23 Congenital Complete Heart Block
I. CASE
A. Fetal echocardiography findings
1. The fetal echo reveals situs solitus of the atria, levocardia, and left aortic arch.
2. The ventricular rate is 58 bpm and the atrial rate is 150 to 160 bpm, showing complete atrioventricular (AV) dissociation (congenital complete heart block [CCHB]).
3. The intracardiac anatomy is normal, with normal cardiac axis and position and equal-sized ventricles but mildly increased heart size (cardiothoracic ratio = 0.37).
4. There is no evidence of endocardial fibroelastosis (echogenic walls of one or more of the cardiac chambers).
5. The ductal and aortic arches are of similar size, and both arches have antegrade flow.
6. The pulmonary venous flow pattern is normal.
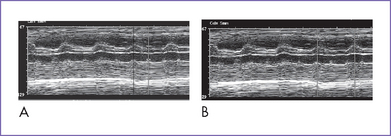
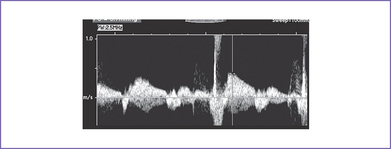
7. M-mode echocardiography confirmed the presence of atrial contractions that are at a normal rate and unrelated to a much slower ventricular rate (Fig. 23-1).
8. There is no evidence of valvar regurgitation (AV or semilunar). However, there is occasional intermittent tricuspid valve regurgitation related to the AV sequence. There is also a very low velocity diastolic regurgitation, which is normal in complete heart block (CHB).
9. The right ventricle (RV) and left ventricle (LV) Tei indices (myocardial performance index) are normal.
10. There is no evidence of heart failure.
11. The pulsatility index on the umbilical arterial Doppler (1.3) and middle cerebral artery Doppler (1.9) are normal.
D. Fetal management and counseling
1. Amniocentesis is not offered because the risk from amniocentesis is higher than the risk of abnormal karyotype in isolated CCHB.
a. Fetal drug therapy has been mainly directed toward three different features of the disease entity.
3. Follow-up included serial antenatal studies at 1- to 2-week intervals.
b. Fetus’s follow-up: A fetal ECG and general fetal surveillance should be provided every 1 to 2 weeks. The following should be serially evaluated:
E. Delivery
1. In isolated CHB, delivery should be near term (36 to 38 weeks) in a tertiary care center given the risk of hydrops fetalis and fetal demise from more severe bradycardia.
2. Premature delivery should normally be planned (for maternal or fetal indications) so that preparations can be made for care of the baby and mother.
F. Follow-up
1. Before the widespread application of pacemakers, infant mortality in isolated CCHB was estimated to be 8% to 16%.
2. The majority of newborns with isolated CCHB are hemodynamically stable.
3. Permanent pacing is required in a minority because of:
c. Mean heart rate less than 55 bpm.
d. LV dilation and mitral regurgitation.
2. Many asymptomatic patients reach adult life without pacing, but the risk of sudden death is 5% during adult life. For this reason, dual-chamber pacemaker implantation is increasingly advocated routinely in adolescence.
3. Following pacemaker implantation, the prognosis is good, with almost normal exercise tolerance in the majority.
4. Immune-positive patients can develop cardiomyopathy, requiring transplantation.
5. Rarely, LV function deteriorates in spite of adequate pacing. This could be explained by an accompanying myocarditis from the original insult, leading to cardiac dysfunction.
6. Pacemaker checks should be every 6 months in the first year and yearly thereafter.
7. If there is any history of unexplained palpitations or abnormal heart rhythm, a metabolic stress test with a 24-hour Holter monitor is recommended.
8. Subacute bacterial endocarditis (SBE) prophylaxis is required for life.
G. Risk of recurrence
1. The risk for women with known anti-SSA/Ro or anti-SSB/La antibodies of having a child with CCHB is only about 5%.
2. Even after having a child with CCHB, the recurrence rate might only be about 15% to 20% in subsequent pregnancies.
3. In one study, high levels of anti-SSA/Ro and anti-SSB/La by practically all assays were associated with a significantly increased risk of having a CCHB child. The relative risk of CCHB for a female child compared with a male child was increased by 1.9 times (1.2-2.9, P = 0.009).
