tomography: imaging congenital coronary artery anomalies in adults. Heart. 2005;91:1515-1522.
McConnell MV, Stuber M, Manning WJ. Clinical role of coronary magnetic resonance angiography in the diagnosis of anomalous coronary arteries. J Cardiovasc Magn Reson. 2000;2:217-224.
Welker M, Salanitri J, Deshpande VS, et al. Coronary artery anomalies diagnosed by magnetic resonance angiography. Australas Radiol. 2006;50:114-121.
1 Angelini P, Villason S, Chan A, et al. Normal and anomalous coronary arteries in humans. In: Coronary Artery Anomalies, A Comprehensive Approach. Philadelphia: Lippincott Williams & Wilkins; 1999.
2 Click RL, Holmes DR, Vlietstra RE, et al. Anomalous coronary arteries: location, degree of atherosclerosis and effect on survival—a report from the Coronary Artery Surgery Study. J Am Coll Cardiol. 1989;13:531-537.
3 Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Catheter Cardiovasc Diagn. 1990;21:28-40.
4 Levin DC, Fellows KE, Abrams HL. Hemodynamically significant primary anomalies of the coronary arteries: angiographic aspects. Circulation. 1978;58:25-34.
5 Liberthson RR, Dinsmore RE, Fallon JT. Aberrant coronary artery origin from the aorta: report of 18 patients, review of literature and delineation of natural history and management. Circulation. 1979;59:748-754.
6 Baltaxe HA, Wixson D. The incidence of congenital anomalies of the coronary arteries in the adult population. Radiology. 1977;122:47-52.
7 Chaitman BR, Lesperance J, Saltiel J, et al. Clinical, angiographic, and hemodynamic findings in patients with anomalous origin of the coronary arteries. Circulation. 1976;53:122-131.
8 Engel HJ, Torres C, Page HL. Major variations in anatomical origin of the coronary arteries: angiographic observations in 4,250 patients without associated congenital heart disease. Cathet Cardiovasc Diagn. 1975;1:157-169.
9 Garg N, Tewari S, Kapoor A, et al. Primary congenital anomalies of the coronary arteries: a coronary arteriographic study. Int J Cardiol. 2000;74:39-46.
10 Kimbiris D, Iskandrian AS, Segal BL, et al. Anomalous aortic origin of coronary arteries. Circulation. 1978;58:606-615.
11 Basso C, Maron BJ, Corrado D, et al. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493-1501.
12 McConnell MV, Stuber M, Manning WJ. Clinical role of coronary magnetic resonance angiography in the diagnosis of anomalous coronary arteries. J Cardiovasc Magn Reson. 2000;2:217-224.
13 Virmani R, Burke AP, Farb A. Sudden cardiac death. Cardiovasc Pathol. 2001;10:275-282.
14 Cox ID, Bunce N, Fluck DS. Failed sudden cardiac death in a patient with an anomalous origin of the right coronary artery. Circulation. 2000;102:1461-1462.
15 Wesselhoeft H, Fawcett JS, Johnson AL. Anomalous origin of the left coronary artery from the pulmonary trunk: its clinical spectrum, pathology and pathophysiology, based on a review of 140 cases with seven further cases. Circulation. 1968;38:403-425.
16 Eckart RE, Scoville SL, Campbell CL, et al. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann intern Med. 2004;141:829-834.
17 Ropers D, Moshage W, Daniel WG, et al. Visualization of coronary artery anomalies and their anatomic course by contrast-enhanced electron beam tomography and three-dimensional reconstruction. Am J Cardiol. 2001;87:193-197.
18 Yoshimura N, Hamada S, Takamiya M, et al. Coronary artery anomalies with a shunt: evaluation with electron-beam CT. J Comput Assist Tomogr. 1998;22:682-686.
19 Post JC, van Rossum AC, Bronzwaer JG, et al. Magnetic resonance angiography of anomalous coronary arteries: a new gold standard for delineating the proximal course? Circulation. 1995;92:3163-3171.
20 Bunce NH, Lorenz CH, Keegan J, et al. Coronary artery anomalies: assessment with free-breathing three-dimensional coronary MR angiography. Radiology. 2003;227:201-208.
21 McConnell MV, Stuber M, Manning WJ. Clinical role of coronary magnetic resonance angiography in the diagnosis of anomalous coronary arteries. J Cardiovasc Magn Reson. 2000;2:217-224.
22 Taylor AM, Thorne SA, Rubens MP, et al. Coronary artery imaging in grown up congenital heart disease: complementary role of magnetic resonance and x-ray coronary angiography. Circulation. 2000;101:1670-1678.
23 Shi H, Aschoff AJ, Brambs HJ, et al. Multislice CT imaging of anomalous coronary arteries. Eur Radiol. 2004;14:2172-2181.
24 van Ooijen PMA, Dorgelo J, Zijlstra F, et al. Detection, visualization and evaluation of anomalous coronary anatomy on 16-slice multidetector-row CT. Eur Radiol. 2004;14:2163-2171.
25 Schmid M, Achenbach S, Ludwig J, et al. Visualization of coronary artery anomalies by contrast-enhanced multi-detector row spiral computed tomography. Int J Cardiol. 2006;111:430-435.
26 Schmitt R, Froehner S, Brunn J, et al. Congenital anomalies of the coronary arteries: imaging with contrast-enhanced, multidetector computed tomography. Eur Radiol. 2005;15:1110-1121.
27 Deibler AR, Kuzo RS, Vohringer M, et al. Imaging of congenital coronary anomalies with multislice computed tomography. Mayo Clin Proc. 2004;79:1017-1023.
28 Datta J, White CS, Gilkeson RC, et al. Anomalous coronary arteries in adults: depiction at multi-detector row CT angiography. Radiology. 2005;235:812-818.
29 Roberts WC, Siegel RJ, Zipes DP. Origin of the right coronary artery from the left sinus of Valsalva and its functional consequences: analysis of 10 necropsy patients. Am J Cardiol. 1982;49:863-868.
30 Cheitlin MD, De Castro CM, McAllister HA. Sudden death as a complication of anomalous left coronary origin from the anterior sinus of Valsalva: a not-so-minor congenital anomaly. Circulation. 1974;50:780-787.
31 Chaitman BR, Lesperance J, Saltiel J, et al. Clinical, angiographic, and hemodynamic findings in patients with anomalous origin of the coronary arteries. Circulation. 1976;53:122-131.
32 Dodge-Khatami A, Mavroudis C, Backer CL. Anomalous origin of the left coronary artery from the pulmonary artery: collective review of surgical therapy. Ann Thorac Surg. 2002;74:946-955.
33 Wesselhoeft H, Fawcett JS, Johnson AL. Anomalous origin of the left coronary artery from the pulmonary trunk: its clinical spectrum, pathology and pathophysiology, based on review of 140 cases with seven further cases. Circulation. 1968;38:403-425.
34 Greenberg MA, Fish BG, Spindola-Franco H. Congenital anomalies of coronary artery: classification and significance. Radiol Clin North Am. 1989;27:1127-1146.
35 Vlodaver Z, Neufeld HN, Edwards JE. Pathology of coronary disease. Semin Roentgenol. 1972;7:376-394.
36 Danias PG, Stuber M, McConnell MV, et al. The diagnosis of congenital coronary anomalies with magnetic resonance imaging. Coron Artery Dis. 2001;12:621-626.
37 Sajja LR, Farooqi A, Shaik MS, et al. Dual left anterior descending coronary artery: surgical revascularization in 4 patients. Tex Heart Inst J. 2000;27:292-296.
38 Desmet W, Vanhaecke J, Vrolix M, et al. Isolated single coronary artery: a review of 50,000 consecutive coronary angiographies. Eur Heart J. 1992;13:1637-1640.
39 Smith JC. Review of single coronary artery with report of 2 cases. Circulation. 1950;1:1168-1175.
40 Ghahrani A, Iyengar R, Cunha D, et al. Myocardial infarction due to congenital coronary artery aneurysm (with successful saphenous vein bypass graft). Am J Cardiol. 1972;29:863.
41 Mohlenkamp S, Hort W, Ge J, et al. Update on myocardial bridging. Circulation. 2002;106:2616-2622.
42 Said SA, el Gamal MI, van der Werf T. Coronary arteriovenous fistulas: collective review and management of six new cases—changing etiology, presentation, and treatment strategy. Clin Cardiol. 1997;20:748-752.