H. Outcome of this case
a. At the first visit at 22 weeks’ gestation, the mother was started on oral dexamethasone 4 mg daily. She was followed at 2-week intervals.
b. At 28 weeks, there was evidence of increased heart size, with holosystolic tricuspid regurgitation and atrial wave reversal in the ductus venosus (cardiovascular profile score 7/10).
c. The mother had a baseline ECG, which was normal. She was then started on an oral sympathomimetic, terbutaline at 2.5 mg every 6 h, which was increased to 5 mg every 6 h later in gestation based on changes in the signs of fetal heart failure.
d. Throughout gestation, the ventricular heart rate ranged between 60 and 72 bpm.
e. At 34 weeks’ gestation, there was evidence of increased heart size and ventricular dysfunction (cardiovascular profile score 6/10) despite adequate medication dosage.
f. The mother developed gestational diabetes.
a. The mother was admitted to the hospital for monitoring.
b. The baby was delivered by cesarean section at 36 weeks with a birth weight of 2000 g and Apgar scores of 5 at 1 minute and 8 at 5 minutes.
c. Postnatal ECG confirmed the diagnosis of CCHB; echocardiography showed normal cardiac anatomy and function apart from septal hypertrophy.
e. He was resuscitated and started on isoproterenol drip (0.05 μg/kg/min) and IV methylprednisolone (1mg/kg q6h) because the mother was on steroids and to treat possible myocarditis in the baby.
f. The arterial lactate was elevated (3 mmol/L).
g. A VVI (ventricular-demand) epicardial pacemaker was implanted on day 1 of postnatal life.
a. The baby was switched to oral prednisolone 1m/kg per day, which was tapered slowly over 3 weeks.
b. The baby is now 4 months old and thriving with a pacemaker and evidence of mild ventricular dysfunction.
II. YOUR HANDY REFERENCE
A. Prevalence
1. The prevalence of fetal CCHB and/or cardiomyopathy is not entirely known.
a. This is in part due to lack of data regarding the prevalence of autoantibodies in all pregnancies.
b. Women with autoantibodies and autoimmune disease typically are screened for fetal cardiac pathology.
c. The risk of having an affected fetus for women with collagen vascular disease is 1% to 6%. However, 70% to 80% of affected babies are conceived by clinically asymptomatic mothers.
2. The estimated incidence of CCHB among live births is 1 in 20,000. The incidence may be higher in prenatal life, because some fetuses with CHB do not survive to term.
B. Outcome
1. The prenatal loss rate among antenatally diagnosed cases of CCHB is reportedly about 45% to 50%. Thus, the incidence of CCHB among conceptions may be at least 1 in 10,000.
2. The outcome of CCHB recognized postnatally was found to be worse in the presence of structural heart disease.
a. In a series of 37 patients by Machado and colleagues (1988), 57% (21 of the 37) had structural heart diseases, mainly LA isomerism (86%).
b. In this series, because of the severity of the lesions and concomitant hydrops, pregnancy was terminated in 43%. Of the remainder, 70% died in utero or postnatally.
3. The outcome for isolated CCHB was generally considered favorable until the study by Machado and colleagues (1988) revealed a 25% fetal and neonatal death rate.
4. Schmidt and colleagues (1991) found an 18% fetal and early neonatal death rate.
5. Jaeggi and colleagues (2002) found a mortality of 55% with prenatally diagnosed isolated CCHB. With more aggressive management of at-risk pregnancies, however, which includes use of corticosteroids, β-sympathomimetics, and earlier delivery, the mortality has decreased to 17%.
C. Associated syndromes and extracardiac anomalies
1. Isolated CCHB and CCHB with structural /myocardial heart are rarely associated with extracardiac malformations or with chromosomal anomalies.
2. CCHB with structural or myocardial heart disease is mainly seen in fetuses with complex cardiac lesions, usually left atrial isomerism (LAI) or disconcordant AV connection (L-transposition of the great arteries).