43 McNamara JJ, Gross RE. Congenital coronary artery fistula. Surgery. 1969;65:59-69.

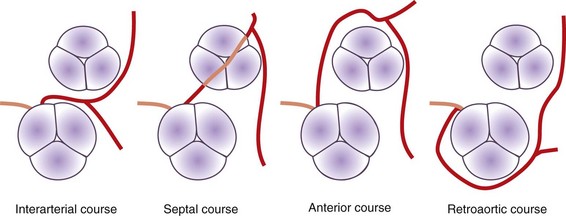
 FIGURE 33-1
FIGURE 33-1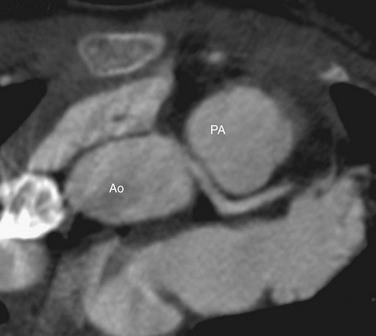
 FIGURE 33-2
FIGURE 33-2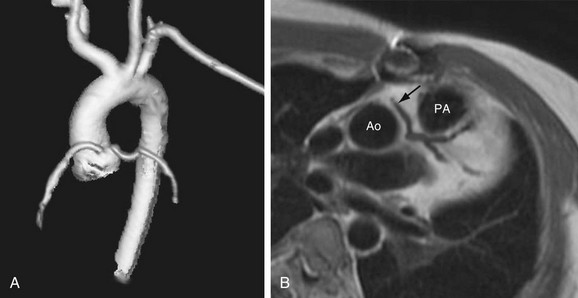
 FIGURE 33-3
FIGURE 33-3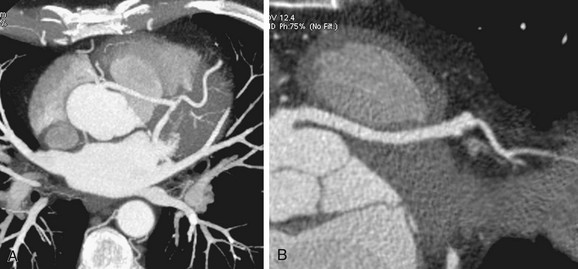
 FIGURE 33-4
FIGURE 33-4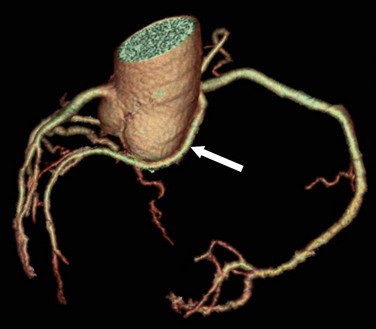
 FIGURE 33-6
FIGURE 33-6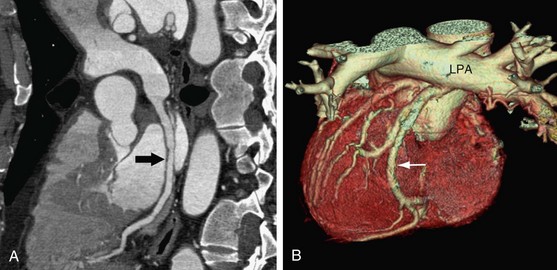
 FIGURE 33-7
FIGURE 33-7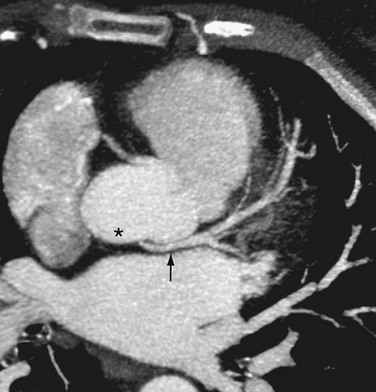
 FIGURE 33-8
FIGURE 33-8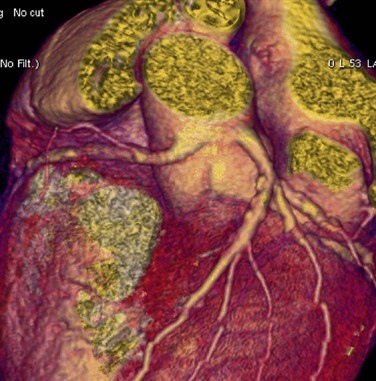