D. Clues to fetal sonographic diagnosis
a. Because of the difficulties with the fetal ECG, it has become a common practice to analyze the fetal atrial and ventricular cardiac activity by sonographic methods mainly during the second and third trimesters. However, the cardiac rhythm analysis based on ultrasonography uses an indirect approach because the observer draws conclusions on electrical events, and this can limit the precision in defining fetal dysrhythmias.
b. Both the fetal cardiac rate and rhythm can be assessed using fetal pulsed Doppler signals obtained from sampling sites within the heart (mitral or tricuspid inflow) or SVC (representing atrial activation) and arterial vessel outflow (pulmonary or aorta) representing the ventricular activation.
c. Using M-mode techniques, atrial and ventricular activity can be displayed simultaneously by placing the sampling line across the atrial and ventricular walls (see Fig. 23-1).
d. Tissue Doppler demonstrating atrial and ventricular movement has recently been demonstrated for rapid assessment of fetal heart rhythm.
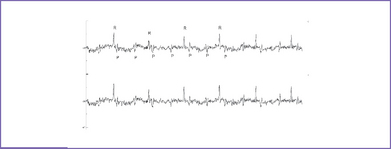
e. The new technique of magnetocardiography allows the P and QRS complexes to be assessed in the awake fetus before birth (Fig. 23-2). This technique is limited because it is only available in a few centers in the United States.
f. Exclude sinus bradycardia; it may be present if the fetal heart rate is less than 100 bpm.
h. Confirm normal four-chamber view including cardiac axis (normal venous atrial and AV connections and normal outflow tracts).
2. For CCHB and structural or myocardial heart disease, refer to Chapter 20, Left Atrial Isomerism.
E. Cardiovascular profile score
1. Cardiovascular profile score can be used to assess:
b. Maternal drug dose adjustment.
c. Planning and timing of delivery.
2. Sustained fetal bradycardia with a very slow ventricular rate of less than 50 bpm and dissociation of atrial and ventricular contractions due to CCHB is commonly not well tolerated by the fetus.
3. Schmidt and colleagues (1991) showed, in one fetal series with isolated CCHB, that fetal hydrops was associated with 100% mortality. This observation justifies serial prenatal follow-up with the cardiovascular profile for fetuses who have CCHB without structural heart defect and who could be at risk for CHF.
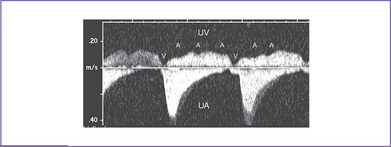
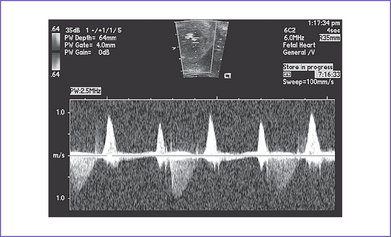
4. Venous Doppler is assessed in the ductus venosus and in the umbilical vein in the free loop of the cord. Umbilical venous pulsations are seen at the atrial rate (Figs. 23-3 and 23-4).
5. Intermittent AV valve regurgitation within the same study is normal in CHB, and no points are taken off for these findings. Diastolic regurgitation is an expected finding and should be differentiated from systolic regurgitation by using pulsed Doppler and not depending on color Doppler (Fig. 23-5). It occurs immediately after a cannon A wave.
F. Progression in utero
a. Isolated CCHB is usually associated with maternal autoantibodies in up to 95% of fetal and 90% of neonatal cases. The onset is typically after 16 to 17 weeks’ gestation, when maternal immunoglobulin G (IgG) (anti-Ro and anti-La antibodies) crosses the placenta. Isolated CCHB develops between 18 and 24 weeks of gestation, and progression from second-degree block to CHB has been observed in utero.
b. In some cases of prenatal treatment with dexamethasone, improvement in the degree of heart block has been reported.
G. Immediate postnatal management for patients without prenatal diagnosis
1. No treatment is required for asymptomatic neonates. Atropine or isoproterenol is used in symptomatic neonates.
2. If the infant is clinically unstable and is not responsive to β-sympathomimetics, a temporary transvenous ventricular pacemaker may need to be placed to stabilize the baby before placing a permanent pacemaker.