 FIGURE 33-9
FIGURE 33-9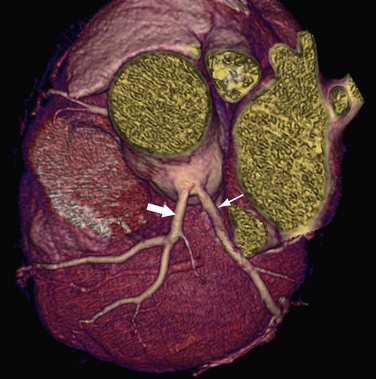
 FIGURE 33-10
FIGURE 33-10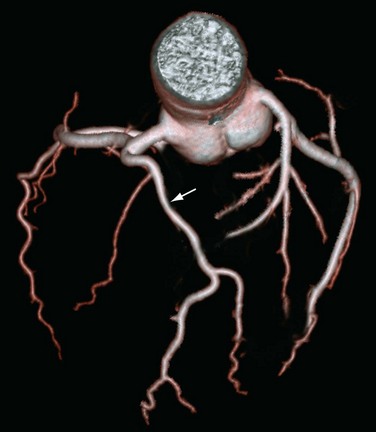
 FIGURE 33-11
FIGURE 33-11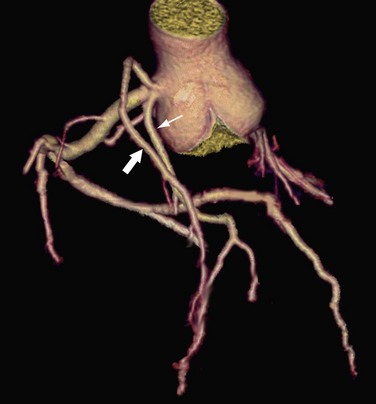
 FIGURE 33-12
FIGURE 33-12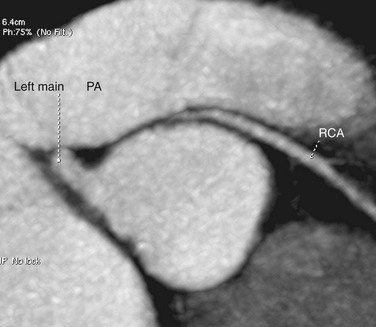
 FIGURE 33-13
FIGURE 33-13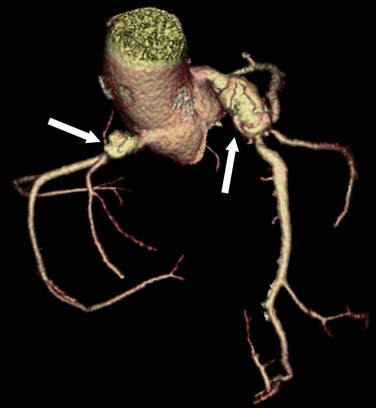
 FIGURE 33-14
FIGURE 33-14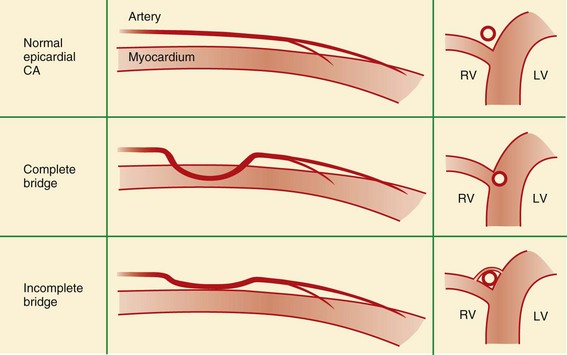
 FIGURE 33-15
FIGURE 33-15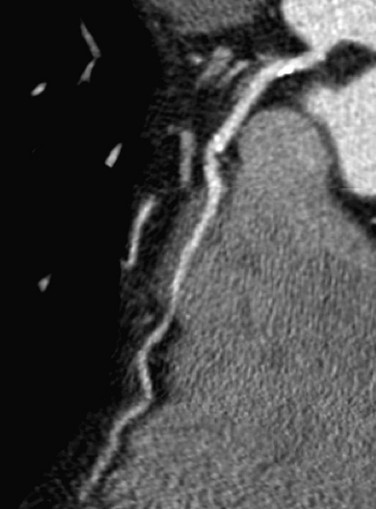
 FIGURE 33-16
FIGURE 33-16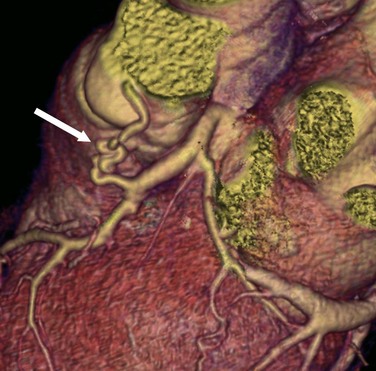
 FIGURE 33-17
FIGURE 33-17