3. About 50% of patients with CCHB require pacing in the newborn period. Dual-chamber pacing (VVIR) is preferred.
4. There is a 5% to 10% risk of sudden death in the first year of life. Risk factors are:
a. Heart rate persistently below 55 bpm.
b. CHF resulting from associated structural heart defects.
c. Presence of significant cardiac anomalies.
5. Some neonates with isolated CCHB do well until childhood, but most (if not all) require pacemaker therapy prior to adulthood.
H. Pathophysiology
1. The normal fetal cardiac rhythm is regular, and the normal fetal heart rates range from 120 to 160 bpm during the second half of pregnancy, depending on gestational age and degree of fetal activity.
a. In the first trimester the rates tend to be higher, ranging from 140 to 180 bpm.
b. In bradydysrhythmia the fetal heart rate is less than 120 bpm. It may be sinus bradycardia resulting from abnormally slow atrial pacemaker activity with a normal 1:1 AV conduction, or it may be due to various forms of block at the AV junction.
2. Morphologically, CHB can result from a lack of fusion between nodal tissue and the His bundle, which develops separately, or it can result from secondary interruption of the AV conduction axis; evidence has been presented for both views. From the clinical point of view, CCHB may be found as an isolated lesion in a fetus with a structurally normal heart (as in the case of maternal connective tissue disease), or it may be associated with structural or myocardial heart diseases (as in LAI or congenitally corrected transposition). Both entities have a different etiology and natural history that affect the management approach and outcome.
3. A fetus that has a structurally normal heart can develop CHB from anti-Ro and anti-La.
a. Pathologic studies suggest that these antibodies cross the placental barrier to damage the fetal conductive system by fibrofatty replacement of the atrial connections to the AV node after about 16 weeks of gestation.
b. These antibodies also cause an inflammatory myocarditis and fibrosis of the conducting tissue in some cases.
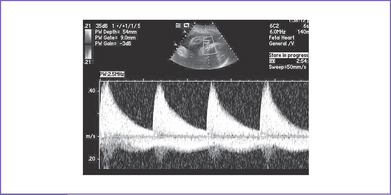
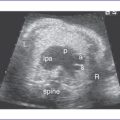
c. CHB can be diagnosed early in the second trimester with Doppler echocardiography (Fig. 23-6).
4. In LAI, histology has revealed discontinuity between the AV node and the ventricular conducting tissue. This is thought to be due to an abnormal development of the central fibrous body through which the penetrating bundle of His passes, which is then surgically vulnerable.
5. CCHB in congenitally corrected transposition can occur prenatally but is often not present at birth and increases in incidence with age, being common after surgical intervention. This is thought to be related to an abnormal length and location of the His bundle.
6. The diastolic ventricular function in the fetal heart depends to a large degree on the atrial contraction.
a. A normal AV activation sequence during the cardiac cycle is particularly important for the fetal circulation.
b. Sustained fetal bradydysrhythmia with a very slow ventricular rate of less than 50 bpm and complete dissociation of atrial and ventricular contractions due to complete AV block is commonly not well tolerated by the fetus.
7. A slow ventricular escape rate of less than 55 bpm is another poor prognostic factor in isolated CCHB.
I. Permanent pacing in children
1. Permanent pacing is indicated in children who have:
a. Symptomatic sinus bradycardia.
b. Recurrent bradycardia–tachycardia syndromes.
d. Advanced second- or third-degree AV block, either surgical or acquired.
2. There are several important additional considerations in young patients.
a. An increasing number of patients are surviving complex surgical procedures for congenital heart disease that result in palliation rather than correction of circulatory physiology.
b. The residua of impaired ventricular function and abnormal physiology can result in symptomatic bradycardia at rates that do not produce symptoms in persons with normal cardiovascular physiology.
3. The indications for pacemaker implantation in these patients need to be based on correlation of symptoms with relative bradycardia rather than absolute heart rate criteria.
4. The clinical significance of bradycardia depends on age. A heart rate of 45 bpm may be a normal finding in an adolescent, whereas the same rate in a newborn or infant indicates profound bradycardia.
III. TAKE-HOME MESSAGE
B. Treatment
1. In isolated CCHB with proven maternal autoantibodies, treatment with fluorinated glucocorticoids that cross the placenta has been used with variable success.
2. Treatment of the hydropic fetus in utero with digoxin (controversial), steroids, β-sympathomimetic agents, and drainage of fluids has some role in allowing pregnancy to continue and perhaps prevent extreme prematurity with attendant morbidity and mortality.
3. Experimental treatment with prenatal pacing has been applied and might prove to be the best therapy in fetuses with isolated CCHB.
C. Prognosis
1. The prenatal and perinatal course as well as the outcome at the end of the neonatal period with CCHB depend on the cardiac output and the presence of heart failure.
2. Fetal bradydysrhythmia may be a benign condition involving short episodes of sinus bradycardia or bigeminal blocked premature atrial contractions that have little or no consequence for treatment or further management (Fig. 23-7).
3. Of the bradydysrhythmias, CCHB can compromise the fetus to the greatest extent.
4. The prognosis for such a fetus depends on the associated structural or myocardial heart disease, and the degree of CHF can be assessed with the cardiovascular profile score rather than the presence or absence of hydrops alone. The onset of hydrops in a fetus with CCHB and severe bradycardia and structural heart disease before 24 weeks’ gestation is an ominous sign, and termination of pregnancy could be considered.
5. In isolated CCHB there is a significant incidence of fetal or early neonatal death if fetal hydrops develops or if the ventricular heart rate is less than 50 to 55 bpm.
Anderson RH, Wenick AC, Losekoot TG, Becker AE. Congenitally complete heart block. Developmental aspects. Circulation. 1977;56(1):90-101.
Assad RS, Zielinsky P, Kalil R, et al. New lead for in utero pacing for fetal congenital heart block. J Thorac Cardiovasc Surg. 2003;126(1):300-302.
Carpenter RJJr, Strasburger JF, Garson AJr, et al. Fetal ventricular pacing for hydrops secondary to complete atrioventricular block. J Am Coll Cardiol. 1986;8(6):1434-1436.
Copel JA, Buyon JP, Kleinman CS. Successful in utero therapy of fetal heart block. Am J Obstet Gynecol. 1995;173(5):1384-1390.
Jaeggi ET, Fouron JC, Silverman ED, et al. Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation. 2004;110(12):1542-1548.
Jaeggi ET, Hamilton RM, Silverman ED, et al. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. A single institution’s experience of 30 years. Am Coll Cardiol. 2002;39(1):130-137.
James TN. Cardiac conduction system: Fetal and postnatal development. Am J Cardiol. 1970;25(2):213-226.
Julkunen H, Kaaja R, Siren MK, et al. Immune-mediated congenital heart block (CHB): identifying and counseling patients at risk for having children with CHB. Semin Arthritis Rheum. 1998;28(2):97-106.
Machado MV, Tynan MJ, Curry PV, Allan LD. Fetal complete heart block. Br Heart J. 1988;60(6):512-515.
Michaelsson M, Engle MA. Congenital complete heart block: An international study of the natural history. Cardiovasc Clin. 1972;4(3):85-101.
Moak JP, Barron KS, Hougen TJ, et al. Congenital heart block: Development of late-onset cardiomyopathy, a previously underappreciated sequela. J Am Coll Cardiol. 2001;37(1):238-242.
Ramsey-Goldman R, Hom D, Deng JS, et al. Anti-SS-A antibodies and fetal outcome in maternal systemic lupus erythematosus. Arthritis Rheum. 1986;29(10):1269-1273.
Rein AJ, O’Donnell C, Geva T, et al. Use of tissue velocity imaging in the diagnosis of fetal cardiac arrhythmias. Circulation. 2002;106(14):1827-1833.
Saleeb S, Copel J, Friedman D, Buyon JP. Comparison of treatment with fluorinated glucocorticoids to the natural history of autoantibody-associated congenital heart block: Retrospective review of the research registry for neonatal lupus. Arthritis Rheum. 1999;42(11):2335-2345.
Schmidt KG, Ulmer HE, Silverman NH, et al. Perinatal outcome of fetal complete atrioventricular block: A multicenter experience. J Am Coll Cardiol. 1991;17(6):1360-1366.
Scott JS, Maddison PJ, Taylor PV, et al. Connective-tissue disease, antibodies to ribonucleoprotein, and congenital heart block. N Engl J Med. 1983;309(4):209-212.